Abstract
Ricin is a potent A-B toxin that is transported from the cell surface to the cytosol, where it inactivates ribosomes, leading to cell death. Ricin enters cells via endocytosis, where only a minute number of ricin molecules reach the endoplasmic reticulum (ER) lumen. Subsequently, the ricin A chain traverses the ER bilayer by a process referred to as dislocation or retrograde translocation to gain access to the cytosol. To study the molecular processes of ricin A chain dislocation, we have established, for the first time, a human cell system in which enzymatically attenuated ricin toxin A chains (RTAE177D and RTAΔ177–181) are expressed in the cell and directed to the ER. Using this human cell-based system, we found that ricin A chains underwent a rapid dislocation event that was quite distinct from the dislocation of a canonical ER soluble misfolded protein, null Hong Kong variant of α1-antitrypsin. Remarkably, ricin A chain dislocation occurred via a membrane-integrated intermediate and utilized the ER protein SEL1L for transport across the ER bilayer to inhibit protein synthesis. The data support a model in which ricin A chain dislocation occurs via a novel strategy of utilizing the hydrophobic nature of the ER membrane and selective ER components to gain access to the cytosol.
Keywords: Endoplasmic Reticulum (ER), Membrane Bilayer, Membrane Trafficking, Protein Processing, Toxins, Ricin
Introduction
Ricin toxin is a toxic polypeptide found in castor beans (Ricinus communis) (1) comprised of a catalytically active polypeptide (A chain or RTA)5 covalently linked by a disulfide bond to a lectin-binding B subunit (B chain). Like most A-B toxins, the specificity of the B chains for cell surface receptors dictates cell entry. In the case of ricin toxin, the galactose-specific lectin-binding domain of the B chain interacts with cell surface glycolipids and glycoproteins, allowing the toxin to enter the cell via clathrin-dependent and clathrin-independent endocytosis (2).
Following endocytosis, ricin toxin is transported in a retrograde manner through the Golgi and ER and into the cytosol by a similar manner as Shiga toxin, cholera toxin, Pseudomonas exotoxin A, and pertussis toxin (3). These toxins are proposed to utilize protein kinases during the endocytosis step (4). In contrast, other A-B toxins such as diphtheria toxin and anthrax toxin enter the cytoplasm following a low pH-induced translocation event directly from endocytic vesicles (3).
The retrograde trafficking of ER-directed toxins requires numerous cellular factors to reach the ER lumen (4). Both ricin and Shiga toxin are dependent on Vps34, a PI3K, and members of the sorting nexin family of proteins. In the case of ricin toxin, it is independent of Rab-9 and Rab-11 (5) and dependent on dynamin (6) and cellular cholesterol levels (7). A recent high-throughput screen identified small molecule inhibitors that demonstrate the ability to protect cells from ricin toxicity by targeting endosome-to-Golgi retrieval factors, specifically syntaxins 5 and 6 (8).
Despite the lack of a KDEL sequence, the heterodimer is transported from the Golgi apparatus to the ER lumen. While in the ER, the ricin A chain disassociates from the ricin toxin B chain and is transported across the membrane by a process referred to as dislocation or retrograde translocation (9). Once in the cytosol, the A chain acts as an N-glycosidase, which inactivates the ribosome and eventually causes cell death. However, ricin-induced cell death may also be due to inhibition of processes other than translation shutdown (10).
Ricin takes advantage of the ER quality control machinery, a cell system that normally scrutinizes nascent polypeptides and disposes of misfolded proteins by proteasome destruction (11). The ER chaperones PDI and calreticulin are proposed to play a role in ricin transport across the ER membrane (12, 13). In addition, EDEM1 (ER degradation-enhancing α-mannosidase I-like protein 1), which is involved in the degradation of misfolded ER proteins (14), also participates in ricin dislocation (15). Recently, ricin studies in yeast cells demonstrated that Hrd1p (HMG-CoA reductase degradation-1 protein) is implicated in ricin dislocation (16). The toxin is proposed to be extracted through the ER membrane via the Sec61 translocon (17), but a recent study suggests that the Hrd1p complex may act as the dislocon (18). Once dislocated, the Hsc70 cytosolic chaperone machinery protects the ricin A chain from Hsp90-assisted cytosolic degradation (19). The mechanism of ricin dislocation in human cells has not been well defined despite the identification of cellular factors that ricin utilizes for dislocation.
The potency of ricin to inactivate 2000 ribosomes/min precludes the need for a large number of molecules to reach the cytoplasm (20, 21). To characterize one of the rate-limiting steps of ricin entry, dislocation, a human cell system has been created, for the first time, that expresses an enzymatic mutant of the ricin A chain in the ER. Using this model system, ricin A chain dislocation occurs in a unique manner, utilizing the ER membrane itself as well as ER proteins.
EXPERIMENTAL PROCEDURES
Cell Lines and Antibodies
Human U373-MG astrocytoma-expressing RTA polypeptides (U373RTAΔ and U373 RTA-E177D) were generated and maintained as described (22). Anti-ricin toxin, anti-GAPDH, anti-α1-antitrypsin, anti-SEL1L (suppressor of lin-12-like), anti-γ-tubulin, anti-GFP, and anti-p97 antibodies were purchased from Biodesign International, Millipore Corp., Chemicon International Inc., Sigma, Santa Cruz Biotechnology, and Abcam, respectively. Anti-HA (12CA5) antibodies were purified from hybridoma cells (23). The anti-calnexin monoclonal antibody (AF8) and anti-PDI antibody were gifts from Dr. Brenner (Harvard Medical School, Boston, MA) and Dr. Ploegh (Whitehead Institute, Cambridge, MA), respectively.
cDNA Constructs
Ricin toxin mutant chimeras RTAE177D and RTAΔ177–181 (RTAΔ) (24) were generated from PCR fragments (22) using full-length wild-type ricin toxin A chain (FL-RTAWT) cDNA as a template. RTAE177D represents a site-directed point mutant in which residue 177 (glutamic acid) was changed to aspartic acid. The RTAΔ mutant is a deletion construct lacking residues 177EAARF181. Finally, the chimeras consisted of an N-terminal murine MHC class I heavy chain H2-Kb signal peptide (Kb) followed by the HA epitope tag (AYPYDVPDYA), linker region (15 amino acids), and RTAWT, RTAE177D, or RTAΔ. The Derlin-1-GFP construct was generated as a chimera by fusing the Derlin-1 cDNA to enhanced GFP cDNA. The shRNA construct against SEL1L was a kind gift from Dr. Ploegh. SEL1L knockdown in HeLa cells was performed using shRNAs from Santa Cruz Biotechnology.
Cell Lysis, Immunoprecipitation, SDS-PAGE, and Endoglycosidase H and Peptide N-Glycanase Assays
Briefly, cells (1 × 106) were lysed in 0.5% Nonidet P-40 lysis mixture, followed by incubation with the respective antibody and protein A-agarose beads (22). The immunoprecipitates were subjected to immunoblot analysis using the respective immunoglobulin. Endoglycosidase H and peptide N-glycanase (New England Biolabs) assays were performed at 37 °C for 1.5 h (100 units of enzyme/reaction).
Pulse-Chase Analysis
Briefly, cells were labeled with [35S]methionine (PerkinElmer Life Sciences) and chased in unlabeled methionine (25 mm) (22). RTA proteins were recovered from Nonidet P-40 cell lysates using anti-HA antibodies and resolved by SDS-PAGE (12.5%). The polyacrylamide gels were dried and exposed to autoradiography film. The polypeptide levels were quantified by densitometry analysis using an AlphaImager 3400 system (Alpha Innotech).
Subcellular Fractionation
RTA-expressing cells were mechanically homogenized using a 12-μm ball-bearing homogenizer (12 passes; Isobiotec, Heidelberg, Germany) in homogenization buffer (100 mm Tris, 150 mm NaCl, 250 mm sucrose, 1.5 μg/ml aprotinin, 1 μm leupeptin, and 200 μm phenylmethylsulfonyl fluoride) (25). Unbroken cells and other debris were pelleted at 15,000 × g for 10 min at 4 °C, and the supernatants were untreated or treated with 4.5 m urea (final concentration) for 10 min at 4 °C. Supernatants were centrifuged at 100,000 × g for 1 h at 4 °C. 100,000 × g supernatants and pellets were resolved by SDS-PAGE (12.5%).
Isoelectric Focusing
The RTA precipitates were incubated with isoelectric focusing sample buffer (57% (w/v) urea, 2% (v/v) Nonidet P-40, 0.02% ampholytes (pH 3.5–10; Amersham Biosciences), and 0.025% 2-mercaptoethanol) and resolved on a 17-cm one-dimensional isoelectric focusing gel (57% (w/v) urea, 2% (v/v) Nonidet P-40, 15.5% acrylamide (30:1.6 acrylamide/bisacrylamide), 4% ampholytes (pH 5–7), 1% ampholytes (pH 3.5–10), and 0.4% ampholytes (pH 7–9)) for 14 h. After electrophoresis, the gel was soaked in 50% methanol, 1% (w/v) SDS, and 5 mm Tris-Cl (pH 8.0) for 2 h and subjected to immunoblot analysis (25).
Ricin Activity Assays
HEK-293T cells were cotransfected with RTA constructs and a GFP expression plasmid (pCAGGS-GFP) using Lipofectamine 2000 (Invitrogen). Thirty hours post-transfection, GFP-positive cells were examined using a Beckman Coulter Cytomics FC 500 flow cytometer, and the data were analyzed using FlowJo software. HeLa cell lines seeded in 96-well plates at 2 × 104 cells/well were incubated with doubling dilutions of ricin in quadruplicate for various times. The cells were incubated with 35S-Pro-mix (PerkinElmer Life Sciences). The amount of radioactivity incorporated into TCA-precipitable proteins was measured by scintillation counting in a MicroBeta TriLux 1450 counter, and IC50 values (representing the concentration of toxin reducing protein synthesis by 50% relative to untreated control cells) were recorded.
RESULTS
Ricin A Chains Directed to the ER Block Protein Synthesis
The investigation of ricin toxin transport across the ER membrane has been hampered by the low number of toxin molecules that reach the ER lumen when added extracellularly (17). Hence, we aimed to create a human cell-based assay to specifically study RTA transport across the ER membrane. In line with this objective, we initially confirmed that RTA molecules directed to the ER (10, 26) in human cells limited protein expression. FL-RTAWT and wild-type mature RTA equipped with a murine MHC class I molecule (Kb) signal peptide and an HA epitope tag at its N terminus (RTAWT) were cotransfected with a GFP expression plasmid in HEK-293T cells and evaluated for GFP fluorescence using flow cytometry (Fig. 1, A–D). The lack of GFP fluorescent cells is indicative of functionally active RTA molecules. FL-RTAWT and RTAWT limited the expression of GFP in a significant percentage of cells (2 and 15%, respectively) (Fig. 1, C and D) compared with cells transfected with GFP alone (85%) (Fig. 1B). These data suggest that ER-directed RTA molecules are dislocated across the ER and to the cytosol, where they inhibit protein synthesis.
FIGURE 1.
RTA inhibits protein expression in human cells. HEK-293T cells transfected with an empty plasmid (Control; A) or a GFP-expressing plasmid (B) with FL-RTAWT (C), RTAWT (D), RTAΔ (E), and RTAE177D (F) were analyzed for GFP expression using flow cytometry. The values in the quadrants represent percentage of total cells.
Ricin A Chain Expression in Human Cells
Because of the toxic nature of wild-type RTA, we cloned ricin A chain mutants (RTAE177D and RTAΔ; see “Experimental Procedures”) that are enzymatically attenuated to study A chain dislocation (27, 28). RTAE177D is a site-directed mutant with attenuated activity that is structurally similar to wild-type RTA (29), whereas RTAΔ has deletion of residues 177–181. Like RTAWT, these constructs were also engineered with the N-terminal Kb signal peptide and HA epitope tag. An initial experiment was performed to determine whether RTAE177D and RTAΔ inhibit protein synthesis. GFP-positive cells were examined in HEK-293T cells cotransfected with RTAE177D and RTAΔ and a GFP expression plasmid (Fig. 1, E and F). The RTAE177D and RTAΔ mutants had a diminished ability to affect GFP expression (Fig. 1, E and F), with RTAE177D retaining some capacity to inhibit GFP expression. These data demonstrate that ricin A chain mutants have a limited capacity to prevent protein expression.
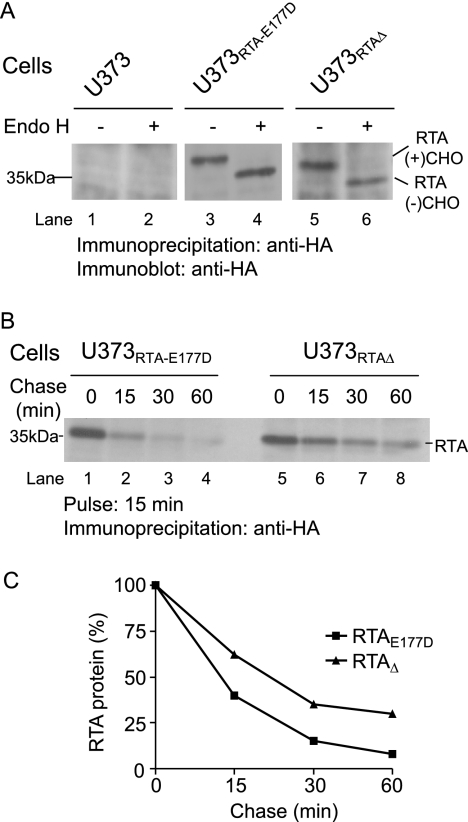
To study RTA dislocation, human U373 cells that stably express RTAE177D and RTAΔ (see “Experimental Procedures”) (Fig. 2) were created. Our initial experiment examined the acquisition of an N-linked glycan onto RTAE177D and RTAΔ to confirm their localization to the ER. RTAE177D and RTAΔ molecules recovered from these cells were subjected to endoglycosidase H (Endo H) treatment, followed by immunoblot analysis (Fig. 2A). Endo H preferentially cleaves high mannose-containing N-linked oligosaccharides characteristic of ER-resident molecules (30). RTAE177D and RTAΔ polypeptides with the predicted molecular mass were recovered from cell lysates (Fig. 2A, lanes 3 and 5). As expected, faster migrating RTA polypeptides were observed upon treatment with Endo H (Fig. 2A, lanes 4 and 6). The difference in the relative molecular mass of the two species (∼3–4 kDa) confirmed that the RTA polypeptides acquired a single N-linked glycan and were retained in the ER. These data demonstrate that this human cell-based assay can be utilized to study ricin dislocation.
FIGURE 2.
RTA polypeptides are unstable ER proteins. A, U373, U373RTA-E177D, and U373RTAΔ cell lysates were incubated with anti-HA antibodies. The recovered precipitates were subjected to Endo H digestion and analyzed by immunoblot analysis. RTA(+)CHO and RTA(−)CHO, glycosylated and deglycosylated molecules, respectively. B, U373RTA-E177D and U373RTAΔ cells were pulsed with [35S]methionine for 15 min and chased for up to 60 min. The recovered RTA polypeptides were resolved by SDS-PAGE and detected by autoradiography. C, the levels of RTA polypeptides were quantified by densitometry and are plotted as a percentage to the 0-min chase point (100%). RTA polypeptides and molecular mass markers are indicated.
Ricin A Chains Are Subjected to Proteasome Degradation
We next investigated the stability of the RTA polypeptides in U373RTA-E177D and U373RTAΔ cells by pulse-chase analysis (Fig. 2, B and C). The cells were metabolically labeled with [35S]methionine for 15 min and chased for up to 60 min. Strikingly, the half-life of RTAE177D (<10 min) was less than that of RTAΔ (∼20 min) (Fig. 2, B, lanes 1–8, and C). Given that ricin utilizes ER quality control to gain access to the cytosol (9, 16), it is expected that a population of ricin would be degraded. However, the rapid half-life of RTAE177D expressed in human cells compared with yeast cells (16, 26) and plant cells (31, 32) suggests that RTA is efficiently transported out of the ER in human cells.
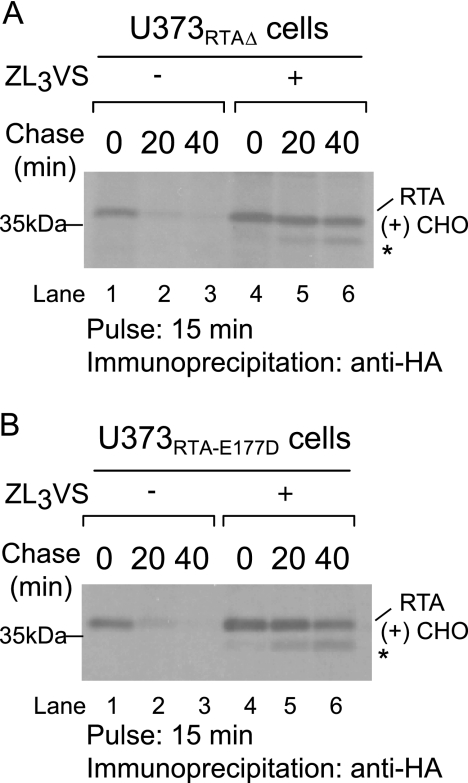
Are RTA polypeptides targeted for proteasome-dependent degradation? To address this question, U373RTA-E177D and U373RTAΔ cells treated with or without the proteasome inhibitor carboxybenzyl-leucyl-leucyl-leucyl vinyl sulfone (ZL3VS; 25 μm, 1 h) were subjected to pulse-chase analysis (Fig. 3). As expected, RTA polypeptides decreased over the chase period (Fig. 3, A and B, lanes 1–3). Strikingly, inclusion of the proteasome inhibitor stabilized both RTAE177D and RTAΔ molecules (Fig. 3, A and B, lanes 4–6). In addition, proteasome inhibition enabled the recovery of a faster migrating polypeptide intermediate during the chase, most likely deglycosylated RTA species (Fig. 3, A and B, lanes 4–6, *). Collectively, the data demonstrate that ricin A chains are eventually degraded in a proteasome-dependent manner.
FIGURE 3.
RTA polypeptides are stabilized in the presence of the proteasome inhibitor. U373RTAΔ (A) and U373RTA-E177D (B) cells treated with or without the proteasome inhibitor ZL3VS were pulsed with [35S]methionine for 15 min and chased for up to 40 min. RTA polypeptides were recovered with an anti-HA antibody, resolved by SDS-PAGE, and detected by autoradiography. RTA polypeptides and molecular mass markers are indicated. The asterisks indicate faster migrating species. RTA(+)CHO, glycosylated molecules.
Characterization of RTAE177D and RTAΔ Intermediates
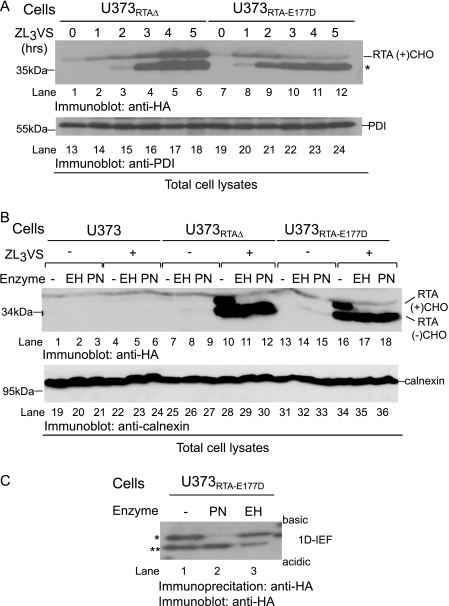
We next optimized conditions to observe the faster migrating RTA species. Total cell lysates from U373RTA-E177D and U373RTAΔ cells treated with ZL3VS (10 μm) for up to 5 h were subjected to immunoblot analysis (Fig. 4A). Strikingly, RTAE177D proteins were almost completely converted to the faster migrating species after 2 h of ZL3VS treatment (Fig. 4A, lanes 9–12). In contrast, RTAΔ molecules displayed a biphasic conversion in which an increase in glycosylated RTAΔ was observed during the early times of proteasome inhibition (Fig. 4A, lanes 2 and 3), followed by an accumulation of glycosylated and faster migrating species during later times of ZL3VS treatment (lanes 4–6). Interesting, the ratio of glycosylated (RTA(+)CHO) to faster migrating species following proteasome treatment significantly varied between the RTA mutants (Fig. 4A, lanes 1–6 and 7–12, respectively). These results suggest that RTAE177D and RTAΔ are likely processed differently due to the folding status of the polypeptide.
FIGURE 4.
RTA molecules accumulate as deglycosylated intermediates. A, U373RTAΔ and U373RTA-E177D cells treated with ZL3VS for up to 5 h were subjected to immunoblot analysis for RTA (lanes 1–12) and PDI (lanes 13–24).The asterisk indicates the faster migrating polypeptides. B, total cell lysates from U373, U373RTA-E177D, and U373RTAΔ cells untreated or treated with the proteasome inhibitor were subjected to Endo H (EH) or PNGase (PN). Immunoblot analysis was performed for RTA (lanes 1–18) and calnexin (lanes 19–36) proteins. RTA(+)CHO and RTA(−)CHO, glycosylated and deglycosylated molecules, respectively. C, RTA polypeptides recovered from U373RTA-E177D cells treated with ZL3VS were subjected to PNGase or Endo H treatment. Subsequently, the samples were resolved by one-dimensional isoelectric focusing (1D-IEF) and subjected to immunoblot analysis. The respective polypeptides and molecular mass markers are indicated. The asterisks (* and **) represent RTA molecules with different charges.
To address the N-linked glycosylation status of the RTAΔ and RTAE177D intermediates, we examined their sensitivity to in vitro glycosidase treatment (Fig. 4B). RTA polypeptides recovered from U373RTA-E177D and U373RTAΔ cells treated with the proteasome inhibitor (2.5 μm, 10 h) were undigested or digested with Endo H or peptide N-glycanase (PNGase), followed by immunoblot analysis (Fig. 4B). U373 cells were used as a negative control. As expected, two species of RTA polypeptides were observed exclusively from proteasome inhibitor-treated cells (Fig. 4B, lanes 10 and 16). Moreover, only the slower migrating glycosylated species were sensitive to Endo H and PNGase digestion, resulting in the faster migrating form of the RTA-reactive polypeptides (Fig. 4B, lanes 11, 12, 17, and 18). Calnexin levels demonstrated equivalent protein loading (Fig. 4B, lanes 19–36). These data suggest that the faster migrating RTA species are deglycosylated intermediates generated from the dislocation of ricin polypeptides.
To confirm that the N-terminal epitope tag does not affect the processing of the RTA polypeptides, we examined the accumulation of untagged RTA mutants upon proteasome inhibition (supplemental Fig. 1). Cell lysates from proteasome inhibitor-treated U373, U373RTA-E177D, U373KbRTAΔ, and U373KbRTA-E177D cells were subjected to immunoblot analysis. Two species of RTA molecules, glycosylated and deglycosylated, accumulated in proteasome inhibitor-treated cells, demonstrating that the stability of both forms of RTA is independent of the N-terminal epitope tag.
To confirm that the faster migrating RTA species were indeed digested by cellular PNGase, the RTA polypeptides were resolved on a one-dimensional isoelectric focusing gel. PNGase cleavage results in the conversion of asparagine to aspartic acid, causing a more acidic species, thus changing the overall charge of the polypeptide (33). RTA polypeptides recovered from U373RTA-E177D cells treated with the proteasome inhibitor (2.5 μm, 10 h) were untreated or treated with Endo H or PNGase, resolved on a one-dimensional isoelectric focusing gel, and subjected to immunoblot analysis (Fig. 4C). RTAE177D polypeptides from ZL3VS-treated cells migrated as two discrete bands likely corresponding to species with different charges (Fig. 4C, lane 1, * and **). Strikingly, in vitro PNGase digestion of RTAE177D polypeptides caused the polypeptides to migrate at a similar position as the more acidic RTA molecules (Fig. 4C, lane 2, **), whereas Endo H treatment did not alter the migration pattern of the RTAE177D polypeptides (lane 3). The same result was observed for RTAΔ.6 These results demonstrate that RTA polypeptides are subjected to cellular deglycosylation during dislocation.
Ricin A Chains Utilize ER Membrane Factors for Efficient Dislocation
To characterize the membrane topology of stabilized RTA polypeptides, we performed a subcellular fractionation protocol (see “Experimental Procedures”). Membrane microsomes from U373RTA-E177D and U373RTAΔ cells treated with the proteasome inhibitor (2.5 μm, 10 h) were incubated with 4.5 m urea and subjected to high-speed centrifugation (100,000 × g) to separate the membrane fraction from the supernatant fraction. The 100,000 × g supernatant, 100,000 × g pellet, and total cell lysates were subjected to immunoblot analysis for RTA mutants (Fig. 5, lanes 1–10) and p97 (lanes 11–20). The p97 polypeptide is a soluble cytosolic protein that associates with the ER membrane (34) and, as expected, was completely extracted from the bilayer upon treatment with urea (Fig. 5, lanes 12 and 14). As expected, some of the stabilized RTAE177D and RTAΔ molecules were found in the 100,000 × g supernatant (Fig. 5, lanes 6 and 8). Strikingly, a significant population of RTAE177D and RTAΔ was observed with the membrane fraction (Fig. 5, lanes 2 and 4), demonstrating that these RTA molecules are integrated into the bilayer. These results imply that ricin A chains are efficiently dislocated via membrane-integrated intermediates.
FIGURE 5.
RTA dislocation utilizes membrane components. Microsomal preparations from subcellular homogenates of U373RTAΔ and U373RTA-E177D cells were treated with or without 4.5 m urea and centrifuged at 100,000 × g. Total cell lysates, 100,000 × g pellets, and the 100,000 × g supernatant were subjected to immunoblot analysis against RTA (lanes 1–10) and p97 (lanes 11–20). The respective polypeptides and molecular mass markers are indicated. RTA(+)CHO and RTA(−)CHO, glycosylated and deglycosylated molecules, respectively.
Ricin A Chains Are Dislocated Utilizing Selective Components of ER Quality Control
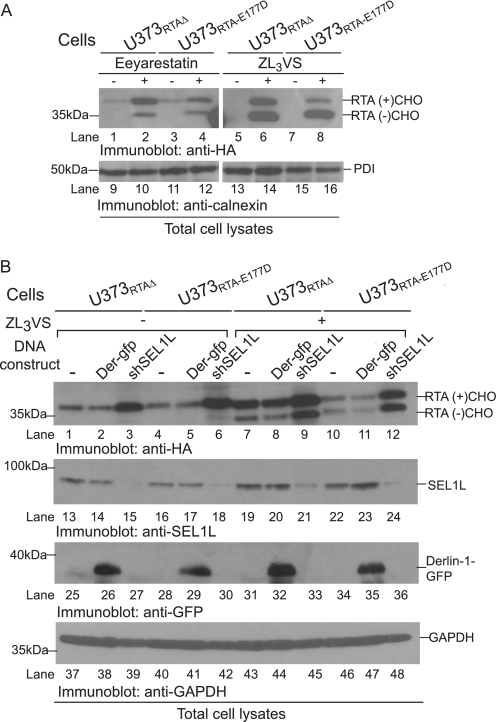
To validate that RTA polypeptides were dislocated utilizing the ER quality control machinery, we examined the effect of a dislocation inhibitor, eeyarestatin I (35), on the stability of ricin A chains. U373RTA-E177D and U373RTAΔ cells treated with or without eeyarestatin I (5 μg/ml) or the proteasome inhibitor ZL3VS (2.5 μm) were subjected to immunoblot analysis (Fig. 6A). PDI levels assured equivalent protein loading (Fig. 6A, lanes 9–16). As expected, treatment with ZL3VS caused a significant increase in both RTAΔ and RTAE177D polypeptides (Fig. 6A, lanes 5–8), with a difference between the levels of glycosylated (RTA(+)CHO) and deglycosylated (RTA(−)CHO) molecules. Interestingly, mostly glycosylated RTAΔ and RTAE177D polypeptides accumulated from cells treated with eeyarestatin I (Fig. 6A, lanes 2 and 4). However, because eeyarestatin I attenuates the process of dislocation and not proteasome function (35), it was interesting that deglycosylated forms of the ricin A chain were observed (Fig. 6A, lanes 2 and 4). Therefore, ricin A chain dislocation probably occurs using a distinct mechanism compared with ER-associated degradation substrates.
FIGURE 6.
RTA is dislocated utilizing selective ER proteins. A, U373RTAΔ and U373RTA-E177D cells untreated or treated with eeyarestatin I and ZL3VS were subjected to immunoblot analysis for RTA (lanes 1–8) and PDI (lanes 9–16) polypeptides. B, total cell lysates from U373RTAΔ and U373RTA-E177D cells knocked down for SEL1L or overexpressing Derlin-1-GFP, untreated or treated with proteasome inhibitor (ZL3VS), were subjected to immunoblot analysis for RTA (lanes 1–12), SEL1L (lanes 13–24), GFP (lanes 25–36), and GAPDH (lanes 37–48). The respective polypeptides and molecular mass markers are indicated. RTA(+)CHO and RTA(−)CHO, glycosylated and deglycosylated molecules, respectively.; Der-gfp, Derlin-1-GFP; shSEL1L, SEL1L shRNA.
To further explore the involvement of dislocation-associated cellular proteins in the transport of ricin toxin across the ER membrane, we investigated the role of SEL1L and Derlin-1 in the dislocation of RTAE177D and RTAΔ polypeptides. SEL1L functions as part of the mammalian HRD1 ligase complex and is proposed to be involved in the recognition of ER degradation substrates (36, 37). Derlin-1 plays a role in the extraction of certain misfolded ER proteins, and a dominant-negative form, Derlin-1-GFP, can impede substrate dislocation (38–40). U373RTA-E177D and U373RTAΔ cells transduced with Derlin-1-GFP and shRNA against SEL1L were untreated or treated with ZL3VS (2.5 μm, 10 h) and subjected to immunoblot analysis (Fig. 6B). The expression of the constructs was confirmed by anti-SEL1L (Fig. 6B, lanes 13–24) and anti-GFP (lanes 25–36) immunoblotting. Derlin-1-GFP expression did not alter the levels of RTA polypeptides regardless of proteasome inhibition (Fig. 6B, lanes 1, 2, 4, 5, 7, 8, 10, and 11). These results are consistent with published findings implying that ricin toxin transport across the ER membrane is Derlin-1-independent (15). On the other hand, silencing of SEL1L resulted in a substantial increase in RTA polypeptides in both the presence and absence of proteasome inhibition (Fig. 6B, lanes 3, 6, 9, and 12). Anti-GAPDH immunoblotting indicated equal protein loading (Fig. 6B, lanes 37–48). These results imply that ricin can utilize specific components of ER quality control machinery to be dislocated across the ER membrane.
We next examined whether deficiency in SEL1L levels affects ricin toxicity (Fig. 7). Ricin holotoxin was exogenously administered to HeLa cells stably expressing shRNA against SEL1L or a control shRNA (Fig. 7A). A control cell line (C1), a cell line revealing no SEL1L knockdown (120% of the control; S8), and a cell line showing 53% knockdown (S24) were utilized in subsequent cytotoxicity experiments (Fig. 7A). After inclusion of ricin holotoxin, the incorporation of radiolabeled amino acids into nascent polypeptides was measured by scintillation counting. Protection from toxin challenge would be demonstrated if the IC50 ratio of SEL1L knockdown cells to control cells is >1. The ricin IC50 for S8 cells was equivalent to that for the C1 cell line (Fig. 7B). In contrast, a significant protection from ricin was observed in the SEL1L knockdown cell line (S24), ranging from 1.6- to 1.4-fold during the time course of ricin challenge (Fig. 7C). Collectively, the results indicate that ricin requires SEL1L for the dislocation of its A chain from the ER to the cytosol and subsequently induces toxicity.
FIGURE 7.
SEL1L knockdown protects cells from ricin. HeLa cells were transfected with shRNA targeted against SEL1L and a control shRNA. A, SEL1L levels from lysates of cells expressing a control shRNA (C1) or shRNAs targeting SEL1L (S8 and S24) were analyzed by immunoblot analysis. SEL1L and γ-tubulin were quantified to assess levels of SEL1L knockdown (n = 8). B and C, the cytotoxicity of ricin against the stable cell lines was measured, and the mean IC50 values for different times of toxin incubation are plotted (nanograms/ml; n = 2). The respective polypeptides and molecular mass markers are indicated.
The Ricin A Chain Is Not Dislocated as a Canonical Soluble Misfolded ER Protein
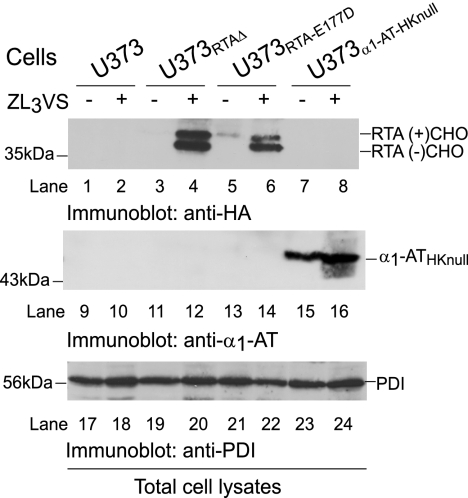
We compared the degradation intermediates of RTA and a canonical soluble ER degradation substrate, null Hong Kong variant of α1-antitrypsin (α1-ATHKnull) (41, 42). The total cell lysates of U373, U373RTA-E177D, and U373RTAΔ cells and U373 cells that stably express the α1-ATHKnull variant (U373α1-AT-HKnull) were untreated or treated with ZL3VS (2.5 μm, 10 h) and subjected to immunoblot analysis (Fig. 8). PDI levels confirmed equal protein loading (Fig. 8, lanes 17–24). As expected, both glycosylated and deglycosylated RTAE177D and RTAΔ molecules accumulated upon inclusion of the proteasome inhibitor (Fig. 8, lanes 4 and 6). In sharp contrast, only an increase in glycosylated α1-ATHKnull species was observed in ZL3VS-treated cells (Fig. 8, lane 16). These results indicate that even though both RTA and α1-ATHKnull polypeptides represent soluble substrates of dislocation, RTA extraction across the ER membrane probably occurs in a directed manner to increase the likelihood of gaining access to the cytosol.
FIGURE 8.
RTA is not degraded as a canonical misfolded ER protein. Total cell lysates from U373, U373RTAΔ, U373RTA-E177D, and U373α1-AT-HKnull cells treated with ZL3VS were subjected to immunoblot analysis for RTA (lanes 1–8), α1-ATHKnull (lanes 9–16), and PDI (lanes 17–24). The respective polypeptides and molecular mass markers are indicated. RTA(+)CHO and RTA(−)CHO, glycosylated and deglycosylated molecules, respectively.
DISCUSSION
Ricin toxin co-opts the trafficking machinery and ER quality control to gain access to the cytosol. In this study, we established a human cell-based assay to study ricin dislocation by generating, for the first time, human cells that stably express enzymatic RTA mutants in the ER (Fig. 2). Using this human cell-based system, we demonstrated that ricin A chains are rapidly extracted from the ER in a directed manner utilizing specific ER factors to enhance their dislocation efficiency. These ER factors include the ER membrane itself (Fig. 5) and SEL1L, a component associated with the degradation of ER proteins (Figs. 6 and 7). These data support a model in which RTA is transported out of the ER by a novel strategy of co-opting specific ER proteins and the ER bilayer to gain access to the cytosol.
Ricin toxin has evolved to selectively utilize cellular proteins to promote its dislocation across the membrane bilayer. The toxin likely undergoes a conformational change induced by the interaction with ER chaperones such as calreticulin and PDI (12, 13), thus allowing its engagement with specific factors of the dislocation apparatus, SEL1L (Figs. 6 and 7) and probably Hrd1p (16); yet this process seems to act independently of Derlin-1 (Fig. 6) (15), which is unlike cholera toxin, whose dependence on Derlin-1 for dislocation remains in question (43). SEL1L is an auxiliary protein for the E3 ubiquitin ligase HRD1 (37), and the dependence of RTA dislocation on SEL1L would typically suggest that RTA is ubiquitinylated prior to dislocation. Despite the low number of lysines within ricin as observed for other toxins (44), ubiquitin conjugation to ricin A chains would be selective for the purposes of dislocation (3, 45). Therefore, ricin can utilize SEL1L for dislocation, and yet a population of the A chains can escape degradation to block protein synthesis (Fig. 1).
Once targeted for extraction out of the ER, we propose that ricin A chains utilize the hydrophobic nature of the bilayer to catalyze their dislocation (Fig. 5). The recovery of membrane-integrated glycosylated A chains suggests that RTA is inserted into the bilayer due to a conformational change induced by its engagement with ER factors. In fact, the association of RTA molecules with ER microsomes upon an increase in temperature suggests that extrinsic factors can induce a partial conformational change in ricin A chains (46). The ability of ricin to insert into the bilayer would afford ricin dislocation to be less dependent on cellular proteins. Yeast cell studies demonstrate that wild-type RTA dislocation is independent of the p97-Npl4p-Ufd1p complex (16). Therefore, membrane integration of RTA may provide some of the driving force for extraction out of the ER. Alternatively, ricin A chains may be extracted from the ER as a protein/lipid micelle (47). In either case, RTA has evolved a unique strategy to efficiently cross the ER bilayer.
The ricin A chain contains only two lysines as a possible strategy to limit ubiquitin-dependent degradation (45). Despite the low lysine number and the use of the protective nature of Hsc70, a population of ricin A chains is degraded by the proteasome (Figs. 2–4) (26). However, there are distinct differences between ricin A chains and misfolded ER proteins that demonstrate that ricin co-opts select components of ER quality control to gain access to the cytosol. A major difference is observed in the dislocation kinetics of ricin as evaluated by the stability of glycosylated proteins and the accumulation of deglycosylated intermediates. This is apparent when compared with the levels of the canonical soluble misfolded ER-associated degradation substrate α1-ATHKnull in proteasome inhibitor-treated cells (Fig. 8). Another factor may be the ability of ricin to integrate into the bilayer. Membrane integration may allow limited exposure to the degradation machinery and permit RTA to escape destruction for a period of time to inhibit protein synthesis (Fig. 1). Thus, the ricin has evolved strategies inherent to its sequence and structure to bypass ER-associated degradation to reach the cytosol and inhibit protein synthesis.
Our human cell-based ricin dislocation assay is a powerful system to elucidate the molecular details of how ricin co-opts ER quality control, bypassing the ubiquitin-proteasome degradation process and gaining access to the cytosol. Our future objectives will be focused on delineating the components of the ER that ricin toxin co-opts to gain access to the cytosol.
Supplementary Material
Acknowledgment
We thank Dr. Matthew Bogyo (Stanford University) for the generous gift of the proteasome inhibitor.
This work was supported, in whole or in part, by National Institutes of Health Grant AI060905. This work was also supported by Defense Threat Reduction Agency Contract W81XWH-10-2-0048 and the Irma T. Hirschl Trust.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
K. Oresic and D. Tortorella, unpublished data.
- RTA
- ricin toxin A chain
- ER
- endoplasmic reticulum
- PDI
- protein-disulfide isomerase
- Endo H
- endoglycosidase H
- ZL3VS
- carboxybenzyl-leucyl-leucyl-leucyl vinyl sulfone
- PNGase
- peptide N-glycanase
- α1-ATHKnull
- null Hong Kong variant α1-antitrypsin.
REFERENCES
- 1. Lord J. M., Roberts L. M., Robertus J. D. (1994) FASEB J. 8, 201–208 [PubMed] [Google Scholar]
- 2. Lehar S. M., Pedersen J. T., Kamath R. S., Swimmer C., Goldmacher V. S., Lambert J. M., Blättler W. A., Guild B. C. (1994) Protein Eng. 7, 1261–1266 [DOI] [PubMed] [Google Scholar]
- 3. Hazes B., Read R. J. (1997) Biochemistry 36, 11051–11054 [DOI] [PubMed] [Google Scholar]
- 4. Sandvig K., Torgersen M. L., Engedal N., Skotland T., Iversen T. G. (2010) FEBS Lett. 584, 2626–2634 [DOI] [PubMed] [Google Scholar]
- 5. Iversen T. G., Skretting G., Llorente A., Nicoziani P., van Deurs B., Sandvig K. (2001) Mol. Biol. Cell 12, 2099–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Llorente A., Rapak A., Schmid S. L., van Deurs B., Sandvig K. (1998) J. Cell Biol. 140, 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grimmer S., Iversen T. G., van Deurs B., Sandvig K. (2000) Mol. Biol. Cell 11, 4205–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stechmann B., Bai S. K., Gobbo E., Lopez R., Merer G., Pinchard S., Panigai L., Tenza D., Raposo G., Beaumelle B., Sauvaire D., Gillet D., Johannes L., Barbier J. (2010) Cell 141, 231–242 [DOI] [PubMed] [Google Scholar]
- 9. Spooner R. A., Smith D. C., Easton A. J., Roberts L. M., Lord J. M. (2006) Virol. J. 3, 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X. P., Baricevic M., Saidasan H., Tumer N. E. (2007) Infect. Immun. 75, 417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vembar S. S., Brodsky J. L. (2008) Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Day P. J., Owens S. R., Wesche J., Olsnes S., Roberts L. M., Lord J. M. (2001) J. Biol. Chem. 276, 7202–7208 [DOI] [PubMed] [Google Scholar]
- 13. Spooner R. A., Watson P. D., Marsden C. J., Smith D. C., Moore K. A., Cook J. P., Lord J. M., Roberts L. M. (2004) Biochem. J. 383, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hosokawa N., Wada I., Hasegawa K., Yorihuzi T., Tremblay L. O., Herscovics A., Nagata K. (2001) EMBO Rep. 2, 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slominska-Wojewodzka M., Gregers T. F., Wälchli S., Sandvig K. (2006) Mol. Biol. Cell 17, 1664–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li S., Spooner R. A., Allen S. C., Guise C. P., Ladds G., Schnöder T., Schmitt M. J., Lord J. M., Roberts L. M. (2010) Mol. Biol. Cell 21, 2543–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wesche J., Rapak A., Olsnes S. (1999) J. Biol. Chem. 274, 34443–34449 [DOI] [PubMed] [Google Scholar]
- 18. Carvalho P., Stanley A. M., Rapoport T. A. (2010) Cell 143, 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spooner R. A., Hart P. J., Cook J. P., Pietroni P., Rogon C., Höhfeld J., Roberts L. M., Lord J. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 17408–17413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eiklid K., Olsnes S., Pihl A. (1980) Exp. Cell Res. 126, 321–326 [DOI] [PubMed] [Google Scholar]
- 21. Olsnes S., Fernandez-Puentes C., Carrasco L., Vazquez D. (1975) Eur. J. Biochem. 60, 281–288 [DOI] [PubMed] [Google Scholar]
- 22. Oresic K., Noriega V., Andrews L., Tortorella D. (2006) J. Biol. Chem. 281, 19395–19406 [DOI] [PubMed] [Google Scholar]
- 23. Harlow E., Franza B. R., Jr., Schley C. (1985) J. Virol. 55, 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaddock J. A., Roberts L. M. (1993) Protein Eng. 6, 425–431 [DOI] [PubMed] [Google Scholar]
- 25. Baker B. M., Tortorella D. (2007) J. Biol. Chem. 282, 26845–26856 [DOI] [PubMed] [Google Scholar]
- 26. Simpson J. C., Roberts L. M., Römisch K., Davey J., Wolf D. H., Lord J. M. (1999) FEBS Lett. 459, 80–84 [DOI] [PubMed] [Google Scholar]
- 27. Frankel A., Schlossman D., Welsh P., Hertler A., Withers D., Johnston S. (1989) Mol. Cell. Biol. 9, 415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schlossman D., Withers D., Welsh P., Alexander A., Robertus J., Frankel A. (1989) Mol. Cell. Biol. 9, 5012–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allen S. C., Moore K. A., Marsden C. J., Fülöp V., Moffat K. G., Lord J. M., Ladds G., Roberts L. M. (2007) FEBS J. 274, 5586–5599 [DOI] [PubMed] [Google Scholar]
- 30. Maley F., Trimble R. B., Tarentino A. L., Plummer T. H., Jr. (1989) Anal. Biochem. 180, 195–204 [DOI] [PubMed] [Google Scholar]
- 31. Di Cola A., Frigerio L., Lord J. M., Ceriotti A., Roberts L. M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14726–14731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Cola A., Frigerio L., Lord J. M., Roberts L. M., Ceriotti A. (2005) Plant Physiol. 137, 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wiertz E. J., Jones T. R., Sun L., Bogyo M., Geuze H. J., Ploegh H. L. (1996) Cell 84, 769–779 [DOI] [PubMed] [Google Scholar]
- 34. Patel S., Latterich M. (1998) Trends Cell Biol. 8, 65–71 [PubMed] [Google Scholar]
- 35. Fiebiger E., Hirsch C., Vyas J. M., Gordon E., Ploegh H. L., Tortorella D. (2004) Mol. Biol. Cell 15, 1635–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mueller B., Klemm E. J., Spooner E., Claessen J. H., Ploegh H. L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12325–12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mueller B., Lilley B. N., Ploegh H. L. (2006) J. Cell Biol. 175, 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lilley B. N., Ploegh H. L. (2004) Nature 429, 834–840 [DOI] [PubMed] [Google Scholar]
- 39. Sun F., Zhang R., Gong X., Geng X., Drain P. F., Frizzell R. A. (2006) J. Biol. Chem. 281, 36856–36863 [DOI] [PubMed] [Google Scholar]
- 40. Ye Y., Shibata Y., Yun C., Ron D., Rapoport T. A. (2004) Nature 429, 841–847 [DOI] [PubMed] [Google Scholar]
- 41. Qu D., Teckman J. H., Omura S., Perlmutter D. H. (1996) J. Biol. Chem. 271, 22791–22795 [DOI] [PubMed] [Google Scholar]
- 42. Teckman J. H., Qu D., Perlmutter D. H. (1996) Hepatology 24, 1504–1516 [DOI] [PubMed] [Google Scholar]
- 43. Bernardi K. M., Forster M. L., Lencer W. I., Tsai B. (2008) Mol. Biol. Cell 19, 877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. London E., Luongo C. L. (1989) Biochem. Biophys. Res. Commun. 160, 333–339 [DOI] [PubMed] [Google Scholar]
- 45. Deeks E. D., Cook J. P., Day P. J., Smith D. C., Roberts L. M., Lord J. M. (2002) Biochemistry 41, 3405–3413 [DOI] [PubMed] [Google Scholar]
- 46. Mayerhofer P. U., Cook J. P., Wahlman J., Pinheiro T. T., Moore K. A., Lord J. M., Johnson A. E., Roberts L. M. (2009) J. Biol. Chem. 284, 10232–10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ploegh H. L. (2007) Nature 448, 435–438 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.