Abstract
Sialyl Lewis antigens, sialyl Lewis a and sialyl Lewis x, are utilized as tumor markers, and their increase in cancer is associated with tumor progression by enhancement of cancer cell adhesion to endothelial E-selectin. However, regulation mechanisms are not fully understood. We previously demonstrated that NEU4 is the only sialidase efficiently acting on mucins and it is down-regulated in colon cancer. To elucidate the significance of NEU4 down-regulation, we investigated sialyl Lewis antigens as endogenous substrates for the sialidase. NEU4 was found to hydrolyze the antigens in vitro and decrease cell surface levels much more effectively than other sialidases. Western blot, thin layer chromatography, and metabolic inhibition studies of desialylation products revealed NEU4 to preferentially catalyze sialyl Lewis antigens expressed on O-glycans. Cell adhesion to and motility and growth on E-selectin were significantly reduced by NEU4. E-selectin stimulation of colon cancer cells enhanced cell motility through activation of the p38/Hsp27/actin reorganization pathway, whereas NEU4 attenuated the signaling. On immunocytochemical analysis, some NEU4 molecules were localized at cell surfaces. Under hypoxia conditions whereby the antigens were increased concomitantly with several sialyl- and fucosyltransferases, NEU4 expression was markedly decreased. These results suggest that NEU4 plays an important role in control of sialyl Lewis antigen expression and its impairment in colon cancer.
Keywords: Carbohydrate Complex, Cell Adhesion, Cell Migration, Colon Cancer, Glycosylation, Tumor Marker, Sialic Acid, Sialidase, Sialyl Lewis a, Sialyl Lewis x
Introduction
Aberrant glycosylation is a characteristic feature of cancer cells, and especially alteration in sialylation during malignant transformation has been proposed to be associated with the malignant phenotype, including metastatic potential and invasiveness (1–3). Sialic acids, in fact, often include tumor-specific carbohydrates as tumor markers. In particular, sialyl Lewis a (sialyl-Lea)2 and sialyl Lewis x (sialyl-Lex), known, respectively, as clinical markers CA19-9 and NCC-ST-439, are utilized because they are useful for monitoring recurrence after surgery and predicting the prognosis (4–6). Expression of Lewis antigens is increased significantly in cancer compared with non-malignant epithelial cells, and induction has been suggested to be attributable to specific alteration of transcription of genes for several glycosyltransferases and sugar transporters, which are induced by hypoxia-inducible factor, hypoxia-induced factor, and hypoxic culture conditions (7, 8). In normal colon mucosa, the expression of sialyl-Lea and sialyl-Lex is quite low, whereas more complicated carbohydrates, such as disialyl-Lea and sialyl-6-sulfo-Lex, are major components (9, 10). Sialyl-Lea and sialyl-Lex play an important role, as carbohydrate ligands for E-selectin, in E-selectin-mediated cancer cell adhesion to vascular endothelial cells during the course of hematogenous metastasis (11), through activation of the SAPK/p38 signaling pathway (12, 13). On the other hand, disialyl-Lea, sialyl-6-sulfo-Lex, Lea, and Lex do not bind to E-selectin. Control of sialyl-Lea and sialyl-Lex expression may therefore have important implications for the development of anti-metastatic therapy.
To understand the causes of aberrant sialylation and the consequences for cancer, we have focused on mammalian sialidases, which control cellular sialic acid contents in collaboration with sialyltransferases. Human sialidases are classified into four groups according to their enzymatic properties and subcellular localization (abbreviated to NEU1, NEU2, NEU3, and NEU4), and their expression levels indeed change in response to various cellular phenomena and especially in relation to cancer phenotype (14, 15). We previously demonstrated that NEU1 shows down-regulation in cancers, promoting anchorage-independent growth and contributing to metastatic ability (16), whereas NEU3 exhibits marked up-regulation, playing as an essential factor for cancer cell survival (17, 18). Among them, NEU4 is a unique sialidase efficiently removing sialic acid residues from mucins. It possesses two splicing isoforms, NEU4L and NEU4S, differing in the existence of 12 N-terminal amino acids (19). NEU4L is dominantly expressed in brain, whereas NEU4S levels are relatively high in liver, kidney, and colon mucosa. NEU4S can be recovered in membrane fractions, and recently was suggested to be present in endoplasmic reticulum (20), whereas NEU4L is likely to be localized in mitochondria (19, 20). Although the functions of NEU4 remain largely unclear, the murine form seems to be involved in neuronal differentiation (21).
We previously showed that NEU4S is down-regulated in colon cancer as compared with normal mucosa and that the expression ratio of tumor to non-tumor in patient samples is significantly linked with venous invasion (22) as an indicator of the development of hematogenous metastasis (23). To elucidate the significance of NEU4S down-regulation and its close relationship with venous invasion in colon cancer, we investigated whether the sialidase might influence expression of sialyl-Lea and sialyl-Lex that is associated with tumor progression by enhancement of cancer cell adhesion. Here we found that NEU4S efficiently cleaves sialic acids from these carbohydrates, leading to alteration of cancer cell phenotypes. The present results suggest an essential biological role of NEU4S in homeostasis of colon mucosa through regulating sialyl Lewis antigen expression and the functional loss by its down-regulation in the cancer.
EXPERIMENTAL PROCEDURES
Cells, Transfection, and Sialidase Activity
Human colon cancer HT29 cells (ATCC), DLD-1 cells (Health Science Research Sources Bank, Osaka, Japan), human hepatoma HepG2 cells (Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer of Tohoku University), and human embryonic kidney HEK293T cells (a gift from M. Sugai, Kyoto University School of Medicine, Japan) were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (FBS). The ORF of human NEU4S tagged with HA at the C-terminal was subcloned into pcDNA 3.1 (Invitrogen), and a pCAGGS expression plasmid (a generous gift from Dr. Miyazaki, Osaka University School of Medicine), and then transfected with Lipofectamine 2000 reagent (Invitrogen). Expression vectors for NEU1, NEU2, and NEU3 were prepared as previously reported (24), and for NEU1 expression, the plasmid for the protective protein (carboxypeptidase A) pp11 gene was co-transfected (16). To obtain stable transfectants, G418 (Sigma) was used with HT29 cells at 600 μg/ml and with DLD-1 at 300 μg/ml. For NEU4 gene knockdown, NEU4 siRNA (22) was transfected with Lipofectamine RNAiMAX (Invitrogen). For sialidase activity assays, homogenates were prepared in PBS buffer containing leupeptin, pepstatin, PMSF, and 1 mm EDTA using transfected cells as the enzyme source. In some experiments, HEK293T cells were transfected with the respective sialidase cDNAs and their homogenates were used (24). Sialidase activity was measured with 4-methylumbelliferyl-neuraminic acid (4MU-NeuAc), GM3, sialyl-Lea, and sialyl-Lex (6 sugars, ganglioside-type, Wako Pure Industries, Osaka, Japan) as substrates. Released 4-methylumbelliferylone (4MU) and sialic acids were measured by fluorescence spectrophotometry and HPLC, respectively (25). Protein concentrations were measured by dye-binding assay (Bio-Rad). One unit (U) was defined as the release of 1 nmol of sialic acid over 1 h.
Cell Treatments
To characterize glycans bearing sialyl-Lea and sialyl-Lex epitopes, cells were cultured for 72 h at 37 °C in medium containing 2 mm benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside (benzyl-GalNAc) to inhibit O-linked glycosylation (26) or 10 μg/ml of castanospermine to inhibit N-linked processing of glycoproteins (27). For culture under hypoxia conditions, cells were treated for 72 h in the presence of hypoxia mimetic, 100 μm deferoxamine (Sigma).
Flow Cytometric Analysis
Cell surface sialyl-Lea, sialyl-Lex, Lea, Lex, and disialyl-Lea were determined by flow cytometric analysis as described previously (28). Briefly, detached cells were incubated with the respective antibodies or control IgG on ice for 45 min, and then incubated with FITC-conjugated anti-mouse IgG/M antibodies (Dako). Washed cells were analyzed using a flow cytometer (BD Bioscience). The antibodies for sialyl-Lea (1H4, 7LE), sialyl-Lex (2H5), Lea (2–25LE), and Lex (H18A) were from Seikagaku Biobusiness (Tokyo, Japan), and those for sialyl-Lex (CSLEX and HECA-452) were from BD Pharmingen and Biolegend, respectively, and for disialyl-Lea (FH7) from Chemicon.
Quantitative Reverse Transcription-PCR Analysis
Quantitative analysis of endogenous mRNA levels of NEU4, β-galactoside α-2,3-sialyltransferase 1 (ST3 Gal I), β-galactoside α-2,3-sialyltransferase 3 (ST3 Gal III), ST3 β-galactoside α-2,3-sialyltransferase 4 (ST3 Gal IV), fucosyltransferase 3 (FUT3), and fucosyltransferase 7 (FUT7) were performed by real time PCR using a Light Cycler rapid thermal cycler system (Roche Applied Science) as described previously (29). NEU4 primers were designed as detailed in a previous report (22). Primers were as follows: ST3 Gal I, sense (5′-ATGAGGTGGACTTGTACGGC-3′) and antisense (5′-AACGGCTCCAGCAAGATG-3′); ST3 Gal III, sense (5′-TGAGACTGAATTCAGCACCAG-3′) and antisense (5′-TCAGATGCCACTGCTTAGATC-3′); ST3 GalIV, sense (5′-ACACACTCCTCGTCCTGGTAGCT-3′) and antisense (5′-CTACAGCTCTTGCCCAGGTCAGAA-3′); FUT3, sense (5′-GCCGACCGCAAGGTGTAC-3′) and antisense (5′-TGACTTAGGGTTGGACATGATATCC-3′); FUT7, sense (5′-CTCGGACATCTTTGTGCCCTATG-3′) and antisense (5′-CGCCAGAATTTCTCCGTAATGTAG). GAPDH was used as an internal control to correct for expression between samples.
Immunoblotting
Cells were lysed with modified RIPA buffer (50 mm Hepes, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 2 mm EDTA, 7.5 μg/ml of aprotinin, 10 μg/ml of leupeptin, 10 mm NaF, 2 mm orthovanadate, 0.25% sodium deoxycholate, and 2 mm PMSF). The lysates were clarified by centrifugation at 10,000 × g for 10 min and then resolved on SDS-PAGE. After transfer to polyvinylidene difluoride membranes, blocking with 1% BSA in TBST, and incubation with primary antibodies, binding was visualized using the appropriate peroxidase-coupled secondary antibodies with ECL detection (Amersham Biosciences). Antibodies for anti-phospho-p38 (Thr180/Tyr182) and anti-p38, were from Cell Signaling Technology. Anti-phospho-Hsp27 (Ser78) and anti-Hsp27 antibodies were from Stressgen and Santa Cruz Biotechnology, respectively. Recombinant human E-selectin/Fc chimera was a product of R&D Systems. Densitometric analyses were carried out with Quantity One one-dimensional analysis software (Bio-Rad).
Thin Layer Chromatography (TLC) Immunostaining
Glycolipids were extracted from cells and fractionated by thin layer chromatography on HPTLC plates (Baker, Phillipsburg, NJ) in chloroform/methanol/H2O (60:35:8, v/v/v), as described previously. For detection of sialyl Lewis antigens, immunostaining of thin layer chromatography was performed using the respective antibodies with an avidin-biotin immunoperoxidase staining kit (Vector).
Indirect Immunofluorescence Microscopy
Cells grown on glass coverslips were fixed with 4% paraformaldehyde for 15 min and then incubated with or without 0.1% Triton X-100. After blocking with 1% BSA, cells were incubated with anti-HA (Roche Applied Science) and anti-sialyl-Lea antibody for 1 h, followed by incubation with Alexa 488 anti-rat IgG and 594 anti-mouse IgG. FITC-phalloidin (Sigma) was used to visualize F-actin. Preparations were examined by confocal microscopy (LSM5, Carl Zeiss, Germany).
Cell Surface Biotinylation
Cell surface biotinylation was carried out as described previously (16). Cell surface proteins were labeled with sulfo-NHS-LC-Biotin (Pierce) according to the manufacturer's instructions. After quenching the biotinylation with glycin/PBS, cells were lysed with RIPA buffer followed by separation of biotinylated proteins with streptavidin-agarose resin. Collected proteins were analyzed by immunoblotting with antibodies for HA, Met (as a control of cell surface proteins), and caveolin-1 (as a control for intracellular proteins).
E-selectin Stimulation
Cells were maintained under serum-depleted conditions for 16 h before stimulation. E-selectin/Fc chimera was adjusted to 1 μg/ml with medium and then supplemented to culture dishes. At the indicated time points, cells were collected, and signaling alterations were analyzed by Western blotting.
Cell Motility Assays
Cell motility assays were carried out using non-coated cell culture inserts (Falcon) (30). Cells were seeded at 2.5 × 105 cells/well onto the upper chamber, and the lower chamber was filled with medium. After 24 h cells were fixed and stained with Wright-Giemsa solution and the migrated cells on the lower membrane surface were counted under a microscope. E-selectin/Fc chimera (1 μg/ml) was added to the upper chamber. To confirm the specificity of effects, cells were incubated with anti-sialyl-Lea and sialyl-Lex antibodies (5 μg/ml each) for 30 min on ice before seeding.
Cell Adhesion and Cell Proliferation
Cell adhesion and growth were analyzed as described previously (31). Briefly, 96-well plates were coated with 1 μg/ml of recombinant E-selectin/Fc chimera, and then blocked with 1% BSA in PBS. Cell suspensions were then applied to these coated 96-well plates. For cell adhesion, after incubation for 30 min and fixation, attached cells were stained with 0.1% crystal violet for 20 min, and incorporated dye was eluted with 10% acetic acid, then measured spectrophotometrically at A550. Cell growth in vitro was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. After 0–120 h, formazan produced by live cells was measured at absorbance 450 nm (reference 630 nm). To analyze cell growth in vivo, DLD-1 cells (5.0 × 106) were injected subcutaneously into the lower flanks of 6-week-old scid mice obtained from CLEA Japan, Inc. (Tokyo). Tumor diameters were measured at maximum length and width with digital calipers, and the tumor volume was calculated from the equation: volume = (width)2 × length/2.
Statistical Analysis
Results are expressed as mean ± S.D. The differences between the data from the experimental groups were analyzed for statistical significance by Student's or Welch's t tests.
RESULTS
NEU4 Down-regulates Cell Surface Sialyl-Lea and Sialyl-Lex
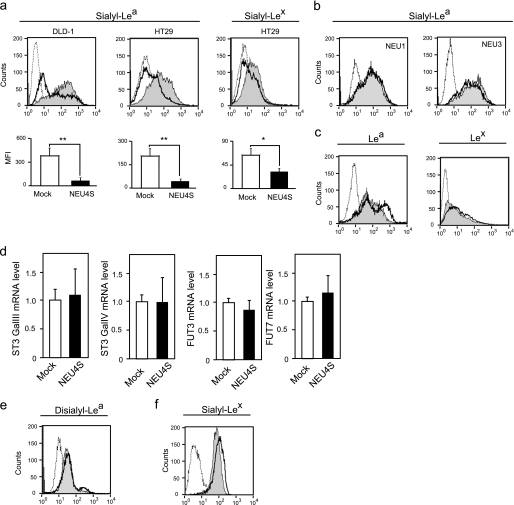
To examine the possibility of an involvement of the sialidase(s) in the expression of sialyl-Lea and sialyl-Lex, sialidase genes were transfected into DLD-1 expressing sialyl-Lea and into HT29 possessing both sialyl-Lea and sialyl-Lex (32, 33). Because we previously found that expression of NEU2 and NEU4L was hardly detectable in colon mucosa and cancer (22, 24), NEU1, NEU3, or NEU4S genes were introduced. First, we observed NEU4S effects on the sialyl-Lea and sialyl-Lex expression, because of its broad substrate specificity (19). Stable transfectants of NEU4S were obtained in DLD-1 and HT29 cells with G418 selection, and their sialidase activities were increased toward 4MU-NeuAc in DLD-1 cells (7.1 ± 1.0 units/mg in Mock, 16.1 ± 1.2 units/mg in NEU4S#1, 32.2 ± 3.0 units/mg in NEU4S#2) and in HT29 cells (9.9 ± 0.3 units/mg in Mock, 19.9 ± 2.2 units/mg in NEU4S#11, 36.0 ± 4.0 units/mg in NEU4S#12). Evaluation of cell surface sialyl-Lea and sialyl-Lex levels was carried out by flow cytometric analysis. As shown in Fig. 1a, sialyl-Lea and sialyl-Lex levels were significantly down-regulated in the transfectants. NEU4S decreased sialyl-Lea by 86% in DLD-1 (NEU4S#1), and sialyl-Lea by 80% and sialyl-Lex by 50% in HT29 cells (NEU4S#11) as compared with mock cells. Similar results were obtained in the other transfectants in three experiments (lower panel in Fig. 1a). However, unlike NEU4S, NEU1 hardly exerted any effects and only a slight change was noted with NEU3 (Fig. 1b), even they showed a 3–5-fold increase in sialidase activity. We used two different antibodies for the estimation of sialyl-Lea and three antibodies for sialyl-Lex, and obtained essentially similar results. When cell surface Lea and Lex were measured in NEU4S overexpressing cells, a slight but significant increase was observed (Fig. 1c), possibly produced by desialylation of sialyl-Lea or sialyl-Lex. To verify whether the levels of biosynthetic enzymes were changed by NEU4S overexpression, expression of ST3 Gal III, ST3 Gal IV, FUT3, and FUT7 were estimated by quantitative PCR. NEU4S transfection was without apparent influence (Fig. 1d), suggesting that NEU4S decreased sialyl-Lea and sialyl-Lex without affecting the expression of sialyl Lewis antigen synthetic enzymes.
FIGURE 1.
Down-regulation of sialyl-Lea and sialyl-Lex in DLD-1 and HT29 colon cancer cells by NEU4S overexpression. a, flow cytometric analysis of cell surface sialyl-Lea in DLD-1 (left), HT29 (middle) cells, and sialyl-Lex in HT29 (right) (dotted line, mouse IgG; line filled with gray, Mock; black line, NEU4S). The data are mean ± S.D. from three experiments (*, p < 0.05; **, p < 0.01). b, effects of NEU1 (left) and NEU3 (right) overexpression on cell surface sialyl-Lea in HT29 cells (dotted line, mouse IgG; line filled with gray, Mock; black line, NEU1 or NEU3). c, altered expression of Lea (left panel in DLD-1) and Lex (right panel in HT29) by NEU4S (dotted line, mouse IgG; line filled gray, Mock; black line, NEU4S). d, relative expression of ST3 GalIII, ST3 Gal IV, FUT3, and FUT7 in NEU4-overexpressing HT29 cells. mRNA levels were assessed by real time PCR. e, no significant change in disialyl-Lea expression by NEU4S (dotted line, mouse IgG; line filled gray, Mock; black line, NEU4S). f, up-regulation of sialyl-Lex in HepG2 cells by NEU4S knockdown (dotted line, mouse IgG; line filled gray, scramble control; black line, NEU4S RNAi).
As mentioned earlier, NEU4S is relatively rich in non-cancerous mucosa, where the expression of sialyl-Lea and sialyl-Lex is quite low and disialyl-Lea is preferentially expressed. In this context, we examined whether NEU4S altered disialyl-Lea levels in DLD1 cells expressing the disialyl-Lea (10). Interestingly, the sialidase did not change the level of this normal glycan generally expressed in non-malignant epithelial cells (Fig. 1e). It is feasible that desialylation by NEU4S may occur specifically with cancer-related sialyl Lewis antigens and thus maintenance of the normal glycan level can be achieved in colon mucosa even highly expressing NEU4. To confirm NEU4 function for the desialylation of sialyl Lewis antigens, endogenous NEU4 was knocked down with the specific siRNA for NEU4 and alteration of the sialyl-Lex expression was assessed. However, due to the extremely low expression of NEU4 in cancer cells (22), the knockdown showed no apparent change in sialyl-Lea and sialyl-Lex in DLD-1 and HT29 cells (data not shown). We then employed human hepatoma HepG2 cells, possessing sialyl-Lex on the cell surface (34), because the endogenous NEU4 mRNA level is far higher than in DLD-1 and HT29 cells. At 120 h after treatment with siRNA for NEU4, cell surface sialyl-Lex was slightly but significantly increased by 90% knockdown (Fig. 1f). These results indicate that NEU4S regulates the expression of sialyl-Lea and sialyl-Lex, but not disialyl-Lea, in colon mucosa.
Sialyl-Lea and Sialyl-Lex Are Substrates for NEU4S
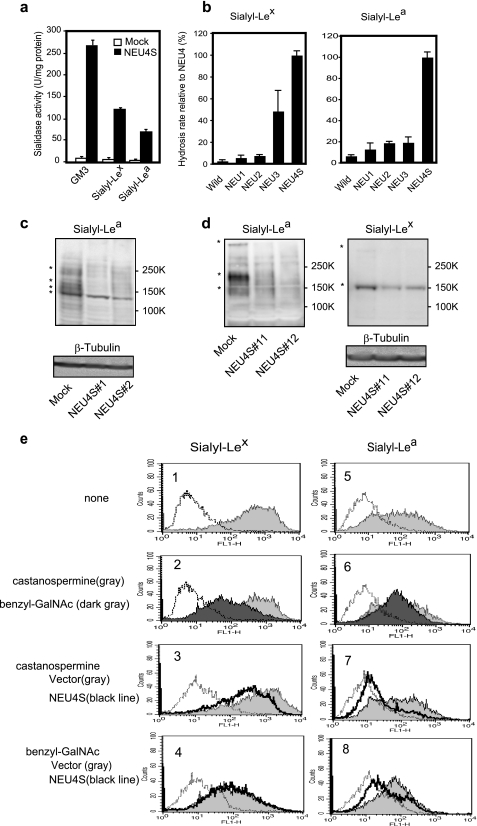
As NEU4S was found to reduce the expression of sialyl-Lea and sialyl-Lex in colon cancer cells, we next examined whether sialyl-Lea and sialyl-Lex could be substrates for NEU4S. Using synthesized sialyl-Lea and sialyl-Lex, homogenates of sialidase gene-transfected cells were incubated in the reaction mixture for the sialidase activity assay. NEU4S effectively hydrolyzed these carbohydrates to an extent comparable with GM3, a good substrate for NEU4 (Fig. 2a). Consistent with the results obtained above by flow cytometric analysis, three sialidases other than NEU4S only poorly catalyzed the removal of sialic acids from sialyl-Lex and sialyl-Lea with and without detergents including Triton X-100 and sodium cholate, except NEU3 showing a considerable activity toward sialyl-Lex (Fig. 2b). To obtain further information about endogenous substrates bearing the cancer-associated carbohydrates, lysates from NEU4S-transfected cells were analyzed by immunoblotting and thin layer chromatography, because our previous studies demonstrated removal of sialic acids from both lipid- and protein-bound carbohydrates by NEU4S (19). The levels of protein-bound sialyl-Lea and sialyl-Lex were first compared between the mock and NEU4S transfectants by immunoblotting with the respective antibodies, because NEU4 can efficiently hydrolyze mucin-type glycoprotein in vitro (19, 35). As shown in Fig. 2, c and d, the levels of sialyl-Lea and sialyl-Lex on several glycoproteins were reduced by NEU4S overexpression in DLD-1 and HT29 cells. In DLD-1 cell lysates, glycoproteins of ∼140, 160, and 200 kDa bearing sialyl-Lea were reduced by NEU4S (Fig. 2c). In HT29 cells, NEU4S decreased the levels of protein-bound sialyl-Lea 140, 200, and over 300 kDa, and also the sialyl-Lex on glycoproteins by nearly 150 kDa (Fig. 2d). We used another antibody for sialyl-Lex (HECA-452) and found that about 100, 140, 160, 180, and over 300 kDa proteins were also decreased (data not shown). These results showed that the several glycoproteins are the targets for NEU4S, suggesting that desialylation of cancer-associated glycans by the sialidase might cause functional changes accompanying cancer progression. As it was reported that these E-selectin ligands are carried by CD44, especially CD44v (36) and Mac1 (37), we attempted to identify the glycoproteins observed above by immunoprecipitation using antibodies for CD44S (2C5, R&D Systems), CD44v6 (2F10, R&D Systems), Mac1 (VU4H5, Santa Cruz), Mac3 (M3.1, Abcam), Mac4 (1G8, Zymed Laboratories Inc.), and Mac5AC (CLH2, Chemicon), followed by immunoblotting with antibodies for sialyl-Lea and sialyl-Lex. However, the proteins immunoprecipitated from the cell lysates by the respective antibodies for CD44 and Mac did not correspond exactly to the sialyl Lewis antigens. We then characterized whether the sialyl Lewis antigen structures presented on N-linked and/or O-linked glycans. Cells were cultured in the presence of benzyl-GalNAc or castanospermine to inhibit synthesis of O-linked or N-linked glycans, respectively. In Fig. 2e, in control cells, benzyl-GalNAc diminished the expression levels of both sialyl-Lex and sialyl-Lea at cell surfaces, whereas castanospermine hardly changed either. Consistent with these results, the NEU4S-mediated decrease in the glycan expression, especially in sialyl-Lex, was abrogated profoundly when benzyl-GalNAc was present, but castanospermine did not block the reduction by the sialidase. These data indicate sialyl Lewis structures hydrolyzed by NEU4S to be mostly expressed on O-glycans. Regarding lipid-bound sialyl-Lea, DLD-1 cells exhibited no detectable change by NEU4S as assessed by TLC immunostaining. Even endogenous sialyl-Lea failed to be detected in glycolipids from DLD-1 cells, possibly due to low expression (data not shown).
FIGURE 2.
Desialylation of sialyl-Lea and sialyl-Lex by NEU4 and characterization of these glycans. Substrate specificity of NEU4S toward GM3, sialyl-Lex and sialyl-Lea in vitro (a). Different substrate specificity of sialidases NEU1, NEU2, NEU3, and NEU4S toward sialyl-Lex (b, left panel) and sialyl-Lea (b, right). Homogenates from HEK293T cells transfected with the respective sialidase cDNAs were used as enzyme sources, and essentially equal amounts for each sialidase were applied. According to their known substrate specificity, the activity levels were determined in advance with 4MU-NeuAc for NEU1, NEU2, NEU4 and with GM3 for NEU3 and NEU4 as substrates with Triton X-100 (0.1%) or sodium cholate (0.05%). Released sialic acids were estimated by HPLC as described under “Experimental Procedures.” Immunoblot analysis of sialyl-Lea in DLD-1 (c), HT29 (d, left panel) cells, and sialyl-Lex in HT29 cells (d, right). Equal protein loading was confirmed by immunoblots of β-tubulin. Marked decrease in sialyl Lewis antigens is shown with an asterisk. To characterize glycans bearing sialyl Lewis antigens, cells were cultured in the presence of benzyl-GalNAc and castanospermine to inhibit synthesis of O-linked or N-linked glycans, respectively. In both DLD-1 (e, right panel) and HT29 colon cancer cells (e, left panel), treatment with benzyl-GalNAc diminished most of the expression level of both sialyl-Lex (dark gray in 2) and sialyl-Lea (dark gray in 6) at cell surfaces as compared with non-treated cells (gray in 1 and 5), whereas castanospermine (gray in 2 and 6) hardly changed either. The NEU4S-mediated decrease in the glycan expression, especially in sialyl-Lex, was abrogated by treatment with benzyl-GalNAc (black lines in 4 and 8) but castanospermine did not block the reduction by the sialidase (black lines in 3 and 7).
NEU4S Suppresses Cell Attachment to and Cell Growth on E-selectin
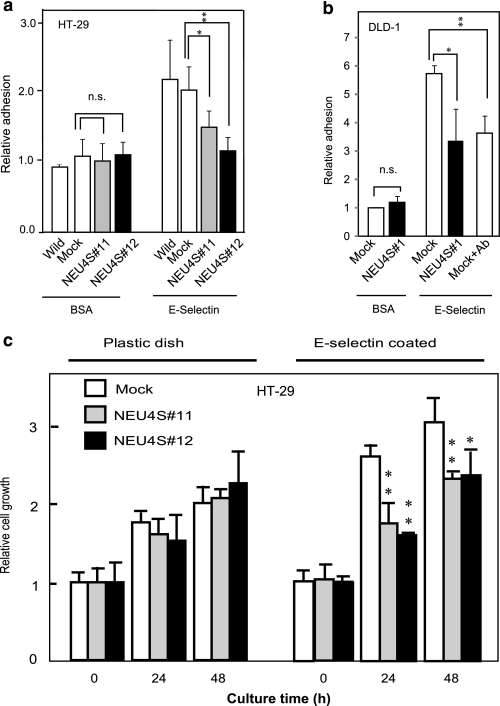
Interaction of tumor cell surface sialyl-Lex and sialyl-Lex with endothelium E-selectin is a major component of cancer invasion and metastasis. To explore the physiological significance of the decreased sialyl Lewis antigens by NEU4S, cell adhesion to E-selectin was examined. NEU4S showed a significant decrease in HT29 cell attachment onto E-selectin-coated plates, whereas there was no significant change on BSA-coated plates with mock and NEU4S transfectants (Fig. 3a). NEU4S also suppressed cell attachment to E-selectin in NEU4S-transfected DLD-1 cells (Fig. 3b). To confirm whether these alterations are caused by hydrolysis of sialyl-Lea and sialyl-Lex, cells were pre-treated with anti-sialyl-Lea and/or anti-sialyl-Lex antibodies before attachment (33). Antibody treatment suppressed cell attachment to E-selectin in both cells, indicating that sialyl Lewis antigen-mediated cell attachment was negatively modified by NEU4S. Next, the cell growth rate on E-selectin-coated dishes was assessed in HT29 cells, as Yusa et al. (38) reported that induction of sialyl-Lex accelerates proliferation of colon cancer cells. Significant inhibition was observed with a concomitant decrease of sialyl-Lex in NEU4S-transfected cells as compared with that in the mock cells (Fig. 3c).
FIGURE 3.
Effect of NEU4S on cell adhesion to and proliferation on E-selectin. HT29 (a) and DLD-1 (b) cell adhesion on BSA and E-selectin-coated plates. Open bars, wild and mock; gray and black bars, NEU4S transfectants. As a positive control, cells were preincubated with anti-sialyl-Lea antibody for 30 min, and then seeded. c, growth of HT29 cells cultured on E-selectin (5 μg/ml) or non-treated plastic dishes assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The curves are compared for mock and NEU4S (#11 and #12) transfectants. *, p < 0.05; **, p < 0.01. n.s., not significant.
To examine growth effects of the sialidase in vivo, NEU4S-transfected DLD-1 cells (NEU4S#1 and NEU4S#2, 5 × 106 each) were implanted subcutaneously into scid mice (four mice for each group). Surprisingly, NEU4S overexpression showed almost complete inhibition of tumor growth in both transfectants even after 6 weeks of inoculation, whereas the wild type and mock transfectants produced a considerable volume of tumors that continued to increase in size (3867 ± 1115 and 4786 ± 1428 mm3, respectively). These results suggest a strong growth inhibitory effect of NEU4S in vivo, although it may not be a direct E-selectin-mediated event but possibly a result of acceleration of apoptosis as described in our previous report (22). Observations on in vivo liver metastasis by injecting the cancer cells transsplenically into mice would give further insight into a possible role of the sialidase in the development of hematogenous metastasis.
NEU4S Suppresses E-selectin-induced Cell Motility through Action on the p38/Hsp27 Pathway
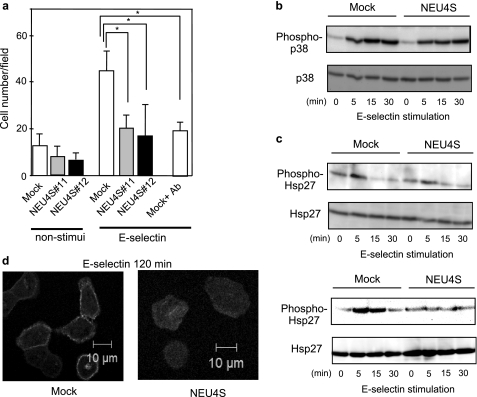
Previous observations have showed that sialyl-Lea and sialyl-Lex regulate cell motility (39). To test whether NEU4 affects cell migration through desialylation of sialyl-Lea and sialyl-Lex, cell motility assays were performed in the cells stimulated with E-selectin using a Boyden chamber. Because E-selectin is known to work not as a chemoattractant (39), alteration of cell motility could be regulated only by E-selectin and sialyl-Lea and sialyl-Lex binding. Under conditions of no stimulation, both mock and NEU4S-transfected cells migrated, and there was no significant difference between the two cases, as in previous reports (22). On the other hand, E-selectin stimulation only markedly provoked motility in mock cells (Fig. 4a), but NEU4S showed little effect on E-selectin-stimulated cell migration. These results indicate that decrease of sialyl-lea and sialyl-Lex by NEU4S led to suppression of E-selectin-mediated cell motility. To clarify details with reference to signal transduction, we focused attention on the p38/Hsp27 pathway. It is known that binding of sialyl-Lea and sialyl-Lex to E-selectin activates p38 signaling in cancer cells (12, 39), resulting in stimulation of phosphorylation of various transcription factors and MAPKAP kinase 2, with subsequent activation of MAPKAP kinase 2 and Hsp27, the actin polymerizing factor (40). To investigate the influence of NEU4S on cell motility, HT29 cells were stimulated with E-selectin and the phosphorylation levels were assessed. The stimulation caused phosphorylation of p38 after 5–10 min, whereas NEU4S suppressed this E-selectin-induced p38 phosphorylation (Fig. 4b). Moreover, Hsp27 phosphorylation was also suppressed by NEU4S in HT29 cells (Fig. 4c). Similar phenomena were also observed in DLD-1 cells. To assess actin reorganization, fluorescence microscopy was conducted with FITC-phalloidin. At 120 min after E-selectin stimulation, F-actin accumulated at the cell periphery in mock cells (Fig. 4d) but NEU4S hardly exerted any influence, suggesting that the decrease of sialyl-Lea and sialyl-Lex caused by NEU4S leads to suppression of signaling followed by decreased cell motility.
FIGURE 4.
Effects of NEU4S on cell motility and p38/Hsp27 signaling induced by E-selectin stimulation. a, alteration of cell motility in HT29 cells, with or without E-selectin stimulation. Open bars, mock; gray and black bars, NEU4S transfectants. As a positive control, cells were preincubated with antibodies for sialyl-Lex and sialyl-Lea 30 min before seeding onto Boyden chambers (*, p < 0.05; **, p < 0.01). b, phosphorylation of p38 in HT29 cells with E-selectin stimulation. c, phosphorylation of Hsp27 in HT29 (upper) and DLD-1 (lower) cells with E-selectin stimulation. d, actin localization in HT29 cells at 120 min after E-selectin stimulation (left panel, mock; right panel, NEU4S). Cells were fixed and stained with FITC-phalloidin.
NEU4S May Hydrolyze Sialyl Lewis Antigens at Cell Surfaces
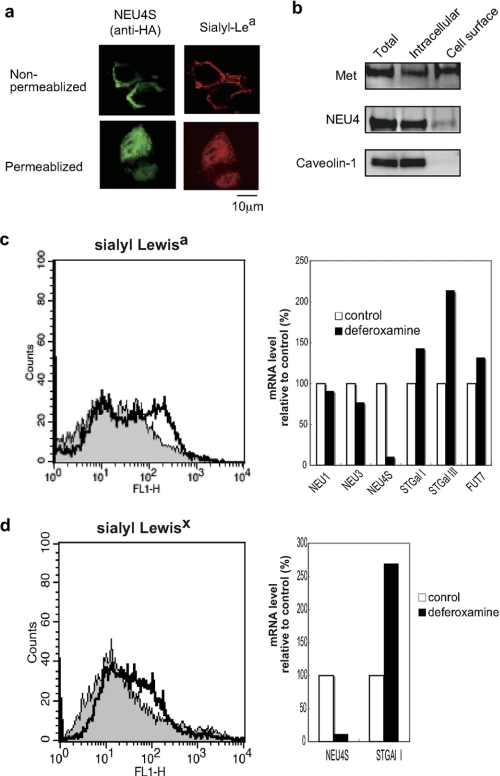
The intracellular localization of NEU4S has not been detailed, although the presence as extrinsic proteins in the ER was reported by Bigi et al. (20). We then studied the possibility that NEU4S might localize at plasma membranes with access to sialyl Lewis antigens in DLD-1 cells. With 0.1% Triton X-100 permeabilization, NEU4S was almost entirely intracellular, but in its absence some NEU4S was localized at the plasma membrane, along with sialyl-Lea (Fig. 5a). Biotinylation of cell surface proteins confirmed the existence of NEU4S. In NEU4S-transfected cells, some NEU4 molecules were found in the biotinylated fractions, in which Met was collected but not caveolin-1, an intracellular control (Fig. 5b). These results imply that NEU4S could be present functionally at plasma membranes and desialylate the sialyl-Lea and sialyl-Lex of cell surface glycans.
FIGURE 5.
Functional localization of NEU4S at plasma membranes and decreased expression of endogenous NEU4S under conditions of hypoxia. a, intracellular localization of NEU4S in DLD-1 cells with (upper panel) or without permeabilization (lower panel). Indirect immunofluorescence for HA-tagged NEU4S and sialyl-Lea using anti-HA (left) and sialyl-Lea (right) antibodies, respectively. b, biotinylation of the NEU4S protein. DLD-1 cells were subjected to protein biotinylation and labeled proteins were collected by affinity chromatography followed by immunoblotting analysis with antibodies. c and d, NEU4S down-regulation in the presence of deferoxamine, a hypoxia mimetic. HCT-15 (c) and SW-480 (d) colon cancer cells were cultured in the presence of deferoxamine (100 μm), and expression of sialyl Lewis antigens was assessed by flow cytometrically. mRNA levels were evaluated by quantitative RT-PCR, and the values represent means from two independent experiments.
Endogenous NEU4S Is Down-regulated under Hypoxic Conditions
Induction of sialyl Lewis antigens has been reported when cells were cultured in the presence of hypoxia mimetics such as deferoxamine, an iron-chelating agent known to activate hypoxia-inducible factor-1 (41), concomitant with increased expression of several sialyl- and fucosyltransferases responsible for the antigen synthesis. To establish whether endogenous NEU4S is linked to these events, expression was therefore assessed under hypoxic culture conditions by adding deferoxamine to cultures of HCT-15 and SW480 colon cancer cells expressing relatively higher NEU4S levels than HT29 and DLD-1 cells. Under conditions whereby sialyl-Lea expression was up-regulated (Fig. 5c, left panel) accompanied by ST3Gal I and FUT7 elevation, endogenous NEU4S was dramatically down-regulated in HCT-15 cells (Fig. 5c, right panel), despite only a slight change in NEU1 and NEU3 expression. Similar results were obtained with SW-480 cells for sialyl-Lex expression (Fig. 5d). These data suggest that NEU4S may be negatively linked to regulation of sialyl Lewis antigen expression and adhesion of cancer cells to endothelial E-selectin.
DISCUSSION
Sialyl-Lea and sialyl-Lex are widely used as cancer markers, and a positive correlation between their expression and cancer cell metastasis or invasion has been reported. In the present study, we demonstrated that human sialidase NEU4S efficiently catalyzes the removal of sialic acids from sialyl-Lea and sialyl-Lex, causing reduction of malignant phenotypes including cell attachment, cell migration, and cell proliferation, probably by lowering binding of cancer cells to E-selectin on endothelial cells. These findings are likely to account for the tumor progression by down-regulation of NEU4S in colon cancer, as described in our previous report (22).
Hydrolysis of the antigens by NEU4S was observed in cells by flow cytometric analysis and in in vitro sialidase assays. To our knowledge, this is the first description of regulation of sialyl-Lea and sialyl-Lex expression by NEU4 in a cancer. Although it has been proposed that glycosyltransferases are responsible for synthesis of these antigens, expression levels of the encoding genes have not always been found to correlate with sialyl Lewis antigen contents, with even contradictory expression noted in various cells, including normal colon FHC cells (42). One report indicated that ST3Gal IV, Fuc-T3, and Fuc-T6 are mainly responsible for synthesis of the sialyl Lewis antigens in colon tissues, but are not for their augmented expression in colon cancers (43). In this context, it is important to consider sialidase as a critical regulator of the cancer-associated antigens. In particular, it may be feasible that lack of NEU4S contributes to metastatic events in colon cancer, because the sialidase is down-regulated (22) and desialylates only sialyl-Lea and sialyl-Lex but not disialyl-Lea in normal mucosa. Interestingly, the NEU4S expression ratio of tumor to non-tumor tissues was previously found to be statistically linked (p = 0.025) to venous invasion (22) that is known to be closely related to the development of hematogenous metastasis in patients with colorectal carcinoma (23). Although we found a marked inhibition of tumor growth in vivo by NEU4S, it is uncertain whether the effect is directly related to reduction of sialyl Lewis antigens. To confirm further the role of NEU4S in liver metastasis, experiments of in vivo metastasis with the mock and NEU4S-transfected cells would be necessary.
NEU4S down-regulation here was marked in the presence of the hypoxia mimetics, deferoxamine, as well as in colon cancer tissues, where the expression of sialyl Lewis antigens was augmented, along with ST3Gal I, STGal III, and FUT7, responsible for their synthesis. It should be noted that under these conditions NEU1 and NEU3 showed no significant changes in expression. The molecular mechanisms remain unclear, but action of transcription factors including hypoxia-inducible factor-1 may be indirectly involved. We surveyed possible transcription factors in the upstream region of the NEU4 gene using TF search3 and found a transcription factor, P300, to be down-regulated in colon cancer. The gene has also been identified as a co-activator of hypoxia-inducible factor-1α and a stimulating factor of hypoxia-induced genes (44), although we did not estimate the functional transcriptional activity of the gene in the cells used here. One possibility is that NEU4S is down-regulated physiologically under hypoxic conditions or pathologically in colon cancer by the action(s) of transcription factors and then plays a critical role in controlling sialyl-Lea and sialyl-Lex through desialylation of the antigens.
In a recent report, Gadhoum and Sackstein (45) presented evidence that NEU1 up-regulates Lex through desialylation of sialyl-Lex during differentiation of human myeloid cells, although they did not cover or discuss NEU4. Rat Neu2 also decreased sialyl-Lex in mouse colon 26 cells (28), whereas human NEU2 is almost absent in colon mucosa. DR3 glycoproteins of the death receptor family may include sialyl-Lea and sialyl-Lex, and could bind to E-selectin in HT29 and Lovo cells (39), impacting on cell motility and cell survival. As our previous study (19) showed that NEU4S enhanced TRAIL-induced apoptosis in colon cancer cells, it is conceivable that it desialylates the sialyl Lewis antigens on DR3 glycoproteins, thus reducing their binding to E-selectin and inhibiting cell motility and survival. However, we were not able to identify DR3 as a sialyl Lewis positive glycoprotein by immunoblot analysis using antibodies for sialyl Lewis and DR3. We also failed to demonstrate the presence of either CD44v (36) or Mac1 (37) carrying sialyl-Lex and/or sialyl-Lea epitope(s) in HT29 and DLD-1 cells. At present, little is known about the endogenous glycoprotein molecules as targets for NEU4S, but it is obvious that most sialyl-Lea and sialyl-Lex are detectable as mucin-type glycans. Consistent with this conclusion, differing from other sialidases, NEU4 isoforms can cleave sialic acids from mucins in vitro (19, 35).
To ascertain whether endogenous NEU4S actually plays an essential role in regulation of the expression of sialyl Lewis antigens, we evaluated the expression level of NEU4S in human colon mucosa and made a comparison with those of NEU4S-transfected cells. Because in general, a good correlation has so far been observed between the activity and mRNA levels in sialidase expression, the activity was estimated according to these levels. It appears that the NEU4S level in mucosa is roughly comparable with those of overexpressing NEU4S in the transfected DLD-1 and HT29 cells, based on the following estimation. We previously described the sialidase activity toward 4MU-NeuAc in normal colon mucosa to be 12–17 units/mg (29). The activity includes those of NEU1 as well as NEU4S due to their similar substrate preference to 4MU-NeuAc (14), and the relative mRNA level of NEU4S to NEU1 in colon mucosa was found ∼⅓ by quantitative analysis (19), suggesting that the activity for NEU4S might be 4–6 units/mg in mucosa. On the other hand, colon cancer cells increased the apparent activity by NEU4S transfection, as described earlier (7.1 and 9.9 to 16.1 and 19.9 units/mg in transfectants NEU4S#1 and NEU4S#11, respectively). However, because of an extreme reduction of NEU4S in the colon cancer cells, the ratio of endogenous NEU4S to the NEU1 mRNA level was quite low (1/100–1/500) and the activity for NEU4S is predicted to be 0.01–0.2 units/mg before the transfection, where almost all activity toward 4MU-NeuAc is probably derived from endogenous NEU1. The NEU4S activity after transfection then becomes 9–10 units/mg by subtracting the endogenous NEU1 activity, as the NEU1 level is not affected by the transfection. Altogether, the increased activity level after transfection can be considered in a range similar to those in normal colon. These data support that endogenous NEU4S in normal mucosa is probably enough to hydrolyze and physiologically regulate the expression of sialyl Lewis antigens effectively as in the case of the NEU4S transfectants used in this study. Because of the lack of NEU4L in colon mucosa and cancer tissues, it is likely that NEU4S dominantly contributes to control of the cancer-related antigens, especially on O-glycans, and that its reduced expression in cancer probably causes impairment of their control and thus accumulation.
Although subcellular localization of NEU4S has not been thoroughly clarified, it has been recovered in intracellular membrane fractions (19) and in endoplasmic reticulum (20). We observed here some portion of NEU4S molecules localized at plasma membranes as assessed by immunostaining and biotinylation. Similar to the case of human myeloid cell differentiation (45), desialylation of sialyl-Lea and sialyl-Lex by the sialidase might occur at cell surfaces. Taken together, the present results revealed that NEU4S plays a physiological role in control of sialyl Lewis antigens through desialylation of O-glycans, reinforcing our previous evidence that down-regulation of the sialidase linked to venous invasion in colon cancer might contribute to invasive properties of the cancer. Regulation of NEU4 gene expression could be of potential utility in the development of novel cancer therapies.
Acknowledgment
We appreciate the expert technical assistance of Momo Shiozaki.
This work was supported in part by Grants-in-aid for Scientific Research on Priority Areas in Cancer (20013047) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Y. Akiyama, unpublished observations.
- sLea
- sialyl Lewis a
- sLex
- sialyl Lewis x
- FUT3
- fucosyltransferase 3
- FUT7
- fucosyltransferase 7
- ST3 Gal I
- β-galactoside α-2,3-sialyltransferase 1
- ST3 Gal III
- β-galactoside α-2,3-sialyltransferase 3
- ST3 GalIV
- β-galactoside α-2,3-sialyltransferase 4
- 4MU
- 4-methylumbelliferylone
- GM3
- N-acetylneuraminylgalactosylceramide.
REFERENCES
- 1. Dennis J. W., Granovsky M., Warren C. E. (1999) Biochim. Biophys. Acta 1473, 21–34 [DOI] [PubMed] [Google Scholar]
- 2. Hakomori S. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10231–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varki N. M., Varki A. (2007) Lab. Invest. 87, 851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakagoe T., Sawai T., Tsuji T., Jibiki M. A., Nanashima A., Yamaguchi H., Yasutake T., Ayabe H., Arisawa K., Ishikawa H. (2002) J. Clin. Gastroenterol. 34, 408–415 [DOI] [PubMed] [Google Scholar]
- 5. Steplewska-Mazur K., Gabriel A., Zajecki W., Wylezoł M., Glück M. (2000) Hybridoma 19, 129–133 [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi M., Lee H., Schaffer L., Gilmartin T. J., Head S. R., Takaishi S., Wang T. C., Nakayama J., Fukuda M. (2007) J. Histochem. Cytochem. 55, 263–274 [DOI] [PubMed] [Google Scholar]
- 7. Koike T., Kimura N., Miyazaki K., Yabuta T., Kumamoto K., Takenoshita S., Chen J., Kobayashi M., Hosokawa M., Taniguchi A., Kojima T., Ishida N., Kawakita M., Yamamoto H., Takematsu H., Suzuki A., Kozutsumi, Kannagi R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 8132–8137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kannagi R., Sakuma K., Miyazaki K., Lim K. T., Yusa A., Yin J., Izawa M. (2010) Cancer Sci. 101, 586–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Izawa M., Kumamoto K., Mitsuoka C., Kanamori C., Kanamori A., Ohmori K., Ishida H., Nakamura S., Kurata-Miura K., Sasaki K., Nishi T., Kannagi R. (2000) Cancer Res. 60, 1410–1416 [PubMed] [Google Scholar]
- 10. Miyazaki K., Ohmori K., Izawa M., Koike T., Kumamoto K., Furukawa K., Ando T., Kiso M., Yamaji T., Hashimoto Y., Suzuki A., Yoshida A., Takeuchi M., Kannagi R. (2004) Cancer Res. 64, 4498–4505 [DOI] [PubMed] [Google Scholar]
- 11. Berg E. L., Robinson M. K., Mansson O., Butcher E. C., Magnani J. L. (1991) J. Biol. Chem. 266, 14869–14872 [PubMed] [Google Scholar]
- 12. Laferriere J., Houle F., Taher M. M., Valerie K., Huot J. (2001) J. Biol. Chem. 276, 33762–33772 [DOI] [PubMed] [Google Scholar]
- 13. Laferrière J., Houle F., Huot J. (2004) Clin. Exp. Metastasis 21, 257–264 [DOI] [PubMed] [Google Scholar]
- 14. Miyagi T. (2008) Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 84, 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monti E., Bonten E., D'Azzo A., Bresciani R., Venerando B., Borsani G., Schauer R., Tettamanti G. (2010) Adv. Carbohydr. Chem. Biochem. 64, 403–479 [DOI] [PubMed] [Google Scholar]
- 16. Uemura T., Shiozaki K., Yamaguchi K., Miyazaki S., Satomi S., Kato K., Sakuraba H., Miyagi T. (2009) Oncogene 28, 1218–1229 [DOI] [PubMed] [Google Scholar]
- 17. Kakugawa Y., Wada T., Yamaguchi K., Yamanami H., Ouchi K., Sato I., Miyagi T. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10718–10723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wada T., Hata K., Yamaguchi K., Shiozaki K., Koseki K., Moriya S., Miyagi T. (2007) Oncogene 26, 2483–2490 [DOI] [PubMed] [Google Scholar]
- 19. Yamaguchi K., Hata K., Koseki K., Shiozaki K., Akita H., Wada T., Moriya S., Miyagi T. (2005) Biochem. J. 390, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bigi A., Morosi L., Pozzi C., Forcella M., Tettamanti G., Venerando B., Monti E., Fusi P. (2010) Glycobiology 20, 148–157 [DOI] [PubMed] [Google Scholar]
- 21. Shiozaki K., Koseki K., Yamaguchi K., Shiozaki M., Narimatsu H., Miyagi T. (2009) J. Biol. Chem. 284, 21157–21164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamanami H., Shiozaki K., Wada T., Yamaguchi K., Uemura T., Kakugawa Y., Hujiya T., Miyagi T. (2007) Cancer Sci. 98, 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ouchi K., Sugawara T., Ono H., Fujiya T., Kamiyama Y., Kakugawa Y., Mikuni J., Tateno H. (1996) Cancer 78, 2313–2317 [DOI] [PubMed] [Google Scholar]
- 24. Hata K., Koseki K., Yamaguchi K., Moriya S., Suzuki Y., Yingsakmongkon S., Hirai G., Sodeoka M., von Itzstein M., Miyagi T. (2008) Antimicrob. Agents Chemother. 52, 3484–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shiozaki K., Yamaguchi K., Sato I., Miyagi T. (2009) Cancer Sci. 100, 588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huet G., Hennebicq-Reig S., de Bolos C., Ulloa F., Lesuffleur T., Barbat A., Carrière V., Kim I., Real F. X., Delannoy P., Zweibaum A. (1998) J. Cell Biol. 141, 1311–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saul R., Chambers J. P., Molyneux R. J., Elbein A. D. (1983) Arch. Biochem. Biophys. 221, 593–597 [DOI] [PubMed] [Google Scholar]
- 28. Sawada M., Moriya S., Saito S., Shineha R., Satomi S., Yamori T., Tsuruo T., Kannagi R., Miyagi T. (2002) Int. J. Cancer 97, 180–185 [DOI] [PubMed] [Google Scholar]
- 29. Miyagi T., Wada T., Yamaguchi K., Shiozaki K., Sato I., Kakugawa Y., Yamanami H., Fujiya T. (2008) Proteomics 8, 3303–3311 [DOI] [PubMed] [Google Scholar]
- 30. Ueno S., Saito S., Wada T., Yamaguchi K., Satoh M., Arai Y., Miyagi T. (2006) J. Biol. Chem. 281, 7756–7764 [DOI] [PubMed] [Google Scholar]
- 31. Kato K., Shiga K., Yamaguchi K., Hata K., Kobayashi T., Miyazaki K., Saijo S., Miyagi T. (2006) Biochem. J. 394, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsuchida A., Okajima T., Furukawa K., Ando T., Ishida H., Yoshida A., Nakamura Y., Kannagi R., Kiso M., Furukawa K. (2003) J. Biol. Chem. 278, 22787–22794 [DOI] [PubMed] [Google Scholar]
- 33. Srinivas U., Påhlsson P., Lundblad A. (1996) Scand. J. Immunol. 44, 197–203 [DOI] [PubMed] [Google Scholar]
- 34. Yang W., Fan H., Gao X., Gao S., Karnati V. V., Ni W., Hooks W. B., Carson J., Weston B., Wang B. (2004) Chem. Biol. 11, 439–448 [DOI] [PubMed] [Google Scholar]
- 35. Seyrantepe V., Landry K., Trudel S., Hassan J. A., Morales C. R., Pshezhetsky A. V. (2004) J. Biol. Chem. 279, 37021–37029 [DOI] [PubMed] [Google Scholar]
- 36. Hanley W. D., Napier S. L., Burdick M. M., Schnaar R. L., Sackstein R., Konstantopoulos K. (2006) FASEB J. 20, 337–339 [DOI] [PubMed] [Google Scholar]
- 37. Inata J., Hattori N., Yokoyama A., Ohshimo S., Doi M., Ishikawa N., Hamada H., Kohno N. (2007) Int. J. Cancer 120, 2643–2649 [DOI] [PubMed] [Google Scholar]
- 38. Yusa A., Miyazaki K., Kimura N., Izawa M., Kannagi R. (2010) Cancer Res. 70, 4064–4073 [DOI] [PubMed] [Google Scholar]
- 39. Gout S., Morin C., Houle F., Huot J. (2006) Cancer Res. 66, 9117–9124 [DOI] [PubMed] [Google Scholar]
- 40. Laferriere J., Houle F., Huot J. (2002) Ann. N.Y. Acad. Sci. 973, 562–572 [DOI] [PubMed] [Google Scholar]
- 41. Denko N. C. (2008) Nat. Rev. Cancer 8, 705–713 [DOI] [PubMed] [Google Scholar]
- 42. Bardoni A., Valli M., Trinchera M. (1999) FEBS Lett. 451, 75–80 [DOI] [PubMed] [Google Scholar]
- 43. Kudo T., Ikehara Y., Togayachi A., Morozumi K., Watanabe M., Nakamura M., Nishihara S., Narimatsu H. (1998) Lab. Invest. 78, 797–811 [PubMed] [Google Scholar]
- 44. Arany Z., Huang L. E., Eckner R., Bhattacharya S., Jiang C., Goldberg M. A., Bunn H. F., Livingston D. M. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 12969–12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gadhoum S. Z., Sackstein R. (2008) Nat. Chem. Biol. 4, 751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]