Abstract
Sterol regulatory element binding proteins (SREBPs) regulate the expression of a number of enzymes, which catalyze the synthesis of fatty acids, cholesterol, triglycerides, and phospholipids. SREBP1c is the most relevant isoform in the adult liver, and its expression is controlled by the nutritional state. Transcriptional regulation studies into the SREBP1c gene, performed in the last few years, have improved our knowledge of the variability of signals that converge on its promoter region. Insulin, cholesterol derivatives, T3 and other endogenous molecules have been demonstrated to regulate the SREBP1c expression, particularly in rodents. The present study aimed to perform a detailed analysis of the human SREBP1c gene promoter structure in liver cells by focusing on responses to diverse metabolic signals. Serial deletion and mutation assays reveal that both SREBP (SRE) and LXR (LXRE) response elements are involved in SREBP1c transcription regulation mediated by insulin and cholesterol derivatives. We discovered that peroxisome proliferation-activated receptor alpha (PPARα) agonists enhance the activity of the SREBP1c promoter; a DR1 element, at −453 in the human promoter was involved in this activation. Moreover, PPARα agonists act in cooperation with LXR or insulin to induce lipogenesis. Collectively, our results identify PPARα as a novel regulatory factor in SREBP1c regulation which plays a relevant role in the interplay between lipids and insulin metabolic regulation.
Keywords: Gene Regulation, Liver, PPAR, Promoters, Transcription, LXR, SREBP1
Introduction
The prevalence of overweight and obesity is increasing worldwide at an alarming rate. An excess amount of body fat not only leads to reduced quality of life and immense healthcare-associated costs but also increases risk of death. Indeed, obesity has been related to a number of cardiovascular and metabolic disorders such as hypertension, type 2 diabetes, hyperinsulinemia, dyslipidemia, and atherosclerosis, all of them defining features of the metabolic syndrome. Beyond obesity and a number of independent factors, the other etiological factor of metabolic syndrome is insulin resistance, commonly considered to be of greater priority in pathogenesis (1, 2).
The discovery of sterol regulatory element binding proteins (SREBPs)4 was critical for our understanding of hepatic cholesterol homeostasis. SREBP1c, one of three SREBPs members of the basic helix-loop-helix family of transcription factors, is essential for the genomic actions of insulin on both carbohydrate and lipid metabolism (3) and plays a central role in the molecular biochemistry of metabolic syndrome. The SREBP1c expression is controlled by nutritional status. Fasting lowers SREBP1c mRNA and protein levels, whereas they are strongly induced in a fed state, followed by a compatible pattern of nutritional changes in lipogenic genes (4). Accordingly, changes in the activity of this transcription factor may be the key to linking insulin resistance with other obesity-associated metabolic disorders.
Liver X receptors (LXRs) belong to the nuclear hormone receptor superfamily. The LXR subfamily consists of two members, LXRα and LXRβ, which are activated by oxysterols. The LXRα expression pattern is mainly restricted to the liver, adipocytes, the small intestine, and macrophages, whereas LXRβ is expressed ubiquitously (5). LXRs directly bind the cis elements in the Srebp1c promoter as heterodimers with RXR, leading to transcriptional activation. Lipogenesis regulation by LXR is mediated through this effect on SREBP1c expression (6).
Along with LXR, other members of this superfamily of nuclear hormone receptors, peroxisome proliferator-activated receptors (PPARs) play a major role in lipid metabolism. The PPAR family is represented by three members: PPARα, the predominant form in the liver, PPARδ, and PPARγ. Different PPARs can be considered key messengers responsible for the translation of nutritional, pharmacological, and metabolic stimuli into changes in the expression of those genes specifically involved in lipid metabolism (7). Like LXRs, activated PPARs also heterodimerize with RXR and alter the transcription of target genes. These heterodimers bind to specific peroxisome proliferator response elements (PPREs) consisting of a direct repeat of a hexameric DNA core recognition motif spaced by one nucleotide (8). The overexpression of PPARα in HEK293 cells has been shown to inhibit mouse Srebp1c promoter activity through competition with LXR/RXR heterodimerization (9). Thus, hepatic lipid homeostasis is a result of a complex cross-talk between a number of transcription factors, including LXR, PPARs, and SREBPs.
In order to understand the molecular mechanism behind the nutritional regulation of the SREBP1c expression, the Srebp1c gene rodent promoter and, to a much lesser extent, the human regulatory region, has been previously characterized (10, 11). In the proximal region of the mice Srebp1c promoter, SP1, NFY, Upstream Stimulatory Factor (USF), SREBP, and LXR-binding sites have been identified (6, 12). An SRE element together with two LXREs motifs have proved indispensable for the insulin response (13). The sequence of the rat Srebp1c proximal promoter is 97% identical to its murine counterpart (14). Experiments carried out in the Marshall B. Elam laboratory have revealed that at least four unique transcription factor-binding elements recognized by LXRα, SREBP1, NFY, and SP1 constitute the insulin-response unit of the rat Srebp1c promoter (14, 15).
Sequence alignments show that the human SREBP1c promoter presents only 42.0% similarity to the mouse promoter, suggesting that promoters might be regulated by different pathways and mechanisms. In the present study, we extensively characterized the human proximal SREBP1c promoter by identifying the nutritional regulation mechanism in liver cells. Moreover, we identified a PPRE element in the proximal human sequence. In vitro and in vivo studies show the direct interaction of the PPAR receptor with the human SREBP1c promoter and propose a novel aspect of the network of transcription factors regulating human fatty acid metabolism.
EXPERIMENTAL PROCEDURES
Plasmids
A DNA fragment containing 1801 bp corresponding to the 5′ upstream region of the human SREBP1c gene was amplified by PCR and cloned into the pCR2.1-TOPO vector (Invitrogen) to construct pPro1c-TOPO. A 1564-bp fragment was obtained by NcoI digestion and subcloned in the NcoI site of pGL3-basic luciferase vector (Promega) to construct the −1564/+1-luc vector. The −520/+1-luc vector was prepared by PCR from the −1564/+1-luc vector using the forward primer 5′-GGAGGGTACCAGGCTCGCTCAGGGTGCCAGC-3′ and the reverse primer GLprimer2 (Promega) to be then inserted into the KpnI/NcoI site of the pGL3-basic vector. −310/+1-luc was prepared by XhoI digestion and religation from 1564/+1-luc. Mutagenesis was performed by means of the QuikChange site-directed Mutagenesis kit (Stratagene, La Jolla, CA) using pPro1c-TOPO as a template. All of the constructions were confirmed by nucleotide sequencing. The expression vectors pCMX-PPARα, pCMX-LXRα, pCMX-LXRβ, and pCMX-RXRα were obtained from Dr. Antonio Castrillo (Facultad de Medicina, Universidad de Las Palmas, Las Palmas de Gran Canaria, Spain).
Cell Culture and Luciferase Assay
Rat and mouse hepatocytes in primary culture were prepared from adult rats or mice by collagenase perfusion (16) and seeded in Williams' E medium, supplemented with glutamine, 100 nm insulin, 1 μm dexametasone, 5% FBS, and antibiotics. Transfection assays were performed using Lipofectamine 2000 (Invitrogen) in six-well culture dishes with 3.8 μg of SREBP1c reporter plasmids and 200 ng of Renilla luciferase vector (pRL-TK) 6 h after seeding. At 16 h post-transfection, hepatocytes were left for 24 h in basal induction medium (Williams' E, supplemented with glutamine, 0.75% BSA, 100 nm dexametasone, and 20 mm glucose). When indicated, cells were treated with the following: (a) 100 nm insulin, (b) 10 μm or 10 nm TO901317, (c) 30 μm WY14643, and (d) 1 μm GW7647. In some experiments, cells were co-transfected with 200 ng of the indicated expression vector, whereas the pCMX empty vector was used to normalize the amount of DNA. Transactivation activities were measured at 24 h post-transfection in a Wallac 1420 VICTOR luminometer according to the technical manual for the Dual-Luciferase Reporter Assay System (Promega).
Human hepatocytes were prepared from the liver biopsies obtained from patients submitted to a surgical resection of a liver tumor after obtaining patients' written consent. Hepatocytes isolation was based on the two-step collagenase procedure (17). Cell viability was consistently >85%, as determined by trypan blue exclusion. Hepatocytes (8 × 106 cells; 150,000 cells/cm2) were seeded at confluence on type I collagen-coated dishes (Iwaki, Gyouda, Japan) and maintained in a DMEM-Ham-F12:William's E (1:1) medium supplemented with 26 mm NaHCO, 15 mm HEPES, 0.29 g/liter glutamine, 50 mg/liter vitamin C, 0.04 mg/liter dexametasone, 2 mg/liter insulin, 200 μg/liter glucagon, 50 mg/liter transferrin, and 4 ng/liter ethanolamine containing 5% fetal calf serum for 12 h. Afterward, the medium was removed and replaced with a fresh culture medium supplemented, when indicated, with: (a) 100 nm insulin, (b) 10 μm or 10 nm TO901317, (c) 50 μm WY14643, and (d) 1 μm GW7647.
Electrophoretic Mobility Shift Assays
EMSAs were performed using double-stranded DNA oligonucleotides end-labeled with [γ-32P]dATP. The oligonucleotides corresponding to the DR1 binding site (5′-CCCTTCGTTAAAGGGTCAAAGCAGAGAAGTCCTGGCCC-3′), the LXRE1 binding site (5′-GGAGCTGAGGGCCAGTGACCGCCAGTAACCCCGGCAGACGCTGG-3′), and the LXRE2 binding site (5′-CGGGTTAAAGGCGGACGTCCGCTAGTAACCCCAACCCCATTCAGC-3′) were annealed with the complementary sequence by incubation at 85 °C for 10 min in 70 mm Tris-HCl (pH 7.5), 13 mm MgCl, 1.3 mm EDTA, 1.3 mm spermidine, and 6.7 mm DTT with overnight cooling. A 300-ng aliquot of the probe was labeled with 20 units of T4 polynucleotide kinase in the presence of 40 μCi [γ-32P]dATP at a final volume of 10 μl for 30 min at 37 °C and purified in Sephadex G25 columns. Binding reactions were carried out for 20 min at room temperature using in vitro-translated human PPARα, LXRα and RXRα prepared with the TNT T7-coupled reticulocyte lysate system (Promega), 9 fmol of probe, and 2 μg poly (dI/dC) in the binding buffer (20 mm HEPES (pH 8.0), 0.1 mm EDTA, 50s mm ClNA, 1 mm DTT, 5% glycerol, and 5 mm Cl2Mg). Each protein expression was confirmed by Western blot as described previously (18). Supershifts assays were carried out using specific α-PPARα (sc-9000), α-LXRα/β (sc-13068), and α-RXRα (sc-553) antibodies in the reaction mix for 30 min on ice before adding the probe. DNA-protein complexes were resolved on a 6% (w/v) non-denaturing polyacrylamide gels in 0.5× TBE buffer (1× TBE is 90 mm Tris, 90 mm boric acid, and 1 mm EDTA). The dry gel was exposed overnight in an immunoprecipitation screen and analyzed in a FLA5000 (Fujifilm).
Total RNA Preparation and RT-PCR
Total RNA was isolated from hepatocytes by using TRIzol reagent (Invitrogen). First-strand cDNA was synthesized from 1 μg of total RNA using a random hexamer and expand reverse transcriptase (Roche Diagnostics). The cDNA was used as a template for quantitative PCR using Syber Green reagent (Applied Biosystem) and specific primers for rat and mouse Srebp1c (forward primer, 5′-CCATGGATTGCACATTTGAA-3′ and reverse primer 5′-GGCCAGGGAAGTCACTGTCTT-3′) or human SREBP1c (forward primer, 5′-CCATGGATTGCACTTTCGAA-3′ and reverse primer 5′- GGCCAGGGAAGTCACTGTCTT-3′). The amount of total cDNA in the sample was analyzed in the same reaction using specific primers for mouse Gapdh (forward primer, 5′-GTATTGGGCGCCTGGTCAC-3′ and reverse primer, 5′-AATCTCCACTTTGCCACTGCA-3′), rat β-actin (forward primer, 5′-TTCACCACCCCAGCCATGT-3′ and reverse primer 5′-GTGGTACGACCAGAGGCATACA-3′) or human 36B4 (forward primer, 5′-AGATGCAGCAGATCCGCAT-3′ and reverse primer, 5′-GTTCTTGCCCATCAGCACC-3′) as a control. Fatty acid synthase (Hs00188012_m1) and acetyl-coA carboxylase (Hs00172885_m1) mRNA expression levels were quantified using the ABI 7500 fast instrument and Taqman technology (Assays-on-demand gene expression product; Applied Biosystems).
Chromatin Immunoprecipitation
ChIP assays were performed as described previously (19) using the isolated nuclei from the formaldehyde cross-linked human and rat hepatocytes. Immunoprecipitation was performed with the α-PPARα (sc-9000), α-RXRα (sc553), α-SREBP1 (sc-8984), and α-RNA pol II (sc-899) (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. Normal rat IgG (sc-2026) was employed as a negative control for rat hepatocytes. Purified samples were analyzed by PCR. The primers used to detect target sequences were as follows: human DR1/LXRE site (SREBP1c promoter), 5′-GCCAGGACTTCTCTGCTTTG-3′ and 5′-GGGTTGGGGTTACTAGCGGACG-3′; human SREBP1c coding region (exon 9), 5′-GGCTGCTGCCCCCAGT-3′ and 5′-GACAAAGAGAAGCACCAAGGAGAC-3′; human HMGCR (promoter), 5′-ACGCTGATTTGGGTCTATGG-3′ and 5′-GTGTAAATGGCTCCGGTCAC-3′; human α-actin (coding region), 5′-CTTCTGCCCTCCGCAGCTGA-3′ and 5′-GTGAATGCCCGCCGACTCCA-3′; rat LXRE site (Srebp1c promoter), 5′-CTGGCGCAGTTGCGGTTAAA-3′ and 5′-GCCGCGCCGCGCCCCAATAA-3′, rat SRE site (Srebp1c promoter), 5′-CTGCTGATTGGCCATGTGC-3′ and 5′-GCTACCCCTACAGCGTCCG-3′; rat Srebp1 coding region (exon 4), 5′-GCCCATCCACCGACTAGCAG-3′ and 5′-GGAACGGTAGCGCTTCTCAA-3′; and rat β-actin (coding region), 5′-TTCACCACCCCAGCCATGT-3′ and 5′-GTGGTACGACCAGAGGCATACA-3′. PCR fragments were generated with a 5-min melting step at 94 °C, followed by 45 cycles of amplification (94 °C for 30 s, 56 °C for 30 s, and 72 °C for 45 s) and a terminal extension. Each ChIP assay was performed at least twice to ensure reproducibility.
Confocal Microscopy
Human hepatocytes grown in 35-mm glass-bottomed dishes no. 1.5 (MatTek Corp., Ashland, MA) were used for live cell imaging. Cells were treated for 24 h under basal conditions or with 1 μm GW7647, 100 nm insulin, or a combination of both. Cells were fixed in 4% PFA (pH 7) for 10 min and washed three times with PBS 1×. Nile red staining was performed using the modified protocol described previously (20). Briefly, fixed cells were incubated for 4 h with 1 μg/ml of Nile red solution and washed in PBS 1× overnight. Afterward, cells were stained for 10 min with 8 μg/ml DAPI to visualize cell nuclei. Confocal images were obtained with a Leica TCS SP2 spectral microscope and a 63×/1.40 numerical aperture oil objective (Leica Microsystems, Wetzlar, Germany). Fluorescence intensity was measured with the ImageJ program.
Statistical Analysis
Values are expressed as mean ± S.D. (n ranged from three to five independent experiments). Statistical significance was estimated with the Student's two-tailed t test for unpaired observations. A p value of < 0.05 was considered significant.
RESULTS
Sequence Alignment of Rodents and Human SREBP1c Promoters
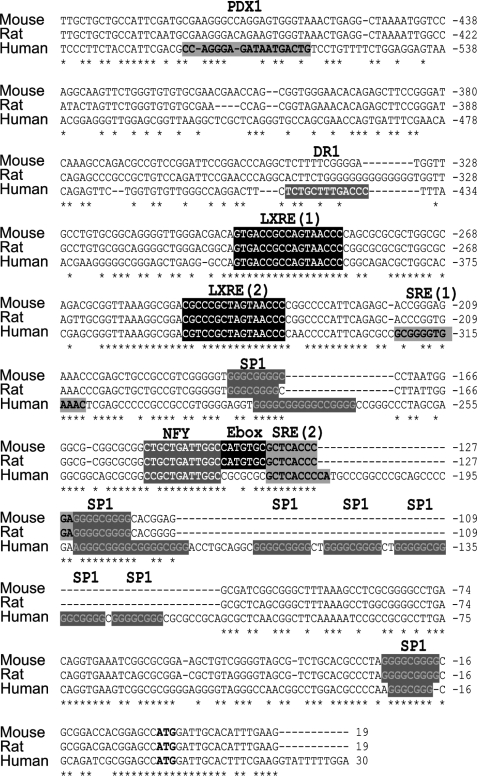
A comparative analysis of the human, rat, and mouse genomic sequences at the 5′ upstream position from the SREBP1c isoform transcriptional start site reveals a high degree of similarity, particularly in the first 300 bp (Fig. 1). However, some critical differences along the promoter region can be identified. First, the human sequence presents an expansion of 67 bp that contains at least five SP1 binding sites. Second, the E-box present in the mouse and rat promoters is absent in the human sequence. Third, the rat and mouse promoters present a unique SRE binding site, whereas an additional SRE element is found 100 bp upstream in the human promoter, as described previously (10). Two human exclusive regulatory sequences were observed in the distal region: a previously described PDX1 binding site (21) and a DR1 binding site predicted by an in silico analysis.
FIGURE 1.
Sequence analysis of the 5′-flanking sequence of the human SREBP1c promoter. Sequence alignment of the proximal promoter region of human, rat and mouse SREBP1c, including the transcriptional start site (boldface letters). A schematic representation of previously demonstrated binding sites in mice and rats, and the homolog sites in the human sequence are shown. Human specific predicted binding sites are also shown. *, conserved nucleotides in all three species.
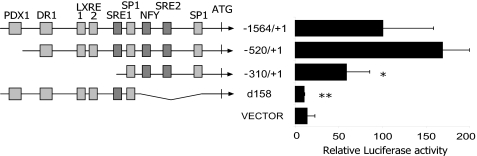
We first confirmed the identity of the basal transcription regulatory elements, which were predicted on the basis of the similarity between species of the proximal promoter region. A deletion analysis of 1500 bp of the human SREBP1c promoter cloned in a luciferase reporter vector was performed in transiently transfected rat primary hepatocytes. A construct containing 300 bp, which includes one SRE site, the NFY site, and all of the SP1 sites, was able to maintain almost 60% of the activity observed with the complete 1500-bp promoter luciferase construction (Fig. 2). The −520/+1 vector, which lacks only the PDX1 binding site, showed no diminished activity. Furthermore, a construction with a 158-bp deletion without the SRE2, the NFY, and all of the proximal SP1 binding sites, displayed no luciferase activity. This deletion analysis indicates that the PDX1, DR1, and LXRE sites are not relevant for the basal activity in hepatocytes of the human SREBP1c promoter.
FIGURE 2.
Identification of the minimal human SREBP1c promoter region. Serial of luciferase reporter constructs containing various lengths of the human SREBP1c promoter, from −1564 to +1, were analyzed in primary culture rat hepatocytes. The d158-luc construction has a deletion of 158 bp, which contains NFY, SRE2, and multiple SP1 sites corresponding to the region responsible for basal activity in mice and rats. Firefly and Renilla luciferase activities were measured in cell lysates. The results represent relative Firefly/Renilla luciferase activities by considering the −1564/+1 construct as 100% fold activation. Error bars represent the mean ± S.E. of four separate experiments. *, p < 0.05; **, p < 0.01.
Insulin Regulation Is Mediated by SRE and LXRE Sites
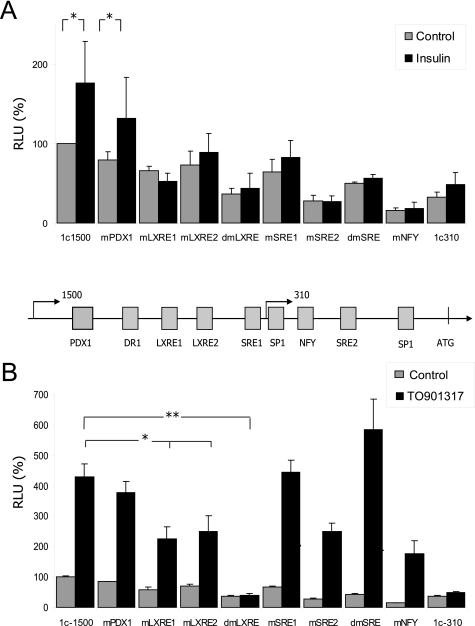
The insulin-mediated regulation of the SREBP1 pathway has been studied extensively in recent years. This hormone is the most important factor to specifically regulate the SREBP1c isoform, which reflects the relevance of this protein in the interplay between glucose and lipid metabolism (3). Although it is clear that insulin activates the murine Srebp1c promoter by acting on SRE sites and is mediated by SREBP itself, there has been some controversy about the relevance of LXRE sites in this regulation (10). To clarify this issue, we analyzed the insulin-mediated regulation of the human SREBP1c promoter in rat primary hepatocytes. The single and double mutants for all the relevant binding sites in the promoter were generated on the −1564/+1-luc construction. The activity of each construct was studied under basal conditions or after 100 nm insulin stimulation by luciferase assays in transiently transfected rat hepatocytes. Insulin increased WT human promoter construction activation by 1.75-fold (Fig. 3A). The single and double SRE mutants in the human promoter abolished the insulin response. The same effect was seen in the single and double LXRE mutants or in the 1c310 construction, which lacks both LXREs and one SRE binding site. This result suggests that the human SREBP1c promoter regulation by insulin was mediated by both the SRE and LXRE elements in the liver and that the absence of any of these sites abolishes SREBP1c activation. Deletion of the NFY binding site also led to a loss of insulin response, but this effect was probably associated with the diminished basal activity in this mutant.
FIGURE 3.
Nutritional regulation of the human SREBP1c promoter. Insulin- and LXR-mediated regulation of the human SREBP1c promoter were analyzed using −1564/+1-luc (1c1500), −310/+1-luc (1c310) plasmids or mutants of the different cis elements in the −1564/+1-luc vector. The relevance of each predicted binding site for insulin and TO901317 (LXR agonist) regulations was analyzed by luciferase assays. Activities of the WT and mutated constructs transfected in rat primary hepatocytes under basal conditions (gray bars) and 100 nm insulin (black bars) (A) or under basal conditions (gray bars) and 10 μm TO901317 (black bars) (B) are shown. The results represent relative Firefly/Renilla luciferase activities (RLU) considering the wild-type construct under basal conditions as 100% fold activation. Values are the mean ± S.E. of four separate experiments. Differences between the control and the TO901317 treatment significantly differ for all the constructions except for dmLXRE and 1c310 (*, p < 0.05; **, p < 0.01). A scheme of the predicted binding sites in the human promoter is shown.
Both LXRE Sites Are Necessary for Complete Promoter Activation
Activation of the Srebp1c mouse promoter at the LXRE sites is mediated by LXR nuclear receptors, as shown previously (6). To study this regulation on the human promoter, we performed a luciferase assay in transiently transfected rat hepatocytes with various human SREBP1c reporter vectors. The −1564/+1-luc construction showed a >4-fold activation in the presence of LXR synthetic agonist TO901371 (Fig. 3B). This activation lowered by 50% when one of the LXRE sites was deleted, and it was completely abolished when both sites were muted. The same effect was seen in the −310/+1 construct, which does not contain the LXRE binding sites. The SRE double mutant and the NFY mutant displayed a normal LXR response despite their diminished basal activity. This experiment demonstrates that the two LXR binding sites are functional for the human SREBP1c promoter stimulatory effect of TO901317 and in the same way as in the rat and mouse promoters.
Human SREBP1c Promoter Is Activated by PPARα Agonists
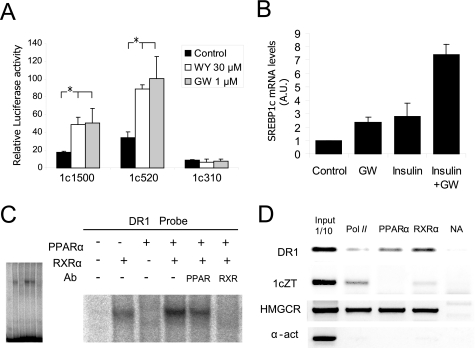
The in silico analysis of the human SREBP1c promoter predicted a DR1 motif, recognized as a PPAR/RXR or a HNF4 binding site according to the Motifviz and Matinspector programs, respectively. A comparative analysis with different databases revealed that the DR1 site present in SREBP1c is very similar to that of the active PPRE in the regulatory region of the malic enzyme promoter (22). Although there are several members of the PPAR family, PPARα is the most abundant in the liver. To evaluate the relevance of PPARα on the SREBP1c promoter regulation, we performed a reporter assay using rat hepatocytes transiently transfected with human SREBP1c luciferase constructs. The cells were treated with two PPARα agonists: WY14643 and GW7647. Both agonists increased the SREBP1c promoter activity of either the −1564/+1 or the −520/+1 constructs. No activation was observed using the −310/+1-luciferase vector, which lacks DR1 and both LXREs motifs (Fig. 4A).
FIGURE 4.
PPARα regulates human SREBP1c promoter activity. A, PPARα-dependent regulation of the SREBP1c promoter was analyzed by luciferase assays using the −1564/+1-luc (1c1500), −520/+1-luc (1c520), and −310/+1-luc (1c310) constructs in the presence of WY14643 (WY) or GW7647 (GW) as selective PPARα agonists. Activities of the promoter constructs transfected in rat primary hepatocytes under basal conditions (black bars), 30 μm WY14643 (white bars) or 1 μm GW7647 (gray bars) are shown. The results represent relative Firefly/Renilla luciferase activities considering the wild-type construct under basal conditions as the reference value. Values are the mean ± S.E. of four separate experiments. *, p < 0.05. B, the endogenous SREBP1c expression in cultured human hepatocytes was analyzed by quantitative PCR. Cells were seeded under basal conditions (control) or treated with 1 μm GW7647 (GW) and/or 100 nm insulin. Total RNA was extracted 24 h after treatment, and cDNA was synthesized by reverse transcriptase. The relative amount of the SREBP1c/36B4 expression was determined in each condition by taking the basal condition as the reference value. The results are the mean ± S.E. of three separate experiments. Differences between the control and the treatments were statistically significant. C, analysis of PPAR binding in vitro to the SREBP1c promoter. EMSA was performed with recombinant human proteins incubated with 32P-labeled oligonucletide encoding for the DR1 element of the human SREBP1c promoter. α-PPAR α or α-RXR α antibodies were used for the supershift assays. The complete EMSA gel (left panel) shows that an equal amount of the probe was loaded in all the lanes. D, analysis in vivo of PPAR binding to the SREBP1c promoter. The ChIP assay was performed in human hepatocytes treated with 1 μm GW7647. Immunoprecipitation of samples was performed with the α-PPARα antibody. A positive control of transcriptionally active genes was performed using the α-RNA pol II antibody and a negative control with no antibody. An α-RXR antibody was used as a positive control for the PPARα activated promoters. An analysis of the HMGCR promoter was used as a positive control for PPARα and RXR binding. The α-actin primers were used as negative control for all the antibodies. The results are representative of the PCR fragments analyzed by agarose gel electrophoresis of two independent experiments.
Regulation of the SREBP1c expression by PPARα agonists was assessed in human hepatocytes primary culture by a quantitative PCR analysis after 24 h of treatment with GW7647. The SREBP1c expression significantly increased by 2-fold in the presence of the PPARα agonist (Fig. 4B). As expected, a similar effect was seen when cells were treated with 100 nm insulin for 24 h. Moreover, the PPARα agonist increased the insulin effect with more than a 7-fold activation in the presence of both GW7647 and insulin.
To determine whether the activation of SREBP1c by PPARα is due to its binding to the predicted DR1 site in the human promoter, we performed an EMSA assay using in vitro synthesized PPARα and RXRα proteins. A PPAR/RXR heterodimer was found to bind to the DR1 probe with partial competition in the presence of the anti-PPARα antibody and an extinction of binding in the presence of the anti-RXR antibody. When adding synthesized RXRα protein alone, the presence of a single retarded band indicated that an RXR homodimer was also capable of binding to this sequence in vitro (Fig. 4C). An EMSA assay was performed using a PPRE probe as a positive control and a mutated PPRE as a negative control; the expression of each recombinant protein was confirmed by Western blot analysis (data not shown).
The gel shift data were supported with a ChIP assay using human primary hepatocytes. Monolayer cells were fixed, and total chromatin was extracted 24 h after incubation with 1 μm GW7647. The binding of PPARα to the SREBP1c promoter was analyzed. An RNA pol II antibody was used as a control of the active gene expression, whereas an RXRα antibody was used as a positive control. After DNA purification, we analyzed the binding of PPARα, RXRα and RNA pol II by PCR. Both PPARα and RXRα were seen to bind to the SREBP1c promoter (Fig. 4D). We analyzed the binding of PPARα and RXRα to the HMGCR promoter, which contains an active PPRE and, as expected, presented a positive binding to both proteins. No enrichment was seen when we used primers to amplify the α-actin or the SREBP1c coding region. The α-RNA polymerase antibody mainly precipitated in the coding chromatin fragments of HMGCR and SREBP1c and was absent in α-actin, which was not expressed under the experimental conditions (Fig. 4D).
LXRE-mediated Activation of SREBP1c Promoter by PPARα Agonists
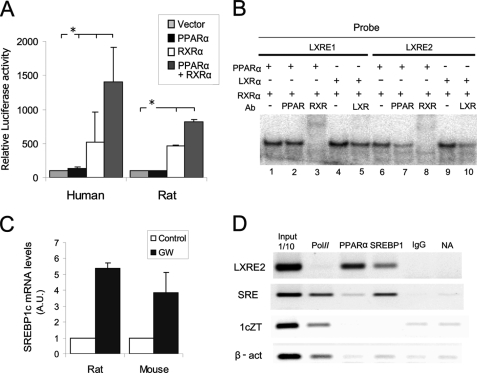
Considering that the human SREBP1c expression was induced by PPARα agonists in human hepatocytes and that the SREBP1c promoter was able to bind to PPAR/RXR heterodimers in vitro and in vivo, we analyzed the regulation of the human SREBP1c promoter in the presence of the PPARα and RXRα overexpression. The overexpression of RXR alone activated the promoter in rat primary hepatocytes, whereas PPARα overexpression was only able to produce a slight, but not significant, increase in SREBP1c activity. It was the coexpression of both proteins that brought about the greater activation of the human SREBP1c promoter (Fig. 5A). To evaluate whether SREBP1c activation was present only in the human promoter, we performed the same analysis using a rat Srebp1c promoter luciferase reporter vector. An unexpected result was obtained given that the PPAR/RXR heterodimer overexpression also activated the rat Srebp1c promoter (Fig. 5A).
FIGURE 5.
PPARα-mediated SREBP1c activation through an LXRE binding site. A, PPARα-mediated regulations of the human and rat SREBP1c promoters were analyzed using rat primary hepatocytes. Cells were transiently transfected with a plasmid containing 1500 bp of a human or rat SREBP1c promoter sequence linked to the luciferase reported plasmid pGL3-basic together with the (a) PPARα, (b) RXRα, and (c) PPARα and RXRα expression vectors. An empty vector was used to normalize the amounts of total transfected DNA. Firefly and Renilla luciferase activities were measured in cell lysates. The results represent relative Firefly/Renilla luciferase activities by considering the empty vector condition as 100% fold activation. Values are the mean ± S.E. of four separate experiments. *, p < 0.05. B, EMSA was performed with the recombinant human proteins incubated with 32P-labeled oligonucletides encoding for LXRE1 and LXRE2 sites. α-PPARα, α-LXRα, or α-RXRα antibodies were used for the supershift assays. C, the endogenous SREBP1c expression in cultured rat and mouse hepatocytes was analyzed by quantitative PCR. Cells were seeded under basal conditions (control) or treated with 1 μm GW7647 (GW). Total RNA was extracted 24 h after treatment, and cDNA was synthesized by reverse transcriptase. The relative amount of the SREBP1c/GAPDH expression was determined under each condition by taking the basal condition as the reference value. The results are the mean ± S.E. of three separate experiments. D, the ChIP assay was performed with rat primary hepatocytes. Immunoprecipitation of samples was performed with the α-PPARα and α-SREBP1 antibodies. A positive control of the transcriptionally active genes was performed using the α-RNA pol II antibody, and a negative control was normal rat IgG and no antibody. The results are representative of the PCR products analyzed by agarose electrophoresis of three independent experiments.
Taking into account the activation of the rat promoter by PPAR/RXR and the absence of a DR1 binding site in the sequence, we considered an LXRE binding site implication in this activation. We performed a shift assay to analyze the binding of PPAR/RXR and LXR/RXR to two separate probes that contained all the SREBP1c promoter LXRE sites (Fig. 5B) and observed an in vitro binding of the LXR/RXR heterodimer to both probes (lanes 4 and 9), which was partially competed by the anti-LXR antibody (lanes 5 and 10). Surprisingly, when we incubated both probes in the presence of PPAR and RXR, a retarded band appeared (lanes 1 and 6), which was totally competed with the α-RXR antibody (lanes 3 and 8). In the presence of the α-PPARα antibody, however, we observed competition for the PPAR/RXR heterodimer only with the LXRE2 probe (lane 7). This result suggests that the PPAR/RXR heterodimer is capable of binding in vitro to the LXRE2 site, but not to the LXRE1 site. Moreover, the PPARα agonist significantly increased the liver SREBP1c endogenous expression in rat and mouse cultured hepatocytes treated with 1 μm GW7647 for 24 h (Fig. 5C).
To determine the in vivo binding of PPARα to the rat Srebp1c promoter, we performed a ChIP assay using rat primary hepatocytes. Monolayer cells were fixed and total chromatin was extracted 24 h after incubation with 1 μm GW7647. The binding of PPARα and SREBP1 to the Srebp1c promoter was analyzed. An RNase pol II antibody was used as a control of the active gene expression. A positive binding of PPARα to the LXRE region of the SREBP1c promoter was observed (Fig. 5D). SREBP1 binding was detected mainly in the SRE binding site together with RNA pol II, which also precipitated with the SREBP1c and β-actin-coding chromatin fragments. No PPARα binding was observed in the absence of GW7647 (data not shown).
Cross-regulation between PPAR and LXR on SREBP1c Promoter
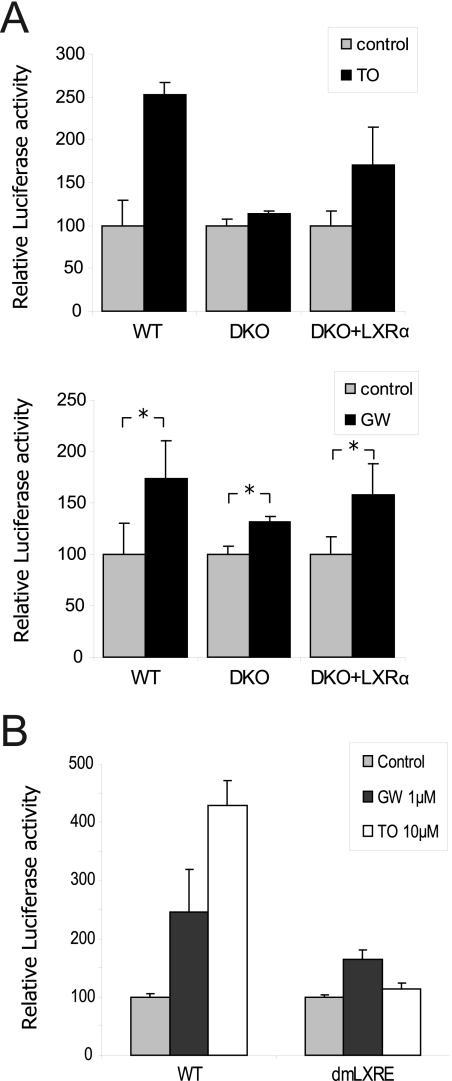
We have demonstrated above an interaction between PPARα and the LXRE binding site in the SREBP1c promoter. We next studied the cross-regulation between LXR and PPARα, which participate together in SREBP1c activation. We evaluated the effect of LXR knocking on human SREBP1c promoter activity by transfecting WT and LXRα/β double knock-out (DKO) mouse hepatocytes with the human SREBP1c reporter promoter construct. The −1564/+1 construct of the human SREBP1c promoter was activated in the presence of the LXR and PPARα agonists, TO901317 and GW7647, respectively. The LXR-mediated response was abolished in the DKO hepatocytes and was rescued when an LXRα expression vector was added to the transfection mix, as expected. However, we observed a diminished but still significant activation of the human promoter in the DKO hepatocytes in the presence of 1 μm GW7647, with a normal response noted in the presence of the exogenous LXRα protein (Fig. 6A).
FIGURE 6.
Cross-regulation between PPARα and LXR on the SREBP1c promoter. A, human SREBP1c promoter regulation was analyzed using the wild-type (WT) and LXRα/LXRβ double-mutant mice (DKO) primary hepatocytes transfected with the −1564/+1-luc vector either with or without an LXRα expression vector. An empty vector was used to normalize the amounts of total transfected DNA. Firefly and Renilla luciferase activities were measured in cell lysates after treatment with 10 μm TO901317 (TO; upper panel) or 1 μm GW7647 (GW; lower panel) for 24 h. The results represent relative Firefly/Renilla luciferase activities by considering the control condition as 100% fold activation. Values are the mean ± S.E. of four separate experiments. *, p < 0.05. B, luciferase assay was performed in the rat hepatocytes transfected with −1564/+1-luc (WT) and LXRE double mutant-luc (dmLXRE). Cells were seeded under basal conditions (control) or treated with 1 μm GW7647 (GW) or 10 μm TO901317 (TO). The results represent relative Firefly/Renilla luciferase activities by considering the control condition for each construction as 100%. Values are the mean ± S.E. of three separate experiments.
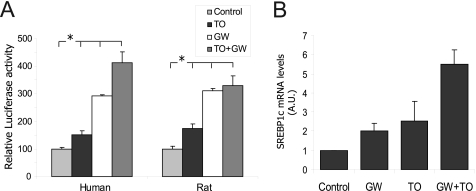
A luciferase assay with the WT and LXRE double mutant constructions of the human SREBP1c promoter was performed to further analyze the interaction between PPARα and LXR. This assay revealed that TO901317 activation was eliminated in the double LXRE mutant construction, but GW7647 significantly activated both the WT and the mutant constructions. However, the double LXRE mutant led to diminished activation in the presence of the PPARα agonist if compared with the WT. This result corroborates the data obtained in DKO mice, demonstrating that LXR is necessary for SREBP1c promoter maximum activation in the presence of a PPARα ligand (Fig. 6B). Furthermore, a synergic effect with combined LXR and PPARα agonists was observed in the human promoter, but not in the rat promoter (Fig. 7A). This difference between species was also observed when using the PPARα and LXR overexpression (data not shown). To corroborate this synergic effect produced by the LXR and PPARα agonists, we analyzed the activation of endogenous SREBP1c in cultured human hepatocytes treated with the respective agonists. The SREBP1c gene expression was activated 2-fold in the presence of 1 μm GW7647. A concentration of 10 nm TO901317 activated SREBP1c to the same extent and boosted GW7647 activation with a 5.5-fold induction in the presence of both agonists (Fig. 7B).
FIGURE 7.
Additive effect of PPARα and LXR on SREBP1c promoter regulation. A, the additive PPARα- and LXR-mediated regulations of human and rat SREBP1c promoters were analyzed using rat primary hepatocytes. Cells were transiently transfected with a plasmid containing 1500 bp of the human or rat SREBP1c promoter sequence linked to the luciferase reported plasmid pGL3-basic treated for 24 h with 1 μm GW7647 (GW) and/or 10 nm TO901317 (TO). Firefly and Renilla luciferase activities were measured in cell lysates. The results represent relative Firefly/Renilla luciferase activities by considering the control condition as 100% fold activation. Values are the mean ± S.E. of four separate experiments (*, p < 0.05). B, endogenous SREBP1c expression in the cultured human hepatocytes was analyzed by quantitative PCR. Cells were seeded under basal conditions (control) or treated with 1 μm GW7647 (GW) and/or 10 nm TO901317 (TO). Total RNA was extracted 24 h after treatment, and cDNA was synthesized by reverse transcriptase. The relative amount of the SREBP1c/36B4 expression was determined under each condition by considering the basal condition as the reference value. The results are the mean ± S.E. of three separate experiments. Differences between the control and the treatments were statistically significant.
Lipogenesis Activation by PPARα Agonists
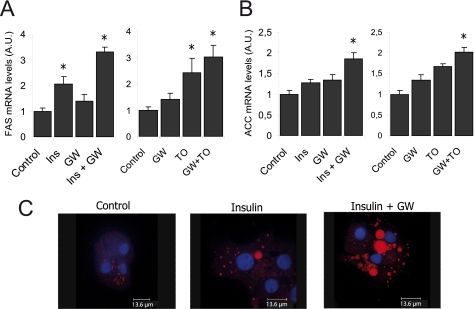
To evaluate the effect of the PPARα-mediated activation of SREBP1c on the lipogenic pathway, we analyzed the expression of fatty acid synthase and acetyl-coA carboxylase lipogenic genes. GW7647, insulin, and TO901317 slightly activated both fatty acid synthase and acetyl-coA carboxylase in human hepatocytes; an additive effect was seen with insulin and GW7647 and also with GW7647 and TO901317 (Fig. 8, A and B). This effect on GW7647-mediated insulin lipogenic potentiation was confirmed by confocal microscopy. Freshly isolated human hepatocytes were treated as indicated previously and were subjected to Nile red and DAPI staining. Under these conditions, we were observed a cytosolic lipids accumulation in the presence of 100 nm insulin with a synergic effect when GW7647 was also present (Fig. 8B). Fluorescence intensity in the insulin- and GW7647-treated hepatocytes was significantly enhanced (data not shown).
FIGURE 8.
Lipogenic effect of PPARα agonists in the liver. A, the endogenous fatty acid synthase and acetyl-coA carboxylase expression in human cultured hepatocytes were analyzed by quantitative PCR. Cells were seeded under basal conditions (control) or treated with 1 μm GW7647 (GW), 10 nm TO901317 (TO), 100 nm insulin (Ins), or with a combination of GW7647 with TO901317 or insulin. Total RNA was extracted 24 h after treatment, and cDNA was synthesized by reverse transcriptase. The relative amount of the SREBP1c/36B4 expression was determined under each condition by considering the control condition as the reference value. The results are the mean ± S.E. of three separate experiments. (*, p <0.05). B, lipid accumulation in human hepatocytes was analyzed by Nile red staining under a confocal microscope. The images are representative of at least 10 different images under each condition. All images were obtained under the same zoom, wavelength, and intensity conditions.
DISCUSSION
Insulin and lipid mediators are signaling molecules that regulate cellular responses in a wide range of physiological and pathological nutritional disorders. The SREBP1c transcriptional factor is a convergence point from starving to post-prandial phases. To coordinate these signals, the promoter region of SREBP1c contains multiple and complex regulatory elements modulated by cholesterol, fatty acids and carbohydrates (11). In this report, we have identified the most relevant regulatory sequences in the human SREBP1c promoter structure.
We have clearly demonstrated that the insulin-responsive elements in the SREBP1c promoter involve both LXRE and SRE binding sites, which are essential for maximum activation in hepatocytes. Different binding sites, such as LXRE, SRE, SP1, and NFY, have been shown to be involved in the regulation of the mouse and rat Srebp1c promoters in different cell lines during a full insulin response (15). It is important to stress that SP1 and NFY are responsible for almost 60% of basal activity; this sometimes makes it difficult to elucidate their implication in insulin activation. An SRE binding site implicates an auto-regulatory loop mediated by SREBP1c itself and by other SREBP family members. The presence of a second active SRE binding site in the human promoter suggests that this loop plays an important role in human metabolism regulation. Studies performed in skeletal muscle have demonstrated that this loop is more relevant for insulin regulation than LXR (10). However, our results prove the essential role of LXR signaling in insulin regulation in hepatocytes. Different mechanisms have been described to be implicated in the insulin mediated activation of LXR and its target genes. In addition, insulin has been seen to activate the LXR expression in the liver (23), whereas others have hypothesized that insulin may stimulate the production of an oxygenated sterol ligand for this nuclear receptor (13). Furthermore, physiological glucose concentration activates LXR in the liver and induces the expression of LXR target genes, whose efficacy is similar to that of oxysterols, the well known LXR ligands (24). LXR could, therefore, be a transcriptional switch that integrates hepatic glucose metabolism and fatty acid synthesis.
Many aspects of hepatic lipid metabolism are under the transcriptional control of PPARα. It is well established that an impaired PPARα function is associated with hepatic lipid accumulation. Moreover, synthetic agonists for PPARα have been explored for the treatment of fatty liver disease (25). The discovery of a DR1 putative binding site in the human promoter prompted us to study the response of the human SREBP1c promoter to PPARα ligands. We found a positive response to WY14643 and GW7647 agonists together with activation in the presence of an exogenous PPAR expression. Moreover, a synergic effect was observed in the presence of insulin or LXR agonists. PPARα seems to be an activator of the SREBP1c promoter in vivo, as predicted by chip-chip analyses (26).
Crosstalk between PPAR and LXR at the transcriptional level has been previously demonstrated. An active PPRE element present in the LXRα promoter region is able to respond to PPAR agonists (27). Given the fact that there are two LXRE active sites in the SREBP1c promoter, we considered the possibility of an indirect activation of this promoter mediated by LXRα activation. However, SREBP1c promoter activation in the presence of the PPARα/RXR heterodimer was seen in either hepatocytes from double LXR knock-out mice (DKO) or rat hepatocytes transfected with the LXRE double mutant luciferase construction. These experiments demonstrate a direct activation mediated by PPAR itself.
The effect of the PPARα nuclear receptor on the control of Srebp1c mouse promoter activity has been studied previously (9). The authors showed that PPARα inhibited the mouse Srebp1c promoter LXR-mediated activation in HEK293 and HepG2 cells; moreover, they observed no activation in rat hepatocytes treated with WY14643. The authors suggested that this inhibition was mediated by the sequestration of RXR avoiding the LXR-mediated activation. This discrepancy may relate to the use of fasted mice in Yosikawa's study (9). König et al. (28) have also shown that PPARα agonists reduce triglycerides synthesis in rat hepatoma cells, which is partially caused by inhibited SREBP1 activation. The above experiments were performed under low glucose conditions to mimic the fasted condition. However, our experiments were performed in cells maintained at high glucose concentrations or in hepatocytes obtained from ad libitum-fed animals. Our results suggest that PPARα-mediated SREBP1c promoter activation is dependent on the presence of the LXR nuclear receptor, and probably on nutritional status. Recently, Chakravarhy et al. (29) have identified a physiologically relevant endogenous ligand for PPARα in the liver that induces the expression of those genes involved in fatty acid metabolism, but only in the presence of active fatty acid synthase. Furthermore, the SREBP1c mRNA levels were lower in the livers of PPARα-null mice than in the WT mice (30). Accordingly, the level of SREBP1c significantly increased in fenofibrate-treated WT mice (31), but not in PPARα-null mice (32). Thus, it is feasible to hypothesize that PPARα gene expression regulation in general, and the SREBP1c expression in particular, may depend on an acting ligand nature.
PPARα has been shown to govern the expression of numerous genes involved in fatty acid oxidation, ketogenesis, gluconeogenesis, cholesterol catabolism, and lipoprotein metabolism (33). However, the data obtained from transgenic mice (34) and our results achieved with human hepatocytes report that PPARα does not merely serve as a transcriptional activator of fatty catabolism but plays a much broader role in lipid metabolism. An important question to clarify is why a transcription factor, whose primary role is to coordinate fatty acid oxidation in the starved state, is required to facilitate fatty acid synthesis during the refeeding period. We have shown that PPARα agonists cooperate with insulin to induce hepatic lipid synthesis. Our results also demonstrate that LXR is essential for SREBP1c regulation by insulin through its LXR elements present in the promoter. Moreover, the maximum effect of PPARα is obtained when the LXR element remains unmodified. PPARα may be required for the production of an endogenous LXR ligand. In line with this, the original PPARα dependence of insulin action in PPARα-deficient mice was abolished under conditions in which LXR activation was not limiting (35).
Important differences between rodents and humans have been demonstrated previously in terms of gene regulation by PPAR and LXRs, especially in the field of macrophage homeostasis (36). It is important to bear in mind not only that the expression levels of PPAR and LXR can differ between humans and mice, but levels of RXR expression may vary and could influence the activity of both LXR and PPAR, and their cross-talk (7).
Ours results provide an insight into the role of the insulin-, LXR-, and PPAR-mediated regulation of human SREBP1c and its relation with nutritional status. PPARα and LXR act as lipid-glucose sensors and bind to ligands deriving from either intracellular metabolism or dietary lipids. Endogenous ligands for PPAR include fatty acids and eicosanoids, whereas metabolites of oxidized cholesterol activate LXRs. Moreover, it has been demonstrated there is no single endogenous ligand for PPARα; thus, PPAR could play a role as a nutritional status sensor in the cell to regulate the SREBP1c-dependent pathway by controlling the fasting/fed transition.
Acknowledgments
We thank Dr. David J. Mangelsdorf (Howard Hughes Medical Institute, University of Texas Southwestern Medical Center) for the generous gift of double LXR knock-out mice, Dr. Domingo Barettino for providing us with the RXR antibody, and Benito Alarcon for technical assistance in the confocal analysis.
This work was supported by the Spanish Ministry of Science and Innovation (SAF2006-06760; SAF2009-12602) and the Carlos III Health Institute (Red de Centros FIS-RECAVA RD06/0014/0025).
- SREBP
- sterol regulatory element binding protein
- PPAR
- peroxisome proliferator-activated receptor
- PPRE
- peroxisome proliferator response element
- DKO
- double knock-out
- LXR
- liver X receptor
- pol II
- polymerase II
- luc
- luciferase
- RXR
- retinoid X receptor
- NFY
- nuclear transcription factor Y
- LXRE
- liver X receptor element
- SRE
- sterol regulatory cis element.
REFERENCES
- 1. Grundy S. M., Brewer H. B., Jr., Cleeman J. I., Smith S. C., Jr., Lenfant C. (2004) Circulation 109, 433–438 [DOI] [PubMed] [Google Scholar]
- 2. Reaven G. M. (2005) Annu. Rev. Nutr. 25, 391–406 [DOI] [PubMed] [Google Scholar]
- 3. Foufelle F., Ferré P. (2002) Biochem. J. 366, 377–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horton J. D., Goldstein J. L., Brown M. S. (2002) J. Clin. Invest. 109, 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Janowski B. A., Grogan M. J., Jones S. A., Wisely G. B., Kliewer S. A., Corey E. J., Mangelsdorf D. J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshikawa T., Shimano H., Amemiya-Kudo M., Yahagi N., Hasty A. H., Matsuzaka T., Okazaki H., Tamura Y., Iizuka Y., Ohashi K., Osuga J., Harada K., Gotoda T., Kimura S., Ishibashi S., Yamada N. (2001) Mol. Cell. Biol. 21, 2991–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rakhshandehroo M., Hooiveld G., Müller M., Kersten S. (2009) PloS one 4, e6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. (1992) Nature 358, 771–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshikawa T., Ide T., Shimano H., Yahagi N., Amemiya-Kudo M., Matsuzaka T., Yatoh S., Kitamine T., Okazaki H., Tamura Y., Sekiya M., Takahashi A., Hasty A. H., Sato R., Sone H., Osuga J., Ishibashi S., Yamada N. (2003) Mol. Endocrinol. 17, 1240–1254 [DOI] [PubMed] [Google Scholar]
- 10. Dif N., Euthine V., Gonnet E., Laville M., Vidal H., Lefai E. (2006) Biochem. J. 400, 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raghow R., Yellaturu C., Deng X., Park E. A., Elam M. B. (2008) Trends Endocrinol. Metab. 19, 65–73 [DOI] [PubMed] [Google Scholar]
- 12. Amemiya-Kudo M., Shimano H., Yoshikawa T., Yahagi N., Hasty A. H., Okazaki H., Tamura Y., Shionoiri F., Iizuka Y., Ohashi K., Osuga J., Harada K., Gotoda T., Sato R., Kimura S., Ishibashi S., Yamada N. (2000) The Journal of biological chemistry 275, 31078–31085 [DOI] [PubMed] [Google Scholar]
- 13. Chen G., Liang G., Ou J., Goldstein J. L., Brown M. S. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11245–11250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng X., Cagen L. M., Wilcox H. G., Park E. A., Raghow R., Elam M. B. (2002) Biochem. Biophys. Res. Commun. 290, 256–262 [DOI] [PubMed] [Google Scholar]
- 15. Cagen L. M., Deng X., Wilcox H. G., Park E. A., Raghow R., Elam M. B. (2005) Biochem. J. 385(Pt 1), 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Casado M., Callejas N. A., Rodrigo J., Zhao X., Dey S. K., Boscá L., Martín-Sanz P. (2001) FASEB J. 15, 2016–2018 [DOI] [PubMed] [Google Scholar]
- 17. Ferrini J. B., Ourlin J. C., Pichard L., Fabre G., Maurel P. (1998) Methods Mol. Biol. 107, 341–352 [DOI] [PubMed] [Google Scholar]
- 18. Fernández-Alvarez A., Tur G., López-Rodas G., Casado M. (2008) FEBS Lett. 582, 177–184 [DOI] [PubMed] [Google Scholar]
- 19. Fernández-Alvarez A., Soledad Alvarez M., Cucarella C., Casado M. (2010) The J. Biol. Chem. 285, 11765–11774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McMillian M. K., Grant E. R., Zhong Z., Parker J. B., Li L., Zivin R. A., Burczynski M. E., Johnson M. D. (2001) In Vitro Mol. Toxicol. 14, 177–190 [DOI] [PubMed] [Google Scholar]
- 21. Tarling E., Salter A., Bennett A. (2004) Biochem. Soc. Trans. 32, 107–109 [DOI] [PubMed] [Google Scholar]
- 22. Castelein H., Gulick T., Declercq P. E., Mannaerts G. P., Moore D. D., Baes M. I. (1994) J. Biol. Chem. 269, 26754–26758 [PubMed] [Google Scholar]
- 23. Tobin K. A., Ulven S. M., Schuster G. U., Steineger H. H., Andresen S. M., Gustafsson J. A., Nebb H. I. (2002) J. Biol. Chem. 277, 10691–10697 [DOI] [PubMed] [Google Scholar]
- 24. Mitro N., Mak P. A., Vargas L., Godio C., Hampton E., Molteni V., Kreusch A., Saez E. (2007) Nature 445, 219–223 [DOI] [PubMed] [Google Scholar]
- 25. Fernández-Miranda C., Pérez-Carreras M., Colina F., López-Alonso G., Vargas C., Solís-Herruzo J. A. (2008) Dig. Liver Dis. 40, 200–205 [DOI] [PubMed] [Google Scholar]
- 26. van der Meer D. L., Degenhardt T., Väisänen S., de Groot P. J., Heinäniemi M., de Vries S. C., Müller M., Carlberg C., Kersten S. (2010) Nucleic Acids Res. 38, 2839–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tobin K. A., Steineger H. H., Alberti S., Spydevold O., Auwerx J., Gustafsson J. A., Nebb H. I. (2000) Mol. Endocrinol. 14, 741–752 [DOI] [PubMed] [Google Scholar]
- 28. König B., Koch A., Spielmann J., Hilgenfeld C., Hirche F., Stangl G. I., Eder K. (2009) European journal of pharmacology 605, 23–30 [DOI] [PubMed] [Google Scholar]
- 29. Chakravarthy M. V., Lodhi I. J., Yin L., Malapaka R. R., Xu H. E., Turk J., Semenkovich C. F. (2009) Cell 138, 476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel D. D., Knight B. L., Wiggins D., Humphreys S. M., Gibbons G. F. (2001) J. Lipid Res. 42, 328–337 [PubMed] [Google Scholar]
- 31. Oosterveer M. H., van Dijk T. H., Tietge U. J., Boer T., Havinga R., Stellaard F., Groen A. K., Kuipers F., Reijngoud D. J. (2009) PloS One 4, e6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le Jossic-Corcos C., Duclos S., Ramirez L. C., Zaghini I., Chevillard G., Martin P., Pineau T., Bournot P. (2004) Biochim. Biophys. Acta 1683, 49–58 [DOI] [PubMed] [Google Scholar]
- 33. Mandard S., Müller M., Kersten S. (2004) Cell Mol. Life Sci. 61, 393–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chakravarthy M. V., Pan Z., Zhu Y., Tordjman K., Schneider J. G., Coleman T., Turk J., Semenkovich C. F. (2005) Cell Metab. 1, 309–322 [DOI] [PubMed] [Google Scholar]
- 35. Hebbachi A. M., Knight B. L., Wiggins D., Patel D. D., Gibbons G. F. (2008) J. Biol. Chem. 283, 4866–4876 [DOI] [PubMed] [Google Scholar]
- 36. Rigamonti E., Chinetti-Gbaguidi G., Staels B. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1050–1059 [DOI] [PubMed] [Google Scholar]