Abstract
During the early development of the nervous system, γ-aminobutyric acid (GABA) type A receptor (GABAAR)-mediated signaling parallels the neurotrophin/tropomyosin-related kinase (Trk)-dependent signaling in controlling a number of processes from cell proliferation and migration, via dendritic and axonal outgrowth, to synapse formation and plasticity. Here we present the first evidence that these two signaling systems regulate each other through a complex positive feedback mechanism. We first demonstrate that GABAAR activation leads to an increase in the cell surface expression of these receptors in cultured embryonic cerebrocortical neurons, specifically at the stage when this activity causes depolarization of the plasma membrane and Ca2+ influx through L-type voltage-gated Ca2+ channels. We further demonstrate that GABAAR activity triggers release of the brain-derived neurotrophic factor (BDNF), which, in turn by activating TrkB receptors, mediates the observed increase in cell surface expression of GABAARs. This BDNF/TrkB-dependent increase in surface levels of GABAARs requires the activity of phosphoinositide 3-kinase (PI3K) and protein kinase C (PKC) and does not involve the extracellular signal-regulated kinase (ERK) 1/2 activity. The increase in GABAAR surface levels occurs due to an inhibition of the receptor endocytosis by BDNF, whereas the receptor reinsertion into the plasma membrane remains unaltered. Thus, GABAAR activity is a potent regulator of the BDNF release during neuronal development, and at the same time, it is strongly enhanced by the activity of the BDNF/TrkB/PI3K/PKC signaling pathway.
Keywords: Calcium Imaging, Cell Surface Receptor, CREB, Endocytosis, GABA Receptors, Intracellular Trafficking, Neurodevelopment, Neurotrophic Factor, PI 3-Kinase, Protein Kinase C (PKC)
Introduction
Fast synaptic inhibition in the adult brain is largely mediated by GABAA receptors, members of a large family of GABA2-gated Cl−/HCO3−-permeable ion channels (1). GABAARs are heteropentameric assemblies of subunits classified as α (1–6), β (1–3), γ (1–3), δ, ϵ, θ, and π (2). Increasingly, experimental evidence supports the central role of these receptors as mediators of GABAergic transmission in the developing brain where GABA acts as a trophic signal by exciting neurons. Thus, excitatory GABAAR activity regulates neuronal proliferation (3, 4), migration (5, 6), differentiation (7), and neuronal network formation (8) and refinement (9–11). The depolarizing activity of GABA activates voltage-gated Ca2+ channels (VGCCs) (9, 12), relieves Mg2 blockade of NMDA receptors (13), and can lead to generation of action potentials (13–17). Although anatomically defined connections are still not established (18, 19), activation of GABAARs occurs due to a tonic, Ca2+- and soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-independent release of GABA (20).
A depolarization-dependent increase in intracellular Ca2+ downstream of GABAARs has been suggested to play a key role in the trophic effects of GABA (9, 21). However, the transient nature of the Ca2+ increase suggests that other, still unknown mechanisms must be triggered to mediate the long term regulation of neuronal development by GABA. We have hypothesized that one of the mechanisms involved may be mediated by GABAAR-evoked Ca2+-dependent release of BDNF, which parallels GABA as a key regulator of neuronal survival (22–24), morphological development (25, 26), synaptic connectivity (27, 28), and activity-dependent maturation of synapses (29, 30). BDNF operates through a complex web of signaling pathways that are initiated upon its binding to TrkB receptors with a high affinity and to a p75 pan-neurotrophin receptor (p75NTR) with a lower affinity (31). Long term effects of BDNF during development are mediated by the activation of specific transcription factors, including cAMP response element-binding protein (CREB) (29). BDNF has been shown to modulate the efficacy of transmission at GABAergic synapses (33–36) by regulating GABAAR phosphorylation (37, 38) and cell surface expression (39–42) and to promote GABAergic synaptogenesis (43–46).
Here we provide evidence for a positive feedback mechanism operating between the depolarizing activity of GABAARs and the secretion of BDNF in developing cerebrocortical neurons. We demonstrate that activation of GABAARs triggers the Ca2+-dependent release of BDNF, which is coupled to the activation of L-type voltage-gated Ca2+ channels. This, in turn, leads to an increase in the cell surface expression of GABAARs via the activation of TrkB receptors and downstream signaling pathways mediated by PI 3-kinase and PKC. By specifically inhibiting GABAAR endocytosis, BDNF-dependent signaling increases the responsiveness of developing neurons to GABA and, in turn, enhances its own release. The reciprocal augmentation operating between GABA and BDNF/TrkB signaling is specific for developing neurons and may facilitate the establishment and functional maturation of GABAergic synapses.
EXPERIMENTAL PROCEDURES
Cell Culture
Primary cortical neuronal cultures were prepared using E16-E17 Sprague-Dawley rats as described previously (47) with minor modifications (38). Dissociated neurons were plated at a density of 60,000 cells/cm2 in Neurobasal medium containing B27 supplement, glutamine (2 mm), penicillin (100 units), streptomycin (100 μg), and glucose (6 mm; all from Invitrogen) on either 0.1 mg/ml poly-d-lysine-coated culture dishes or 0.1 mg/ml poly-l-lysine-coated glass coverslips. Cultures were incubated in a humidified 37 °C, 5% CO2 incubator for 6 or 14 days in vitro (DIV) before experimentation.
Immunocytochemistry and Confocal Microscopy
Cortical cultures plated on glass coverslips (40,000 cells/cm2; 6 or 14 DIV) were treated; fixed in 4% paraformaldehyde/4% sucrose/PBS (PFA-sucrose) for 10 min; and processed as described previously (48) using a mouse anti-GABAAR β2/3-specific antibody (1:200; clone bd17, Millipore) to label the receptors expressed at the cell surface followed by permeabilization and incubation with a guinea pig anti-vesicular glutamate transporter (VGlut; 1:2000; Millipore) antibody and a chicken anti-microtubule-associated protein (MAP) antibody (1:2000; Sigma). Appropriate secondary antibodies conjugated to Alexa Fluor 488, Alexa Fluor 555, and Cy5 (3 μg/ml; Millipore) were incubated in 1% BSA/PBS for 60 min. Cultures were washed, and coverslips were mounted using Vectashield (Vector Laboratories). Immunofluorescence was visualized using a laser scanning confocal microscope (Zeiss LSM 510 Meta) with ×63 oil immersion objective and ×3 digital zoom. In each image, laser light levels and detector gain and offset were adjusted to avoid any saturation. Confocal micrographs (Figs. 1 and 5) represent digital composites of a Z-series scan of four to six optical sections through a depth of 4–5 μm. For each experimental condition, three-color images from a total of 25 VGlut-immunopositive cells (collected from three independent experiments) were analyzed quantitatively using ImageJ software (NIH, Bethesda, MD). Briefly, for each cell stack, the VGlut labeling was subtracted from the GABAAR labeling to remove any cytoplasmic labeling that could occur after PFA fixation. The labeled area fraction of positive pixels for GABAAR β2,3 distributed throughout the cell membrane was measured. This value was divided by the area fraction of positive pixels for MAP2 in the stack and expressed as the GABAAR β2,3/MAP2 surface ratio.
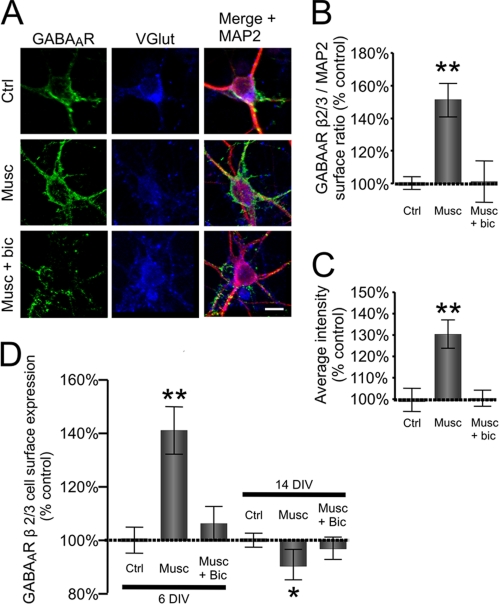
FIGURE 1.
Muscimol-dependent activation of GABAARs leads to increase in cell surface expression of these receptors in developing glutamatergic cerebrocortical neurons. A, cultured neurons (6 DIV) in control conditions (Ctrl) or treated with muscimol (Musc) or muscimol plus bicuculline (Musc + bic) immunolabeled with GABAAR β2,3-subunit-specific mouse monoclonal antibody (MAB 314, bd17) to reveal expression at the cell surface (left panel; green) and, following permeabilization, immunolabeled with anti-VGlut guinea pig antibody (middle panel; blue) and anti-MAP2 rabbit polyclonal antibody (right panel; red) to reveal the intracellular distribution of these proteins. The right panels were obtained by merging GABAAR β2,3, VGlut, and MAP2 labeling. Scale bar, 20 μm. The quantification of the surface GABAAR β2,3/intracellular MAP2 fluorescence ratio (B) and the average intensity of GABAAR β2,3 fluorescence at the cell surface of VGlut-positive cortical neurons (C) treated with muscimol alone (50 μm; 10 min; Musc) or muscimol plus bicuculline (10 μm; 10 min; Musc + bic) in comparison with the vehicle-treated control (Ctrl) is shown. Means ± S.E. are given (25 neurons were analyzed per condition per experiment; n = 3; **, p < 0.001; paired Student's t test). D, cell surface levels of GABAARs measured using ELISA with GABAAR β2,3-subunit specific antibody. Surface levels were obtained from 6- and 14-DIV cultured cortical neurons treated with muscimol alone (50 μm; 10 min; Musc) or in the presence of bicuculline (10 μm; 10 min; Musc + Bic) and expressed as a percentage of vehicle-treated controls. Means ± S.E. are given (n = 4; *, p < 0.05; **, p < 0.001; Student's paired t test). Error bars represent standard error of the mean.
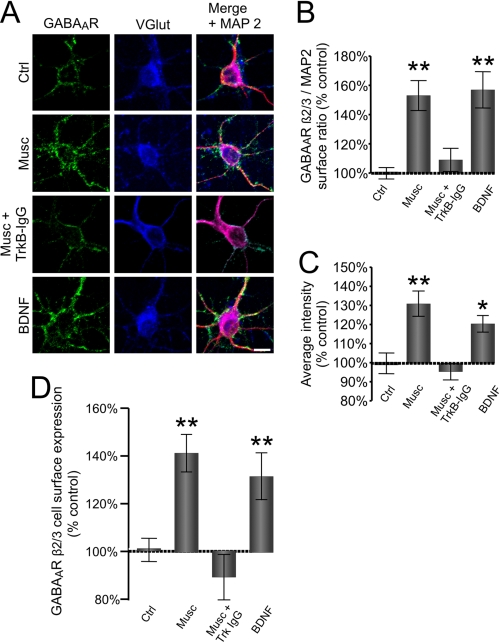
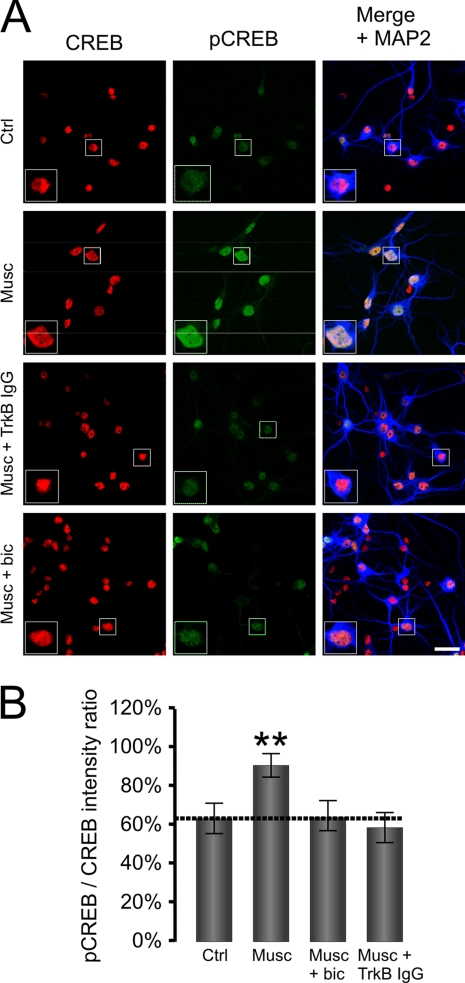
FIGURE 5.
GABAAR-dependent release of endogenous BDNF mediates increase in cell surface expression. A, cultured cerebrocortical neurons (6 DIV) in control conditions (Ctrl) or treated with muscimol in the absence (Musc) or presence of TrkB-IgG (Musc + TrkB-IgG) or with the exogenous BDNF (100 ng/ml). Neurons were immunolabeled with GABAAR β2,3-subunit-specific mouse monoclonal antibody (MAB 314, bd17) (left panel; green) to reveal expression at the cell surface and, following permeabilization, immunolabeled with anti-VGlut guinea pig antibody (middle panel; blue) or anti-MAP2 rabbit polyclonal antibody (right panel; red) to reveal the intracellular distribution of these proteins. The right panels were obtained by merging GABAAR β2,3, VGlut, and MAP2 labeling. Scale bar, 20 μm. The quantification of the surface GABAAR β2,3/intracellular MAP2 fluorescence ratio (B) and the average intensity of GABAAR β2,3 fluorescence (C) at the surface of VGlut-positive neurons treated with vehicle (Ctrl), muscimol alone (50 μm; 10 min; Musc), with muscimol plus TrkB-IgG (10 μm; 10 min; Musc + TrkB IgG), or with the exogenous BDNF. Means ± S.E. are given (25 neurons were analyzed per condition per experiment; n = 3; *, p < 0.05; **, p < 0.001; Student's paired t test). D, cell surface levels of GABAARs measured using ELISA with GABAAR β2,3-subunit specific antibody in neurons treated with muscimol alone (50 μm; 10 min; Musc), with muscimol plus TrkB-lgG (10 μm; 10 min; Musc + TrkB-lgG), or with the exogenous BDNF (100 ng/ml) and expressed as a percentage of vehicle-treated controls. Means ± S.E. are given (n = 3; **, p < 0.001; Student's paired t test). Error bars represent standard error of the mean.
Fluorescence Measurement of Intracellular Ca2+ Concentration ([Ca2+]i)
Cortical neurons (6 DIV) plated on glass bottom dishes (Mattek) were loaded with the Ca2+ indicator Fluo-4-AM (5 μm; Invitrogen) by bath application for 30 min at 37 °C in loading buffer (10 mm HEPES, pH 7.4, 150 mm NaCl, 3 mm KCl, 2 mm MgCl2, 0.1 mm CaCl2, 5 mm glucose) with TTX (0.5 μm; Sigma) and CNQX (33 μm; Tocris). GABA (50 μm) was applied to the bath in the presence or absence of bicuculline (10 μm). Ca2+ responses were recorded at 37 °C as digitized images acquired using a Nikon Diaphot 300 inverted microscope with epifluorescence attachments. Data were collected with a Hamamatsu ORCA 2 cooled charge-coupled device camera using MetaFluor software (Universal Imaging) with images acquired every 5 s. The series of digitized fluorescence images was analyzed by MetaFluor software to determine the average level of fluorescence of each cell at each time point sampled. ΔF/F was calculated as follows. The basal fluorescence (Fbas) was obtained by averaging the neuronal fluorescence intensity of the last five frames before application of GABA. Frame fluorescence (Ffr) was the neuronal fluorescence intensity of the frame. Therefore, ΔF/F = (Ffr − Fbas)/Ffr. The pick value of ΔF/F following GABA application in the absence or presence of bicuculline was used for the statistical analysis.
Surface GFP-BDNF Immunofluorescence Analysis
Cortical neurons (4 DIV) plated on glass coverslips were transfected with cDNAs encoding the BDNF-GFP (49) (kindly provided by Dr. V. Lessmann, Institute of Physiology, Otto von Guericke University, Magdeburg, Germany) using LipofectamineTM 2000 (Invitrogen). Cultures were incubated at 37 °C in 5% CO2 for 24 h. The procedure for surface BDNF-GFP immunostaining was similar to that described previously (50). Briefly, after incubation of cells in the absence or presence of muscimol (50 μm; Tocris), TTX (0.5 μm), or muscimol/bicuculline (10 μm; Tocris) for 10 min and a wash with Hanks' balanced salt solution, the living cultures were incubated at 4 °C for 1 h in the presence of an anti-GFP antibody (10 μg/ml; Invitrogen). Cultures were then washed with 0.1 m PBS (4 °C; pH 7.4) and fixed for 10 min with PFA-sucrose. After fixation, the neurons were exposed to a saturating concentration (10 μg/ml) of Alexa Fluor 555-conjugated anti-rabbit IgG (Invitrogen) for 1.5 h. Immunolabeling confirmed that BDNF-GFP was stored in secretory granules of the regulated pathway of secretion as shown previously (50). Quantification was performed using ImageJ software. The ratio of surface-bound BDNF-GFP to total BDNF-GFP was estimated as the ratio of the area of co-localized Alexa Fluor 555 and BDNF-GFP/total area of BDNF-GFP and expressed as “percentage of co-localized signals” (Fig. 3).
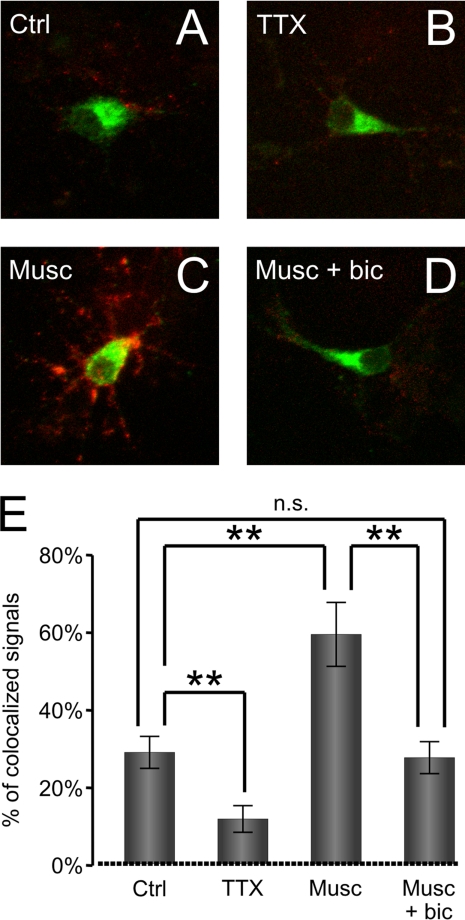
FIGURE 3.
GABAAR activation triggers secretion of BDNF-GFP. A–D (top), superimposed images showing the intracellular BDNF-GFP fluorescence (green) and secreted BDNF-GFP detected using anti-GFP antibody (red) under non-permeable conditions in vehicle-treated controls (Ctrl) (A), in the presence of TTX (0.5 μm) (B), in the presence of muscimol (50 μm; Musc) alone (C), or in the presence of muscimol plus bicuculline (10 μm; Musc + bic) (D). E (bottom), quantitative analysis of BDNF-GFP signal bound to the cell surface and overlapping (yellow) with the signal from BDNF-GFP-expressing neurons (green) in vehicle-treated controls (Ctrl) or TTX-, muscimol (Musc)-, or muscimol + bicuculline (Musc + bic)-treated cultures. Means ± S.E. are given (total of 11 cells analyzed per treatment in n = 3 independent experiments; **, p < 0.001; Student's independent t test). n.s., not significant. Error bars represent standard error of the mean.
Phospho-CREB Activation
The procedure for phospho-CREB activation and immunocytochemistry was similar to that described previously (50). To reduce the basal level of CREB phosphorylation, cultures (5 DIV) were incubated overnight in TTX (0.5 μm). Cultures were then stimulated with BDNF (100 ng; Alomone Labs) or muscimol (50 μm) in the absence or presence of TrkB-IgG (2 μg/ml; Regeneron) or bicuculline (10 μm). Five to 10 min after stimulation, neurons were fixed for 10 min with PFA-sucrose at 4 °C and rinsed several times with PBS. Coverslips were then preincubated in 0.1% Triton, 3% goat serum, PBS for 1 h at room temperature and incubated overnight with mouse anti-CREB (1:1000), rabbit anti-phospho-CREB (pCREB; 1:1000; both from Cell Signaling Technology), and chicken anti-MAP2 (1:2000) antibodies. Immunoreactivities for pCREB, CREB, and MAP2 were visualized and analyzed as described above using the laser scanning confocal microscope (Zeiss LSM 510 Meta). The optical sections were digitized (1024 × 1024 pixels) and processed using ImageJ software. For analysis of the intensity of pCREB staining in neuronal cells, we first created a binary mask from MAP2-positive cells and then analyzed pCREB intensity only in regions overlapping the binary mask. Acquisition parameters were the same for every set of experiments. The pCREB to CREB intensity ratio was expressed as the mean ratio of the pCREB-Alexa Fluor 488/CREB-Alexa Fluor 555 staining intensity. All data were expressed as percentage of control values obtained from sister non-stimulated cultures.
Determination of GABAA Receptor Cell Surface Levels Using ELISA
Changes in surface and total levels of GABAARs were analyzed using a cell surface ELISA as described previously (38, 51). Cortical neurons were cultured in 24-well plates at a density of 60,000 cells/cm2 for 6 DIV. Following treatments, cultures were fixed using PFA-sucrose for 10 min. After washing with Hanks' balanced salt solution (Invitrogen) and blocking with Hanks' balanced salt solution/1% BSA/10% rabbit serum for 30 min, cultures were incubated with anti-GABAAR β2/3 antibody (2 μg/ml; MAB341, bd17 clone, Millipore). In those cultures where total levels of GABAAR β2/3-subunits were evaluated, cells were permeabilized using Hanks' balanced salt solution/0.5% Triton/1% BSA/10% rabbit serum for 30 min prior to the addition of the primary antibody. Cultures were extensively washed and incubated with a rabbit anti-mouse IgG conjugated to horseradish peroxidase (HRP; 1:5000; Pierce) followed by the addition of 3,3,5,5-tetramethylbenzidine (Sigma-Aldrich). Absorbance was read at λ = 655 nm using a spectrophotometer (Duo 800, Beckman Coulter). Controls lacking the primary antibody were routinely used to determine background levels of peroxidase and the nonspecific binding of the secondary antibody.
Cell Surface Biotinylation, Endocytosis, and Recycling Assays
Biotinylation assays were performed as described previously (38, 52). Cultured neurons (6 DIV) were treated, and cell surface proteins were biotinylated using sulfo-NHS-SS-biotin (1 mg/ml; Pierce) at 4 °C. Cells were lysed, and biotinylated proteins were precipitated using UltraLink Immobilized NeutrAvidin biotin-binding protein (Pierce) and resolved by SDS-PAGE. The amount of biotinylated GABAA receptor β3-subunit was determined by quantitative immunoblotting with a β3-specific antibody (0.5 μg/ml; Phosphosolutions) followed by 125I-coupled anti-rabbit IgG (Amersham Biosciences) and subsequent analysis using a phosphorimaging system (Bio-Rad). In endocytosis assays, plasma membrane proteins were first biotinylated using sulfo-NHS-SS biotin at 4 °C and then incubated in the absence (control) or presence of BDNF for 15 or 30 min at 37 °C. In recycling assays, cell surface proteins were first biotinylated using sulfo-NHS-SS biotin and incubated at 37 °C for 30 min to allow the endocytosis to occur. After removing the residual biotin from the cell surface with the reduced glutathione at 4 °C (first cleavage), cells were incubated in the absence (control) or presence of BDNF for 15 or 30 min at 37 °C to allow reinsertion of endocytosed receptors followed by a second round of biotin cleavage from the cell surface with the reduced glutathione. Cells were subsequently lysed, and biotinylated proteins were analyzed as described above. The amount of endocytosed and reinserted GABAAR β3 was calculated as described previously (53).
Metabolic Labeling
Cultures were incubated in methionine-free DMEM containing 0.8 mCi of [35S]methionine (PerkinElmer Life Sciences) for 12–14 h followed by lysis in phosphate buffer (PB; 10 mm Na3PO4, pH 7.4, 5 mm EDTA, 5 mm EGTA, 100 mm NaCl, 10 mm sodium pyrophosphate, 50 mm NaF) containing protease inhibitors (100 μm PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin) and 1% SDS. SDS was neutralized by 5 volumes of ice-cold PB containing 2% Nonidet P-40 followed by incubation with nonspecific IgG or GABAAR β3-, β2-, or γ2-specific antibody (10 μg; kindly provided by Prof. W. Sieghart, Centre for Brain Research, Vienna, Austria). Immunoprecipitates were analyzed by SDS-PAGE and visualized using a phosphorimaging system.
Statistical Analysis
If not stated otherwise, all population data are expressed as mean ± S.E. A paired Student's t test was used to determine statistical significance between groups in ELISA experiments, and an unpaired Student's t test was used to examine the statistical significance of the differences between groups in microscopy analysis; an analysis of variance was used for multiple comparisons. Significantly different values (*, p < 0.05; **, p < 0.001) are indicated by asterisks in figures.
RESULTS
Cell Surface Expression of GABAARs Is Increased upon Their Activation in Developing Cerebrocortical Neurons
Disassociated embryonic cerebrocortical tissue forms a heterogenous population of neurons in vitro, the majority of which are glutamatergic, pyramidal-like neurons that are immunopositive for V-Glut and MAP2. These cells also exhibit a diffuse GABAAR immunolabeling at the cell surface with sporadic cluster-like accumulations (Fig. 1A, Ctrl). To investigate whether the activity of GABAARs affects their stability at the cell surface, we used immunocytochemistry and cell surface ELISAs using an antibody specific for the extracellular domain of the GABAAR β2/3-subunits (MAB314, bd17). At 5 DIV, cerebrocortical cultures were incubated overnight with TTX (0.5 μm) to reduce the spontaneous activity of these neurons. This treatment was followed by the addition of the GABAAR agonist muscimol (50 μm) for 10 min. Immunocytochemistry using the GABAAR β2,3-specific antibody in combination with anti-VGlut and anti-MAP2 antibodies allowed a semiquantitative analysis of the GABAAR levels at the surface of glutamatergic neurons (see “Experimental Procedures”) in which the GABAAR β2,3/MAP2 ratio and the average intensity were quantified. GABAAR β2,3 cell surface expression in the region of soma and proximal dendrites of these neurons was estimated in control conditions (25 ± 7% surface ratio, n = 24; Fig. 1, A and B). The application of muscimol resulted in a significant increase of GABAAR β2,3 surface levels (153 ± 21%, p < 0.001 compared with control, n = 26; Fig. 1, A and B, Musc), and this effect was abolished in the presence of the GABAAR antagonist bicuculline (10 μm; 101 ± 27%, p > 0.05 compared with control, n = 25; Fig. 1, A and B, Musc + bic). The average intensity of GABAAR surface labeling was also increased in response to muscimol (130 ± 12%, p < 0.001 compared with control), and this increase was abolished in the presence of bicuculline (100 ± 6%, p > 0.05 compared with control; Fig. 1, A and C).
To obtain a quantitative measure of these changes, we carried out cell surface ELISA experiments using the same GABAAR β2,3-specific antibody (38). The addition of muscimol (50 μm; 10 min) led to an increase in cell surface expression of the β2,3-subunit (141 ± 19% of control non-stimulated cultures, p < 0.001, n = 4; Fig. 1D). This effect was abolished in the presence of bicuculline (10 μm; 106 ± 17% of control non-stimulated cultures, p > 0.05, n = 4; Fig. 1D). This increase in GABAAR surface expression was detected only at the early stage of neuronal differentiation (up to 7 DIV). In neurons cultured for 14 days, the addition of muscimol caused a small decrease in GABAAR cell surface expression (14 DIV; 89 ± 11% of control non-stimulated cultures, p < 0.05, n = 4; Fig. 1D). No significant difference was observed in the total levels of GABAAR β2,3-subunit between control and treated cultures (data not shown). Thus, GABAAR activity leads to an overall increase in their cell surface expression in developing cortical neurons.
Influx of Ca2+ through L-type VGCCs Promotes Increase in GABAAR Cell Surface Expression
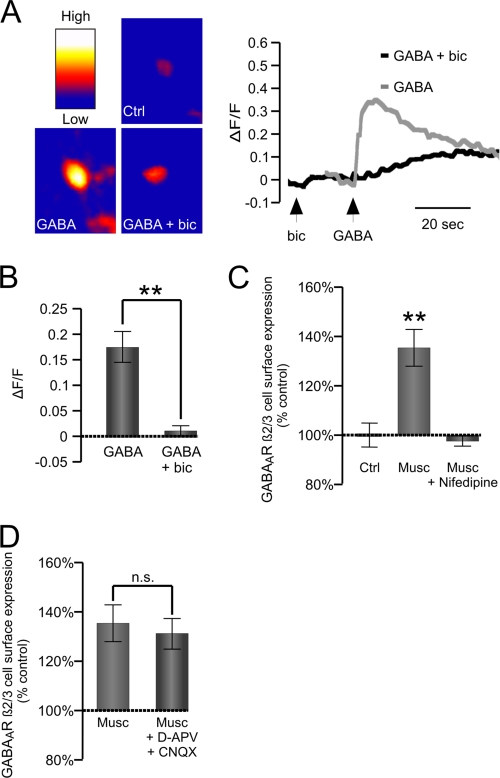
Activation of GABAARs in developing neurons is known to lead to a depolarization-dependent increase in [Ca2+]i (54). To establish whether this is indeed the case in our cultures, we labeled cortical neurons (6 DIV) with the fluorescent Ca2+ indicator Fluo-4. Application of GABA (50 μm) led to a transient increase in [Ca2+]i (Fig. 2, A and B). The effect of GABA was abolished in the presence of bicuculline (50 μm), indicating that GABAARs were involved in this process (Fig. 2, A and B).
FIGURE 2.
GABAAR-dependent increase in [Ca2+]i is required for increase in cell surface expression. A, representative images of labeled cells (left) and average change in Fluo-4 signal (ΔF/F) produced by bath application of GABA (50 μm; Musc) alone or GABA plus bicuculline (50 μm; GABA + bic) (right). B, graph showing the maximum change in fluorescence in the presence of GABA (ΔF/F; GABA) alone or GABA plus bicuculline (ΔF/F; GABA + bic). Means ± S.E. are given (n = 3; **, p < 0.001; Student's paired t test). C and D, cell surface levels of GABAARs measured using ELISA with GABAAR β2,3-subunit-specific antibody in cultured cortical neurons treated with muscimol alone (50 μm; 10 min; Musc) or in the presence of the L-type VGCC blocker nifedipine (10 μm; 10 min; Musc + Nifedipine) (C) or muscimol alone (50 μm; 10 min; Musc) or in the presence of d-APV and CNQX (10 μm; 10 min; Musc + d-APV + CNQX) (D) and expressed as the percentage of vehicle-treated controls. Means ± S.E. are given (n = 4; **, p < 0.001; Student's paired t test). n.s., not significant; Ctrl, control. Error bars represent standard error of the mean.
To test whether the rise in [Ca2+]i plays a role in the observed increase in GABAAR surface levels, we treated our cultures with muscimol (50 μm; 10 min) in the absence or presence of nifedipine (10 μm), an inhibitor of L-type VGCCs, or d-APV/CNQX (both at 10 μm) to inhibit the NMDA and AMPA receptor activity. Cell surface ELISAs using GABAAR β2,3-specific antibody demonstrated that the muscimol-dependent increase in GABAAR surface levels was prevented by the application of nifedipine (Fig. 2C) and unaffected by d-APV/CNQX (Fig. 2D), suggesting that [Ca2+]i influx through L-type VGCCs plays a critical role in this process.
Activation of GABAARs Triggers Secretion of BDNF
BDNF is a neurotrophic factor known to regulate GABAAR trafficking and cell surface expression (38–42). To determine whether the activation of GABAARs could, in turn, regulate the Ca2+-dependent secretion of BDNF, GFP-tagged BDNF was expressed in cultured cerebrocortical neurons (50, 55). In the vicinity of neurons expressing BDNF-GFP (Fig. 3, A–D, green), the addition of muscimol (50 μm; 10 min) resulted in an increase in immunolabeled extracellular GFP-BDNF (Fig. 3C, red), reflecting an increase in BDNF release. The quantification of this labeling demonstrated that the effect of muscimol was significant (n = 11 cells per condition, three independent experiments, p < 0.001 compared with control; Fig. 3E, Musc). This effect was prevented by the addition of bicuculline (10 μm; Fig. 3, D and E, Musc + bic). In the presence of TTX (0.5 μm), a significant reduction in the release of BDNF-GFP was observed (Fig. 3, B and E, TTX; p < 0.001), indicating that the release of BDNF-GFP is also regulated by the spontaneous activity of neurons.
To determine whether GABAAR activation can trigger secretion of the endogenous BDNF, we used an indirect approach based on an increase in BDNF/TrkB/ERK-dependent pCREB (50, 56). We therefore tested whether the addition of muscimol (50 μm; 10 min) induces an increase in pCREB immunoreactivity in MAP2-positive neurons (Fig. 4A). Cortical neuronal cultures were stimulated, and the immunofluorescence ratio between pCREB and CREB was quantified. Muscimol (50 μm) induced a significant increase (p < 0.0001; Fig. 4, A and B, Musc) of the pCREB/CREB ratio (91 ± 14%, n = 51 cells) in comparison with the controls (63 ± 18%, n = 39 cells). This increase was prevented by bicuculline (10 μm; n = 37 cells, p < 0.0001 compared with muscimol alone), demonstrating that GABAARs indeed mediate this effect (Fig. 4, A and B, Musc + bic).
FIGURE 4.
GABAAR activation induces BDNF/TrkB-dependent phosphorylation of CREB. A, immunofluorescence of CREB (left panels; red) and pCREB (middle panels; green) in cortical neurons in control conditions (Ctrl) or treated with muscimol alone (50 μm; Musc), muscimol plus TrkB-IgG (2 μg/ml; Musc + TrkB IgG), or muscimol plus bicuculline (10 μm; Musc + bic). Merged images with MAP2 staining (blue) are shown on the right. Scale bar, 20 μm. B, average pCREB/CREB ratio in four different conditions. Means ± S.E. are given (total of 12–17 cells analyzed per treatment in n = 3 independent experiments; **, p < 0.001; Student's independent t test). Error bars represent standard error of the mean.
Importantly, this increase in pCREB was also prevented by the addition of the BDNF “scavenger” TrkB-IgG, a fusion protein expressing the extracellular domain of TrkB (2 μg/ml; p < 0.0001 compared with muscimol alone, n = 46 cells, all from three independent experiments; Fig. 4, A and B, Musc + TrkB IgG). Thus, activation of GABAARs leads to an increase in the secretion of the endogenous BDNF, which activates TrkB receptors to increase the phosphorylation of CREB.
GABAAR-dependent Release of Endogenous BDNF Leads to Increase in Cell Surface Expression
To investigate whether endogenous BDNF, released in response to GABAAR activation, can, in turn, regulate their cell surface levels, we performed immunocytochemistry and cell surface ELISAs in cultures treated with muscimol (50 μm) in the absence or presence of TrkB-IgG. The observed increase in the cell surface staining ratio, GABAAR β2,3/MAP2 (153 ± 21% increase in surface ratio, p < 0.001 compared with control, n = 26; Fig. 5, A and B, Musc), was abolished by TrkB-IgG (2 μg/ml; 109 ± 17%, p < 0.05 compared with muscimol alone, n = 21; Fig. 5, A and B, Musc + TrkB IgG) and was mimicked by the addition of the exogenous BDNF (100 ng/ml; 157 ± 26% increase in surface ratio, p < 0.001 compared with control, n = 20; Fig. 5, A and B, BDNF). The increase in the average intensity of cell surface labeling in the presence of muscimol was also prevented by the addition of TrkB-IgG to the medium (97 ± 10% of control, p > 0.05) and mimicked by the addition of the exogenous BDNF (120 ± 8%, p < 0.05 compared with control; Fig. 5C).
In cell surface ELISA experiments, the increase in surface levels of GABAAR β2,3-subunit in response to muscimol (141 ± 19% of control unstimulated cultures, p < 0.001, n = 4) was also prevented by TrkB-IgG (2 μg/ml TrkB; 86 ± 13% of control non-stimulated cultures, p < 0.05, n = 4) and mimicked by the exogenous BDNF (100 ng/ml; 131 ± 22% of control non-stimulated cultures, p < 0.001, n = 4; Fig. 5D). No difference was observed in the total levels of GABAAR β2,3-subunits between control and treated cultures (data not shown).
BDNF-dependent Increase in Surface Levels of GABAARs Is Mediated by PKC and PI 3-Kinase Signaling Pathways
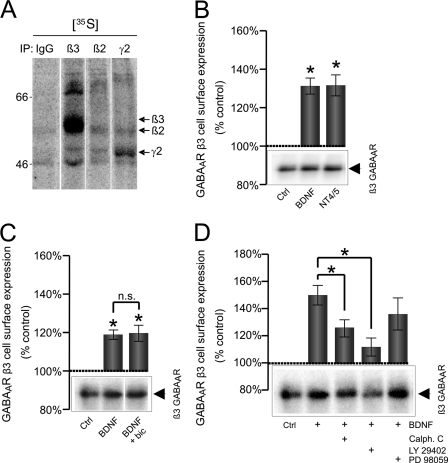
To investigate which of the three main signaling pathways activated downstream of TrkB receptors, the PKC, PI 3-kinase, or ERK 1/2 pathway, mediates the effects of BDNF on GABAARs, we carried out cell surface biotinylation and quantitative immunoblotting assays using a β3-subunit-specific antibody as this subunit was found to be significantly more abundant than the β2-subunit in our cultures (Fig. 6A).
FIGURE 6.
BDNF-dependent increase in cell surface expression of GABAA receptor β3-subunit is mediated by PI 3-kinase and PKC signaling pathways. A, [35S]methionine labeling of cortical neurons and immunoprecipitation (IP) with control rabbit IgG or antibodies specific for the β3-, β2-, or γ2-subunit of GABAARs. Arrows indicate migration of individual GABAAR subunits. Cortical neurons were treated with vehicle (Ctrl), BDNF (100 ng/ml), or neurotrophin 4/5 (100 ng/ml; NT4/5) (B); with vehicle (Ctrl), BDNF alone, or BDNF plus bicuculline (BDNF + bic) (C); and with vehicle (Ctrl), BDNF alone, BDNF plus calphostin C (Calph. C; 0.5 μm), BDNF plus LY 294002 (2 μm), or BDNF plus PD 98059 (50 μm) (D). These treatments were followed by biotinylation of cell surface proteins using sulfo-NHS-SS-biotin (1 mg/ml) and precipitation using NeutrAvidin-agarose. Protein samples were resolved by SDS-PAGE, and the amount of biotinylated β3-subunit was determined by quantitative immunoblotting with a specific antibody followed by 125I-conjugated secondary antibody and analysis using a phosphorimaging system. Means ± S.E. are given (n = 3–4; *, p < 0.05; Student's paired t test). n.s., not significant. Error bars represent standard error of the mean.
We first confirmed that activation of TrkB receptors by BDNF (100 ng/ml; 132 ± 8% of control, n = 7, p < 0.01) or neurotrophin-4/5 (100 ng/ml; 135 ± 9% of control, n = 5, p < 0.01) causes a measurable increase in the surface expression of GABAARs using this assay (Fig. 6B). This increase was insensitive to GABAAR blockade with bicuculline (10 μm; BDNF alone: 119 ± 3% of control; BDNF/bicuculline: 120 ± 9% of control; Fig. 6C) as expected given that the effects of the exogenous BDNF do not depend on GABAAR activation (Figs. 1 and 5).
The BDNF/TrkB-dependent increase in surface levels of GABAARs (150 ± 15% of control, n = 5, p < 0.05; Fig. 6D) was significantly attenuated by the PKC inhibitor calphostin C (0.5 μm; 126 ± 20% of control, n = 5, p < 0.05; Fig. 6D) and the PI 3-kinase inhibitor LY 29402 (2 μm; 112 ± 12% of control, n = 5, p < 0.01; Fig. 6D). However, it was unaffected by the presence of PD 98059 (135 ± 24% of control, n = 5, p > 0.05; Fig. 6D), an inhibitor of ERK 1/2. Control experiments using the phosphorylation state-specific antibody for ERK 1/2 confirmed that the inhibitor was effective in blocking the activity of these kinases (data not shown).
BDNF Inhibits Endocytosis of GABAARs but Has No Effect on Their Reinsertion
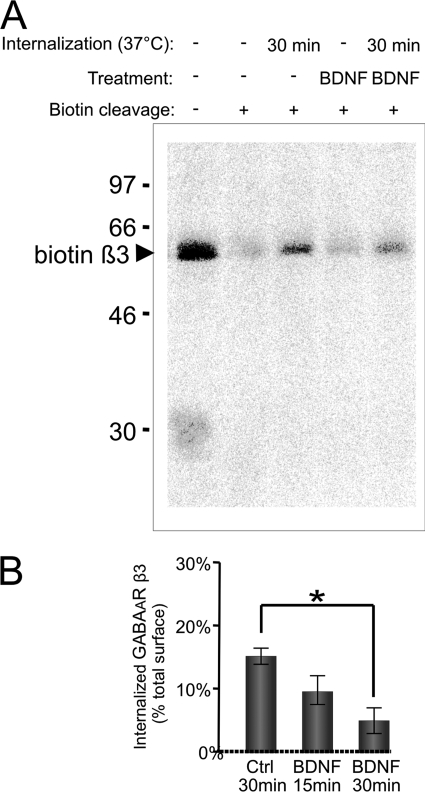
To define which steps in GABAAR trafficking may be regulated by BDNF, we carried out the endocytosis and recycling assays using cell surface biotinylation approaches as described previously (53). In controls, 15 ± 2% of the total surface GABAARs was internalized within 30 min of incubation at 37 °C. Following incubation with BDNF (100 ng/ml), internalization of GABAARs was decreased to 5 ± 2% (Fig. 7, A and B). The inhibition of GABAAR endocytosis was already evident within 15 min of incubation with BDNF (10 ± 2% of total surface pool; Fig. 7B, BDNF 15 min). In these experiments, we also monitored the endocytosis of the GluR1 subunit of AMPA receptors. We found that, albeit significantly more robust than GABAAR endocytosis (42 ± 6% of the total surface pool was endocytosed within 30 min), the endocytosis of GluR1 was not affected by BDNF (36 ± 2% of the total surface pool was internalized within 30 min; data not shown).
FIGURE 7.
BDNF-dependent decrease in endocytosis of GABAARs. Cell surface proteins were biotinylated using sulfo-NHS-SS biotin (1 mg/ml) at 4 °C followed by incubation at 37 °C in the absence (Ctrl) or presence of BDNF for 15 or 30 min. Control biotinylated samples that were not incubated at 37 °C but kept at 4 °C were processed in parallel. Residual biotin was removed from the cell surface with reduced glutathione, cells were lysed, and biotinylated proteins were precipitated using NeutrAvidin-agarose. Protein samples were resolved by SDS-PAGE, and the amount of endocytosed GABAAR β3-subunit was determined by quantitative immunoblotting with a specific antibody followed by incubation with 125I-conjugated secondary antibody and analysis using a phosphorimaging system. A, representative immunoblot. B, quantification of GABAAR endocytosis. Means ± S.E. are given (n = 4; *, p < 0.05; Student's paired t test). Error bars represent standard error of the mean.
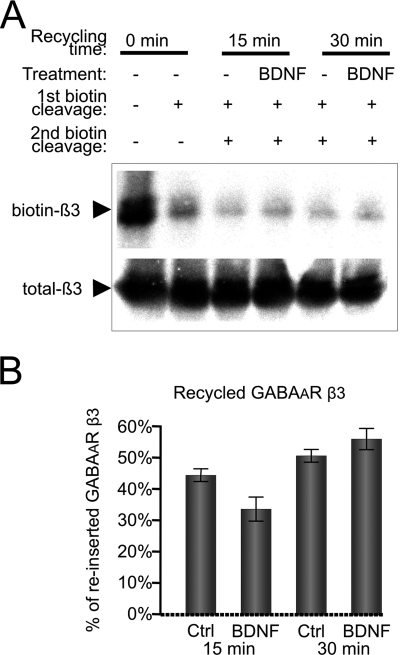
In neurons, a significant pool of endocytosed GABAARs is known to be targeted for reinsertion into the plasma membrane (57). These receptors are expected to follow the same intracellular trafficking routes that mediate insertion of newly synthesized receptors (58). In our cultures (at 6 DIV), the reinsertion of endocytosed GABAAR β3-subunit into the plasma membrane was significant with 44 ± 4 and 51 ± 4% of the total endocytosed pool being reinserted within 15 and 30 min of incubation at 37 °C, respectively (Fig. 8, A and B). This process, however, was unaffected by the BDNF (Fig. 8, A and B). In parallel experiments, the amount of the reinserted AMPA receptor GluR1 subunit within 15 or 30 min of incubation at 37 °C was 58 ± 7 or 72 ± 2% of the total endocytosed pool, respectively. This process was also unaffected in the presence of BDNF (data not shown).
FIGURE 8.
Recycling of endocytosed GABAA receptors is not regulated by BDNF. Cell surface proteins were first biotinylated using sulfo-NHS-SS biotin (1 mg/ml) at 4 °C and then incubated at 37 °C for 30 min to allow endocytosis to occur. After removing residual biotin from the cell surface with reduced glutathione (first cleavage), cells were incubated in the absence (Ctrl) or presence of BDNF for 15 or 30 min to allow reinsertion into the plasma membrane followed by a second round of biotin cleavage from the cell surface with glutathione. Cells were lysed, and residual biotinylated proteins were precipitated using NeutrAvidin-agarose. Protein samples were resolved by SDS-PAGE, and the amount of endocytosed GABAAR β3-subunit was determined by quantitative immunoblotting with a specific antibody followed by incubation with 125I-conjugated secondary antibody and analysis using a phosphorimaging system. A, representative immunoblot. B, quantification of biotinylated GABAARs that were reinserted into the plasma membrane. Means ± S.E. are given (n = 4; Student's paired t test). Error bars represent standard error of the mean.
DISCUSSION
In the developing brain, GABA acts as a trophic factor to regulate multiple steps of neuronal differentiation (59, 60). This activity is primarily mediated by GABAARs, which generate depolarization of the plasma membrane and transient increases in the intracellular calcium (54, 61). Although demonstrably necessary, changes in intracellular calcium appear insufficiently long lasting to support these GABAAR-regulated developmental processes. This ultimately raises a question as to what could be the missing regulatory link operating in this system. Here we provide evidence that at least one such regulatory mechanism involves a positive feedback interaction between the depolarizing activity of GABAARs and the regulated pathway of BDNF secretion.
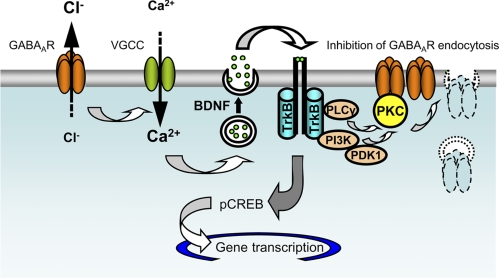
As schematically depicted in Fig. 9, we demonstrate that activation of GABAARs triggers the Ca2+-dependent release of BDNF. This, in turn, leads to an increase in the cell surface levels of GABAARs via activation of TrkB receptors and the downstream signaling pathways mediated by PI 3-kinase and PKC. By inhibiting specifically GABAAR endocytosis, BDNF/TrkB-dependent signaling increases the responsiveness of developing neurons to GABA. However, we hypothesize that this mechanism may be limited by a number of processes, including desensitization of GABAARs (62), rundown of the chloride gradient due to prolonged activation of these receptors (1), limitations in the amount of BDNF available for release and/or the amount of TrkB receptors available to bind BDNF given that these receptors rapidly internalize upon activation (63, 64), etc.
FIGURE 9.
Positive feedback mechanisms operating between GABAAR activity and BDNF release in developing cerebrocortical neurons. Activation of GABAARs leads to a depolarization of the plasma membrane, activation of VGCCs, and Ca2+-dependent release of BDNF. BDNF activates TrkB receptors and, via downstream signaling pathways mediated by PI 3-kinase and PKC, leads to an inhibition of GABAAR endocytosis and a consequent increase in the cell surface expression of these receptors. PLC, phospholipase C.
Changes in the overall number or the surface density of GABAARs potently modulate the postsynaptic response to GABA (65). This may be particularly important for the developing brain, which lacks tight temporal and spatial control of GABA concentrations before the anatomically defined synapses (18, 19) and the GABA uptake mechanisms are established (20). Under these conditions, GABA is known to operate in a paracrine fashion (20). Embryonic GABAARs are adapted to such conditions functionally, showing a high affinity for GABA and slow desensitization (66, 67). This is also correlated with a specific repertoire of GABAAR subunits expressed at this stage with α4/β1/γ2-subunits being abundant in proliferating neuronal precursors (68, 69) and α3/β3/γ2-subunits being abundant in early differentiating neurons (66, 68, 70). Although mechanisms that regulate embryonic GABAARs are mostly unknown, it is evident that they can have a significant impact on many aspects of neuronal development known to be regulated by GABA.
BDNF is of particular interest in this regard as it is released in response to GABAAR activation, and in return, it is known to regulate the efficacy of transmission at GABAergic synapses (37, 38, 40–42, 44, 71) as well as GABAergic synaptogenesis both in vitro (73–77) and in vivo (46, 78, 79). The increase in cell surface expression of GABAARs at the postsynaptic membrane reported here may be particularly important during the formation of GABAergic synapses as the postsynaptic expression of GABAARs and their clustering are the key steps in this process (80). Conversely, the release of BDNF triggered by GABAAR activity can have pronounced effects on the presynaptic counterparts as both axonal growth and structural maturation of presynaptic nerve terminals are known to be regulated by BDNF (81). This is in an agreement with the previously published evidence that activity-dependent release of BDNF occurs predominantly from the postsynaptic sites (82).
As demonstrated here, an increase in cell surface expression of GABAARs results from the inhibition of the receptor endocytosis by BDNF and is mediated by the activation of the TrkB/PI 3-kinase/PKC signaling pathway. It is important to note that the same signaling pathway was found previously to mediate the BDNF-dependent increase in GABAAR phosphorylation at Ser-408/409 residues (38). Although phosphorylation of these residues has a positive effect on the gating properties of GABAARs (83), their dephosphorylation was shown to be directly responsible for a decrease in GABAergic currents in our previous study (38). In the same study, we have also reported an increase in cell surface expression of GABAARs by BDNF that was long lasting and therefore not correlative with a decrease in GABAergic currents. Thus, our findings described here are consistent with our previously published results. It is also well established that an increase in phosphorylation of Ser-408/409 inhibits the interaction between GABAAR subunits and the clathrin AP2 adaptor protein, which mediates the dynamin-dependent endocytosis of these receptors (84). Thus, the inhibition of the receptor endocytosis by BDNF reported here is likely to occur due to an increase in GABAAR phosphorylation (38) and subsequent inhibition of their interaction with the AP2 complex.
The BDNF-dependent increase in cell surface expression is specific for early developing neurons where GABAAR activation leads to a depolarization of the plasma membrane. In more mature neurons, BDNF appears to reduce the cell surface expression of GABAARs (40, 41). This shift in the regulation of GABAAR surface expression by BDNF (from an increase to a decrease) is accompanied by a shift in the GABAAR-dependent regulation of BDNF expression (from an increase to a decrease (85)) as well as its secretion. Importantly, these processes also coincide with the transition in the functional outcome of GABAAR activation (depolarization to hyperpolarization) and with the formation of GABAergic synapses (59). As GABA and BDNF are components of the positive feedback mechanism described here, it is possible that they in fact synergize to drive this transition. This may lead to an increase in the expression of the K+-Cl− cotransporter (KCC2) (12, 86). Consequently, later in development, a KCC2-driven decrease in [Cl−]i and a shift in the reversal potential for GABAergic currents toward more hyperpolarized levels (15, 54, 87) would result in termination of Ca2+-dependent signaling downstream of GABAARs.
We hypothesize that the positive feedback mechanism operating between GABAARs and BDNF/TrkB may be restricted to those synapses that show the first signs of activity. By increasing the expression and accumulation of GABAAR at the postsynaptic sites, BDNF may stabilize these synapses and promote their functional maturation. Emphasizing the crucial role of GABA and BDNF in development, transgenic mouse models with genetic ablation of proteins involved in these signaling cascades display severe physiological and behavioral deficits, severe epilepsy, and abnormal neural activity or neonatal death (32, 88–90). As the brain develops further and GABA becomes hyperpolarizing, balancing the maturation of glutamatergic transmission, this feedback loop falls into abeyance. The control of BDNF release comes under the regulation of other signals and GABAAR expression, and clustering at the postsynaptic membrane comes under the control of transsynaptic and intracellular protein-protein interactions (72).
Acknowledgments
We thank Prof. Werner Sieghart for help in obtaining GABAAR subunit-specific antibodies and A. L. K. Sihra-Jovanovic for technical assistance.
This work was supported by Biotechnology and Biological Sciences Research Council UK New Investigator Grant BB/C507237/1 (to J. N. J.), Medical Research Council Grant G0800498 (to J. N. J. and A. M. T.), and INSERM and Agence Nationale pour la Recherche (to C. P.).
- GABA
- γ-aminobutyric acid
- GABAAR
- GABAA receptor
- BDNF
- brain-derived neurotrophic factor
- VGCC
- voltage-gated Ca2+ channel
- KCC2
- K+-Cl− cotransporter
- VGlut
- vesicular glutamate transporter
- MAP
- microtubule-associated protein
- TrkB
- tropomyosin-related kinase B
- PI
- phosphoinositide
- CREB
- cAMP response element-binding protein
- pCREB
- phospho-CREB
- DIV
- days in vitro
- PFA
- paraformaldehyde
- TTX
- tetrodotoxin
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- sulfo-NHS-SS-biotin
- sulfosuccinimidyl-2-(biotinamido)-ethyl-1,3′-dithiopropionate
- d-APV
- d-2-amino-5-phosphonovalerate.
REFERENCES
- 1. Farrant M., Kaila K. (2007) Prog. Brain Res. 160, 59–87 [DOI] [PubMed] [Google Scholar]
- 2. Whiting P. J., Bonnert T. P., McKernan R. M., Farrar S., Le Bourdellès B., Heavens R. P., Smith D. W., Hewson L., Rigby M. R., Sirinathsinghji D. J., Thompson S. A., Wafford K. A. (1999) Ann. N.Y. Acad. Sci. 868, 645–653 [DOI] [PubMed] [Google Scholar]
- 3. LoTurco J. J., Owens D. F., Heath M. J., Davis M. B., Kriegstein A. R. (1995) Neuron 15, 1287–1298 [DOI] [PubMed] [Google Scholar]
- 4. Haydar T. F., Wang F., Schwartz M. L., Rakic P. (2000) J. Neurosci. 20, 5764–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behar T. N., Li Y. X., Tran H. T., Ma W., Dunlap V., Scott C., Barker J. L. (1996) J. Neurosci. 16, 1808–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barker J. L., Behar T., Li Y. X., Liu Q. Y., Ma W., Maric D., Maric I., Schaffner A. E., Serafini R., Smith S. V., Somogyi R., Vautrin J. Y., Wen X. L., Xian H. (1998) Perspect. Dev. Neurobiol. 5, 305–322 [PubMed] [Google Scholar]
- 7. Belhage B., Hansen G. H., Elster L., Schousboe A. (1998) Perspect. Dev. Neurobiol. 5, 235–246 [PubMed] [Google Scholar]
- 8. Davies P., Anderton B., Kirsch J., Konnerth A., Nitsch R., Sheetz M. (1998) Prog. Neurobiol. 55, 651–658 [DOI] [PubMed] [Google Scholar]
- 9. Ben-Ari Y. (2002) Nat. Rev. Neurosci. 3, 728–739 [DOI] [PubMed] [Google Scholar]
- 10. Fagiolini M., Fritschy J. M., Löw K., Möhler H., Rudolph U., Hensch T. K. (2004) Science 303, 1681–1683 [DOI] [PubMed] [Google Scholar]
- 11. Hensch T. K., Stryker M. P. (2004) Science 303, 1678–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganguly K., Schinder A. F., Wong S. T., Poo M. (2001) Cell 105, 521–532 [DOI] [PubMed] [Google Scholar]
- 13. Leinekugel X., Tseeb V., Ben-Ari Y., Bregestovski P. (1995) J. Physiol. 487, 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mueller A. L., Taube J. S., Schwartzkroin P. A. (1984) J. Neurosci. 4, 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luhmann H. J., Prince D. A. (1991) J. Neurophysiol. 65, 247–263 [DOI] [PubMed] [Google Scholar]
- 16. Obrietan K., van den Pol A. N. (1995) J. Neurosci. 15, 5065–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khazipov R., Leinekugel X., Khalilov I., Gaiarsa J. L., Ben-Ari Y. (1997) J. Physiol. 498, 763–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bourgeois J. P., Rakic P. (1993) J. Neurosci. 13, 2801–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balslev Y., Saunders N. R., Møllgård K. (1996) Acta Anat. 156, 2–10 [DOI] [PubMed] [Google Scholar]
- 20. Demarque M., Represa A., Becq H., Khalilov I., Ben-Ari Y., Aniksztejn L. (2002) Neuron 36, 1051–1061 [DOI] [PubMed] [Google Scholar]
- 21. Wang D. D., Kriegstein A. R. (2009) J. Physiol. 587, 1873–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chao M. V., Rajagopal R., Lee F. S. (2006) Clin. Sci. 110, 167–173 [DOI] [PubMed] [Google Scholar]
- 23. Kalb R. (2005) Trends Neurosci. 28, 5–11 [DOI] [PubMed] [Google Scholar]
- 24. Huang E. J., Reichardt L. F. (2003) Annu. Rev. Biochem. 72, 609–642 [DOI] [PubMed] [Google Scholar]
- 25. Chapleau C. A., Larimore J. L., Theibert A., Pozzo-Miller L. (2009) J. Neurodev. Disord. 1, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen-Cory S., Kidane A. H., Shirkey N. J., Marshak S. (2010) Dev. Neurobiol. 70, 271–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cunha C., Brambilla R., Thomas K. L. (2010) Front. Mol. Neurosci. 3, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu B., Wang K. H., Nose A. (2009) Curr. Opin. Neurobiol. 19, 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoshii A., Constantine-Paton M. (2010) Dev. Neurobiol. 70, 304–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gottmann K., Mittmann T., Lessmann V. (2009) Exp. Brain Res. 199, 203–234 [DOI] [PubMed] [Google Scholar]
- 31. Chao M. V. (2003) Nat. Rev. Neurosci. 4, 299–309 [DOI] [PubMed] [Google Scholar]
- 32. Fujii M., Arata A., Kanbara-Kume N., Saito K., Yanagawa Y., Obata K. (2007) Neuroscience 146, 1044–1052 [DOI] [PubMed] [Google Scholar]
- 33. Tanaka T., Saito H., Matsuki N. (1997) J. Neurosci. 17, 2959–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frerking M., Malenka R. C., Nicoll R. A. (1998) J. Neurophysiol. 80, 3383–3386 [DOI] [PubMed] [Google Scholar]
- 35. Boxall A. R. (2000) J. Physiol. 524, 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wardle R. A., Poo M. M. (2003) J. Neurosci. 23, 8722–8732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanematsu T., Yasunaga A., Mizoguchi Y., Kuratani A., Kittler J. T., Jovanovic J. N., Takenaka K., Nakayama K. I., Fukami K., Takenawa T., Moss S. J., Nabekura J., Hirata M. (2006) J. Biol. Chem. 281, 22180–22189 [DOI] [PubMed] [Google Scholar]
- 38. Jovanovic J. N., Thomas P., Kittler J. T., Smart T. G., Moss S. J. (2004) J. Neurosci. 24, 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hewitt S. A., Bains J. S. (2006) J. Neurophysiol. 95, 2193–2198 [DOI] [PubMed] [Google Scholar]
- 40. Brünig I., Penschuck S., Berninger B., Benson J., Fritschy J. M. (2001) Eur. J. Neurosci. 13, 1320–1328 [DOI] [PubMed] [Google Scholar]
- 41. Cheng Q., Yeh H. H. (2003) J. Physiol. 548, 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mizoguchi Y., Kanematsu T., Hirata M., Nabekura J. (2003) J. Biol. Chem. 278, 44097–44102 [DOI] [PubMed] [Google Scholar]
- 43. Elmariah S. B., Oh E. J., Hughes E. G., Balice-Gordon R. J. (2005) J. Neurosci. 25, 3638–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elmariah S. B., Crumling M. A., Parsons T. D., Balice-Gordon R. J. (2004) J. Neurosci. 24, 2380–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palizvan M. R., Sohya K., Kohara K., Maruyama A., Yasuda H., Kimura F., Tsumoto T. (2004) Neuroscience 126, 955–966 [DOI] [PubMed] [Google Scholar]
- 46. Rico B., Xu B., Reichardt L. F. (2002) Nat. Neurosci. 5, 225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goslin K., Asmusen H., Benker G. (1998) in Culturing Nerve Cells (Banker G., Goslin K. eds) pp. 339–371, MIT Press, Cambridge, MA [Google Scholar]
- 48. Goffin D., Ali A. B., Rampersaud N., Harkavyi A., Fuchs C., Whitton P. S., Nairn A. C., Jovanovic J. N. (2010) J. Neurosci. 30, 2935–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haubensak W., Narz F., Heumann R., Lessmann V. (1998) J. Cell Sci. 111, 1483–1493 [DOI] [PubMed] [Google Scholar]
- 50. Kuczewski N., Porcher C., Ferrand N., Fiorentino H., Pellegrino C., Kolarow R., Lessmann V., Medina I., Gaiarsa J. L. (2008) J. Neurosci. 28, 7013–7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Noel J., Ralph G. S., Pickard L., Williams J., Molnar E., Uney J. B., Collingridge G. L., Henley J. M. (1999) Neuron 23, 365–376 [DOI] [PubMed] [Google Scholar]
- 52. Mammen A. L., Huganir R. L., O'Brien R. J. (1997) J. Neurosci. 17, 7351–7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ehlers M. D. (2000) Neuron 28, 511–525 [DOI] [PubMed] [Google Scholar]
- 54. Owens D. F., Boyce L. H., Davis M. B., Kriegstein A. R. (1996) J. Neurosci. 16, 6414–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fiorentino H., Kuczewski N., Diabira D., Ferrand N., Pangalos M. N., Porcher C., Gaiarsa J. L. (2009) J. Neurosci. 29, 11650–11661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ghosh A., Carnahan J., Greenberg M. E. (1994) Science 263, 1618–1623 [DOI] [PubMed] [Google Scholar]
- 57. Kittler J. T., Thomas P., Tretter V., Bogdanov Y. D., Haucke V., Smart T. G., Moss S. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12736–12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arancibia-Cárcamo I. L., Kittler J. T. (2009) Pharmacol. Ther. 123, 17–31 [DOI] [PubMed] [Google Scholar]
- 59. Ben-Ari Y., Gaiarsa J. L., Tyzio R., Khazipov R. (2007) Physiol. Rev. 87, 1215–1284 [DOI] [PubMed] [Google Scholar]
- 60. Sernagor E., Chabrol F., Bony G., Cancedda L. (2010) Front. Cell. Neurosci. 4, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cancedda L., Fiumelli H., Chen K., Poo M. M. (2007) J. Neurosci. 27, 5224–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hutcheon B., Morley P., Poulter M. O. (2000) J. Physiol. 522, 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kuczewski N., Porcher C., Gaiarsa J. L. (2010) Eur. J. Neurosci. 32, 1239–1244 [DOI] [PubMed] [Google Scholar]
- 64. Greenberg M. E., Xu B., Lu B., Hempstead B. L. (2009) J. Neurosci. 29, 12764–12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nusser Z., Cull-Candy S., Farrant M. (1997) Neuron 19, 697–709 [DOI] [PubMed] [Google Scholar]
- 66. Serafini R., Ma W., Maric D., Maric I., Lahjouji F., Sieghart W., Barker J. L. (1998) Eur. J Neurosci. 10, 1771–1783 [DOI] [PubMed] [Google Scholar]
- 67. Owens D. F., Liu X., Kriegstein A. R. (1999) J. Neurophysiol. 82, 570–583 [DOI] [PubMed] [Google Scholar]
- 68. Laurie D. J., Wisden W., Seeburg P. H. (1992) J. Neurosci. 12, 4151–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ma W., Barker J. L. (1995) J. Neurosci. 15, 2547–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maric D., Maric I., Ma W., Lahojuji F., Somogyi R., Wen X., Sieghart W., Fritschy J. M., Barker J. L. (1997) Eur. J. Neurosci. 9, 507–522 [DOI] [PubMed] [Google Scholar]
- 71. Yamada M. K., Nakanishi K., Ohba S., Nakamura T., Ikegaya Y., Nishiyama N., Matsuki N. (2002) J. Neurosci. 22, 7580–7585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thomson A. M., Jovanovic J. N. (2010) Eur. J. Neurosci. 31, 2193–2203 [DOI] [PubMed] [Google Scholar]
- 73. Mizuno K., Carnahan J., Nawa H. (1994) Dev. Biol. 165, 243–256 [DOI] [PubMed] [Google Scholar]
- 74. Widmer H. R., Hefti F. (1994) Brain Res. Dev. Brain Res. 80, 279–284 [DOI] [PubMed] [Google Scholar]
- 75. Vicario-Abejón C., Collin C., McKay R. D., Segal M. (1998) J. Neurosci. 18, 7256–7271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Seil F. J., Drake-Baumann R. (2000) J. Neurosci. 20, 5367–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Marty S., Wehrlé R., Sotelo C. (2000) J. Neurosci. 20, 8087–8095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bao S., Chen L., Qiao X., Thompson R. F. (1999) Learn. Mem. 6, 276–283 [PMC free article] [PubMed] [Google Scholar]
- 79. Huang Z. J., Kirkwood A., Pizzorusso T., Porciatti V., Morales B., Bear M. F., Maffei L., Tonegawa S. (1999) Cell 98, 739–755 [DOI] [PubMed] [Google Scholar]
- 80. Moss S. J., Smart T. G. (2001) Nat. Rev. Neurosci. 2, 240–250 [DOI] [PubMed] [Google Scholar]
- 81. Vicario-Abejón C., Owens D., McKay R., Segal M. (2002) Nat. Rev. Neurosci. 3, 965–974 [DOI] [PubMed] [Google Scholar]
- 82. Matsuda N., Lu H., Fukata Y., Noritake J., Gao H., Mukherjee S., Nemoto T., Fukata M., Poo M. M. (2009) J. Neurosci. 29, 14185–14198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. McDonald B. J., Amato A., Connolly C. N., Benke D., Moss S. J., Smart T. G. (1998) Nat. Neurosci. 1, 23–28 [DOI] [PubMed] [Google Scholar]
- 84. Kittler J. T., Chen G., Honing S., Bogdanov Y., McAinsh K., Arancibia-Carcamo I. L., Jovanovic J. N., Pangalos M. N., Haucke V., Yan Z., Moss S. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 14871–14876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Berninger B., Marty S., Zafra F., da Penha Berzaghi M., Thoenen H., Lindholm D. (1995) Development 121, 2327–2335 [DOI] [PubMed] [Google Scholar]
- 86. Aguado F., Carmona M. A., Pozas E., Aguiló A., Martínez-Guijarro F. J., Alcantara S., Borrell V., Yuste R., Ibañez C. F., Soriano E. (2003) Development 130, 1267–1280 [DOI] [PubMed] [Google Scholar]
- 87. Cherubini E., Rovira C., Gaiarsa J. L., Corradetti R., Ben Ari Y. (1990) Int. J. Dev. Neurosci. 8, 481–490 [DOI] [PubMed] [Google Scholar]
- 88. Günther U., Benson J., Benke D., Fritschy J. M., Reyes G., Knoflach F., Crestani F., Aguzzi A., Arigoni M., Lang Y. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7749–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Conover J. C., Yancopoulos G. D. (1997) Rev. Neurosci. 8, 13–27 [DOI] [PubMed] [Google Scholar]
- 90. Homanics G. E., DeLorey T. M., Firestone L. L., Quinlan J. J., Handforth A., Harrison N. L., Krasowski M. D., Rick C. E., Korpi E. R., Mäkelä R., Brilliant M. H., Hagiwara N., Ferguson C., Snyder K., Olsen R. W. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4143–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]