Abstract
The activation of renin-angiotensin system contributes to the development of metabolic syndrome and diabetes as well as hypertension. However, it remains undetermined how renin-angiotensin system is implicated in feeding behavior. Here, we show that angiotensin II type 1 (AT1) receptor signaling regulates the hypothalamic neurocircuit that is involved in the control of food intake. Compared with wild-type Agtr1a+/+ mice, AT1 receptor knock-out (Agtr1a−/−) mice were hyperphagic and obese with increased adiposity on an ad libitum diet, whereas Agtr1a−/− mice were lean with decreased adiposity on a pair-fed diet. In the hypothalamus, mRNA levels of anorexigenic neuropeptide corticotropin-releasing hormone (Crh) were lower in Agtr1a−/− mice than in Agtr1a+/+ mice both on an ad libitum and pair-fed diet. Furthermore, intracerebroventricular administration of CRH suppressed food intake both in Agtr1a+/+ and Agtr1a−/− mice. In addition, the Crh gene promoter was significantly transactivated via the cAMP-responsive element by angiotensin II stimulation. These results thus demonstrate that central AT1 receptor signaling plays a homeostatic role in the regulation of food intake by maintaining gene expression of Crh in hypothalamus and suggest a therapeutic potential of central AT1 receptor blockade in feeding disorders.
Keywords: Adipose Tissue, Gene Expression, Neuropeptide, Obesity, Receptors
Introduction
Maintaining energy homeostasis through regulated food intake and body weight is fundamental for survival. However, >1 billion people are now classified as obese or overweight, and the prevalence of these conditions is increasing rapidly (1). As a major risk factor for cardiovascular diseases and diabetes, obesity has become a leading public health threat and economic burden worldwide. On the other hand, weight loss is a serious and occasionally life-threatening problem in patients with anorexia nervosa and in cachectic patients suffering from chronic obstructive pulmonary disease and cancer. Although environmental and lifestyle factors contribute to body weight gain or loss, impaired regulation of food intake also underlies the pathogenesis of these eating disorders (2). Recent studies have demonstrated that feeding behavior is under control of the central neural circuit involving the hypothalamus. Hypothalamus contains several nuclei that exert homeostatic control of food intake through orexigenic and anorexigenic signals (2).
The renin-angiotensin system (RAS)3 plays a crucial role in the physiological control of blood pressure and fluid balance and also participates in the pathological processes in the development of cardiovascular and metabolic diseases. Although the activation of RAS in peripheral organs contributes to the development of obesity and the metabolic syndrome (3–5), it remains undetermined how RAS is implicated in feeding behavior. The effects of angiotensin II (Ang II), a pivotal bioactive molecule of RAS, are mainly mediated through the type 1 (AT1) receptor (6). In rodents, AT1 receptor consists of two subtypes (AT1a and AT1b), but AT1a receptor is predominantly expressed and functionally important in most organs including the heart, blood vessels, kidney, adrenal glands, brain, and adipose tissues (7, 8). Feeding behavior is regulated by multiple orexigenic peptides such as neuropeptide Y (NPY), agouti-related protein (AgRP), orexin, and melanin-concentrating hormone (MCH), and anorexigenic peptides such as corticotropin-releasing hormone (CRH), pro-opiomelanocortin (POMC), and cocaine- and amphetamine-regulated transcript (CART) in hypothalamus (2). The AT1a receptor is expressed in hypothalamus, including paraventricular nucleus (PVN), lateral hypothalamic area, perifornical nucleus, and retrochiasmatic area (9), but the regulatory role of AT1 receptor signaling in the hypothalamic neuronal circuit remains precisely unknown.
In the present study, we demonstrate that AT1a receptor knock-out (Agtr1a−/−) mice were hyperphagic and obese on an ad libitum diet, but not on a pair-fed diet, compared with wild-type Agtr1a+/+ mice and that central AT1 receptor signaling controls food intake by regulating anorexigenic Crh gene expression in hypothalamus.
EXPERIMENTAL PROCEDURES
Animals
Generation of Agtr1a−/− mice on the C57BL/6J Jcl background has been described previously (10). Male mice were used in this study. We maintained mice in individual cages under a temperature- and humidity-controlled condition with a 12 h light-dark cycle. Mice were allowed free access to water and standard chow (343.1 kcal/100 g; crude protein 25.1%; crude fat 4.8%; crude fiber 4.2%; crude ash 6.7%; nitrogen-free extract 50.0%; Clea Japan, Tokyo, Japan) on an ad libitum-feeding. For pair-feeding experiments, Agtr1a−/− mice were restricted to the amount of food consumed by ad libitum-fed Agtr1a+/+ mice, as described previously (11). Pair feeding was started on mice at 5 weeks of age, and continued for a period of 11 weeks. All of the experimental protocols were approved by the Institutional Animal Care and Use Committee of Chiba University.
Cold Exposure Test
Mice were kept at 4 °C for 60 min and then kept at room temperature for 30 min. Rectal body temperatures were measured with a thermometer (BWT-100; Bio Research Center, Nagoya, Japan) at 0, 60, 75, and 90 min during the experiment.
Blood and Urine Analysis
Blood glucose levels were determined by using ACCU-CHEK Blood Glucose Meter (Roche Diagnostics, Basel, Switzerland). For a glucose tolerance test, a glucose load was injected i.p. (1 g/kg body weight) after a 16 h fast. Blood glucose concentrations were measured at 0 (before), 30, 60, 90, and 120 min after the injection. For insulin tolerance test, insulin (Humulin R; Eli Lilly, Indianapolis, IN) was injected i.p. (1 unit/kg body weight) after a 1-h fast. Blood glucose concentrations were measured at 0 (before), 15, 30, 45, and 60 min after the injection. Serum leptin concentrations were assayed by using mouse leptin quantikine ELISA kit (R&D Systems) according to the manufacturer's protocol. Urine concentrations of catecholamines were measured by HPLC in the laboratory of SRL, Inc. (Tokyo, Japan).
Histological Analysis
The liver and epididymal fat were excised, immediately fixed in 10% neutralized formalin, and embedded in paraffin. Sections at 5 μm were stained with hematoxylin and eosin.
In Situ Hybridization
The mice were decapitated, and the brains were rapidly removed and frozen. Frozen sections at 12 μm were used for in situ hybridization, as described previously (12). Antisense probes were 3′-end labeled with 35S by using oligonucleotides complementary to mRNAs of Npy, Agrp, Hcrt, Pmch, Crh, Pomc, and Cartpt. A semi-quantitative image analysis was performed with the MCID Image Analysis System (Imaging Research, St. Catharines, Ontario, Canada).
Intracerebroventricular Administration of CRH
The mice were anesthetized with inhalation of diethyl ether. Vehicle or 0.2 μg of CRH (Sigma-Aldrich, St. Louis, MO) in a total volume of 1 μl was administered intracerebroventricularly (i.c.v.) to the anesthetized mice by using a Hamilton syringe with a catheter PH03 (Hamilton Company, Reno, NV) into the right lateral ventricle using the coordinates: 0.5 mm caudal to bregma, 1 mm lateral to sagital suture, and 2 mm in depth.
Luciferase Assays
HEK293 cells expressing the AT1 receptor were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and were starved under a serum-free condition for 48 h before stimulation with 10−7 m of Ang II (Sigma-Aldrich) (13). The Crh luciferase reporter plasmids, with or without the expression vector for dominant-negative form of cAMP-responsive element (CRE)-binding protein (DN-CREB), was transfected by the FuGENE 6 Transfection Regent (Roche Diagnostics), according to the manufacturer's instructions. pRL-SV40 (Promega, Madison, WI) was co-transfected as an internal control. Luciferase activities were measured 24 h after Ang II stimulation by using the Dual-Luciferase reporter assay system (Promega). Experiments were repeated at least three times in triplicate, and representative data are shown. The Crh luciferase reporter plasmids and the expression vector for dominant-negative form of CRE-binding protein (DN-CREB) (14) were a generous gift from Dr. Y. Iwasaki (Kochi University).
Statistics
The results are expressed as the mean ± S.E. Differences in measured values were evaluated with an analysis of variance using Fisher's t test and an unpaired Student's t test. Values of p < 0.05 were considered statistically significant.
RESULTS
Agtr1a−/− Mice Are Obese and Hyperphagic under ad Libitum Feeding
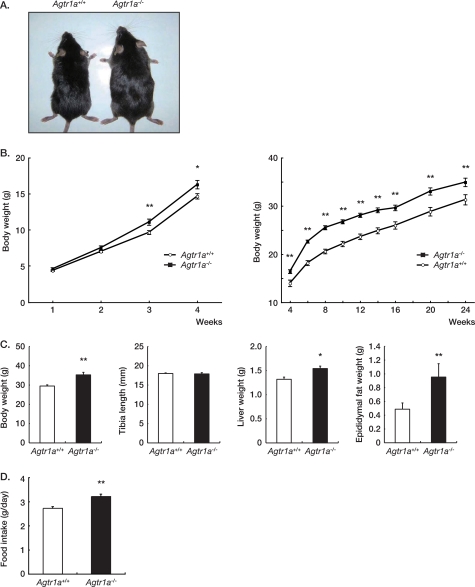
We first compared the body weight between male Agtr1a+/+ and Agtr1a−/− mice on the C57BL/6 background. When maintained ad libitum on standard laboratory chow, Agtr1a−/− mice were significantly heavier than Agtr1a+/+ mice after 3 weeks of age and weighed 11.5 ± 2.9% more than Agtr1a+/+ mice at 24 weeks of age (Fig. 1, A–C). Although the tibial length was indistinguishable, liver and epididymal fat were significantly heavier in Agtr1a−/− mice (Fig. 1C). Because an excess of body weight results from an imbalance between food intake and energy expenditure, we compared the food intake between Agtr1a+/+ and Agtr1a−/− mice. The daily food intake of Agtr1a−/− mice was significantly more throughout the course of observation, than that of Agtr1a+/+ mice (Fig. 1D).
FIGURE 1.
Agtr1a−/− mice are obese and hyperphagic on a standard diet. A, gross appearance of male Agtr1a+/+ and Agtr1a−/− mice at 16 weeks of age. B, growth curves of male Agtr1a+/+ (n = 20) and Agtr1a−/− (n = 25) mice. **, p < 0.01. C, body and organ weight, and tibia length of male Agtr1a+/+ (n = 9) and Agtr1a−/− (n = 9) mice at 24 weeks of age. *, p < 0.05; **, p < 0.01. D, daily food intake of ad libitum-fed male Agtr1a+/+ (n = 20) and Agtr1a−/− (n = 25) mice at 16 weeks of age. **, p < 0.01.
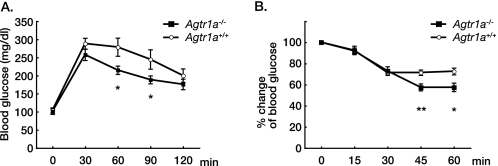
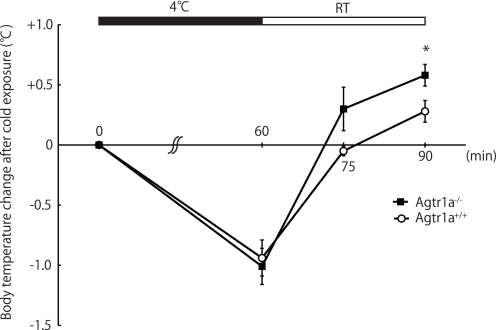
On the other hand, i.p. glucose tolerance test and insulin tolerance test revealed that the glucose disposal and the hypoglycemic effect of insulin were pronounced in Agtr1a−/− mice compared with Agtr1a+/+ mice (Fig. 2). These results suggest that Agtr1a−/− mice have better glucose tolerance and insulin sensitivity despite increased food intake, body weight, and adiposity. Although the core body temperatures were comparable under basal conditions (Agtr1a+/+, 38.5 ± 0.1 °C; Agtr1a−/−, 38.4 ± 0.1 °C; n = 8 per group; p = 0.234), Agtr1a−/− mice showed enhanced thermogenic response after cold exposure, compared with Agtr1a+/+ mice (Fig. 3), suggesting that energy expenditure was increased in Agtr1a−/− mice, as reported previously (15). In addition, clinical and experimental studies have demonstrated that pharmacological inhibition of RAS improves insulin sensitivity in part through increased glucose uptake in skeletal muscle (3). We suppose that the beneficial effect of Agtr1a deficiency on glucose metabolism overcomes the detrimental effect of increased body weight and adiposity in Agtr1a−/− mice.
FIGURE 2.
Agtr1a−/− mice show lower glucose concentrations than Agtr1a+/+ mice after i.p. injection of glucose or insulin. A, glucose tolerance test (Agtr1a+/+, n = 8; Agtr1a−/−, n = 11) at 24 weeks of age. *, p < 0.05. B, insulin tolerance test (Agtr1a+/+, n = 8; Agtr1a−/−, n = 9) at 24 weeks of age. *, p < 0.05; **, p < 0.01.
FIGURE 3.
Agtr1a−/− mice show enhanced thermogenic response after cold exposure. Changes in body temperature after cold exposure in Agtr1a+/+ (n = 8) and Agtr1a−/− (n = 8) mice on an ad libitum diet at 16 weeks of age. *, p < 0.05. RT, room temperature.
Agtr1a−/− Mice Weigh Less Than Agtr1a+/+ Mice under Pair feeding
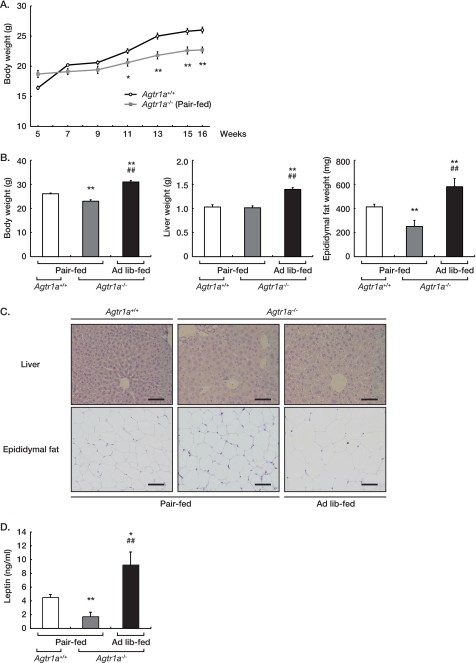
Next, we examined the relevance of hyperphagia to increased body weight gain and adiposity in Agtr1a−/− mice. For this purpose, we restricted 5-week-old Agtr1a−/− mice to the amount of food consumed by ad libitum-fed Agtr1a+/+ littermate mice and continued pair-feeding for 11 weeks. On a pair-fed diet, the body weight gain of Agtr1a−/− mice was less than that of Agtr1a+/+ mice, and Agtr1a−/− mice weighed 11.9 ± 2.3% less than Agtr1a+/+ mice at 16 weeks of age (Fig. 4, A and B). In parallel with body weight, the weights of liver and epididymal fat in pair-fed Agtr1a−/− mice were comparable with or less than those of Agtr1a+/+ mice (liver: p = 0.806; epididymal fat; p < 0.01, Fig. 4B). Histological analysis revealed that hepatic steatosis and adipocyte hypertrophy were prominent in ad libitum-fed Agtr1a−/− mice, but not in pair-fed Agtr1a−/− mice (Fig. 4C). In general, weight gain and fat content are associated with increased levels of leptin (16). The serum leptin concentrations of Agtr1a−/− mice were significantly higher on an ad libitum-fed diet, but lower on a pair-fed diet, than those of Agtr1a+/+ mice (Fig. 4D). In pair-fed Agtr1a−/− mice, sympathetic activation may induce higher levels of energy expenditure, resulting in less body weight. Supporting this hypothesis, pair-fed Agtr1a−/− mice exhibited higher levels of daily urinary catecholamine than Agtr1a+/+ mice (Table 1). Therefore, we suppose that an excess of food intake is causative to increased body weight and adiposity in ad libitum-fed Agtr1a−/− mice.
FIGURE 4.
Pair feeding supernormalizes obesity in Agtr1a−/− mice. A, growth curves of male Agtr1a+/+ (n = 13) and pair-fed Agtr1a−/− (n = 13) mice. Male Agtr1a−/− mice were pair-fed with the same amount of chow consumed by Agtr1a+/+ mice from 5 to 16 weeks of age. *, p < 0.05; **, p < 0.01. B, body and organ weight of male Agtr1a+/+ (n = 10), pair-fed Agtr1a−/− (n = 7), and ad libitum (Ad lib)-fed Agtr1a−/− (n = 10) mice at 16 weeks of age. *, p < 0.05 versus Agtr1a+/+; **, p < 0.01 versus Agtr1a+/+; #, p < 0.05 versus pair-fed Agtr1a−/−; ##, p < 0.01 versus pair-fed Agtr1a−/−. C, histological analysis of liver and epididymal fat of male Agtr1a+/+, pair-fed Agtr1a−/−, and ad libitum-fed Agtr1a−/− mice at 16 weeks of age. All sections were stained with hematoxylin and eosin. Scale bars, 50 μm. D, serum leptin concentrations in male Agtr1a+/+ (n = 7), pair-fed Agtr1a−/− (n = 7), and ad libitum-fed Agtr1a−/− (n = 7) mice at 16 weeks of age. *, p < 0.05 versus Agtr1a+/+; **, p < 0.01 versus Agtr1a+/+; #, p < 0.05 versus pair-fed Agtr1a−/−; ##, p < 0.01 versus pair-fed Agtr1a−/−.
TABLE 1.
Urinary excretions of catecholamines in pair-fed Agtr1a+/+ (n = 7) and Agtr1a−/− (n = 7) mice at 6 weeks of age
| Agtr1a+/+ | Agtr1a−/− | |
|---|---|---|
| Urine volume (μl/day) | 632.0 ± 113.9 | 1836.1 ± 253.3a |
| Urinary norepinephrine (ng/day) | 1.1 ± 0.2 | 5.9 ± 1.3b |
| Urinary epinephrine (ng/day) | 8.5 ± 2.9 | 78.0 ± 20.6b |
| Urinary dopamine (ng/day) | 92.4 ± 9.1 | 345.5 ± 93.6b |
a p < 0.01.
b p < 0.05.
Hypothalamic Crh Expression Is Decreased in ad Libitum- and Pair-fed Agtr1a−/− Mice
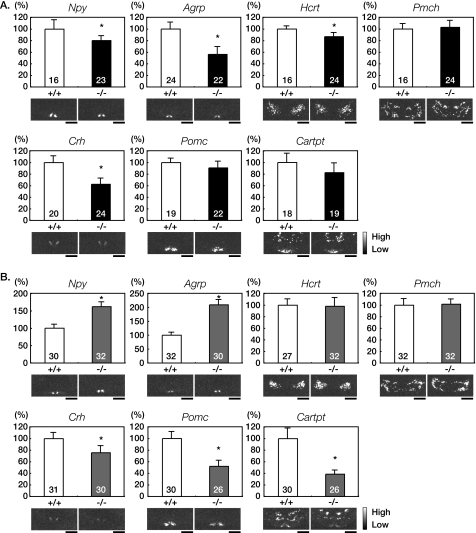
Feeding behavior is regulated by multiple orexigenic and anorexigenic peptides in hypothalamus (2). To elucidate the mechanism underlying hyperphagia in Agtr1a−/− mice, we performed semi-quantitative in situ hybridization analysis of appetite-related peptide genes in the hypothalamus (2). Despite hyperphagia, the expression levels of orexigenic Npy and Agrp in arcuate nucleus and Hcrt in lateral hypotharamic area were lower in ad libitum-fed Agtr1a−/− mice than in Agtr1a+/+ mice (Fig. 5A). In contrast, the expression levels of Npy and Agrp were higher in pair-fed Agtr1a−/− mice (Fig. 5B). It has been reported that Npy and Agrp mRNA expression are suppressed in the state of high leptin levels and enhanced in the state of low leptin levels (17–19). In Agtr1a−/− mice, the Npy and Agrp expressions were in inverse proportion to the levels of leptin concentration and adiposity. The expression levels of anorexigenic Pomc and Cartpt were decreased in Agtr1a−/− mice, which were statistically indistinguishable from those in Agtr1a+/+ mice. Notably, the expression levels of anorexigenic Crh in PVN were significantly decreased in both ad libitum- or pair-fed Agtr1a−/− mice, compared with Agtr1a+/+ mice (Fig. 5, A and B). It has been reported that the Crh expression is stimulated by leptin and that a CRH antagonist attenuates the leptin-induced reduction in food intake and body weight (20, 21). Therefore, these results raised the possibility that a down-regulation of anorexigenic Crh, occurring irrespective of the leptin levels, was the cause of hyperphagia in Agtr1a−/− mice.
FIGURE 5.
Hypothalamic Crh expression is decreased in ad libitum- and pair-fed Agtr1a−/− mice. A, quantitative in situ hybridization analysis of hypothalamic orexigenic and anorexigenic peptides in ad libitum-fed Agtr1a+/+ and Agtr1a−/− mice at 16 weeks of age. The number of sections for each analysis is indicated in the bars. *, p < 0.05. Scale bars, 1 mm. B, quantitative in situ hybridization analysis of hypothalamic orexigenic and anorexigenic peptides in pair-fed Agtr1a+/+ and Agtr1a−/− mice at 16 weeks of age. The number of sections for each analysis is indicated in the bars. *, p < 0.05. Scale bars, 1 mm.
i.c.v. Injection of CRH Reduces Food Intake in Agtr1a−/− Mice
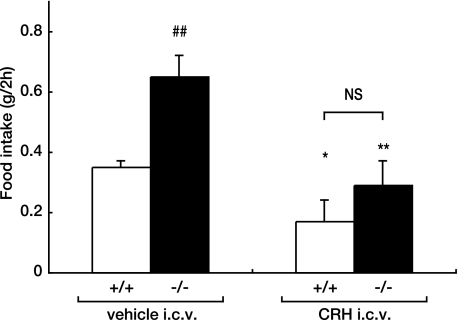
CRH is a major regulator of the hypothalamo-pituitary-adrenocortical axis and also functions as an endogenous inhibitor of food intake (2). Indeed, i.c.v. injection of CRH reduced spontaneous or deprivation-induced feeding (22), but Crh−/− mice did not exhibit an increase in food intake under physiological conditions (23). In general, appetite in animals and humans is closely related to the glucocorticoid levels. Although Crh−/− mice showed a markedly low corticosterone levels (23), the serum corticosterone concentrations were not significantly different between Agtr1a+/+ and Agtr1a−/− mice on an ad libitum diet (supplemental Fig. S1). Interestingly, it has been reported that the food intake of Crh−/− mice was significantly higher than that of Crh+/+ mice, when the corticosterone levels were adjusted to the equal levels through adrenalectomy and corticosterone replacement (24). We speculate that down-regulation of Crh in Agtr1a−/− mice activates downstream satiety and reward circuits to increase food intake without altering the corticosterone levels through undefined compensatory mechanisms. Importantly, administration of CRH by i.c.v. injection reduced food intake during 2 h to the indistinguishable level between Agtr1a−/− and Agtr1a+/+ mice (Fig. 6). These results strongly suggest that down-regulation of anorexigenic Crh is the underlying mechanism of hyperphagia in Agtr1a−/−.
FIGURE 6.
i.c.v. injection of CRH reduces food intake in Agtr1a−/− mice. Food intake over 2 h after i.c.v. administration of CRH or vehicle in Agtr1a+/+ and Agtr1a−/− mice at 8 weeks of age. *, p < 0.05 versus vehicle; **, p < 0.01 versus vehicle; ##, p < 0.01 versus Agtr1a+/+. NS, not significant.
AT1 Receptor Signaling Regulates Crh Gene Transcription via cAMP-responsive Element
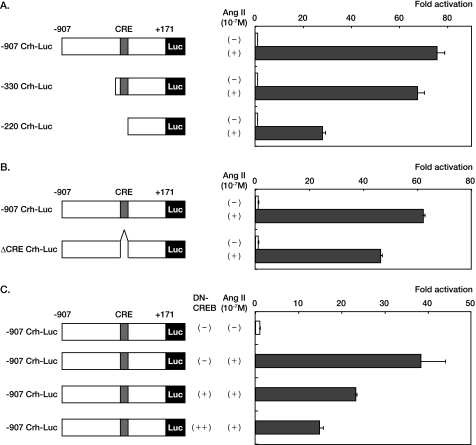
Next, to examine how AT1 receptor signaling regulates Crh gene transcription, we transfected a luciferase reporter containing the −907 bp promoter of the human Crh gene (−907 Crh-Luc) into HEK293 cells expressing AT1 receptor. After stimulation with 10−7 m of Ang II, a strong transactivation of the Crh gene promoter was observed (Fig. 7A). Deletion of the Crh promoter between −907 and −220 markedly reduced the fold activation by Ang II stimulation, although deletion between −907 and −330 had a marginal effect (Fig. 7A). Deletion of the cAMP-responsive element (CRE) located between −330 and −220 of the Crh promoter (14, 25) significantly reduced the fold activation by Ang II stimulation (Fig. 7B), and co-transfection of an expression vector for DN-CREB diminished Ang II-induced fold activation of −907 Crh-Luc in a dose-dependent manner (Fig. 7C). These results suggest that the AT1 receptor signaling regulates Crh gene transcription via CRE.
FIGURE 7.
AT1 receptor signaling regulates Crh gene transcription. A and B, the luciferase reporters containing full-length (−907 Crh-Luc), deleted (−330 Crh-Luc or −220 Crh-Luc), or CRE-mutated (ΔCRE Crh-Luc) were transfected in HEK293 cells expressing AT1 receptor (HEK293-AT1 cells). The cells were stimulated with Ang II (10−7 m) 24 h before the measurement of luciferase activities. C, the luciferase reporters containing full-length Crh promoter (−907 Crh-Luc) was co-transfected in HEK293-AT1 cells with an expression plasmid of DN-CREB. The cells were stimulated with Ang II (10−7 m) 24 h before the measurement of luciferase activities.
DISCUSSION
Our present study demonstrated a hitherto undefined role of the AT1 receptor signaling in the regulation of hypothalamic neurocircuit that is involved in the control of food intake. Ang II is produced by cleavage of angiotensiongen by renin and ACE. Mice deficient for angiotensinogen (Agt) or renin (Ren1c) are similarly resistant to diet-induced weight gain (26, 27). On a high-fat diet, no differences in food intake were observed between these mutant mice and wild-type mice, although the food intake of Agt−/− mice was significantly more than that of Agt+/+ mice on a standard chow diet (26). On the other hand, mice deficient for ACE (Ace) are lean, exhibiting unchanged food intake and increased energy expenditure (28). Insomuch as the renal abnormalities observed in Agt−/− mice are quantitatively similar to those of mutant mice homozygous for both AT1a and AT1b (29, 30), it is possible that the effect of Ang II on AT1b receptor might contribute to feeding behavior in Agtr1a−/− mice. However, the compensatory effect of Ang II receptor subtypes was minimal, because the mRNA levels of the AT1b (Agtr1b) and AT2 (Agtr2) receptors in the hypothalamus did not differ significantly between Agtr1a+/+ and Agtr1a−/− mice (supplemental Fig. S2). Kouyama and colleagues reported that Agtr1a−/− mice showed attenuation of high-fat diet-induced weight gain and adiposity, which was accompanied by unchanged food intake and increased energy expenditure (15). However, they also described that Agtr1a−/− mice ate more on a calorie basis than Agtr1a+/+ mice on a standard chow diet (15). In our hands, Agtr1a−/− mice were initially hyperphagic, compared with Agtr1a+/+ mice, when maintained ad libitum on a high-fat diet (supplemental Fig. S3A). However, the difference in daily food intake between Agtr1a−/− and Agtr1a+/+ mice became insignificant after 6 weeks of high-fat diet (supplemental Fig. S3B). The reasons for this phenomenon are currently unclear, but unpalatable taste of high-fat chow and altered metabolic status due to high-fat diet might potentially contribute to it. As a consequence, Agtr1a−/− weighed 9.8 ± 3.7% more than Agtr1a+/+ mice after 6 weeks of high-fat diet (supplemental Fig. S3B), but the difference in body weight between Agtr1a−/− and Agtr1a+/+ mice was not statistically significant (p = 0.053). We assume that to gain or to lose weight may be determined upon a delicate balance between the counteracting effects of the central versus peripheral function of AT1 receptor signaling in Agtr1a−/− mice. Our Agtr1a−/− mice seem to be more susceptible to body weight gain than those used by Kouyama et al. (15), on a standard or high-fat chow, which might be caused by the differences in the extent of energy expenditure, potentially resulting from different housing conditions. Further studies will be needed to elucidate the differential regulation of feeding behavior by RAS components.
It has been reported that neuroendocrine dysfunction including CRH hyperactivity has been associated with anorexia nervosa (31), and AT1 receptor expression in the PVN is increased in several stress models, contributing to enhanced CRH production and release (32). Although AT1 receptor blockers are widely used to treat hypertension, the effects of AT1 receptor blockers on feeding behavior have not been well documented in clinical practice. Subcutaneous administration of an AT1 receptor blocker olmesartan in wild-type mice lowered blood pressure to the level comparable with that of Agtr1a−/− mice (supplemental Fig. S4A), but the body weight and daily food intake did not differ significantly between olmesartan- and vehicle-treated mice (supplemental Fig. S4B). In addition, the expression levels of hypothalamic appetite-related peptides genes were unchanged (supplemental Fig. S4C). On the other hand, i.c.v. administration of olmesartan significantly increased food intake in wild-type mice (supplemental Fig. S5). These data indicate that peripheral administration of olmesartan is insufficient to inhibit the AT1 receptor signaling in the PVN and thus has little effect on hypothalamic neurocircuits and food intake. However, we propose that the hypothalamic AT1 receptor signaling will be a therapeutic target against neurotic and stress-induced eating disorders, if we develop an AT1 receptor blocker with high ability to penetrate the PVN nuclei and to inhibit the hypothalamic AT1 receptor signaling effectively.
We conclude that the AT1 receptor signaling is involved in feeding behavior by regulating Crh gene expression in the PVN. Elucidation of the mechanism by which the AT1 receptor signaling regulates feeding behavior via neuropeptides circuits will provide a clue to the management of cardiovascular, metabolic, and eating disorders.
Supplementary Material
Acknowledgments
We thank A. Furuyama, M. Ikeda, Y. Ohtsuki, I. Sakamoto, and M. Kikuchi for excellent technical assistance. We thank Dr. M. Nakazato (Miyazaki University) for valuable comments and critical reading of our manuscript.
This work was supported in part by grants from Japan Society for the Promotion of Science (KAKENHI 20390218 and 21229010); Health and Labor Sciences Research Grants (to I. K. and H. A.) and grants from Takeda Science Foundation, Astellas Foundation for Research on Metabolic Disorders, The Uehara Memorial Foundation, The Ichiro Kanehara Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Suzuken Memorial Foundation (to H. A.); and a Research Fellowship from the Japan Association for the Advancement of Medical Equipment (to R. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. S1–S5.
- RAS
- renin-angiotensin system
- AgRP
- agouti-related protein
- AngII
- angiotensin II
- AT1 receptor
- AngII type 1 receptor
- CART
- cocaine- and amphetamine-regulated transcript
- CRE
- cAMP-responsive element
- CREB
- CRE-binding protein
- CRH
- corticotropin-releasing hormone
- Luc
- luciferase
- DN
- dominant-negative
- i.c.v.
- intracerebroventricular
- MCH
- melanin-concentrating hormone
- NPY
- neuropeptide Y
- POMC
- pro-opiomelanocortin
- PVN
- paraventricular nucleus.
REFERENCES
- 1. Haslam D. W., James W. P. (2005) Lancet 366, 1197–1209 [DOI] [PubMed] [Google Scholar]
- 2. Morton G. J., Cummings D. E., Baskin D. G., Barsh G. S., Schwartz M. W. (2006) Nature 443, 289–295 [DOI] [PubMed] [Google Scholar]
- 3. Prasad A., Quyyumi A. A. (2004) Circulation 110, 1507–1512 [DOI] [PubMed] [Google Scholar]
- 4. Hunyady L., Catt K. J. (2006) Mol. Endocrinol. 20, 953–970 [DOI] [PubMed] [Google Scholar]
- 5. Sharma A. M. (2008) Nat. Clin. Pract. Cardiovasc. Med. 5, S3–9 [DOI] [PubMed] [Google Scholar]
- 6. de Gasparo M., Catt K. J., Inagami T., Wright J. W., Unger T. (2000) Pharmacol. Rev. 52, 415–472 [PubMed] [Google Scholar]
- 7. Burson J. M., Aguilera G., Gross K. W., Sigmund C. D. (1994) Am. J. Physiol. 267, E260–267 [DOI] [PubMed] [Google Scholar]
- 8. Gasc J. M., Shanmugam S., Sibony M., Corvol P. (1994) Hypertension 24, 531–537 [DOI] [PubMed] [Google Scholar]
- 9. Lenkei Z., Palkovits M., Corvol P., Llorens-Cortès C. (1997) Front Neuroendocrinol. 18, 383–439 [DOI] [PubMed] [Google Scholar]
- 10. Sugaya T., Nishimatsu S., Tanimoto K., Takimoto E., Yamagishi T., Imamura K., Goto S., Imaizumi K., Hisada Y., Otsuka A., Uchida H., Sugiura M., Fukuta K., Fukamizu A., Murakami K. (1995) J. Biol. Chem. 270, 18719–18722 [DOI] [PubMed] [Google Scholar]
- 11. Song Y. H., Li Y., Du J., Mitch W. E., Rosenthal N., Delafontaine P. (2005) J. Clin. Invest. 115, 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashimoto H., Azuma Y., Kawasaki M., Fujihara H., Onuma E., Yamada-Okabe H., Takuwa Y., Ogata E., Ueta Y. (2007) Clin. Cancer Res. 13, 292–298 [DOI] [PubMed] [Google Scholar]
- 13. Zou Y., Akazawa H., Qin Y., Sano M., Takano H., Minamino T., Makita N., Iwanaga K., Zhu W., Kudoh S., Toko H., Tamura K., Kihara M., Nagai T., Fukamizu A., Umemura S., Iiri T., Fujita T., Komuro I. (2004) Nat. Cell Biol. 6, 499–506 [DOI] [PubMed] [Google Scholar]
- 14. Yamamori E., Asai M., Yoshida M., Takano K., Itoi K., Oiso Y., Iwasaki Y. (2004) J. Mol. Endocrinol. 33, 639–649 [DOI] [PubMed] [Google Scholar]
- 15. Kouyama R., Suganami T., Nishida J., Tanaka M., Toyoda T., Kiso M., Chiwata T., Miyamoto Y., Yoshimasa Y., Fukamizu A., Horiuchi M., Hirata Y., Ogawa Y. (2005) Endocrinology 146, 3481–3489 [DOI] [PubMed] [Google Scholar]
- 16. Maffei M., Halaas J., Ravussin E., Pratley R. E., Lee G. H., Zhang Y., Fei H., Kim S., Lallone R., Ranganathan S., et al. (1995) Nat. Med. 1, 1155–1161 [DOI] [PubMed] [Google Scholar]
- 17. Schwartz M. W., Seeley R. J., Campfield L. A., Burn P., Baskin D. G. (1996) J. Clin. Invest. 98, 1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korner J., Savontaus E., Chua S. C., Jr., Leibel R. L., Wardlaw S. L. (2001) J. Neuroendocrinol. 13, 959–966 [DOI] [PubMed] [Google Scholar]
- 19. Kitamura T., Feng Y., Kitamura Y. I., Chua S. C., Jr., Xu A. W., Barsh G. S., Rossetti L., Accili D. (2006) Nat. Med. 12, 534–540 [DOI] [PubMed] [Google Scholar]
- 20. Uehara Y., Shimizu H., Ohtani K., Sato N., Mori M. (1998) Diabetes 47, 890–893 [DOI] [PubMed] [Google Scholar]
- 21. Masaki T., Yoshimichi G., Chiba S., Yasuda T., Noguchi H., Kakuma T., Sakata T., Yoshimatsu H. (2003) Endocrinology 144, 3547–3554 [DOI] [PubMed] [Google Scholar]
- 22. Heinrichs S. C., Richard D. (1999) Neuropeptides 33, 350–359 [DOI] [PubMed] [Google Scholar]
- 23. Muglia L., Jacobson L., Dikkes P., Majzoub J. A. (1995) Nature 373, 427–432 [DOI] [PubMed] [Google Scholar]
- 24. Jacobson L. (1999) Endocrinology 140, 310–317 [DOI] [PubMed] [Google Scholar]
- 25. Yamamori E., Iwasaki Y., Taguchi T., Nishiyama M., Yoshida M., Asai M., Oiso Y., Itoi K., Kambayashi M., Hashimoto K. (2007) Mol. Cell. Endocrinol. 264, 142–148 [DOI] [PubMed] [Google Scholar]
- 26. Massiera F., Seydoux J., Geloen A., Quignard-Boulange A., Turban S., Saint-Marc P., Fukamizu A., Negrel R., Ailhaud G., Teboul M. (2001) Endocrinology 142, 5220–5225 [DOI] [PubMed] [Google Scholar]
- 27. Takahashi N., Li F., Hua K., Deng J., Wang C. H., Bowers R. R., Bartness T. J., Kim H. S., Harp J. B. (2007) Cell Metab. 6, 506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jayasooriya A. P., Mathai M. L., Walker L. L., Begg D. P., Denton D. A., Cameron-Smith D., Egan G. F., McKinley M. J., Rodger P. D., Sinclair A. J., Wark J. D., Weisinger H. S., Jois M., Weisinger R. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6531–6536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsuchida S., Matsusaka T., Chen X., Okubo S., Niimura F., Nishimura H., Fogo A., Utsunomiya H., Inagami T., Ichikawa I. (1998) J. Clin. Invest. 101, 755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oliverio M. I., Kim H. S., Ito M., Le T., Audoly L., Best C. F., Hiller S., Kluckman K., Maeda N., Smithies O., Coffman T. M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 15496–15501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bailer U. F., Kaye W. H. (2003) Curr. Drug. Targets CNS Neurol Disord 2, 53–59 [DOI] [PubMed] [Google Scholar]
- 32. Sommer W. H., Saavedra J. M. (2008) J. Mol. Med. 86, 723–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.