Abstract
The entry into epithelial cells and the prevention of primary immune responses are a prerequisite for a successful colonization and subsequent infection of the human host by Streptococcus pyogenes (group A streptococci, GAS). Here, we demonstrate that interaction of GAS with plasminogen promotes an integrin-mediated internalization of the bacteria into keratinocytes, which is independent from the serine protease activity of potentially generated plasmin. α1β1- and α5β1-integrins were identified as the major keratinocyte receptors involved in this process. Inhibition of integrin-linked kinase (ILK) expression by siRNA silencing or blocking of PI3K and Akt with specific inhibitors, reduced the GAS M49-plasminogen/plasmin-mediated invasion of keratinocytes. In addition, blocking of actin polymerization significantly reduced GAS internalization into keratinocytes. Altogether, these results provide a first model of plasminogen-mediated GAS invasion into keratinocytes. Furthermore, we demonstrate that plasminogen binding protects the bacteria against macrophage killing.
Keywords: Akt PKB, Cytoskeleton, Integrin, Phagocytosis, siRNA, ILK, Streptococcus Pyogenes, Adherence, Internalization, Keratinocytes
Introduction
Streptococcus pyogenes (group A streptococcus (GAS)2) is an exclusively human pathogen. GAS causes many human diseases ranging from commonly mild superficial infections of the skin and mucous membranes of the naso-pharynxs to severe but rare toxic and invasive diseases (1–3). GAS are equipped with many virulence factors, which allow this pathogen to infect and survive within the host (3–5).
Although GAS had initially been described as an extracellular pathogen, numerous studies have shown that GAS adhere to and internalize into epithelial cells by using matrix proteins, e.g. fibronectin, for bacteria-host cell contact (6–10). Fibronectin-mediated GAS internalization into human epithelial cells is dependent on the intracellular mammalian enzymes phosphatidylinositol 3-kinase (PI3K) and ILK (11, 12). ILK is a serine-threonine kinase, which interacts with cytoplasmic domains of β-integrins. It is an important member of several integrin-dependent pathways. As a catalytic and structural component for actin-cytoskeleton assembly, ILK promotes actin-cytoskeleton rearrangement in a PI3K-dependent manner (13, 14). In addition to matrix proteins, GAS interacts with plasma proteins. Particularly, the binding of plasminogen by GAS is an important virulence attribute (15).
Plasminogen is a single chain glycoprotein found in plasma and extracellular fluids (16). Different mammalian activators cleave plasminogen at a single site between Arg560–Val561. The cleavage results in the formation of the two-chained active plasmin enzyme, containing a serine protease active site in the C-terminal region. Plasminogen interacts with ligands via lysine binding sites in its five kringle domains (17). Plasminogen receptors and activators are expressed by group A, C, and G streptococci (18). GAS interact with plasminogen either by direct binding with specific surface proteins or indirectly by sequential binding of fibrinogen and plasminogen (19). Specific GAS surface proteins involved in plasminogen-binding are glyceraldehyde-3-phosphate dehydrogenase (GAPDH (20, 21); SDH (22)), streptococcal surface enolase (SEN (23)), plasminogen-binding group A streptococcal M protein (PAM (24)), PAM-related protein (Prp (25)), and extracellular protein factor (Epf (26)).
Moreover, the GAS-secreted nephritogenic plasminogen-binding protein is able to interact with plasminogen (27). Another secreted protein, streptokinase, enables GAS to cleave plasminogen to plasmin (18), which degrades connective tissue, extracellular matrix (ECM), and fibrin clots (16, 17).
The redundancy of these secreted and surface-associated GAS plasminogen receptors implies a crucial role of plasminogen binding in GAS pathogenesis. This is underlined by the fact that GAS-plasminogen interaction promotes tissue invasion (28, 29). The exploitation of the human plasminogen systems plays a critical role in GAS systemic disease initiation (30, 31), most likely by allowing GAS to circumvent host defense fibrin networks (32). It has been shown that coating of GAS with plasminogen enhances adherence to Detroit laryngeal epithelial cells and allows transwell penetration through the monolayers by mechanisms not well defined yet (33). However, also a direct binding of SDH to urokinase plasminogen activator receptor (uPAR/CD87) on the surface of Detroit cells has been described (22). Together, these data strongly suggested plasminogen binding to be important for GAS infections through the naso-pharyngeal route. At least the expression of one bacterial receptor, PAM, was found associated with skin infection (34). This leaves the major questions how GAS use surface bound plasminogen/plasmin to adhere to and internalize into skin keratinocytes, a primary event during the skin infection route, what is the nature of the host cell receptor for plasminogen/plasmin on keratinocytes, and which intracellular signaling pathways are exploited for these processes.
To address these open questions we used chemical inhibitors and siRNA silencing to characterize the observed high level keratinocyte invasion by GAS once they are coated with plasminogen/plasmin. These effects can be achieved by intact surface-bound plasminogen as shown with a streptokinase-negative GAS mutant. If, under physiological conditions, plasminogen is cleaved into active plasmin on the bacterial surface, the increased internalization is independent of the serine protease activity of plasmin as we were able to show by aprotinin and α2-antiplasmin inhibition experiments. Because we cannot completely rule out conversion of plasminogen into active plasmin once it is bound to the bacterial surface, we refer to the surface-bound material as plasminogen/plasmin throughout this work. Integrins were identified as binding partners on the keratinocyte surface for plasminogen/plasmin interaction. Inhibition of actin-cytoskeleton dynamics and genetic knock-down of ILK impaired plasminogen/plasmin-mediated GAS keratinocyte invasion. GAS surface-bound plasminogen/plasmin enhanced bacterial blood survival and protected against macrophage killing.
EXPERIMENTAL PROCEDURES
Reagents
Human plasminogen was purchased from Chromogenix and was detected using a monoclonal murine Anti-PLG antibody (Sigma). Cytocalasin D, Wortmannin, Latrunculin B, Akt 1/2 kinase inhibitor, aprotinin, and heparin (sodium salt) were obtained from Sigma. α2-antiplasmin was purchased from antikoerper-online.de. Highly purified whole human integrins αvβ5, α1β1, and α5β1 were obtained from Millipore. SignalSilence ILK1 siRNA, SignalSilence Control siRNA, PI3 Kinase Class III (D4E2) XP Rabbit mAb, Phospho-Akt (Ser-473) antibody, ILK1 antibody, GAPDH (14C10) Rabbit mAb, and anti-rabbit IgG AP-linked antibody were purchased from Cell Signaling Technology. CSPD ready to use was obtained from Roche. LIVE/DEAD Viability/Cytotoxicity kit for mammalian cells was purchased from Invitrogen. In transfection experiments Transpass R2 reagent (NEB) was used.
Bacterial Strains, Eukaryotic Cells, and Culture Conditions
GAS serotype M49 strain 591 was obtained from R. Lütticken (Aachen, Germany). The GAS wild-type strain was cultured in Todd-Hewitt broth (Invitrogen) supplemented with 0.5% (w/v) yeast extract (THY; Invitrogen) at 37 °C under a 5% CO2-20% O2 atmosphere.
A streptokinase-deficient mutant in the GAS M49 chromosomal background was generated by insertional inactivation of the ska gene (data not shown). After plasminogen binding to the mutant surface no plasmin activity above background could be detected (data not shown).
The human keratinocyte cell line HaCaT (Deutsches Krebsforschungszemtrum (DKFZ), Heidelberg) was used for standard adherence and internalization assays. The keratinocytes were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) GlutaMAXTM-I, 10% (v/v) fetal bovine serum (FBS; Invitrogen) in tissue culture flasks (Greiner) at 37 °C under a 5% CO2-20% O2 atmosphere (35).
The mouse monocytes-macrophages J-774A.1 (Deutsche Sammlung von Mikroorganismen und Zellkulturen (DMSZ), DMSZ no.: ACC170) were used for quantitative phagocytosis assays. The cells were routinely maintained in DMEM supplemented with 10% (v/v) FBS (Invitrogen) in tissue culture flasks (Greiner) at 37 °C under a 5% CO2-20% O2 atmosphere.
For all experiments investigating the effects of plasminogen/plasmin human and murine cells were cultured and subsequently infected with bacteria under serum-free conditions. This prevented the potential presence of exogenous plasminogen and other plasma and matrix proteins (particularly fibronectin) in the assays and allowed investigation of the behavior of plasminogen/plasmin-coated bacteria.
siRNA Transfection
Transfections of HaCaT cells with siRNA were promoted with Transpass R2 reagent according to the manufacturer's guidelines.
Eukaryotic Cell Adherence and Internalization
Adherence to and internalization into HaCaT cells was performed in serum-free conditions and quantified using the antibiotic protection assay (36). 24-well plates were inoculated with 2.5 × 105 cells per well in DMEM medium without antibiotics. Cells were allowed to grow to confluence. For the assay, cells were washed with DMEM, and infected with GAS M49 at a multiplicity of infection of 1:10 in DMEM. Two hours after infection, cells were washed extensively with PBS, detached from the wells by trypsin treatment, and lysed with sterile distilled water. The viable counts of GAS (colony forming units (CFU)) released from the lysed cells were determined by serial dilution in PBS and plating on THY agar. For the assessment of bacterial internalization, 2 h after infection the cells were washed with PBS and incubated with DMEM supplemented with penicillin (50 units/ml) and streptomycin (5 mg/ml) for additional 2 h. Subsequently, the cells were washed and lysed and the GAS viable counts were determined as described above. The percentage of attached or internalized bacteria was calculated by relation to the initial inoculum.
To elucidate the effect of bacteria-bound plasminogen/plasmin on adherence and internalization, GAS were incubated for 30 min with human plasminogen (2 μg/ml) at room temperature prior to the adherence and internalization assay. Unbound plasminogen/plasmin was removed by washing the bacteria once with PBS. In inhibition experiments, the plasminogen precoated bacteria were additionally incubated with 2 μg/ml of purified integrins (αvβ5, α1β1, and α5β1) for 15 min or with heparin at room temperature. Unbound integrins or heparin were removed by washing the bacteria once with PBS. To rule out effects of plasmin serine protease activity, the adherence and internalization assay was additionally performed in the presence of plasmin inhibitors aprotinin and α2-antiplasmin (2 μg/ml).
The pharmacological inhibitors used to study the impact of the cytoskeleton or signaling molecules were solved in DMSO. The HaCaT cells were preincubated with the inhibitors for 30 min prior to the infection with GAS. The assay was performed in the presence of the inhibitors. As a control, the cells were preincubated with DMSO at the same concentrations and infected with the bacteria (37). All inhibition experiments were performed in DMEM without FBS supplementation.
Quantitative Phagocytosis Assay
The quantitative phagocytosis assay was carried out as described by Hampton and Winterbourn (38). Briefly, overnight cultures of GAS were inoculated into fresh THY medium and grown to exponential growth phase. Bacteria were harvested by centrifugation and set to an optical density at 600 nm of 1.0, followed by opsonization of 1 × 107/ml of the bacteria in HBSS (10 mm PBS, pH 7.4, containing 1 mm CaCl2, 0.5 mm MgCl2, and 1 mg/ml glucose) containing 10% (v/v) human serum. The opsonized bacteria were incubated with monocytes/macrophages at a ratio of 1:1 for 30 min at 37 °C and slight shaking. The suspension was then harvested by centrifugation at 100 × g for 5 min, and the cell sediment was washed twice with ice-cold PBS. The supernatants were collected. For lysis of the moncytes/macrophages the final pellet was suspended in 1 ml of ice-cold PBS containing saponin (0.05% (w/v)) and homogenized until no visible aggregates remained. Finally, the viable counts of inoculated bacteria, supernatants (extracellular bacteria), and homogenized phagocyte solution (intracellular bacteria) were determined by plating serial dilutions and related to the initial inoculum, which was set to 100%.
Blood Survival Assay
The blood survival assay was performed as described by Nakata et al. (39). Briefly, overnight cultures of GAS were inoculated into fresh THY medium and grown to exponential growth phase. Bacteria were harvested by centrifugation, set to an optical density at 600 nm of 0.25, and further diluted 1:10,000 in PBS. The viable counts of this suspension were determined by plating serial dilutions on THY agar plates. 20 μl of the suspension were incubated with 480 μl of heparinized blood for 3 h at 37 °C with rotation. After this incubation, the remaining CFU were determined by plating and related to the initial inoculum.
Plasminogen Binding Assay
The plasminogen binding assays with intact bacteria were performed by using a method adapted from Ringdahl et al. (40). Overnight cultures grown in THY medium were washed in PBS and suspended in one-tenth of the original volume using PBS. 100 μl of cells, equivalent to 108 CFUs, were added to 4 ml of PBS (negative control) or to 4 ml of PBS supplemented with human plasminogen (2 μg/ml) and incubated at 37 °C for 1 h. Reactions were stopped by centrifugation at 4 °C. The bacterial pellets were washed in ice-cold PBS containing 0.1% Tween 20 and placed on ice. Plasmin activity associated with the bacteria was measured using a method adapted from Kulisek et al. (41). Each pellet was incubated in 100 μl of plasmin substrate solution (2 volumes of chromogenic substrate H-d-Val-Leu-Lys-ρ-nitroanilide (Sigma) stock solution (0.5 mg/ml in water) and 3 volumes of 32 mm Tris, pH 7.5, 1.77 m NaCl solution) for 90 min at 37 °C, followed by absorbance measurement at 405 nm.
Dot Blot Analysis
To show the ability of the bacteria to bind plasminogen, GAS M49 were incubated for 30 min with human plasminogen (2 μg/ml) at room temperature prior to the washing step with ice-cold PBS containing 0.1% Tween 20. The untreated and treated bacteria, plasminogen and fibronectin were transferred to the polyvinylidene fluoride (PVDF) membrane. After drying, the membrane was blocked with 5% (v/v) skim milk prior to antibody incubations. Antibody incubations were performed according to the manufacturer's guidelines.
SDS-PAGE and Immunoblot Analysis
Host cells were lysed with lysis buffer (10 mm Tris-HCl, 5 mm EDTA, 150 mm NaCl, 1% Triton-X 100, pH 7.4 containing protease inhibitor mixture; Sigma). Lysis was performed on ice for 30 min. Protein lysates were then cleared by centrifugation at 13,000 rpm for 20 min. The amount of protein in the samples was determined using the Bradford protein quantification method (Bio-Rad). Lysates were normalized to equal amounts of protein and boiled in sample buffer consisting of 250 mm Tris-HCl (pH 7.6), 20% (w/v) SDS, 2% (v/v) β-mercaptoethanol, 20% (v/v) glycerol, and 0.05% (w/v) bromphenol blue. As molecular mass marker, prestained protein standards were used (Bio-Rad). The protein lysates were separated by 12% SDS-PAGE and transferred to a PVDF membrane. The membranes were blocked with 5% (v/v) skim milk prior to antibody incubations. Antibody incubations were performed according to the manufacturer's guidelines.
Statistical Analysis
The significance of differences between samples in plasminogen/plasmin binding, adherence, and internalization assays was determined using the two-tailed U test. p < 0.05 was defined as marginally significant. p < 0.01 was defined as significant, and p < 0.001 was defined as highly significant. Results are demonstrated as averages and S.D. (n ≥ 5).
RESULTS
Plasminogen Binding by GAS
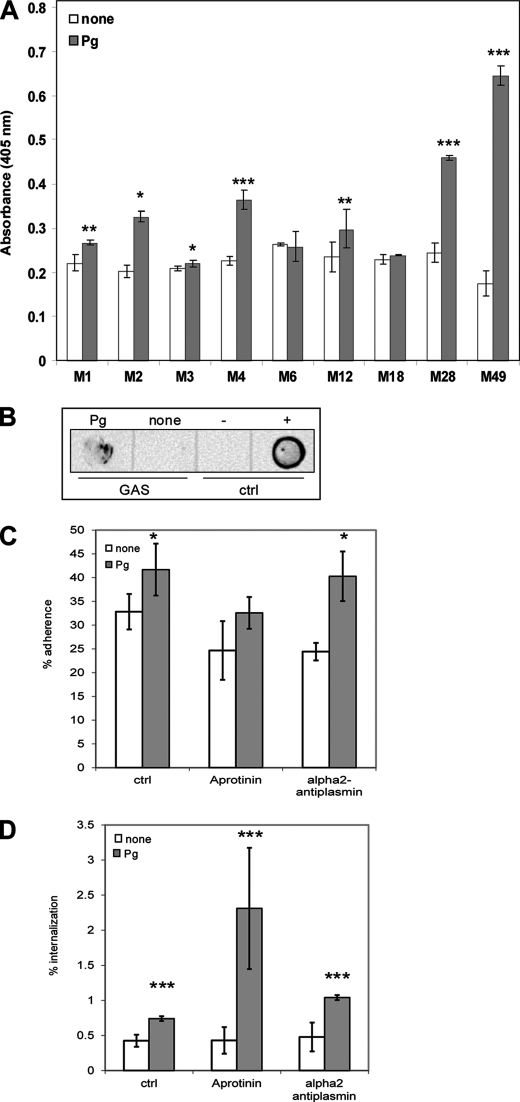
To investigate a possible link between plasminogen/plasmin-binding capacity and serotypes of S. pyogenes, different clinical isolates from invasive diseases and uncomplicated infections were tested. We determined the streptococcal surface plasmin (serine protease) activity after pretreatment of the bacteria with Glu-plasminogen as a measure for the surface-bound plasminogen. Serine protease activity of untreated GAS was measured as background control. Not unexpected, a considerable level of protease activity was noted due to the expression of several other GAS proteases. Plasminogen-coated serotypes M1, M2, M4, M28, and M49 bacteria showed a high level of plasmin activity on their surface (Fig. 1A).
FIGURE 1.
Plasminogen/plasmin promotes adherence to and internalization of S. pyogenes serotype M49 into human keratinocytes. A, measurement of plasmin enzymatic activity of different plasminogen-pretreated and -untreated S. pyogenes serotypes. The data represent the mean values ± S.D. from ten independent experiments. The y axis shows the A405 nm for each serotype. B, dot-blot analysis of plasminogen pretreated and untreated S. pyogenes serotype M49. Plasminogen (+) was used as a positive control and fibronectin (−) as a negative control. The data shown are representative of three independent experiments. Shown is the streptococcal adherence to (C) and internalization into (D) HaCaT cells after 2 h. The percentage of attached or internalized bacteria in the absence of plasminogen (none) was used as a control. To exclude the influence of plasmin serine protease effects aprotinin and α2-antiplasmin were used as inhibitors. The data represent the mean values ± S.D. from five independent experiments. Only significant differences are indicated (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
This assay revealed that the tested GAS serotypes are capable of binding plasminogen, which, once bound on the bacterial surface, can be converted to plasmin within the 4 h of incubation (coating time plus plasmin-assay time). This conversion is most likely achieved by streptokinase, thus it cannot be ruled out that the determined values rather reflect different amounts of secreted streptokinase in the different GAS serotypes.
To prove and detect surface localization of the plasminogen/plasmin recruited by GAS, we performed dot blot analysis with serotype M49 bacteria. The detection of surface-bound plasminogen was performed with a monoclonal anti-PLG antibody (Fig. 1B).
Surface-bound Plasminogen/Plasmin of GAS M49 Enhanced the Adherence to and Internalization into HaCaT Cells
Plasminogen/plasmin-binding is important for adherence of GAS to Detroit laryngeal epithelial cells, suggesting a role in the nasopharyngeal infection route (33).
To study the effect of plasminogen/plasmin on the adherence to and internalization into human keratinocytes, a relevant step during GAS skin infection, HaCaT cells were infected with different plasminogen/plasmin-coated and uncoated GAS serotypes. Supplemental Fig. S1, A and B show the results. All tested serotypes showed increased adherence to HaCaT cells. The interesting result was that serotypes M4 and M49 showed also an increased internalization into human keratinocytes.
Of note, the S. pyogenes serotype M49, derived from a skin infection, exhibited the highest plasmin activity on its surface and showed an increased adherence to keratinocytes (Fig. 1C). More important, a 50% increase in keratinocyte internalization was noted for pretreated bacteria compared with untreated controls (Fig. 1D). From this knowledge, we decided to use this serotype for further studies. To exclude the possibility that these observations resulted from activation of plasminogen to plasmin, the adherence and internalization assay was performed in the presence of the serine protease inhibitor aprotinin and the specific plasmin inhibitor α2-antiplasmin. Again, plasminogen/plasmin-treated GAS M49 showed increased adherence to and internalization into human HaCaT cells (Fig. 1, C and D).
To ask whether intact plasminogen was able to mediate this effect, we performed the adherence and internalization assay with a streptokinase deficient mutant of GAS M49 (ska−). Again, the same effects could be observed (supplemental Fig. S1, C and D).
In addition, the supernatants of untreated and treated HaCaT cells, the bacteria alone and in co-cultivation showed no plasmin activity (supplemental Fig. S1E). This suggested lack of any secreted products which can activate plasminogen into plasmin under our experimental conditions.
These experiments indicate that plasminogen/plasmin-binding of GAS M49 leads to an enhanced host cell adherence and internalization. This effect is based on the surface bound plasminogen/plasmin molecules, and is independent of putative plasminogen cleavage into plasmin and the presence of active serine protease activity in the assays.
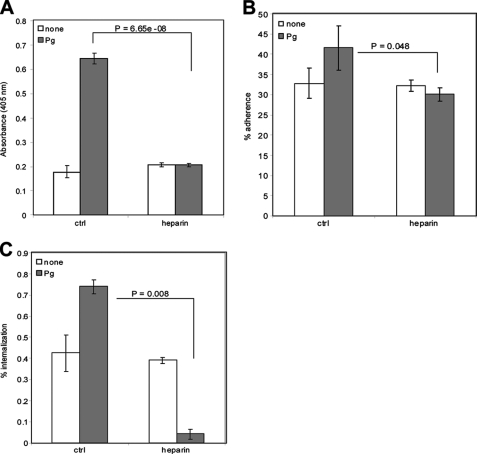
Heparin Inhibits the Binding of Plasminogen/Plasmin-coated GAS M49 to Epithelial Cells
To investigate the specificity of the GAS surface-bound plasminogen/plasmin-host cell interaction we performed inhibition experiments. Serrano et al. (42) described heparin as a potential inhibitor of plasminogen. Thus, we next tested heparin for the ability to inhibit (I) the development of plasmin activity after GAS surface plasminogen binding, and (II) the attachment on and internalization into HaCaT cells of coated GAS. Again, without plasminogen/plasmin-coating GAS M49 showed only background levels of plasmin activity on their surface, which was not further inhibited by heparin treatment (white bars, Fig. 2A). The plasmin activity on the surface of plasminogen-coated GAS M49 was completely (100%) blocked by heparin (gray bars, Fig. 2A). Heparin treatment of plasminogen-free GAS M49 did not influence HaCaT cell adherence and internalization (white bars, Fig. 2, B and C). However, pretreated GAS M49 adherence to HaCaT cells was noticeably decreased (gray bars, Fig. 2B), and HaCaT cell internalization was almost abolished by heparin inhibition (gray bars, Fig. 2C). These results allowed two conclusions. First, other non-plasminogen/plasmin binding-related and heparin-inhibited internalization mechanisms contribute to the overall capacity of GAS M49 to invade into HaCaT cells. Second, a significant proportion of GAS HaCaT cell adherence and internalization is dependent on plasminogen recruited to the bacterial surface.
FIGURE 2.
Influence of heparin on adherence to and internalization into human keratinocytes. A, chromogenic plasminogen binding assay of GAS M49 with and without heparin. The data represent the mean values ± S.D. from twenty independent experiments. B, influence of heparin on the S. pyogenes M49 attachment to and (C) internalization into HaCaT cells in the absence (none) or presence (Pg) of human plasminogen/plasmin. The percentage of attached or internalized bacteria in the absence or presence of plasminogen/plasmin was used as a control. The data represent the mean values ± S.D. from five independent experiments. Only significant differences are indicated.
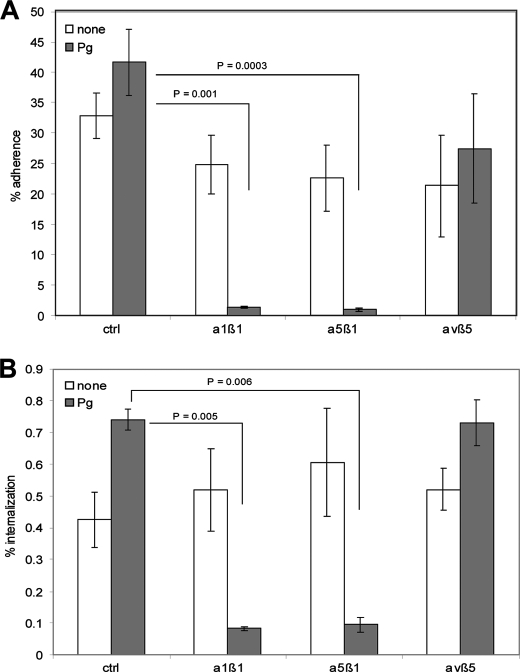
Integrin-Plasminogen/Plasmin-mediated Invasion of Epithelial Cells by GAS
Next we sought to identify the HaCaT cell receptor, which recognizes the GAS surface-bound plasminogen. It is known that immobilized plasminogen supports cell adhesion by interacting with integrins on the surface of monocytoid U937 cells, human embryonic kidney cells (HEK), and also neutrophiles (43, 44). It is also known that the kringle domain of the urokinase-like plasminogen activator (uPA) interacts with cell adhesion receptors of the integrin superfamily, including subfamilies α1, α5, and β1 subunit (45, 46). UPA is involved in (I) the regulation of fibrinolysis, (II) cell surface-focused pericellular proteolysis, and (III) the regulation of intracellular signaling affecting cell-adhesion, migration, and proliferation (47). A sequence alignment of the kringle domains of plasminogen with kringle domains of uPA and the tissue plasminogen activator (tPA) revealed a homology score between 42 and 55.5%. These data strongly suggest that host-cell attachment initiated by GAS surface-bound plasminogen/plasmin could also rely on the interaction with specific integrins. To test this hypothesis we investigated the HaCaT cells used in the previous infection experiments, which are cells known to express a variety of integrins (48). After the pretreatment with plasminogen/plasmin, GAS were incubated with recombinant purified soluble α1β1, α5β1, and αvβ5 (negative control) integrins prior to HaCaT cell infection experiments. In these competitive inhibition experiments, plasminogen/plasmin-promoted GAS adherence to (Fig. 3A, gray bars) and internalization into (Fig. 3B, gray bars) human keratinocytes was significantly reduced after preincubation of plasminogen pretreated GAS with α1β1 and α5β1 integrins. No significant reduction of bacterial attachment and uptake could be detected if αvβ5 integrin was used as competitive inhibitor and if plasminogen/plasmin-free bacteria were pretreated with the recombinant integrins (Fig. 3, A and B, white bars). Together, these data indicate that plasminogen/plasmin present on the GAS surface acts as an adaptor to HaCaT cells via α1β1 and α5β1 integrin interaction. This novel and previously unrecognized GAS-bound plasminogen/plasmin-integrin interaction supports bacterial attachment and invasion.
FIGURE 3.
Integrin-dependent plasminogen/plasmin-mediated adherence to and internalization into human host cells by S. pyogenes. A, influence of different integrins on the adherence to and internalization (B) into HaCaT cells. The percentage of attached or internalized bacteria in the absence or presence of plasminogen/plasmin was used as a control. The data represent the mean values ± S.D. from five independent experiments. Only significant differences are indicated.
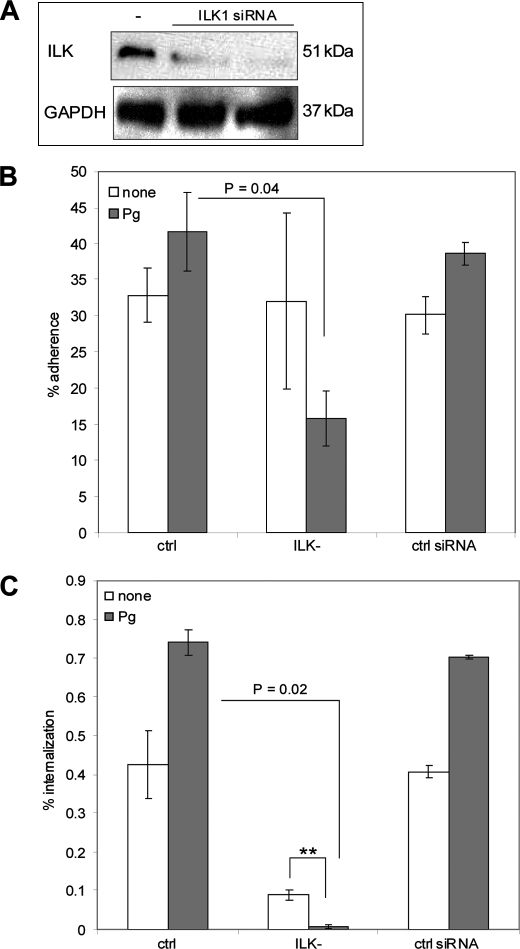
Genetic Knock-down of ILK Expression Reduced GAS Internalization
To understand the cellular cascades downstream of integrin binding we investigated the involvement of ILK in the plasminogen/plasmin-mediated uptake of streptococci into keratinocytes. A siRNA transfection assay was performed to reduce the ILK protein expression. For that purpose HaCaT cells were transfected with SignalSilence ILK1 siRNA and the SignalSilence Control siRNA. To confirm the inhibition of ILK expression by siRNA, Western blot analysis was performed. As shown in Fig. 4A, ILK expression was significantly reduced following siRNA transfection, whereas the expression of GAPDH, which is accepted to be a housekeeping protein, was not affected. ILK knock-down cells were infected with plasminogen/plasmin-treated GAS and the viable counts of attached and internalized bacteria were determined. The adherence and internalization of plasminogen/plasmin-coated GAS was significantly reduced in the ILK knock-down background (Fig. 4, B and C). Also the internalization of untreated streptococci was decreased. These data suggest the presence of redundant plasminogen/plasmin-integrin interaction independent internalization pathways. The control siRNA transfection did not affect the adherence to and internalization into HaCaT cells.
FIGURE 4.
Involvement of ILK in plasminogen/plasmin-dependent invasion into keratinocytes. A, Western blot analysis of ILK-transfected cells. ILK was detected with ILK1 Antibody. GAPDH was used as a negative control. B, adherence and (C) internalization analysis of ILK-transfected cells. The data represent the mean values ± S.D. from five independent experiments. Only significant differences are indicated by a p value or by asterisk (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Disruption of the ILK Pathway by Inhibition of PI3K and Akt Reduced Plasminogen/Plasmin-promoted Invasion of GAS M49
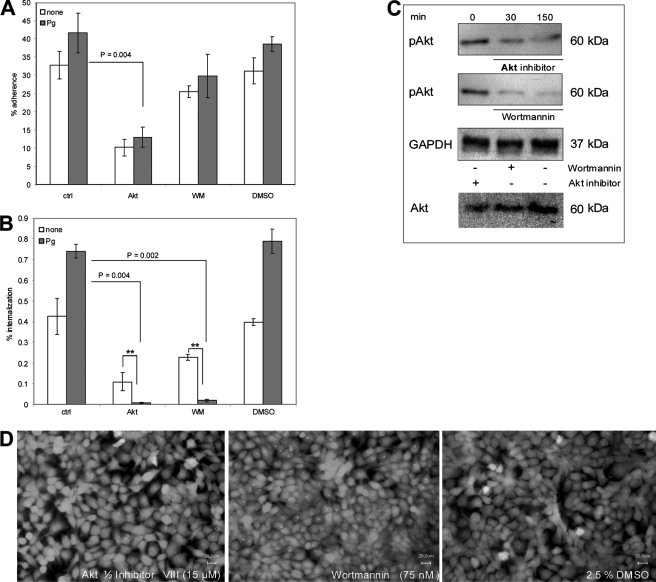
ILK is activated in a PI3K-dependent manner (49). Thus, we investigated the internalization of plasminogen/plasmin-coated GAS M49 in the presence of the PI3K-specific inhibitor Wortmannin. Because Akt is regulated by PI3K and ILK (14), we additionally performed the invasion assay in the presence of the Akt 1/2 kinase inhibitor VIII. While the presence of Wortmannin did not significantly affect the adherence of plasminogen/plasmin-pretreated GAS, a significant reduction of streptococcal attachment to keratinocytes in the presence of the Akt phosphorylation inhibitor was observed (Fig. 5A). In contrast, a significantly decreased bacterial invasion into keratinocytes was detected in the presence of both substances (Fig. 5B). The concentrations of DMSO, Wortmannin, and Akt 1/2 kinase inhibitor VIII used in these inhibition experiments did not affect the viability of host cells (Fig. 5D) and bacteria (data not shown). To confirm the inhibiton of Akt phosphorylation, Western blot analysis was performed. As shown in Fig. 5C, Akt is phosphorylated in the absence of the specific inhibitors (0 min). However, in the presence of both substances (30 min and 150 min after infection) the amount of phosphorylated Akt was reduced. The treatment specifically blocked Akt phosphorylation and did not influence Akt protein amounts. GAPDH protein expression was monitored to exclude a general effect on cellular protein expression by the different treatments. No significant reduction of the GAPDH protein expression was detected after the specific inhibition of both enzymes.
FIGURE 5.
Akt and PI3K-dependent streptococcal attachment and invasion via plasminogen/plasmin integrin pathway. A, plasminogen/plasmin-dependent adherence to and internalization (B) of streptococci into keratinocytes in the absence or presence of Akt 1/2 kinase inhibitor VIII (Akt, 15 μm) and Wortmannin (WM, 75 nm) was measured after 2 h of infection by using antibiotic protection assay. DMSO was used as a control. The data represent the mean values ± S.D. from five independent experiments. Only significant differences are indicated by a p value or by asterisk (*, p < 0.05; **, p < 0.01; ***, p < 0.001). C, phosphorylation of Akt (pAkt) was analyzed 30 and 150 min after infection. Akt and GAPDH were used as a control. D, live/dead scan of treated HaCaT cells.
Inhibition of Actin-Cytoskeleton Dynamics Blocked All Pathways Used by S. pyogenes M49 for Invasion into HaCaT Cells
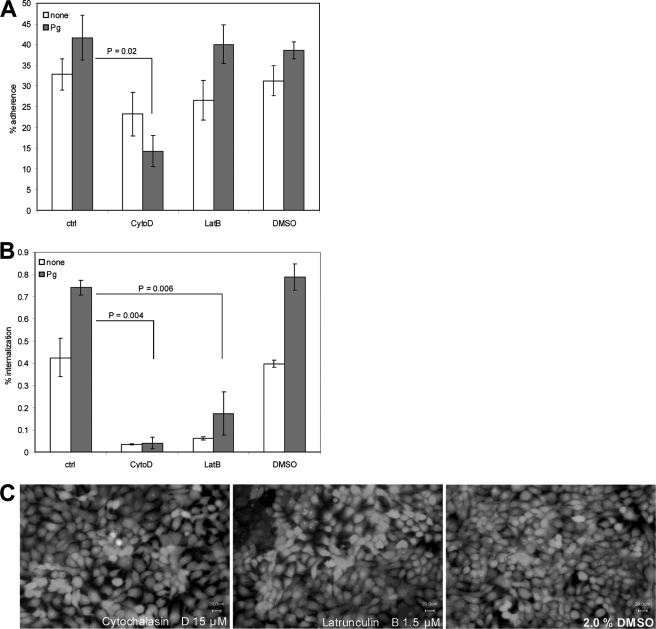
The impact of the actin-cytoskeleton on plasminogen/plasmin-mediated GAS internalization was studied by keratinocyte infection in the presence of the inhibitors cytochalasin D and latrunculin B. Both pharmacological inhibitors affect the actin polymerization. The presence of cytochalasin D significantly blocked the GAS attachment to HaCaT cells. Latrunculin B did not influence the adherence of the bacteria (Fig. 6A). However, both substances also significantly blocked invasion of bacteria, which were not coated with plasminogen/plasmin (Fig. 6B). Again, control experiments showed that the inhibitor concentrations used did not affect the viability of epithelial cells (Fig. 6C) or the M49 strain (data not shown).
FIGURE 6.
Streptococcal invasion requires the dynamics of the actin cytoskeleton. A, plasminogen/plasmin-mediated adherence to and internalization (B) into HaCaT cells in the absence or presence of the actin inhibitors, including cytochalasin D (CytoD, 15 μm) and latrunculin B (LatB, 1.5 μm). The data represent the mean values ± S.D. from five independent experiments. C, live/dead scan of human keratinocytes in the presence of both substances.
Plasminogen/Plasmin Surface Coating Enhances GAS Blood Survival and Protects GAS against Phagocytic Killing
To evaluate whether plasminogen/plasmin binding by GAS M49 contributes to virulence in the blood environment, exponential phase untreated and plasminogen/plasmin-coated GAS were tested for their survival in whole human blood. The binding of plasminogen/plasmin leads to a slightly increased ability of S. pyogenes serotype M49 to survive and multiply in blood (multiplication factor after 3 h of infection: without Pg 47.45 ± 10.5, with Pg 70.04 ± 17.2). However, this tendency is not statistically significant. Additionally we performed phagocytic killing assays with plasminogen/plasmin-treated and uncoated GAS. While only 28.8% (± 12.1) of the plasminogen/plasmin-coated bacteria were killed by murine macrophages/monocytes, 51.1% (± 8.1) of untreated controls were eradicated. Thus, plasminogen/plasmin-coated GAS are significantly protected against phagocytic killing (p = 0.015).
DISCUSSION
The S. pyogenes-plasminogen/plasmin interaction is involved in virulence and pathogenesis on multiple levels, supporting extra- and intracellular GAS lifestyles. This could explain the existing redundancy of GAS plasminogen/plasmin-binding and plasminogen-plasmin converting mechanisms expressed by these bacteria. As central part of the extracellular lifestyle of GAS, plasminogen/plasmin acquisition from the host promotes tissue invasiveness, initiation of systemic spread and disease, and circumvention of fibrin networks as part of the host innate immune response (29–32).
The intracellular lifestyle of GAS has two major target and entry sites in the host. First, epithelial cells of the human larynxs and pharynxs have been extensively studied for plasminogen/plasmin-mediated adherence and internalization mechanisms (22, 33, 50, 51). Although it has been shown that GAS use paracellular transmigration through pharyngeal host cell monolayers (33), nothing is known about cell surface receptors binding to GAS surface-bound plasminogen/plasmin and the intracellular pathways exploited by the bacteria. Second, skin keratinocytes are primary targets for GAS adherence and internalization during skin infection. Here we show that particularly the GAS M49 serotype skin isolate bound high amounts of plasminogen/plasmin, which led to a significantly increased internalization into human keratinocytes. Different explanations for this effect exist. First, intact surface-bound plasminogen could act as bridging molecule for the host cell contact. Second, plasminogen could be converted to its active plasmin form on the GAS surface and the serine protease activity could be responsible for this observation. Third, plasmin as a molecule and not its enzymatic activity could initiate host cell contact. Our results revealed that plasminogen/plasmin as GAS surface-bound molecules mediated this host cell effect, because serine protease activity was ruled out. The general specificity and extension of the increased host cell adherence and internalization phenomenon was investigated and confirmed by latex bead experiments (data not shown) and inhibition studies using human heparin, aprotinin, α2-antiplasmin, and finally a streptokinase negative GAS M49 mutant. In our experiments also plasminogen/plasmin-independent adherence and internalization mechanisms in the GAS-keratinocyte interaction were observed, since only adherence and internalization mediated by the GAS-surface bound plasminogen/plasmin could be blocked by the heparin and α2-antiplasmin treatment.
Such plasminogen/plasmin-independent mechanisms could be mediated by four so far known binding interactions. GAS M protein binding to CD46 on the keratinocyte surface has been described (52, 53). The GAS capsule blocked this interaction (54, 55). The capsule itself has been shown to interact directly with CD44 (55), thereby allowing paracellular transmigration (56). Moreover, fibronectin-binding protein SfbI and the GAS pilus have been described to be involved in keratinocyte adherence (50, 51, 57). However, conflicting data exist and most likely these interactions are not universal but rather serotype dependent (58, 59). Particularly, fibronectin-mediated adherence and internalization pathways were excluded by our serum-free experimental conditions.
We next addressed the question via which pathways the GAS surface-bound plasminogen/plasmin could support the keratinocyte adherence and internalization process. It has been reported that plasminogen deposited in the extracellular matrix supports adhesion of eukaryotic cells by engaging cell-exposed integrins (44). Our study showed that specifically α1β1 and α5β1 integrins significantly inhibited the adherence and internalization processes. Preincubation of the coated bacteria with soluble integrins allowed integrin-binding to the plasminogen/plasmin on the bacterial surface, thereby competitively blocking and inhibiting the plasminogen/plasmin binding sites for the bacterial interaction with the HaCaT cell integrins. That this blocking effect leads to values below those of the controls could be explained by a sterical hindrance exerted by the plasminogen/plasmin-integrin interaction for all other existing adherence/internalization pathways. Both integrins had no inhibitory effect on the keratinocyte adherence and internalization of uncoated GAS, documenting that no direct integrin binding proteins are expressed on the GAS surface. These results allow two conclusions, 1) keratinocyte adherence and internalization of plasminogen/plasmin-free M49 GAS does not rely on any of the integrins tested, and 2), GAS which have sequestered plasminogen/plasmin on their surface can utilize eukaryotic cell adhesion mechanisms to adhere to and internalize into host keratinocytes. Similar mechanisms have been reported for M protein-dependent and fibronectin-binding protein-mediated GAS adherence to and internalization into pharyngeal cells (11, 36, 60).
It is known that kringle domains are responsible for integrin binding of plasminogen, uPA and tPA. Furthermore, it has been shown that lysine-binding sites in the kringle domains of plasminogen are critical for the interaction with integrin (44). Thus, it is likely that also the integrin binding of GAS-bound plasminogen/plasmin relies on kringle domains. The molecular details of this plasminogen/plasmin-integrin interaction during the process of GAS keratinocyte adherence and internalization are currently under investigation.
The benefit for GAS of employing the plasminogen/plasmin-integrin interaction is a measurable increase in keratinocyte adherence and a statistically highly significant increase in keratinocyte internalization. As heterodimeric cell surface receptors, integrins most likely cluster in the keratinocyte membrane and recruit several signaling and adaptor proteins, particularly to link up with the host cell actin cytoskeleton. Our siRNA knockdown experiments in keratinocytes demonstrated an essential role of ILK in this process, However, we observed differential effects for non-coated and plasminogen/plasmin-coated GAS keratinocyte adherence and internalization. ILK knockdown exclusively reduced adherence of plasminogen/plasmin-coated GAS to keratinocytes by 50%, whereas the internalization was completely abolished. Untreated GAS showed unaltered adherence capabilities and still retained a measurable internalization capacity in the ILK knockdown background. This strongly suggests differential uptake mechanisms into the human keratinocytes in the presence or absence of plasminogen/plasmin on the GAS surface.
Wortmannin as inhibitor of PI3K did only marginally influence GAS adherence to keratinocytes irrespective of the presence of plasminogen/plasmin. Nevertheless, internalization defects similar to those caused by Akt inhibition were observed. These results suggest that PI3K activates ILK (49), even under infection conditions. Results with Akt1/2 kinase inhibitors confirmed that Akt is functional downstream of PI3K and ILK (14). Further inhibition experiments with the actin cytoskeleton inhibitors cytochalasin D and latrunculin B supported the notion that plasminogen/plasmin-dependent keratinocyte adherence and internalization required actin polymerization. However, the employed inhibitors blocked also the interaction of uncoated bacteria with keratinocytes. Apparently, cytoskelettal rearrangements are the final consequence of all adherence and internalization pathways used by S. pyogenes M49.
The plasminogen/plasmin-mediated infection of keratinocytes resembles fibronectin-mediated pathways in several aspects. The GAS M1 protein binds fibronectin and leads to pharyngeal cell internalization via α5β1, ILK, and PI3K-dependent pathways (11). SfbI protein-mediated fibronectin-binding and host cell uptake relies on the same pathways (36). A variety of other bacterial pathogens are also able to interact directly and indirectly with integrins to promote invasion into epithelial cells. These are e.g. Streptococcus pneumoniae (37), Staphylococcus aureus (61), or Porphyromonas gingivalis (62).
In addition to supporting keratinocyte adherence and internalization, coating the bacterial surface with plasminogen/plasmin is beneficial for the extracellular lifestyle of the pathogen. Although not statistically significant, a slight increase in the GAS multiplication upon incubation in whole human blood was measured in our experiments. Plasminogen/plasmin on the GAS surface could mask the bacteria and prevent efficient immune recognition. Our results showed that plasminogen/plasmin-coated GAS is less readily killed by murine macrophages. Induction of oncosis has been reported as one GAS phagocyte escape mechanism (63). The main bactericidal activity of murine macrophages against GAS is mediated by phagocyte oxidase (64). Whether plasminogen/plasmin affects one or both of these processes needs to be studied in the future.
We have demonstrated that plasminogen/plasmin-binding by S. pyogenes M49 is an important virulence trait for extra- and intracellular lifestyles (Fig. 7), independent of plasmin serine protease activity. It aids the bacterial attachment to and internalization into host keratinocytes. This process is of importance for the initiation of skin infections. Presence of plasminogen/plasmin allows the bacteria to exploit the eukaryotic intergrin ILK cytoskeletal pathways for their host cell uptake. Whether the intracellular status of GAS is part of an initiation process of aggressive disease, or a fundamental process for persistence and immune evasion is currently under debate. Plasminogen/plasmin binding is further important for survival of phagocytosis, since plasminogen/plasmin-coated GAS are significantly less efficiently killed by macrophages. Taken together, these observations underscore the importance of GAS-plasminogen/plasmin interaction pathways and warrant further investigation.
FIGURE 7.
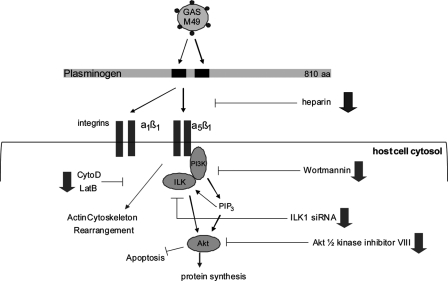
Schematic model of plasminogen/plasmin-mediated internalization into human keratinocytes by S. pyogenes serotype M49 (adapted from Ref. 11). Plasminogen/plasmin binding proteins on the surface of GAS bind to soluble plasminogen/plasmin molecules, which in turn bind to α1β1 and α5β1 integrins. This binding triggers the activation of ILK. ILK activation is dependent on PIP3, a product of PI3K. The directly inhibition of ILK by siRNA or the indirectly inhibition by PI3P inhibitor, Wortmannin, and Akt 1/2 kinase inhibitor prevents GAS invasion into human keratinocytes. The inhibition of actin cytoskeleton dynamics by cytochalasin D (CytoD) and latrunculin B (LatB) prevents the bacterial uptake.
Supplementary Material
This work was supported by a grant from the Medical Faculty of the University of Rostock in the framework of the FORUN program 2010 and by grants from the BMBF (German Federal Ministry of Education and Research) in the framework of the ERANet PathoGenoMics II and SysMO II programs (to B. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- GAS
- group A streptococcus
- uPA
- urokinase-like plasminogen activator
- tPA
- tissue plasminogen activator
- ILK
- integrin-linked kinase.
REFERENCES
- 1. Bisno A. L., Brito M. O., Collins C. M. (2003) Lancet Infect. Dis. 3, 191–200 [DOI] [PubMed] [Google Scholar]
- 2. Carapetis J. R., Steer A. C., Mulholland E. K., Weber M. (2005) Lancet Infect. Dis. 5, 685–694 [DOI] [PubMed] [Google Scholar]
- 3. Cunningham M. W. (2000) Clin. Microbiol. Rev. 13, 470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Courtney H. S., Hasty D. L., Dale J. B. (2002) Ann. Med. 34, 77–87 [DOI] [PubMed] [Google Scholar]
- 5. Kreikemeyer B., Klenk M., Podbielski A. (2004) Int. J. Med. Microbiol. 294, 177–188 [DOI] [PubMed] [Google Scholar]
- 6. Cue D. R., Cleary P. P. (1998) Infect. Immun. 66, 4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greco R., De Martino L., Donnarumma G., Conte M. P., Seganti L., Valenti P. (1995) Res. Microbiol. 146, 551–560 [DOI] [PubMed] [Google Scholar]
- 8. Jadoun J., Ozeri V., Burstein E., Skutelsky E., Hanski E., Sela S. (1998) J. Infect. Dis. 178, 147–158 [DOI] [PubMed] [Google Scholar]
- 9. LaPenta D., Rubens C., Chi E., Cleary P. P. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 12115–12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molinari G., Talay S. R., Valentin-Weigand P., Rohde M., Chhatwal G. S. (1997) Infect. Immun. 65, 1357–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang B., Yurecko R. S., Dedhar S., Cleary P. P. (2006) Cell Microbiol. 8, 257–266 [DOI] [PubMed] [Google Scholar]
- 12. Wang B., Li S., Dedhar S., Cleary P. P. (2007) Cell Microbiol. 9, 1519–1528 [DOI] [PubMed] [Google Scholar]
- 13. Hannigan G. E., Leung-Hagesteijn C., Fitz-Gibbon L., Coppolino M. G., Radeva G., Filmus J., Bell J. C., Dedhar S. (1996) Nature 379, 91–96 [DOI] [PubMed] [Google Scholar]
- 14. Persad S., Dedhar S. (2003) Cancer Metastasis Rev. 22, 375–384 [DOI] [PubMed] [Google Scholar]
- 15. Walker M. J., McArthur J. D., McKay F., Ranson M. (2005) Trends Microbiol. 13, 308–313 [DOI] [PubMed] [Google Scholar]
- 16. Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. (1985) Adv. Cancer Res. 44, 139–266 [DOI] [PubMed] [Google Scholar]
- 17. Ponting C. P., Marshall J. M., Cederholm-Williams S. A. (1992) Blood Coagul. Fibrinolysis 3, 605–614 [PubMed] [Google Scholar]
- 18. Lähteenmäki K., Kuusela P., Korhonen T. K. (2001) FEMS Microbiol. Rev. 25, 531–552 [DOI] [PubMed] [Google Scholar]
- 19. Lottenberg R., Broder C. C., Boyle M. D., Kain S. J., Schroeder B. L., Curtiss R., 3rd (1992) J. Bacteriol. 174, 5204–5210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Broder C. C., Lottenberg R., von Mering G. O., Johnston K. H., Boyle M. D. (1991) J. Biol. Chem. 266, 4922–4928 [PubMed] [Google Scholar]
- 21. Pancholi V., Fischetti V. A. (1992) J. Exp. Med. 176, 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin H., Song Y. P., Boel G., Kochar J., Pancholi V. (2005) J. Mol. Biol. 350, 27–41 [DOI] [PubMed] [Google Scholar]
- 23. Pancholi V., Fischetti V. A. (1998) J. Biol. Chem. 273, 14503–14515 [DOI] [PubMed] [Google Scholar]
- 24. Berge A., Sjöbring U. (1993) J. Biol. Chem. 268, 25417–25424 [PubMed] [Google Scholar]
- 25. Sanderson-Smith M. L., Dowton M., Ranson M., Walker M. J. (2007) J. Bacteriol. 189, 1435–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kreikemeyer B., Nakata M., Köller T., Hildisch H., Kourakos V., Standar K., Kawabata S., Glocker M. O., Podbielski A. (2007) Infect. Immun. 75, 5698–5710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poon-King R., Bannan J., Viteri A., Cu G., Zabriskie J. B. (1993) J. Exp. Med. 178, 759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyle M. D., Lottenberg R. (1997) Thromb. Haemost. 77, 1–10 [PubMed] [Google Scholar]
- 29. Khil J., Im M., Heath A., Ringdahl U., Mundada L., Cary, Engleberg N., Fay W. P. (2003) J. Infect. Dis. 188, 497–505 [DOI] [PubMed] [Google Scholar]
- 30. Cole J. N., McArthur J. D., McKay F. C., Sanderson-Smith M. L., Cork A. J., Ranson M., Rohde M., Itzek A., Sun H., Ginsburg D., Kotb M., Nizet V., Chhatwal G. S., Walker M. J. (2006) FASEB J. 20, 1745–1747 [DOI] [PubMed] [Google Scholar]
- 31. Sanderson-Smith M. L., Dinkla K., Cole J. N., Cork A. J., Maamary P. G., McArthur J. D., Chhatwal G. S., Walker M. J. (2008) FASEB J. 22, 2715–2722 [DOI] [PubMed] [Google Scholar]
- 32. McArthur J. D., McKay F. C., Ramachandran V., Shyam P., Cork A. J., Sanderson-Smith M. L., Cole J. N., Ringdahl U., Sjöbring U., Ranson M., Walker M. J. (2008) FASEB J. 22, 3146–3153 [DOI] [PubMed] [Google Scholar]
- 33. Pancholi V., Fontan P., Jin H. (2003) Microb. Pathog. 35, 293–303 [DOI] [PubMed] [Google Scholar]
- 34. McKay F. C., McArthur J. D., Sanderson-Smith M. L., Gardam S., Currie B. J., Sriprakash K. S., Fagan P. K., Towers R. J., Batzloff M. R., Chhatwal G. S., Ranson M., Walker M. J. (2004) Infect. Immun. 72, 364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. (1988) J. Cell Biol. 106, 761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ozeri V., Rosenshine I., Mosher D. F., Fässler R., Hanski E. (1998) Mol. Microbiol. 30, 625–637 [DOI] [PubMed] [Google Scholar]
- 37. Bergmann S., Lang A., Rohde M., Agarwal V., Rennemeier C., Grashoff C., Preissner K. T., Hammerschmidt S. (2009) J. Cell Sci. 122, 256–267 [DOI] [PubMed] [Google Scholar]
- 38. Hampton M. B., Winterbourn C. C. (1999) J. Immunol. Methods 232, 15–22 [DOI] [PubMed] [Google Scholar]
- 39. Nakata M., Köller T., Moritz K., Ribardo D., Jonas L., McIver K. S., Sumitomo T., Terao Y., Kawabata S., Podbielski A., Kreikemeyer B. (2009) Infect. Immun. 77, 32–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ringdahl U., Svensson M., Wistedt A. C., Renné T., Kellner R., Müller-Esterl W., Sjöbring U. (1998) J. Biol. Chem. 273, 6424–6430 [DOI] [PubMed] [Google Scholar]
- 41. Kulisek E. S., Holm S. E., Johnston K. H. (1989) Anal. Biochem. 177, 78–84 [DOI] [PubMed] [Google Scholar]
- 42. Serrano R. L., Rodriguez P., Pizzo S. V., Gonzalez-Gronow M. (1996) Biochem. J. 313, 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chavakis T., Preissner K. T. (2005) Hamostaseologie 25, 33–38 [DOI] [PubMed] [Google Scholar]
- 44. Lishko V. K., Novokhatny V. V., Yakubenko V. P., Skomorovska-Prokvolit H. V., Ugarova T. P. (2004) Blood 104, 719–726 [DOI] [PubMed] [Google Scholar]
- 45. Wei Y., Eble J. A., Wang Z., Kreidberg J. A., Chapman H. A. (2001) Mol. Biol. Cell 12, 2975–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wei Y., Czekay R. P., Robillard L., Kugler M. C., Zhang F., Kim K. K., Xiong J. P., Humphries M. J., Chapman H. A. (2005) J. Cell Biol. 168, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chapman H. A. (1997) Curr. Opin. Cell Biol. 9, 714–724 [DOI] [PubMed] [Google Scholar]
- 48. Takada Y., Ye X., Simon S. (2007) Genome Biol. 8, 215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abbot E. L., Smith W. D., Siou G. P., Chiriboga C., Smith R. J., Wilson J. A., Hirst B. H., Kehoe M. A. (2007) Cell Microbiol. 9, 1822–1833 [DOI] [PubMed] [Google Scholar]
- 51. Smith W. D., Pointon J. A., Abbot E., Kang H. J., Baker E. N., Hirst B. H., Wilson J. A., Banfield M. J., Kehoe M. A. (2010) J. Bacteriol. 192, 4651–4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Okada N., Pentland A. P., Falk P., Caparon M. G. (1994) J. Clin. Invest. 94, 965–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okada N., Liszewski M. K., Atkinson J. P., Caparon M. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 2489–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schrager H. M., Rheinwald J. G., Wessels M. R. (1996) J. Clin. Invest. 98, 1954–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schrager H. M., Albertí S., Cywes C., Dougherty G. J., Wessels M. R. (1998) J. Clin. Invest. 101, 1708–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cywes C., Wessels M. R. (2001) Nature 414, 648–652 [DOI] [PubMed] [Google Scholar]
- 57. Edwards M. L., Fagan P. K., Currie B. J., Sriprakash K. S. (2004) Microbes Infect. 6, 1156–1162 [DOI] [PubMed] [Google Scholar]
- 58. Darmstadt G. L., Mentele L., Podbielski A., Rubens C. E. (2000) Infect. Immun. 68, 1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Oliver S. L., Wood E., Asobayire E., Wathes D. C., Brickell J. S., Elschner M., Otto P., Lambden P. R., Clarke I. N., Bridger J. C. (2007) J. Clin. Microbiol. 45, 3050–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cue D., Lam H., Cleary P. P. (2001) Microb. Pathog. 31, 231–242 [DOI] [PubMed] [Google Scholar]
- 61. Fowler T., Wann E. R., Joh D., Johansson S., Foster T. J., Höök M. (2000) Eur. J. Cell Biol. 79, 672–679 [DOI] [PubMed] [Google Scholar]
- 62. Yilmaz O., Watanabe K., Lamont R. J. (2002) Cell Microbiol. 4, 305–314 [DOI] [PubMed] [Google Scholar]
- 63. Goldmann O., Sastalla I., Wos-Oxley M., Rohde M., Medina E. (2009) Cell Microbiol. 11, 138–155 [DOI] [PubMed] [Google Scholar]
- 64. Goldmann O., Köckritz-Blickwede M., Höltje C., Chhatwal G. S., Geffers R., Medina E. (2007) Infect. Immun. 75, 4148–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.