Abstract
Polysialic acid (polySia), a unique acidic glycan modifying neural cell adhesion molecule (NCAM), is known to regulate embryonic neural development and adult brain functions. Polysialyltransferase STX is responsible for the synthesis of polySia, and two single nucleotide polymorphisms (SNPs) of the coding region of STX are reported from schizophrenic patients: SNP7 and SNP9, respectively, giving STX(G421A) with E141K and STX(C621G) with silent mutations. In this study, we focused on these mutations and a binding activity of polySia to neural materials, such as brain-derived neurotrophic factor (BDNF). Here we describe three new findings. First, STX(G421A) shows a dramatic decrease in polySia synthetic activity on NCAM, whereas STX(C621G) does not. The STX(G421A)-derived polySia-NCAM contains a lower amount of polySia with a shorter chain length. Second, polySia shows a dopamine (DA) binding activity, which is a new function of polySia as revealed by frontal affinity chromatography for measuring the polySia-neurotransmitter interactions. Interestingly, the STX(G421A)-derived polySia-NCAM completely loses the DA binding activity, whereas it greatly diminishes but does not lose the BDNF binding activity. Third, an impairment of the polySia structure with an endosialidase modulates the DA-mediated Akt signaling. Taken together, impairment of the amount and quality of polySia may be involved in psychiatric disorders through impaired binding to BDNF and DA, which are deeply involved in schizophrenia and other psychiatric disorders, such as depression and bipolar disorder.
Keywords: Carbohydrate-binding Protein, Carbohydrate Function, Glycoprotein, Glycosylation, Neurotransmitters, Polysialic Acid, Neural Cell Adhesion Molecule, Polysialyltransferase, Schizophrenia, Single Nucleotide Polymorphisms
Introduction
PolySia2 is a polymer of sialic acid with a degree of polymerization (DP) ranging from 8 to 400 (1–4) and is mainly attached to neural cell adhesion molecule (NCAM) in vertebrates (1). PolySia is expressed in brains during embryonic and postneonatal development and mostly disappears in adult brains, although NCAM expression levels remain unchanged (1, 5, 6). In adult brains, polySia-NCAM persists in distinct regions where neural plasticity, remodeling of neural connections, or neural generation are ongoing, such as the hippocampus, (7, 8) and olfactory system (9). Due to its bulky polyanionic nature, polySia has an anti-adhesive effect on cell-cell/extracellular matrix interactions mediated by homophilic binding of NCAM as well as heterophilic binding of other cell adhesion molecules (5, 6). Two polysialyltransferases, STX (ST8SiaII/SIAT8B) and PST (ST8SiaIV/SIAT8D), are responsible for the synthesis of polySia on NCAM (10, 11). In both NCAM-deficient (12) and STX- and PST-deficient mice (13–15), anomalous phenotypes in brain development, long term potentiation and depression in the hippocampus CA1 region, lamination of mossy fibers and synapse formation, spatial learning, and various abnormal behaviors are exhibited. Interestingly, these abnormal features of STX- and PST-deficient mice are common to those of schizophrenia patients. Schizophrenia is a severe psychiatric disorder that affects approximately 1% of the population worldwide. Although several factors are associated with an increased risk to develop schizophrenia (16), the overall mechanism resulting in schizophrenia remains unclear. The basic risk profile is mainly dependent upon causative genes, such as those encoding DISC1 (disrupted-in-schizophrenia 1) (17), neuregulin 1 (18), COMP (catechol-o-methyltransferase) (19), dopamine (DA) receptors (20), brain-derived neurotrophic factor (BDNF) (21), etc. It is also considered that schizophrenia is deeply related to neurodevelopmental and neurodegenerative disorders involving disconnectivity and disorder of synapses (22, 23) and that these abnormalities may occur at a very restricted stage during brain development. With regard to the relationship of polySia with schizophrenia patients, abnormalities in polysialylation and polySia-expressing tissues have been reported. For example, a decrease in polySia-NCAM immunoreactivity was observed in schizophrenic hippocampi (24), and patients with schizophrenia often have low olfactory volume (25). Impairment of hippocampal functions and disturbance of its anatomical organization are also involved in the etiology of schizophrenia (18). Recently, the chromosome where STX is localized, 15q26, was reported as a common susceptibility region for both schizophrenia and bipolar disorder in a genome scan of Eastern Quebec families (26). Arai et al. (27) also revealed an association between polymorphisms in the promoter region of the STX gene and schizophrenia in the Japanese population.
To gain further insight into the relationship between psychiatric disorders such as schizophrenia and the polysialylation state of NCAM, we focused on two SNPs of STX, SNP7 (421G→A) and SNP9 (621C→G), which localize to exon 4 and 5 of the STX gene, respectively, and have been identified in a schizophrenia patient (SNP7) and in both schizophrenia and control patients (SNP9). Although these two mutations in the open reading frame have not been reported to be related to schizophrenia due to their low p values (27), it is important to know the structural and functional features of polySia biosynthesized by naturally mutated STX genes. Here we have investigated the enzymatic activities of the mutated STXs and the quantity and quality of polySia structure synthesized by the mutated enzymes. In a previous study, we reported that polySia displays a reservoir function for several neurotrophins, including BDNF, neurotrophin-3, and nerve growth factor (NGF) (28, 29). This was a hitherto unknown polySia function other than anti-cell adhesion activity. Due to this novel capability, we hypothesize that polySia regulates the concentrations of neurological factors whose imbalances are occasionally the cause of psychiatric disorders, such as schizophrenia. In this study, we also focused on DA, whose impaired action is related to schizophrenia and for the first time demonstrated a DA-retaining function of polySia-NCAM. Finally, we examined the effects of the mutated STX-derived polySia-NCAM on the newly identified function toward BDNF and DA.
EXPERIMENTAL PROCEDURES
Materials
The QuikChange site-directed mutagenesis kit was purchased from Stratagene. IgG-Sepharose, CMP-[14C]Neu5Ac, protein G-Sepharose, and ECL reagents were obtained from GE Healthcare. CMP-[14C]Neu5Ac was also obtained from American Radiolabeled Chemicals (St. Louis, MO). Dulbecco's modified medium and colominic acid were obtained from Wako (Tokyo, Japan). Opti-MEM I and Lipofectamine were from Invitrogen. BDNF was purchased from PeproTech Inc. (Rocky Hill, NJ). Rabbit anti-BDNF antibody and anti-NCAM antibody (H300) that recognize both non-polysialylated and polysialylated NCAM were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). anti-Akt and anti-pAkt antibodies were from Cell Signaling Technology (Danvers, MA). GeneJuice transfection reagent was from Novagen (Darmstadt, Germany). The anti-V5 tag antibodies, pBudCE4.1, and pcDNA4-myc/His were purchased from Invitrogen. Horseradish peroxidase-conjugated goat antibodies against human IgG + IgM + IgA and mouse IgG + IgM were obtained from American Qualex (San Clemente, CA). Polyvinylidene difluoride (PVDF) membrane (Immobilon P) was a product of Millipore (Bedford, MA). Sialidase from Arthrobacter ureafaciens were obtained from Nacalai Co. (Kyoto, Japan). Minimum essential medium Eagle α modification was purchased from Sigma. Synthetic oligonucleotide primers were obtained from Rikaken (Nagoya, Japan). Affi-Gel and molecular weight marker were purchased from Bio-Rad. DA was acquired from AbD Serotec (Oxford, UK). 12E3 antibody was a gift from Prof. Tatsunori Seki (Tokyo Medical University). Endo-N-acylneuraminidase (Endo-N) was a generous gift from Prof. Frederic F Troy II (University of California, Davis) and was purified.
Plasmids
pPROTA-STX-V5 (pPROTA-STX(WT)) and pPROTA-PST-V5 (pPROTA-PST) encoding soluble human STX and PST chimeric with protein A and V5, respectively, and pcDNA3.1-STX-V5/His (pcDNA-STX(WT)) and pcDNA3.1-PST-V5/His (pcDNA-PST) encoding full-length human STX and PST, respectively, with V5 and His6 tags, were kindly provided by Prof. Karen Colley (University of Illinois School of Medicine). Mutagenesis of pPROTA-STX(WT) and pcDNA-STX(WT) was performed using the QuikChange site-directed mutagenesis kit (Stratagene, CA) and the primers listed in supplemental Table S1, resulting in the construction of pPROTA-STX(G421A), pPROTA-STX(C621G), pcDNA-STX(G421A), and pcDNA-STX(C621G). We also prepared the plasmid pBudCE4.1-STX(WT)-myc/His-PST-V5/His (pBud-STX(WT)-PST), which encodes STX and PST in tandem with myc/His6 and V5/His6 C-terminal tags, respectively. Using this plasmid as a template, pBudCE4.1-STX(G421A)-PST and pBud-STX(C621G)-PST were constructed using identical primers. A plasmid, pIG-NCAM, containing cDNA encoding NCAM-Fc was a kind gift from Dr. Paul Crocker (University of Dundee) (30). The NCAM-Fc fragment was excised from pIG-NCAM with HindIII and NotI, purified, and then inserted into the HindIII and NotI sites of pcDNA4-myc/His to generate pcDNA4-NCAM-Fc. The sequences of all prepared constructs were confirmed by the deoxynucleotide chain termination method.
Cell Transfection
Cells maintained in DMEM (SK-N-SH) or α-minimum Eagle's medium (CHO) containing 10% FBS (Thermo Scientific, Waltham, MA) were grown in a 37 °C, 5% CO2 incubator until 50–70% confluent. Lipofectamine or GeneJuice transfections were then performed according to the manufacturers' instructions.
Purification of NCAM-Fc Fusion Proteins and a Soluble Form of Polysialyltransferases
The NCAM-Fc chimeric protein and the STX and PST chimeric proteins were purified as described previously, and the purified fusion proteins were used as enzyme sources for the sialyltransferase activity assay (30–32).
Assay for Polysialyltransferase Activity in Vitro
The measurement of enzyme activity was performed at 37 °C for 24 h in 10-μl reaction mixtures containing the target recombinant polysialyltransferase, 50 mm MES buffer (pH 6.0), 10 mm MnCl2, 2 mm CaCl2, 0.5% Triton CF-54, 8.3 μm CMP-[14C]Neu5Ac (0.93 kBq), and 0.1 mg/ml acceptor (NCAM-Fc or pig embryonic brain homogenates). A 5-μl aliquot was spotted on Whatman 3MM paper and developed in ethanol, 1 m ammonium acetate (7:3, v/v), pH 7.5. After air-drying, the amount of incorporated [14C]Neu5Ac remaining at the origin was determined using a BAS 2000 Imaging Analyzer (Fuji Film, Tokyo, Japan) as described previously (30).
Assay for Polysialyltransferase Activity and Product Analysis in Transfected Cells
Stable CHO cell lines that secreted NCAM-Fc were transfected with either pBudCE4.1-STX(WT)-PST, pBudCE4.1-STX(G421A)-PST, pBudCE-STX(C621G)-PST, pcDNA-STX(WT), pcDNA-STX(G421A), or pcDNA-STX(C621G) using Lipofectamine. Stable cell lines that secreted polySia-NCAM were established with G418. Then polySia-NCAM-Fc secreted into culture medium was purified as described above. Purified NCAM-Fc was analyzed by SDS-PAGE/Western blotting using anti-polySia (12E3) and anti-NCAM (H300) antibodies. The polysialylation state was analyzed chemically by the fluorescent C7/C9 analyses (33). In addition, we also analyzed the amount of polySia using anion exchange chromatography. Briefly, the products were applied onto a Mono Q anion exchange column (1 ml) and separated on a JASCO HPLC system to determine the net negative charge (NC) of polySia as described previously (28). The sample was loaded on a column and eluted with 20 mm Tris-HCl (pH 8.0), followed by an NaCl gradient (0–10 min, 0 m; 10–30 min, 0 → 0.3 m; 30–105 min, 0.3 → 0.5 m; 105–115 min, 0.5 → 1 m; 115–125 min, 1 m) in 20 mm Tris-HCl (pH 8.0). The flow rate was 1 ml/min, and 1 ml was collected per fraction. After the samples were dot-blotted onto a PVDF membrane, the amount of polySia in each fraction was determined by the anti-polySia staining (12E3) of the membrane. The column was calibrated with colominic acid (authentic polySia from Escherichia coli) as described (28).
Immunostaining
PVDF membranes were blocked with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and either 1% bovine serum albumin or 5% skim milk at 25 °C for 1 h. The membranes were incubated overnight with the primary antibody, either rabbit polyclonal anti-NCAM (H-300) (0.2 μg/ml) or anti-polySia antibody (12E3) (1 μg/ml), at 4 °C. As the secondary antibody, either peroxidase-conjugated anti-rabbit IgG antibodies (1:4000 dilution) or anti-mouse IgG + IgM antibodies (1:5000 dilution) were applied to the membranes, and after a 60-min incubation at 37 °C, color development was performed using standard reagents.
Frontal Affinity Chromatography (FAC)
The principle of FAC has been well described (34) and performed according to the established method (34, 35). The FAC system consisted of a JASCO pump (PU-980i), JASCO-UV detector (875-UV), and a Chromato-PRO integrator (Run Time Corp., Kanagawa, Japan) equipped with a column oven (Shimadzu, CTO-6A). Sample solutions (neurotransmitters) were injected through a Rheodyne-7125 injector equipped with a 2-ml PEEK sample loop to a polySia- or polySia-NCAM-Fc immobilized column. The flow rate and the column temperature were maintained at 0.125 ml/min and either 25 or 37 °C, respectively.
Horizontal Native PAGE
BDNF (100 ng) in 50 mm Tris-HCl (pH 7.5) containing 0.15 m NaCl was incubated with or without NCAM-Fcs (0–0.9 μg) at 37 °C for 2 h. Samples were subjected to horizontal native PAGE and blotted onto PVDF membranes. The EC50 values were determined as described previously (28).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was prepared from SK-N-SH cells using TRIzol (Invitrogen) according to the manufacturer's instructions. Random-primed cDNA (about 50 ng) and specific primers were used for the PCR.
Effect of PolySia on Akt Signaling
Human neuroblastoma SK-N-SH cells were cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 0.5 mg/ml streptomycin sulfate, 100 units/ml penicillin G, and 10% fetal bovine serum in a 5% CO2 and 95% air humidified atmosphere at 37 °C. For the assay, cells (3 × 105) were plated in 6-well plates and incubated for 24 h. Endo-N (0.9 units/ml), boiled Endo-N (0.9 units/ml) at 100 °C for 10 min, or PBS was then added to the wells, and the plate was incubated for 1 h. After removing the medium, the cells were washed, DMEM without serum was added, and the cells were then incubated for 2 h. DA was then added at a final concentration of 100 μm, and the cells were incubated further for 0.5, 1, 2, and 16 h. The cells were then collected with lysis buffer (50 mm Tris-HCl (pH 8.0) containing 1% Triton X-100; 1 mm PMSF; 1 μg/ml aprotinin, leupeptin, pepstatin; 5 mm EDTA; 10 mm sodium pyrophosphate; 25 mm glycerophosphate; 10 mm NaF; and 1 mm Na3VO4) using a cell scraper. Cell homogenates were left on ice for 1 h and centrifuged at 10,000 × g for 15 min, and the supernatants were used for Western blot analysis with anti-polySia (12E3; 1 μg/ml), anti-pAkt (1:750 dilution), and anti-Akt antibody (1:1000 dilution).
Data Analysis
All values are expressed as the mean ± S.D.
RESULTS
In Vitro Enzyme Activities of STX(WT), STX(G421A), and STX(C621G)
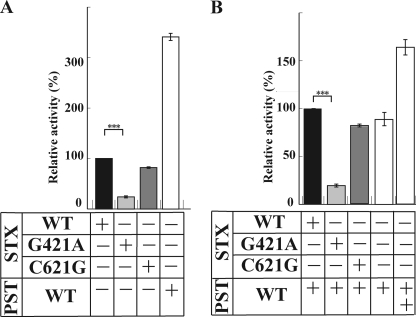
Two point mutants of STX have been recently identified as SNP7 and SNP9 (27). In SNP7, a point mutation of 421G→A occurs to give the E141K mutant, STX(G421A), and was identified from a schizophrenic patient. In SNP9, a point mutation of 621C→G occurs, resulting in a silent P207P mutant, STX(C621G), and was observed in both schizophrenic patients and normal persons. To determine in vitro activities of these enzymes, the soluble forms of enzymes N-terminally fused with protein A were expressed in CHO cells, and the secreted enzymes were purified (supplemental Fig. S1). A purified soluble form of NCAM-Fc (supplemental Fig. S1) and 14C-labeled CMP-Neu5Ac were incubated with the same amount of either of those enzymes, STX(WT), and PST(WT). As shown in Fig. 1A, the wild-type STX(WT) and PST(WT) showed the activity to the NCAM-Fc. The enzyme activity of STX(WT) was 3.4-fold lower than that of PST(WT) (Fig. 1A), consistent with the previous report that in vitro activity of STX is much lower than that of PST (31). STX(C621G) showed much the same activity as STX(WT), whereas STX(G421A) showed dramatically decreased activity, or 22% of the STX(WT) activity (n = 3, p = 0.00022) (Fig. 1A). Previously, the activity of STX was shown to be enhanced in the presence of PST (31), and then we examined the effect of co-incubation of the mutated STX and PST(WT). As shown in Fig. 1B, although the enzyme activity was not enhanced by the co-incubation of STX(WT) and PST(WT) in this experiment, the STX(G421A)/PST(WT) pair displayed only 20% of the STX(WT)/PST(WT) activity (n = 3, p = 0.00012). Thus, the PST(WT) activity was greatly inhibited by the STX(G421A). These results suggest that the STX interacts with PST and that the interaction may be inhibited in the STX(G421A)/PST(WT) pair, leading to the impaired activity. No significant effect was observed for the STX(C621G)/PST(WT) pair. Identical results were obtained in all of the experiments using the pig embryonic brain homogenate, which contains intact NCAM, as an acceptor substrate instead of NCAM-Fc (supplemental Fig. S2).
FIGURE 1.
In vitro enzyme activity of polysialyltransferases. A, in vitro enzyme activity of STX(WT), STX(G421A), STX(C621G), and PST(WT) toward NCAM-Fc. The enzyme reaction was carried out at 37 °C for 3 h in 10-μl reaction mixtures containing each enzyme, 50 mm MES buffer (pH 6.0), 10 mm MnCl2, 0.5% Triton CF-54, 10 μm CMP-[14C]Neu5Ac, and NCAM-Fc. A 5-μl aliquot was spotted on Whatman 3 MM paper and developed in ethanol, 1 m ammonium acetate (7:3, v/v), pH 7.5. After air-drying, the amount of incorporated [14C]Neu5Ac remaining at the origin was determined using a BAS 2000 imaging analyzer (Fuji Film, Tokyo, Japan). The data are expressed as the mean ± S.D. (error bars), with at least 3 replicates/experiment. The relative activity of each polysialyltransferase was determined by comparing with the activity of STX(WT), which was set to 100%. ***, p = 0.00022 (<0.001). B, in vitro enzyme activity of STX(WT), STX(G421A), and STX(C621G), in the presence of PST(WT), toward NCAM-Fc. The data are expressed as the mean ± S.D., with at least 3 replicates/experiment. The relative activity of each pair of polysialyltransferases was determined by comparing to the activity of STX(WT)/PST(WT), which was set to 100%. ***, significance at p = 0.00012 (<0.001). +, presence of enzyme. ++, presence of enzyme in a double amount. −, absence of enzyme.
Enzyme Activities of STX(WT), STX(G421A), and STX(C621G) in Transfected Cells
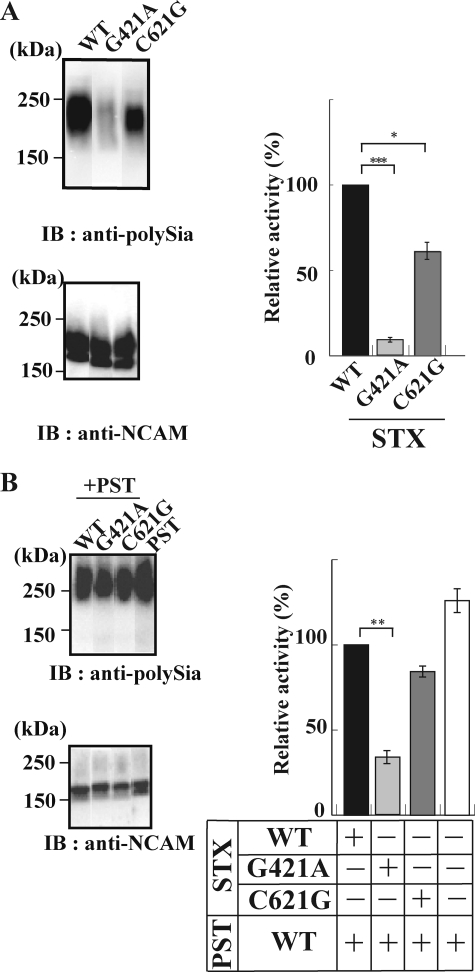
For measuring the polysialyltransferase activity, CHO cells constitutively secreting NCAM-Fc were transfected with a full-length STX(WT), STX(G421A), or STX(C621G) gene. The secreted NCAM-Fcs from those transfectant cells were analyzed for the polysialylation state by anti-polySia antibody (12E3) (7, 36) and anti-NCAM antibody (H-300). The relative activity was expressed as a ratio of the intensity of the immunostaining by anti-polySia to that by anti-NCAM (Fig. 2, A and B, right panels). As shown in Fig. 2A, NCAM was polysialylated by STX(WT) (lane wt). With STX(G421A), however, polysialylation of NCAM dramatically and significantly decreased (lane g421a) to 7.7%, compared with that with STX(WT). In addition, although the reduction was moderate compared with that of STX(G421A), it also decreased to 61% (n = 3, p = 0.054) with STX(C621G). Then we asked what happens upon NCAM polysialylation in STX/PST co-expressing cells. The polySia-NCAMs secreted from the cells co-expressing the same amount of mRNAs for PST(WT) and either STX(WT), STX(G421A), or STX(C621G) using pBudCE-STX-PST plasmids were characterized by the anti-polySia/anti-NCAM immunostaining ratio described above (Fig. 2B). The polysialylation of NCAM in the STX(WT)/PST(WT)-expressing cells was at a level similar to that in PST(WT)-expressing cells. Therefore, the co-expression of STX(WT) and PST(WT) appears not to enhance the NCAM polysialylation compared with PST(WT) alone. However, the extent of NCAM polysialylation in the STX(G421A)/PST(WT) cells relative to that in the STX(WT)/PST(WT) cells was enhanced compared with that in the STX(G421A) relative to STX(WT) cells (Fig. 2, A and B). Notably, the NCAM polysialylation significantly decreased in the STX(G421A)/PST(WT) cells (i.e. to 33% (n = 3, p = 0.0011)) compared with that in the STX(WT)/PST(WT) cells. With the STX(C621G)/PST(WT) cells, the NCAM polysialylation was enhanced by 23% compared with that with the STX(C621G) singly expressing cells. However, it was nearly equal to the case with the STX(WT)/PST(WT) cells (Fig. 2B).
FIGURE 2.
PolySia formation on NCAM by STX(WT), STX(G421A), and STX(C621G) in the absence and presence of PST(WT) in the transfected cells. A, polySia-NCAM-Fc secreted from CHO cells stably expressing NCAM-Fc and each of STX(WT), STX(G421A), or STX(C621G) was purified from the culture medium. The purified polySia-NCAM-Fc was subjected to Western blotting with anti-polySia (12E3) and anti-NCAM (H-300). The relative intensity of polySia-NCAM immunostainings was densitometrically determined. The data are expressed as the mean ± S.D. (error bars), with 3 replicates/experiment. The relative intensity for STX(WT) was set to 100%. ***, significance at p = 0.00075 (<0.001). B, polySia-NCAM-Fc secreted from CHO cells stably expressing NCAM-Fc and each pair of PST(WT) and STX(WT), STX(G421A), or STX(C621G) was purified from culture medium and subjected to Western blotting as described in A. The relative intensity of the STX(WT)/PST(WT) pair was set to 100%. **, significance at p = 0.0011 (<0.01). +, presence of enzyme. −, absence of enzyme.
Chemical Characterization of PolySia-NCAM Synthesized by STX(WT), STX(G421A), and STX(C621G)
The polySia-NCAM-Fcs secreted from the cells expressing STX(WT), STX(G421A), or STX(C621G) were analyzed for the chain length of polySia by the fluorometric C7/C9 analysis. This assay includes periodate oxidation of polySia-NCAM, subsequent reduction, hydrolysis, and fluorescent labeling, followed by the fluorometric HPLC analysis. A non-reducing terminal sialic acid residue gives C7-Neu5Ac, or C7, whereas internal residues of α2–8-linked polySia remain unchanged as C9-Neu5Ac, or C9. Thus, the C9/(C7 + C9) ratio basically reflects the chain length of polySia (33). The results are summarized in Table 1. The C9/(C7 + C9) ratios of polySia-NCAM synthesized by STX(G421A) and STX(C621G) were 0.31 and 0.34, respectively. These values were lower than that of STX(WT), suggesting that the chain length of polySia synthesized by the mutant enzymes are shorter than that by STX(WT). In particular, a significant decrease in the C9/(C7 + C9) ratio was observed for the STX(G421A)-derived polySia (p < 0.042). These values were lower than that of STX(WT), suggesting that the chain length of polySia synthesized by the mutant enzymes is shorter than that of polySia synthesized by STX(WT). In particular, an obvious decrease in the C9/(C7 + C9) ratio was observed for the STX(G421A)-derived polySia. The purified polySia-NCAM-Fcs were further subdivided into two fractions, the 0.3 and 0.4 m fractions, by DEAE-Sephadex A-25 chromatography with sequential elutions with 0.3 and 0.4 m NaCl. Although both fractions contained polySia-NCAM, the polySia immunostaining of the 0.4 m fraction was much stronger than that of the 0.3 m fraction, when the immunostaining for polySia (12E3) and NCAM (H300) and the silver staining were carefully compared (see supplemental Fig. S3 for the STX(WT)-derived polySia-NCAM). The 0.3 and 0.4 m fractions derived from STX(WT)-, STX(G421A)-, or STX(C621G)-expressing cells were also subjected to the fluorometric C7/C9 analysis (supplemental Table S2). The C9/(C7 + C9) ratios for the 0.4 m fractions (0.62–0.70) were larger than those for the 0.3 m fractions (0.42–0.55) in any STX-derived polySia-NCAMs, indicating that the 0.4 m fractions contain longer polySia than the 0.3 m fractions. In both fractions, the C9/(C7 + C9) ratios of the STX mutant-derived polySia were 1.1–1.3-fold lower than those of STX(WT)-derived polySia. These results indicate that the STX mutants synthesize shorter polySia chains than STX(WT).
TABLE 1.
Fluorometric C7/C9 Analysis of NCAM-Fc
| Enzymes (STX) | C9-Neu5Acyla | C7Neu5Acyla | Neu5Aca | C9/(C7 + C9) |
|---|---|---|---|---|
| NCAM-Fc(wt) | 8.63 ± 2.78 | 13.6 ± 0.283 | 22.2 ± 2.96 | 0.39 ± 0.078 |
| NCAM-Fc(g421a) | 4.91 ± 1.14 | 11.0 ± 1.06 | 14.5 ± 1.98 | 0.31 ± 0.071b |
| NCAM-Fc(c621g) | 5.86 ± 0.643 | 12.1 ± 2.28 | 18.0 ± 2.12 | 0.34 ± 0.084 |
a Molar ratio analyzed with 1 mol of polySia-NCAM-Fc (〈Mr(avr)〉 = 200 kDa; see Fig. 2B).
b p < 0.05 (compared with wild type).
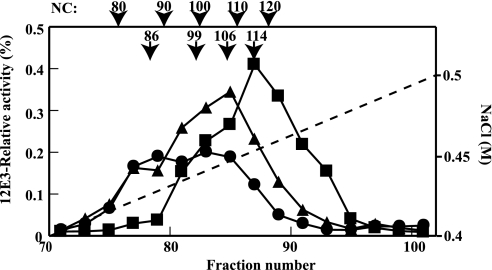
The polysialylation state was also evaluated by applying the same amount of NCAMs derived from two mutant STXs and a wild-type STX to an anion exchange HPLC. With this method, the NC can be indirectly determined based on the relationship between the elution position and NC of a polySia chain (28). As shown in Fig. 3, the predominant form of STX(WT)-derived polySia-NCAM was eluted at 0.451 m NaCl, which corresponds to NC of 114. The dominant NC for STX(C621G)-derived polySia-NCAM was 106 (0.446 m NaCl), which is slightly lower than that for STX(WT)-derived polySia-NCAM. The STX(G421A)-derived polySia-NCAM was eluted as two broad peaks with NC of 86 and 99 (0.430 and 0.440 m NaCl, respectively). These results indicate that the amount of polySia is reduced in STX(G421A)- and STX(C621G)-derived NCAM, compared with in STX(WT)-derived NCAM. The reduction of polySia amount is more prominent for STX(G421A) than for STX(C621G).
FIGURE 3.
Mono Q anion exchange chromatography of polySia-NCAM-Fc synthesized by each STX/PST pair. polySia-NCAM-Fc secreted from CHO cells stably expressing NCAM-Fc and each of STX(WT), STX(G421A), or STX(C621G) in the presence of the PST(WT) was collected from culture medium using a protein G-Sepharose column and subjected to Mono Q anion exchange chromatography. The NaCl gradient is indicated in the graph by the dotted line. The eluate was collected and dot-blotted onto PVDF membrane, followed by immunostaining with anti-polySia (12E3) antibody. The polySia staining exhibited by each sample prior to anion exchange chromatography was set equal to 1.0. The 12E3-relative activity shows the amounts of polySia in each fraction. ■, polySia-NCAM-Fc derived from STX(WT); ●, polySia-NCAM-Fc derived from STX(G421A); ▴, polySia-NCAM-Fc derived from STX(C621G). The relationship between NC and the NaCl concentration at which polySia-NCAM-Fc was eluted is indicated in the figure. The relationship with log(NC) and NaCl concentration was already confirmed (28).
Taken together, it is concluded that the amount and chain length of polySia on NCAM synthesized by STX(G421A) are lower and shorter than that of STX(WT), respectively. This is consistent with the results obtained in the in vitro enzyme activity assay. Interestingly, the silent mutant STX(C621G) also synthesized polySia with a lower amount and shorter DP than STX(WT), inconsistent with the finding that both STXs showed a nearly identical in vitro activity (Fig. 1).
BDNF-retaining Activity of PolySia-NCAM Synthesized by STX(WT), STX(G421A), and STX(C621G)
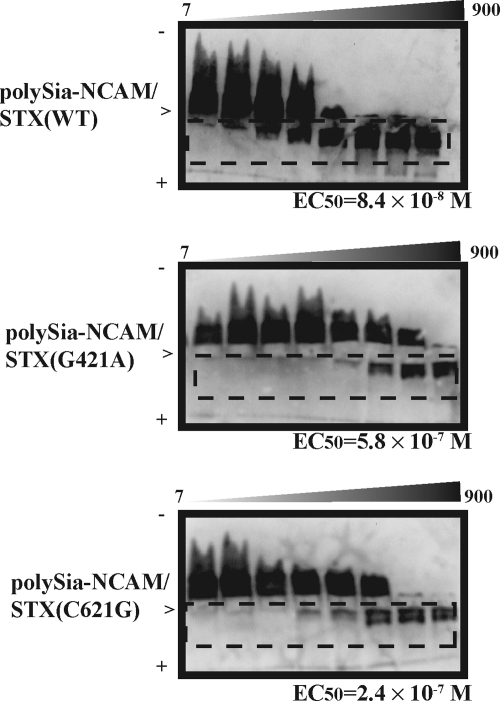
Recently, we demonstrated that polySia directly binds to BDNF and other neurotrophins and proposed a new function of polySia as a reservoir of neurotrophic factors that are involved in the psychiatric disorder and memory (28). The BDNF gene is recognized as one of the causative genes of schizophrenia (21). Therefore, we asked how the BDNF-retaining activity was affected in polySia-NCAM synthesized by the two STX mutants. The binding between polySia-NCAM and BDNF was measured by the horizontal native PAGE, and the complex of polySia-NCAM and BDNF migrated toward the anode region. The amount of BDNF in the cathode region was titrated with the purified polySia-NCAM derived from STX(WT), STX(G421A), and STX(C621G) (Fig. 4). The EC50 was calculated as the concentration of polySia-NCAM that titrated 50% of the 100 ng of BDNF, and the EC50 for polySia (colominic acid) with an average DP of 43 was well characterized to be 7.2 × 10−7 m (28, 29). The EC50 values for the STX(WT)- and STX(G421A)-derived polySia-NCAMs were 8.4 × 10−8 and 5.8 × 10−7 m, respectively, indicating that the STX(G421A)-derived polySia-NCAM showed 7-fold lower binding affinity than STX(WT)-derived polySia-NCAM (Fig. 4, middle panel versus top panel). The EC50 for the STX(C621G)-derived polySia-NCAM was low but not as low as for the STX(G421A)-derived polySia-NCAM (2.4 × 10−7 m). Based on these results, it is clear that STX(G421A)- and STX(C621G)-expressing cells produce polySia-NCAM with lower affinities for BDNF than the polySia-NCAM produced by wild-type STX-expressing cells.
FIGURE 4.
Interaction between polySia-NCAM and BDNF. Top, titration of BDNF with STX(WT)-derived polySia-NCAM (7–900 ng as BSA); middle, titration of BDNF with STX(G421A)-derived polySia-NCAM (7–900 ng as BSA); bottom, titration of BDNF with STX(C621G)-derived polySia-NCAM (7–900 ng as BSA). The principle of this method was well described (28). The calculated EC50 values are indicated below each gel. +, anode region; −, cathode region; >, loaded position. The BDNF-polySia-NCAM complex is boxed by a dotted line.
DA-binding Function of PolySia
Measurement of the Binding between PolySia and Neurotransmitters by FAC
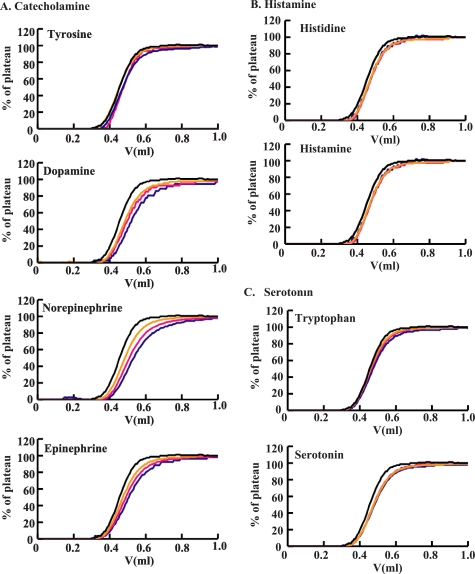
Several neurotransmitters, including DA, are known to be deeply involved in schizophrenia (19, 20, 37). PolySia is known to occur in certain synaptic clefts between neurons (38, 39). These facts led us to ask if polySia can directly bind neurotransmitters at synapses. To analyze the interaction between polySia and neurotransmitters, the FAC (34, 40) on polySia-immobilized columns was performed for three typical types of neurotransmitters and their precursors as analytes: catecholamines, histamine, and serotonin. Prior to the analyses, we tested several materials with these polySia-immobilized columns and several non-binding materials to evaluate their flow-through properties (33, 40) and selected acetylcholine as a non-binding standard molecule. For the analysis, we first used a polySia column immobilized via the reducing terminal end with Affi-Gel-102 and examined the interaction with each of the neurotransmitters in PBS (pH 7.2) at 25 °C (Fig. 5). It was observed that polySia interacted with the catecholamines, DA, norepinephrine, and epinephrine (Fig. 5A). The Kd values calculated from the elution profiles (33, 40) are summarized in Table 2. The Kd values were obtained for DA (17.9 ± 0.4 μm), norepinephrine (11.3 ± 0.3 μm), and epinephrine (12.7 ± 0.6 μm). However, polySia did not interact with tyrosine, the precursor of catecholamine. No interactions of polySia with histamine and its precursor histidine (Fig. 5B) or with serotonin and its precursor tryptophan (Fig. 5C) were observed either. Thus, the positively charged materials do not always bind polySia, indicating that the observed binding is not due to electrostatic interactions. This indication was confirmed by the finding that the interaction between disialic acid, which is found on b-series gangliosides, and catecholamines was below the limit of detection (supplemental Table S3). Therefore, it was concluded that the net negative charge of polySia was not important in the interaction with the catecholamines. Because polySia is known to exist in a helical form, we thought that this conformation was required for DA binding. To gain insight regarding this speculation, polySia was immobilized to Affi-Gel-Hz via its non-reducing terminal end after periodate treatment in order to retain the helical structure of polySia (41). The column thus prepared is shown to retain the helical structure of polySia, because it could retain anti-polySia antibody H.46 that binds to polySia with DP 10 or greater, which are known to take helical conformations (42). With this column, polySia also retained catecholamines with almost the same Kd values as those of polySia immobilized through the reducing terminal end (supplemental Table S3). These results clearly demonstrated that polySia can bind to the catecholamines DA, norepinephrine, and epinephrine, suggesting that the helical form of polySia is important for this interaction.
FIGURE 5.
Interaction between polySia and selected neurotransmitters as measured by frontal affinity chromatography. A, elution profiles of various catecholamines after passing through columns with immobilized polySia. B, histamines. C, serotonins. The neurotransmitters were dissolved in PBS at concentrations of 10 μm (blue), 20 μm (pink), and 30 μm (yellow). Two ml of each solution was applied to the column (126 μl) through a 2-ml loop at a flow rate of 0.125 ml/min at 25 °C. Each elution pattern is superimposed on that of acetylcholine (black). The Kd values obtained by three independent experiments are summarized in Table 2.
TABLE 2.
Kd value between various neurotransmitters and polySia
| Neurotransmitters and their precursors | Kd (m) |
|---|---|
| Catecholamine | |
| Tyrosine | —a |
| Dopamine | 17.9 × 10−6 ± 0.4 × 10−6 |
| Norepinephrine | 11.3 × 10−6 ± 0.3 × 10−6 |
| Epinephrine | 12.7 × 10−6 ± 0.6 × 10−6 |
| Histamine | |
| Histidine | — |
| Histamine | — |
| Serotonin | |
| Tryptophan | — |
| Serotonin | — |
a —, Kd value could not be measured because of weak interaction.
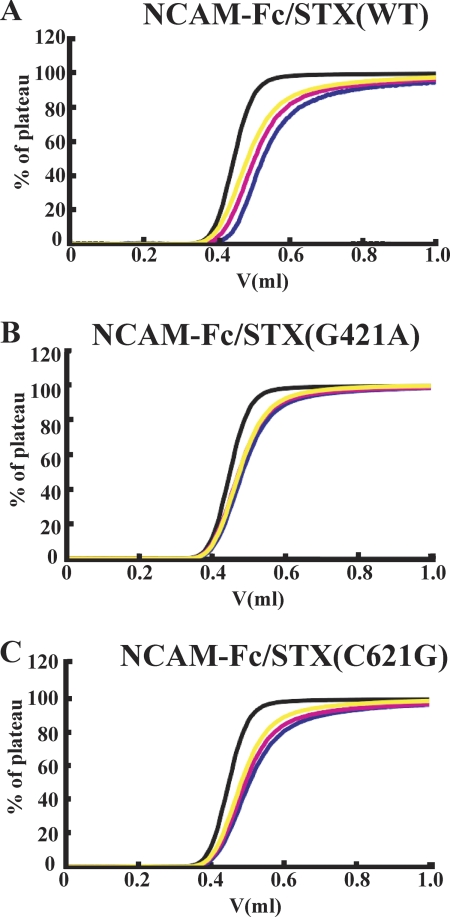
DA Binding Activity of PolySia-NCAM Synthesized by STX(WT), STX(G421A), and STX(C621G)
After demonstrating the new feature of polySia that has affinity for DA, we attempted to measure the Kd values of DA with polySia-NCAM synthesized by STX(WT), STX(G421A), and STX(C621G). The polySia-NCAM-Fcs were immobilized with protein G-Sepharose to be analyzed for DA binding by FAC. As shown in Fig. 6A, an interaction between DA and STX(WT)-derived polySia-NCAM with a Kd of 8.8 ± 0.2 μm was observed. However, the binding of DA with STX(G421A)-derived polySia-NCAM was not detected (Fig. 6B), demonstrating that the DA-binding function of polySia was impaired. The Kd of STX(C621G)-derived polySia-NCAM (17 ± 2.1 μm) was 2-fold lower than that of wild type-derived (Fig. 6C) but not as low as STX(G421A)-derived polySia-NCAM.
FIGURE 6.
Interaction between polySia-NCAM and DA measured by frontal affinity chromatography. Elution profiles of DA after application to a column of immobilized STX(WT)-derived polySia-NCAM (A), STX(G421A)-derived polySia-NCAM (B), and STX(C621G)-derived polySia-NCAM (C). DA was dissolved in PBS at concentrations of 10 μm (blue), 20 μm (pink), and 30 μm (yellow), and 2 ml of each solution was applied to the column (126 μl) through a 2-ml loop at a flow rate of 0.125 ml/min at 25 °C. Each elution pattern is superimposed on that of acetylcholine (black). The Kd values of STX(WT)- and STX(C621G)-derived polySia-NCAM were 8.6 and 19 μm, respectively. The Kd value of STX(G421A)-derived polySia-NCAM could not be calculated.
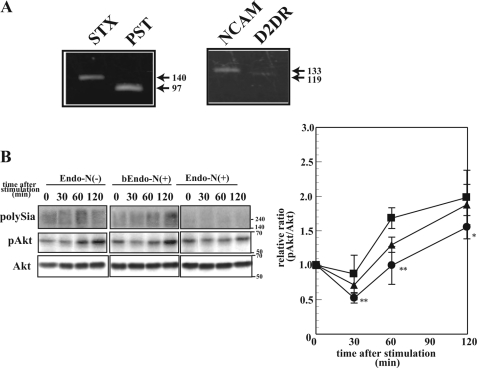
Effects of PolySia-NCAM on DA Signaling
To gain insight into the effects of polySia on DA signaling, the phosphorylation of Akt was investigated because the impairment of Akt signaling is one of the striking phenomena observed in schizophrenia (37) and schizophrenia-causing gene-disrupted cells and animals, such as DISC1 deficiency (43). Because SK-N-SH human neuroblastoma cells endogenously express polySia-NCAM and DA receptor D2 (D2DR) (Fig. 7A), these cells were treated with or without polySia-cleaving enzyme, Endo-N, and also with boiled Endo-N to exclude the possible effects of contaminants or the direct effects of Endo-N itself. As shown in Fig. 7B, about 90% of polySia disappeared after Endo-N treatment (Endo-N(+)), whereas the majority of polySia (75%) remained unchanged after boiled Endo-N treatment (bEndo-N(+)). In the presence or absence of polySia-NCAM on the cell surface, DA was used to treat cells for 0–120 min, and the phosphorylation of Akt was analyzed. Akt phosphorylation showed a decrease between 30 and 120 min after DA stimulation in the absence of polySia-NCAM on the cell surface (Endo-N(−)), compared with non-treated cells (Endo-N(+)) (Fig. 7C). The result obtained after the treatment with boiled Endo-N was nearly identical to that of Endo-N(−), suggesting that polySia-NCAM, especially polySia structure, is involved in alteration of DA signaling through Akt.
FIGURE 7.
Effects of polySia expression on DA-induced Akt signaling. A, RT-PCR of STX, PST, NCAM, and D2DR. The cDNA derived from SK-N-SH cells was used as template for PCR that was performed with specific primers as described in supplemental Table S1. The PCR products were analyzed with 1% agarose gels and visualized with ethidium bromide. B, phosphorylation of Akt after the DA stimulation. SK-N-SH cells were treated with or without Endo-N (Endo-N(+) or Endo-N(−), respectively) or with boiled EndoN (bEndo-N(+)). The polysialylation state was analyzed by the immunostaining of polySia (top, polySia). The cells were then treated with DA for 0, 30, 60, and 120 min, and the homogenates were analyzed by Western blotting with anti-phosphorylated Akt antibody and anti-Akt antibody (middle and bottom, pAkt and Akt). The immunostainings were densitometrically measured, and the relative ratio of pAkt/Akt was plotted for each treatment (right): Endo-N(−) (filled squares), Endo-N(+) (filled circles), and bEndo-N(+) (filled triangles). The pAkt/Akt value at 0 min was set to 1.0. The data are expressed as the mean ± S.D. (error bars), with at least 3 replicates/experiment. ** and *, p < 0.01 and p < 0.1, respectively, compared with the case with Endo-N(−).
DISCUSSION
Polysialic acid has been reported to be involved in schizophrenia and schizophrenia-like symptoms (12, 15, 24–27, 44, 45). In this study, we showed that STX(G421A) that was reported from a schizophrenic patient exhibited a dramatic decrease of the activity to ∼10–30% in polysialylation of NCAM, compared with STX(WT) (Fig. 1 and supplemental Fig. S2), indicating that Glu141 near the sialyl motif L region critical for the substrate CMP-Neu5Ac binding is essential for enzymatic activity. Although the x-ray crystal structure of STX has not been reported, the change of Glu to Lys, which converts the charge from negative to positive, might lead to the conformational perturbation of the sialyl motif L. Alternatively, Glu141 may be involved in the interaction of STX with PST. Our in vitro co-incubation experiments clearly showed that STX(G421A) inhibited the PST(WT) activity, compared with STX(WT) (Fig. 1). The NCAM polysialylation was also suppressed in the STX(G421A)/PST(WT) cells compared with the STX(WT)/PST(WT) cells (Fig. 2). Therefore, STX and PST may interact with each other if both enzymes co-exist, and the Glu141 in STX may be involved in regulation of the polysialylation reaction through the STX and PST interaction.
Unexpectedly, we observed that the amount of polySia on NCAM from the cells expressing a nonsense mutant STX(C621G) significantly decreased compared with that from STX(WT)-expressing cells, although STX(C621G) displayed nearly identical activity in the in vitro assay. Because the primary amino acid sequence and the in vitro enzymatic activity of STX(C621G) are the same as those of STX(WT), the observed in vivo decrease of polySia on NCAM for STX(C621G) may be due to frequency of the codon usage of proline. The codon CCG in STX(C621G) is the least frequent codon for proline in mammals, whereas the codon CCC in STX(WT) is the most frequent one. For example, a codon usage ratio for CCC in hamsters and humans is 17 and 19.8 (frequency per thousand), respectively, whereas that for CCG is only 4.3 and 6.9, respectively, representing 4- and 2.5-fold reductions in the mutation (see the Codon Usage Database on the World Wide Web). The reduced codon usage ratios may lower the translational speed due to an inefficient supply of aminoacyl-tRNAs (46), eventually leading to a decrease in the in vivo activity. Experiments to demonstrate this speculation are under way in our laboratory.
The polySia structure on NCAM synthesized by the mutant STX was also impaired in both quantity and quality (chain length). These impairments of polySia structure greatly affected its biological functions. One function that we focused on is the BDNF-retaining ability, which was recently demonstrated by us (28). Provided that the quality and quantity of polySia might be impaired in a patient expressing STX(G421A), impaired polySia expression may lead to the development of schizophrenia, through the reduction of BDNF in the microenvironment near BDNF receptors. Previously, we demonstrated that glycosaminoglycans like heparan sulfate and chondroitin sulfate also have the ability to bind BDNF (29). The local concentration of BDNF in the microenvironment may also be regulated by such glycosaminoglycans. It is thus interesting to reveal a functional relationship between polySia and glycosaminoglycans.
In this study, we first demonstrated a catecholamine-retaining activity as a new function of polySia using the FAC technique and showed that this activity is completely impaired in the STX(G421A)-derived polySia-NCAM. These findings are significant in two ways. First, this is the first application of FAC to a quantitative analysis of the glycan-small molecule interactions. Second, our FAC analyses successfully demonstrated that polySia binds catecholamines, including DA, norepinephrine, and epinephrine. PolySia structure and the catechol backbone might interact with each other specifically. Based on these observations, polySia may possibly regulate the local concentration of catecholamine around the polySia-NCAM via the binding or releasing of these molecules. Because the Kd between polySia and DA (18 μm) (Table 2) and the reported Kd between DA and D2DR (0.5 μm) (47) are of a similar order, it is reasonable to speculate that polySia retains and releases these molecules toward their receptors depending on the difference between Kd values and that its balance might be fine tuned (28). The polySia-expressing regions in adult brains are typically restricted to the olfactory bulb and hippocampus (48). In humans and primates, it is not restricted to the olfactory region and hippocampus and has also been reported in the prefrontal cortex, the hypothalamus, and the region around the central canal of the spine (48). In addition, weakly polySia-immunostained cells are also observed diffusely in non-neurogenic regions in the brain (48), and tissues other than the brain, including the heart, have been reported to contain polySia (1, 49). Although the role of polySia in these regions has not been well demonstrated, the results presented here suggest that polySia may influence biological signaling through catecholamine binding.
Akt is an important Ser/Thr kinase involved in numerous signaling cascades downstream of neurotrophic factor and monoamine receptors. It is also related to the development of schizophrenia (50). In this study, polySia on NCAM is also demonstrated to be involved in the Akt signaling through DA. Without polySia, DA signaling is enhanced to show the suppression of Akt activation, as reported previously (51). Recently, it was shown that the cytosolic region of NCAM is associated with D2DR in cells, indicating that polySia-NCAM and D2DR are in very close proximity, close enough for polySia to be directly involved in DA signaling (52). The impaired polySia structures on NCAM in the schizophrenia patient with SNP-7 in the STX gene or STX(G421A) might lead to impaired DA signaling, probably because DA directly reaches the receptor without being trapped by STX(G421A)-derived polySia-NCAM. In this regard, it is interesting to note that excess DA is often present in the mesolimbic system in schizophrenic brains (53). Recently, it has been clearly demonstrated with polysialic acid-deficient mice that polysialic acid is dispensable for the formation of the mesencephalic dopaminergic system (54), although the polysialic acids are reported to present during development of the ventral mesencephalone of rats. Therefore, the DA binding feature of polysialic acid might be involved in the adult brain function. It is noteworthy that polysialyltransferase knock-out mice showed impaired social interactions (55).
PolySia is an important molecule for normal brain development. Recently, Angata et al. (56) demonstrated that polySia-directed migration and differentiation of neural precursors are essential for mouse brain development. In addition, polySia is also recognized as a marker of neural stem cells that can still undergo neurogenesis in adult. Interestingly, neural stem cell proliferation is decreased in individuals with schizophrenia (57). We do not know of a direct relationship of the polySia expression with the proliferation rate of the neural stem cells in schizophrenia; however, it is noteworthy that abnormality in polySia expression is one of the factors leading to schizophrenia in the neurodevelopmental hypothesis (56). Because schizophrenia is considered to be a complex disease with multiple factors contributing to pathogenesis (16), the mechanisms by which polySia is involved in schizophrenia are also likely to be complex. The quality and quantity of polySia-NCAM might be strictly regulated. In this regard, imbalances of the polySia expression on NCAM due to low activity or altered expression levels of STX bring about serious consequences for neuronal function and brain diseases other than schizophrenia, such as Alzheimer disease (58, 59), Parkinson disease (60, 61), and drug abuse (62).
Acknowledgments
We thank Dr. Minoru Fukuda (Burnham Institute, La Jolla, CA) and Prof. Sen-ichiroh Hakomori (Washington University, St. Louis, MO) for helpful discussions. We also thank Sayaka Ono, Mizuki Sumida, and Masaya Hane for their assistance.
This research was supported in part by Grants-in-Aid for Scientific Research (C) 20570107 and 23570133 from the Ministry of Education, Science, Sports, and Culture and by the Hayashi Foundation (to C. S.) and by the CREST of Japan Science and Technology Agency (to K. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Tables S1–S3.
- polySia
- polysialic acid
- BDNF
- brain-derived neurotrophic factor
- DA
- dopamine
- DP
- degree of polymerization
- NCAM
- neural cell adhesion molecule
- NC
- net negative charge
- pAkt
- phosphorylated Akt
- FAC
- frontal affinity chromatography
- D2DR
- DA receptor D2
- Endo-N
- endo-N-acylneuraminidase.
REFERENCES
- 1. Troy F. A., II (1996) in Biology of the Sialic Acids (Rosenberg A. ed) pp. 95–144, Plenum Press, New York [Google Scholar]
- 2. Sato C., Kitajima K. (1999) Trends Glycosci. Glycotech. 11, 371–390 [Google Scholar]
- 3. Nakata D., Troy F. A., 2nd (2005) J. Biol. Chem. 280, 38305–38316 [DOI] [PubMed] [Google Scholar]
- 4. Drake P. M., Nathan J. K., Stock C. M., Chang P. V., Muench M. O., Nakata D., Reader J. R., Gip P., Golden K. P., Weinhold B., Gerardy-Schahn R., Troy F. A., 2nd, Bertozzi C. R. (2008) J. Immunol. 181, 6850–6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonfanti L. (2006) Prog. Neurobiol. 80, 129–164 [DOI] [PubMed] [Google Scholar]
- 6. Rutishauser U. (2008) Nat. Rev. Neurosci. 9, 26–35 [DOI] [PubMed] [Google Scholar]
- 7. Seki T., Arai Y. (1991) Neurosci. Res. 12, 503–513 [DOI] [PubMed] [Google Scholar]
- 8. Seki T., Arai Y. (1993) J. Neurosci. 13, 2351–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seki T., Arai Y. (1993) Neurosci. Res. 17, 265–290 [DOI] [PubMed] [Google Scholar]
- 10. Nakayama J., Fukuda M. N., Fredette B., Ranscht B., Fukuda M. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7031–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eckhardt M., Mühlenhoff M., Bethe A., Koopman J., Frosch M., Gerardy-Schahn R. (1995) Nature 373, 715–718 [DOI] [PubMed] [Google Scholar]
- 12. Cremer H., Lange R., Christoph A., Plomann M., Vopper G., Roes J., Brown R., Baldwin S., Kraemer P., Scheff S. (1994) Nature 367, 455–459 [DOI] [PubMed] [Google Scholar]
- 13. Eckhardt M., Bukalo O., Chazal G., Wang L., Goridis C., Schachner M., Gerardy-Schahn R., Cremer H., Dityatev A. (2000) J. Neurosci. 20, 5234–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinhold B., Seidenfaden R., Röckle I., Mühlenhoff M., Schertzinger F., Conzelmann S., Marth J. D., Gerardy-Schahn R., Hildebrandt H. (2005) J. Biol. Chem. 280, 42971–42977 [DOI] [PubMed] [Google Scholar]
- 15. Angata K., Long J. M., Bukalo O., Lee W., Dityatev A., Wynshaw-Boris A., Schachner M., Fukuda M., Marth J. D. (2004) J. Biol. Chem. 279, 32603–32613 [DOI] [PubMed] [Google Scholar]
- 16. Lang U. E., Puls I., Muller D. J., Strutz-Seebohm N., Gallinat J. (2007) Cell Physiol. Biochem. 20, 687–702 [DOI] [PubMed] [Google Scholar]
- 17. Millar J. K., Christie S., Anderson S., Lawson D., Hsiao-Wei Loh D., Devon R. S., Arveiler B., Muir W. J., Blackwood D. H., Porteous D. J. (2001) Mol. Psychiatry 6, 173–178 [DOI] [PubMed] [Google Scholar]
- 18. Harrison P. J. (2004) Psychopharmacology 174, 151–162 [DOI] [PubMed] [Google Scholar]
- 19. Strous R. D., Bark N., Parsia S. S., Volavka J., Lachman H. M. (1997) Psychiatry Res. 69, 71–77 [DOI] [PubMed] [Google Scholar]
- 20. Allen N. C., Bagade S., McQueen M. B., Ioannidis J. P., Kavvoura F. K., Khoury M. J., Tanzi R. E., Bertram L. (2008) Nat. Genet. 40, 827–834 [DOI] [PubMed] [Google Scholar]
- 21. Guillin O., Demily C., Thibaut F. (2007) Int. Rev. Neurobiol. 78, 377–395 [DOI] [PubMed] [Google Scholar]
- 22. Woods B. T. (1998) Am. J. Psychiatry 155, 1661–1670 [DOI] [PubMed] [Google Scholar]
- 23. Ashe P. C., Berry M. D., Boulton A. A. (2001) Prog. Neuropsychopharmacol. Biol. Psychiatry 25, 691–707 [DOI] [PubMed] [Google Scholar]
- 24. Barbeau D., Liang J. J., Robitalille Y., Quirion R., Srivastava L. K. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 2785–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turetsky B. I., Moberg P. J., Roalf D. R., Arnold S. E., Gur R. E. (2003) Arch. Gen. Psychiatry 60, 1193–1200 [DOI] [PubMed] [Google Scholar]
- 26. Maziade M., Roy M. A., Chagnon Y. C., Cliche D., Fournier J. P., Montgrain N., Dion C., Lavallée J. C., Garneau Y., Gingras N., Nicole L., Pirès A., Ponton A. M., Potvin A., Wallot H., Mérette C. (2005) Mol. Psychiatry 10, 486–499 [DOI] [PubMed] [Google Scholar]
- 27. Arai M., Yamada K., Toyota T., Obata N., Haga S., Yoshida Y., Nakamura K., Minabe Y., Ujike H., Sora I., Ikeda K., Mori N., Yoshikawa T., Itokawa M. (2006) Biol. Psychiatry 59, 652–659 [DOI] [PubMed] [Google Scholar]
- 28. Kanato Y., Kitajima K., Sato C. (2008) Glycobiology 18, 1044–1053 [DOI] [PubMed] [Google Scholar]
- 29. Kanato Y., Ono S., Kitajima K., Sato C. (2009) Biosci. Biotechnol. Biochem. 73, 2735–2741 [DOI] [PubMed] [Google Scholar]
- 30. Asahina S., Sato C., Matsuno M., Matsuda T., Colley K., Kitajima K. (2006) J. Biochem. 140, 687–701 [DOI] [PubMed] [Google Scholar]
- 31. Angata K., Suzuki M., Fukuda M. (1998) J. Biol. Chem. 273, 28524–28532 [DOI] [PubMed] [Google Scholar]
- 32. Kojima N., Yoshida Y., Tsuji S. (1995) FEBS. Lett. 373, 119–122 [DOI] [PubMed] [Google Scholar]
- 33. Sato C., Inoue S., Matsuda T., Kitajima K. (1998) Anal. Biochem. 261, 191–197 [DOI] [PubMed] [Google Scholar]
- 34. Tateno H., Nakamura-Tsuruta S., Hirabayashi J. (2007) Nat. Protoc. 2, 2529–2537 [DOI] [PubMed] [Google Scholar]
- 35. Sato C., Yamakawa N., Kitajima K. (2010) Methods Enzymol. 478, 219–232 [DOI] [PubMed] [Google Scholar]
- 36. Sato C., Kitajima K., Inoue S., Seki T., Troy F. A., 2nd, Inoue Y. (1995) J. Biol. Chem. 270, 18923–18928 [DOI] [PubMed] [Google Scholar]
- 37. Tan H. Y., Nicodemus K. K., Chen Q., Li Z., Brooke J. K., Honea R., Kolachana B. S., Straub R. E., Meyer-Lindenberg A., Sei Y., Mattay V. S., Callicott J. H., Weinberger D. R. (2008) J. Clin. Invest. 118, 2200–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uryu K., Butler A. K., Chesselet M. F. (1999) J. Comp. Neurol. 405, 216–232 [DOI] [PubMed] [Google Scholar]
- 39. Schuster T., Krug M., Stalder M., Hackel N., Gerardy-Schahn R., Schachner M. (2001) J. Neurobiol. 49, 142–158 [DOI] [PubMed] [Google Scholar]
- 40. Kasai K., Ishii S. (1978) J. Biochem. 84, 1051–1060 [DOI] [PubMed] [Google Scholar]
- 41. Halberstadt J. B., Flowers H., Glick M. C. (1993) Anal. Biochem. 209, 136–142 [DOI] [PubMed] [Google Scholar]
- 42. Baumann H., Brisson J. R., Michon F., Pon R., Jennings H. J. (1993) Biochemistry 32, 4007–4013 [DOI] [PubMed] [Google Scholar]
- 43. Enomoto A., Asai N., Namba T., Wang Y., Kato T., Tanaka M., Tatsumi H., Taya S., Tsuboi D., Kuroda K., Kaneko N., Sawamoto K., Miyamoto R., Jijiwa M., Murakumo Y., Sokabe M., Seki T., Kaibuchi K., Takahashi M. (2009) Neuron 63, 774–787 [DOI] [PubMed] [Google Scholar]
- 44. Tomasiewicz H., Ono K., Yee D., Thompson C., Goridis C., Rutishauser U., Magnuson T. (1993) Neuron 11, 1163–1174 [DOI] [PubMed] [Google Scholar]
- 45. Angata K., Fukuda M. (2003) Biochimie 85, 195–206 [DOI] [PubMed] [Google Scholar]
- 46. Kimchi-Sarfaty C., Oh J. M., Kim I. W., Sauna Z. E., Calcagno A. M., Ambudkar S. V., Gottesman M. M. (2007) Science 315, 525–528 [DOI] [PubMed] [Google Scholar]
- 47. Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. (1990) Nature 347, 146–151 [DOI] [PubMed] [Google Scholar]
- 48. Seki T. (2004) Trends Glycosci. Glycotech. 16, 319–330 [Google Scholar]
- 49. Lackie P. M., Zuber C., Roth J. (1991) Differentiation 47, 85–98 [DOI] [PubMed] [Google Scholar]
- 50. Datta S. R., Brunet A., Greenberg M. E. (1999) Genes Dev. 13, 2905–2927 [DOI] [PubMed] [Google Scholar]
- 51. Freyberg Z., Ferrando S. J., Javitch J. A. (2010) Am. J. Psychiatry 167, 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiao M. F., Xu J. C., Tereshchenko Y., Novak D., Schachner M., Kleene R. (2009) J. Neurosci. 29, 14752–14763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mackay A. V., Iversen L. L., Rossor M., Spokes E., Bird E., Arregui A., Creese I., Synder S. H. (1982) Arch. Gen. Psychiatry 39, 991–997 [DOI] [PubMed] [Google Scholar]
- 54. Schiff M., Weinhold B., Grothe C., Hildebrandt H. (2009) J. Neurochem. 110, 1661–1673 [DOI] [PubMed] [Google Scholar]
- 55. Calandreau L., Márquez C., Bisaz R., Fantin M., Sandi C. (2010) Genes Brain Behav. 9, 958–967 [DOI] [PubMed] [Google Scholar]
- 56. Angata K., Huckaby V., Ranscht B., Terskikh A., Marth J. D., Fukuda M. (2007) Mol. Cell. Biol. 27, 6659–6668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reif A., Fritzen S., Finger M., Strobel A., Lauer M., Schmitt A., Lesch K. P. (2006) Mol. Psychiatry 11, 514–522 [DOI] [PubMed] [Google Scholar]
- 58. Brocco M., Pollevick G. D., Frasch A. C. (2003) J. Neurosci. Res. 74, 744–753 [DOI] [PubMed] [Google Scholar]
- 59. Mikkonen M., Soininen H., Tapiola T., Alafuzoff I., Miettinen R. (1999) Eur. J. Neurosci. 11, 1754–1764 [DOI] [PubMed] [Google Scholar]
- 60. Oizumi H., Hayashita-Kinoh H., Hayakawa H., Arai H., Furuya T., Ren Y. R., Yasuda T., Seki T., Mizuno Y., Mochizuki H. (2008) Neurosci. Res. 60, 15–21 [DOI] [PubMed] [Google Scholar]
- 61. Yoshimi K., Ren Y. R., Seki T., Yamada M., Ooizumi H., Onodera M., Saito Y., Murayama S., Okano H., Mizuno Y., Mochizuki H. (2005) Ann. Neurol. 58, 31–40 [DOI] [PubMed] [Google Scholar]
- 62. Murphy K. J., Foley A. G., O'connell A. W., Regan C. M. (2006) Neuropsychopharmacology 31, 90–100 [DOI] [PubMed] [Google Scholar]