Abstract
WD repeat-containing protein 5 (WDR5) is a common component of mammalian mixed lineage leukemia methyltransferase family members and is important for histone H3 lysine 4 methylation (H3K4me), which has been implicated in control of activation of cell lineage genes during embryogenesis. However, WDR5 has not been considered to play a specific regulatory role in epigenetic programming of cell lineage because it is ubiquitously expressed. Previous work from our laboratory showed the appearance of histone H3K4me within smooth muscle cell (SMC)-marker gene promoters during the early stages of development of SMC from multipotential embryonic cells but did not elucidate the underlying mechanisms that mediate SMC-specific and locus-selective H3K4me. Results presented herein show that knockdown of WDR5 significantly decreased SMC-marker gene expression in cultured SMC differentiation systems and in Xenopus laevis embryos in vivo. In addition, we showed that WDR5 complexes within SMC progenitor cells contained H3K4 methyltransferase enzymatic activity and that knockdown of WDR5 selectively decreased H3K4me1 and H3K4me3 enrichment within SMC-marker gene promoter loci. Moreover, we present evidence that it is recruited to these gene promoter loci through interaction with a SMC-selective pituitary homeobox 2 (Pitx2). Taken together, studies provide evidence for a novel mechanism for epigenetic control of SMC-marker gene expression during development through interaction of WDR5, homeodomain proteins, and chromatin remodeling enzymes.
Keywords: Chromatin Histone modification, Epigenetics, Gene Regulation, Smooth Muscle, Stem Cell, Histone H3 Lysine 4 Methylation, Pitx2, WDR5
Introduction
A critical question in developmental biology is how cell lineage-specific gene expression patterns are established and propagated during embryogenesis. Although the exact mechanisms by which complex patterns of gene expression are established during development are unclear, epigenetic modification of chromatin appears to play an important role in the transition of embryonic stem cells (ESCs)2 into committed cell lineages (1, 2). Among the epigenetic modifications, histone methylation is an important primary determinant of cellular identity. In pluripotent ESCs, many lineage-specific genes are labeled with a unique bivalent histone modification pattern consisting of “activating” histone H3 lysine 4 methylation (H3K4me) and “repressive” H3 lysine 27 methylation (H3K27me) modifications. During the process of lineage determination from ESCs, these “bivalent domains” are resolved into constitutive H3K4me loci within genes that are actively expressed or H3K27me loci within genes that are repressed in specific cell lineages (2–4). In mammalian cells, H3K4me is mainly carried out by the mixed lineage leukemia (MLL) family members and SET1A/SET1B (5–7). These MLL/SET members have H3K4 methyltransferase (HKMT) activity through a conserved SET domain and form a complex with conserved subunits WDR5, Ash2L, and RbBP5 (8, 9). WDR5 is required to keep the MLL/SET associated with the rest of the complex and is required for its activity (10). Although WDR5 was previously shown to be an H3K4me-binding protein important for recruiting MLL/SET to histone H3 tail and the subsequent transition of H3K4 dimethylation (H3K4me2) to trimethylation (H3K4me3) (8), it is unknown whether H3K4me plays a causal role in cell lineage determination or in cell-selective gene expression. Indeed the general consensus has been that WDR5 is unlikely to contribute to cell-specific gene transcription or epigenetic programming of cell lineage during development because it is expressed in virtually all cell types and there is no known mechanism whereby it might be selectively recruited to gene loci encoding lineage selective-specific genes.
Despite extensive study, the mechanisms of targeting H3K4 HKMTs to lineage-specific gene loci during embryogenesis remain to be established. H3K4 HKMTs can interact with sequence-specific DNA-bound transcription factors (11–13), the estrogen receptor (14), and lymphoid enhancer-binding factors (15). Of special importance related to cell lineage-specific recruitment, Rudnicki and co-workers (11) and Dilworth co-workers (12) reported that during skeletal myogenesis, MLL2 is recruited to myogenic gene promoters through the interaction between its core factors and the transcription factors such as Pax7 and Mef2 (11, 12). These results indicate that H3K4 HKMTs are recruited to cell lineage-specific gene promoters by interacting with lineage-specific transcription factors and contribute to alterations in H3K4me status important in the regulation of differentiation. In this model lineage determination is not controlled by histone methylation per se but, rather, occurs via locus selective binding of cell-specific transcription factors that in turn recruit HKMTs that modify chromatin and increase the rate of transcription of that gene. However, no studies have determined if selective H3K4me itself directly regulates lineage marker gene activation by altering binding of lineage-specific transcription factors, i.e. lies upstream of binding of cell-selective transcription factors.
Recent studies from our laboratory have shown that development of smooth muscle cells (SMCs) from progenitor cells or ESCs within an embryoid body (EB) model provides a unique and powerful model for studying epigenetic control of the early stages of cellular lineage determination during development (16, 17). Specifically, we found that H3K4me2 was introduced without the binding of serum response factor (SRF)-myocardin complexes to their cis element within the SMC-marker gene loci and did not appear on the same gene loci in non-SMCs, suggesting that the SMC- and locus-selective appearance of H3K4me2 may represent an “epigenetic marker” of transcriptional competence and is a primary determinant of SMC identity (18, 19). Moreover, we have shown that H3K4me2 on SMC-marker gene loci, including SM α-actin and SM myosin heavy chain (SM-MHC), persists during reversible phenotypic switching of these cells during vascular repair or in disease, a process in which cells temporarily suppress expression of virtually all marker genes that allow them to be identified as SMC (19, 20). Indeed, we have postulated that H3K4me2 of SMC-marker gene loci represent a mechanism for cell lineage memory during reversible phenotypic switching of SMC and is important for regulating re-differentiation of these cells upon resolution of the stress/injury. However, major unresolved questions include 1) the underlying mechanisms by which H3K4me is established at SMC-marker gene promoters during lineage determination of SMC from multipotential embryonic stem cells and 2) how HKMT complexes are selectively recruited to SMC-marker gene promoter regions during this process.
In the present studies we present evidence showing that WDR5 binds to the bicoid-type homeodomain protein Pitx2 (21), that cell selective recruitment of WDR5 to SMC gene loci is dependent on Pitx2 and binding of Pitx2 to a conserved homeodomain sequence within the SM α-actin promoter, and that suppression of WDR5 significantly decreased SMC-marker gene expression in ESC, SMC progenitor stem cells, and in Xenopus embryos in vivo. In addition, we showed that WDR5 complexes within SMC progenitor cells contained H3K4 HKMT enzymatic activity and that knockdown of WDR5 selectively decreased H3K4me1 and H3K4me3 enrichment within SMC-marker gene promoter loci. Taken together results provide evidence for a novel model for epigenetic control of cell lineage marker gene transcription during early development involving cell type and gene locus selective H3K4me2 that is mediated through the ubiquitously expressed HKMT co-factor WDR5.
EXPERIMENTAL PROCEDURES
Plasmids Construction, siRNA Duplexes, and Antibodies
An expression plasmid for Myc-tagged WDR5 was constructed by inserting WDR5 cDNA into pcDNA3.1-Myc/His (+) (Invitrogen). Pitx2 expression plasmid and its deletion mutants were described previously (21). pTAP-WDR5 was constructed by inserting WDR5 cDNA into pTAP vector (Stratagene). ATTA-mutated SM α-actin_Luc-AttB plasmid was generated by replacing the BstEII and XhoI DNA segment within the wild-type SM α-actin_Luc-ATTB plasmid with that from the ATTA-mutated SM α-actin_lacZ plasmid. siRNAs for WDR5 were purchased from Dharmacon. The siRNA experiments were performed in both the pool of siRNAs and were further confirmed by using two different siRNAs from the pool of four siRNAs. Antibodies were used as follows: SM α-actin (1A4, Sigma), SM22α (Abcam), WDR5 (Abcam), FLAG (M2 Sigma), Myc and SRF (Santa Cruz Biotechnology, Inc.), KLF4 (Chemicon), H3K4me1, H3K4me2, H3K4me3, H3 acetylation, and H4 acetylation (Millipore), cleaved caspase 3 (Cell Signaling Technology), α-tubulin (Abcam), Ash2L and RbBP5 (Bethyl Laboratory).
Cell Culture, ESC-EB SMC Differentiation System, Stable Transfection, and Immunofluorescence
A404 cells were cultured and induced to differentiate into SMCs as described previously (18). Heterozygous WDR5 ES cells were obtained from the Mutant Mouse Regional Resource Center. Neomycin was used to select homozygous WDR5−/− knock-out ES cells as described by Mortensen et al. (22), which also has been successfully used in our laboratory (21). Specific PCR primers were designed to recognize the wild-type and knock-out allele. Procedures for differentiation of ES cells into SMCs in the context of embryoid bodies were described previously (23). The integrase system was employed to generate stable transfection of A404 cells containing the promoter-reporter transgenes (19, 24). A404 cell lines containing Myc-tagged WDR5 or empty vector were generated by stable transfection and G418 selection.
Real-time RT-PCR, Western Blotting, and Coimmunoprecipitation Assays
Total RNA was prepared from cultured cells for real-time RT-PCR using TRIzol as described previously (25). RNA from Xenopus laevis was prepared by using Lysis Matrix D beads in TRIzol. Primers for SM α-actin, SM22α, myocardin, MRTF A and MRTF B, and 18 S ribosomal RNA were described previously (25). Western blotting and co-immunoprecipitation assays were performed as previously described (26).
Tandem Affinity Purification, HKMT Activity Assay, Silver Staining, and Mass Spectrometry
Whole cell extracts were prepared from pTAP-WDR5 or pTAP-empty plasmid-transfected A404 cells. Samples were processed following the protocol recommended in the InterPlay Tandem Purification System kit. The elution sample was incubated with core histones for HKMT activity assay (11). Eluted samples were subjected to SDS-PAGE gel and silver staining. Distinct bands were excised directly from the gel and analyzed at the W. M. Keck Biomedical Mass Spectrometry Laboratory at University of Virginia. The data were analyzed by data base searching using the Sequest search algorithm against the Mouse International Protein Index.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays for cultured A404 cells were performed as previously described (27). For the ChIP assay using EB, the fixed EB was homogenized using Lysis Matrix D beads in lysis buffer using Fastprep in a cold room until the clumps were dissociated. The following steps were the same as described in the ChIP-IT Express kit. Sequential ChIP assay has been described before (26).
Morpholino Design and WDR5 Knockdown
X. laevis WDR5 is encoded by two distinct yet closely related genes. Two morpholino oligonucleotide targeting ATG regions of both putative X. laevis WDR5 mRNAs were synthesized by Gene Tools, LLC (8). For the animal cap assay, two-cell stage embryos were injected with 30 ng of morpholino solution and grown until the blastula stage or stage 17 before dissection (28). For in situ hybridization analysis, microinjected embryos were grown until stage 42 then fixed and processed as described before (28).
Statistical Analyses
Data are presented as the mean ± S.E. Unless mentioned, statistical analyses were performed by Student's t test. p values < 0.05 were considered significant.
RESULTS
WDR5 Knockdown Decreases SMC-marker Gene Expression within in Vitro SMC Differentiation Systems
In mammalian cells H3K4me is carried out mainly by MLL/SET1 family members. These H3K4 HKMTs are active in the context of multimolecular complexes containing three obligatory components including WDR5, RbBP5, and Ash2L. As an initial test if H3K4me is involved in the activation of SMC-marker gene expression during differentiation, we focused on WDR5 because it is required to keep the HKMT associated with the rest of the complex and to bridge them to the H3 tail (8). Two in vitro models of early stages of SMC development were used to test if suppression of WDR5 expression inhibited activation of SMC-marker genes. The first model is a clonal line of SMC progenitor cells derived from multipotential embryonal carcinoma P19 cells (designated A404 cells) (29), in which all known SMC-marker genes are not expressed under basal conditions but are rapidly induced upon retinoic acid (RA) induction (18, 21). RA treatment of A404 cells was not associated with a change in WDR5 expression (supplemental Fig. S1). However, siRNA-mediated repression of WDR5 markedly inhibited RA-induced activation of SMC-marker genes examined, including SM α-actin and SM22α at the RNA (Fig. 1A) and protein levels (Fig. 1B). Similar results were observed using a pool of WDR5 siRNAs as well as two independent siRNAs for WDR5. These effects were not due to general repression of transcription but were selective for SMC genes in that we observed no changes in the expression of ubiquitously expressed SRF and RbBP5. These effects were not associated with alterations in cell apoptosis in that the expression of cleaved caspase 3 was not changed by knockdown of WDR5 (Fig. 1B). In addition, we saw no changes in expression of MCP-1, CX3CL1, and the myocardin-related SRF co-activators A and B (MRTF-A and MRTF-B), which are genes expressed by cultured SMC but which are not cell selective (Fig. 1, A and B). We then tested if WDR5 was also required for development and differentiation of SMC from ESCs using an ESC-EB SMC differentiation system previously described by our laboratory (23, 30). The ESC-EB system recapitulates key inductive signals for SMC lineage determination and differentiation as occurs in vivo in the developing embryo, and SMC within this system can develop contractile forces equivalent to SMC tissues in vivo (23, 30). Despite extensive attempts, we were unable to derive homozygous WDR5 knock-out ESC, suggesting that it is required for ESC survival. Consistent with our observations, it has recently been reported by Stoller et al. (31) that Ash2L, another core factor of MLL complexes, is required for ES cell maintenance and/or proliferation. Nevertheless, consistent with results of WDR5 knockdown studies in A404 cells, heterozygous WDR5 knock-out ESC, which showed a 40–60% reduction in WDR5 expression as compared with wild-type ESC, exhibited attenuated activation of multiple SMC-marker genes including SM α-actin, SM22α, SM-MHC, and myocardin (Fig. 1C) but not of the neuron-specific gene NeuroD at day 7 and day 15 EBs in the ESCs-EB SMC differentiation system. Taken together, the preceding studies suggest that suppression of WDR5 gene expression is associated with impairment of SMC-marker gene expression during the differentiation of SMCs from progenitor cells and stem cells in cultured cell systems.
FIGURE 1.
siRNA-mediated knockdown of WDR5 decreased SMC-marker gene expression in two different SMC differentiation systems. A, expression of WDR5, SMC-marker genes, and control genes was determined by real-time RT-PCR. A404 cells were transfected with control or WDR5 siRNAs and were induced to differentiate into SMCs by RA treatment for 48 h. B, as in A, the protein of WDR5, SMC-marker genes, and control genes was analyzed by Western blot. C, heterozygous knock-out of WDR5 in embryonic stem cells decreased SMC-marker gene expression in an ESC-EB smooth muscle cell differentiation model system at days 7 and 15. Wild-type and heterozygous WDR5 knock-out ES cells were cultured and induced to differentiate into SMCs in the context of embryoid bodies (21, 23), and the level of SMC-marker genes, NeuroD, and WDR5 was determined by real-time PCR at days 7, 15, and 28 EBs. Values represent the mean ± S.E. of three independent experiments.
WDR5 Knockdown Decreases SMC-marker Gene Expression in X. laevis Embryos in Vivo
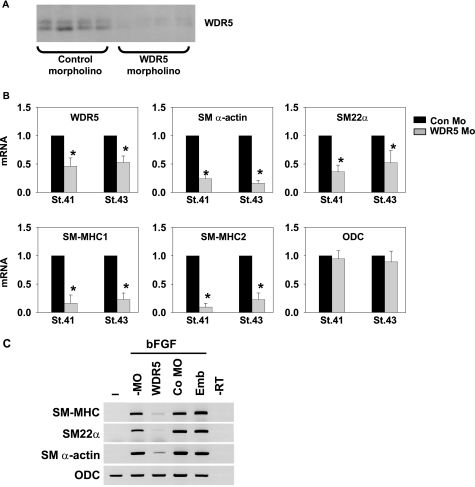
To determine whether WDR5 is important in controlling SMC gene transcription in vivo, we tested the effects of morpholino-induced knockdown of WDR5 in X. laevis embryos. Allis and co-workers (8) had previously shown that knockdown of WDR5 during X. laevis development delayed overall embryonic development. We tested different doses of morpholinos and derived a dosage that resulted in suppression of WDR5 expression by ∼90% (Fig. 2A) and 55% (Fig. 2B) at the protein and mRNA levels, respectively, but that had no effects on overall development stage based on embryo staging. Of interest, morpholino-induced suppression of WDR5 resulted in marked decreases in SM α-actin, SM22α, and SM-MHC RNA expression by real time RT-PCR in whole embryos at developmental stages 41 and 43. Of particular note, we observed a >80% reduction in expression of the most definitive and selective marker of differentiated SMC, the SM2 variant of SM-MHC. These effects were selective, not global, in that there were no changes in the expression of control genes including the ornithine decarboxylase (ODC) gene.
FIGURE 2.
WDR5 knockdown in X. laevis significantly decreased SMC-marker gene expression. A, WDR5 expression at the blastula stage was greatly decreased by WDR5-specific morpholino. Protein from whole embryos at blastula stage was harvested and analyzed by Western blot. Each lane represents for a group of three embryos. B, the expression of WDR5 gene and SMC-marker genes was determined by real-time PCR at two different stages of X. laevis by real-time PCR. C, expression of the SMC-marker genes SM-MHC, SM22α, and SM α-actin was analyzed by semiquantitative RT-PCR. Pluripotent animal cap cells from two-cell-stage X. laevis embryos injected with WDR5 morpholino (WDR5) or control morpholino (CoMO) were treated with bFGF to enhance SMC differentiation. −, without bFGF treatment; −MO, no morpholino injection; Emb, whole embryo; −RT, no reverse transcriptase control; ODC, ornithine decarboxylase.
Basic FGF (bFGF) has been reported to be a potent growth factor for inducing smooth muscle differentiation in X. laevis animal cap explants (28). The pluripotent animal cap was dissected from blastula stage embryos injected with either control or WDR5 morpholinos at the two-cell stage and treated with bFGF to promote SMC differentiation as previously reported (28). Morpholino-mediated knockdown of WDR5 significantly decreased bFGF-induced expression of SMC genes including SM α- actin, SM22α, and SM-MHC (Fig. 2C).
Of major significance, these results were further confirmed by whole-mount in situ hybridization assays. As shown in Fig. 3, WDR5 morpholinos greatly decreased the expression of SM α-actin, SM22α, and SM-MHC within visceral muscle and virtually abolished expression of these genes within the dorsal aorta. Results were highly consistent with >70% of injected embryos (n = 55) showing marked reductions in SMC gene expression when injected with WDR5 versus control morpholinos.
FIGURE 3.
Morpholino-mediated knockdown of WDR5 in X. laevis significantly decreased SMC-marker gene expression in vivo. In situ hybridization analyses of SM α-actin (A–D), SM 22α (E–H), and SM-MHC (I–L) mRNA expression in stage 42 X. laevis embryos injected with either control morpholinos or WDR5 morpholinos at the two-cell stage. The location of visceral muscle and the dorsal aorta are indicated with arrows, whereas arrowheads highlight embryonic regions with either reduced visceral muscle (VM) cells or dorsal aortic (DA) cells expressing SMC-marker genes. B, D, F, H, J, and L represent higher magnifications of panels A, C, E, G, I, and K, respectively.
Taken together, the preceding studies provide compelling evidence that suppression of WDR5 gene expression is associated with selective impairment of SMC-marker gene expression during the early stages of development in vivo in X. laevis embryos.
WDR5 Forms a Multiprotein Complex with H3K4 HKMT Activity and Regulates H3K4 Methylation within SMC-marker Gene Promoter Loci in Differentiated SMCs
It has been previously reported that WDR5 and its associated HKMT complexes can mediate H3K4 methylation (8, 9). Thus, one possible mechanism by which WDR5 may regulate SMC-marker gene expression is through interaction with H3K4 HKMT complexes and subsequent epigenetic remodeling of SMC gene loci. We thus sought to identify factors that interact with WDR5 and regulate its activity and/or gene locus selectivity in differentiated SMC. As an initial test of this hypothesis, an N-terminal TAP-tagged WDR5 expression plasmid was constructed and used to purify WDR5 protein and interacting complexes from A404 SMC progenitor cells (with and without treatment with RA). Purified WDR5 protein complex prepared from A404 SMC progenitor cells either with or without RA treatment were processed for mass spectrometric sequencing. Results showed that at least four distinct bands were evident in 24-h RA-treated A404 cells that were absent in undifferentiated A404 cells or in A404 cells transfected with the empty expression vector (Fig. 4A). Mass spectrometric sequencing analysis of these bands identified them as MybBP1A, ATAC2, Ash2L, and PI3K. Of interest, ATAC2 is the binding protein for CRP2 that has previously been shown to induce expression of multiple SMC-marker genes in fibroblast cells in combination with GATA4 and SRF (32, 33). ATAC2 belongs to a histone acetyltransferase complex and has histone deacetylase activity by itself (34, 35). Consistent with our findings, two different groups provided evidence that WDR5 is contained within the ATAC2 complex within several different cell types (34, 35). Of great interest, Ash2L, one of the subunits within the core of WDR5-RbBP5-Ash2L, appeared in the WDR5 complex in RA-treated A404 cells but not in the undifferentiated cells. It has been reported that Ash2L can regulate the MLL catalytic activity toward H3K4me3 and may function as a transcriptional regulator (36). The appearance of Ash2L only in differentiated A404 cells but not in undifferentiated A404 cells was further confirmed by immunoblot analyses (Fig. 4B). Purified WDR5 protein complex from undifferentiated progenitor A404 cells as well as RA-treated A404 cells at different time points were immunoblotted with RbBP5, Ash2L, and WDR5 antibodies. As shown in Fig. 4B, RbBP5 was detected in the WDR5 protein complex from undifferentiated as well as differentiated A404 cells, whereas Ash2L only appeared in the WDR5 complex in the 24-h RA-treated A404 cells. As shown in supplemental Fig. S1B, Ash2L expression was unchanged during the differentiation of A404 cells. Our results showing that WDR5 always binds with RbBP5 during the differentiation of SMCs from A404 progenitor cells are consistent with reports that WDR5 complexes contain RbBP5 (37, 38). However, the functional significance of the observation that Ash2L only interacts with WDR5 at later stage of RA treatment during the differentiation of SMCs from A404 progenitor cells is still unknown. Taken together, these results suggest that WDR5 complex contains the core factors of MLL/SET1 and that the components of WDR complex are dynamically regulated during the differentiation of SMCs.
FIGURE 4.
WDR5 complex regulated H3K4me within the promoter loci of SMC-marker genes. A, distinct WDR5-interacting proteins in differentiated A404 versus undifferentiated A404 cells were identified by mass spectroscopy. A TAP-WDR5 expression plasmid or a TAP-empty plasmid was transfected into A404 progenitor cells. RA was added to induce SMC differentiation for 24 h. The distinct bands that only appeared in 24-h RA-treated cells on the silver-stained SDS-PAGE gel were subjected to linear trap quadrupole-Fourier transform mass spectrometry analyses as indicated by asterisks. B, the protein samples from more time point treatment as described in A were analyzed by Western blots using RbBP5, WDR5, and Ash2L antibodies. C, the enrichment of SRF and H3K4me1, H3K4me2, and H3K4me3 was determined using ChIP assays within SM α-actin and SM22α promoter loci in control or WDR5 siRNA-transfected A404 cells. D, enrichment of H3K4me1, H3K4me2, and H3K4me3 was detected by ChIP assays in day-15 EBs using primers targeting SM α-actin and SM22α promoters. Values represent the mean ± S.E. of three independent experiments.
To further test the hypothesis that the recruitment of WDR5 and its associated HKMT complex directs H3K4 methylation of SMC-marker genes and stimulates their transcriptional activation, we performed ChIP experiments to assess enrichment of H3K4me1, H3K4me2, and H3K4me3 as well as SRF binding within SMC-marker gene promoter loci in differentiated A404 cells. Results showed that WDR5 knockdown resulted in a significant decrease in H3K4me1 and H3K4me3 within the SM α-actin and SM22α promoter loci (Fig. 4C). At the same time, SRF binding within SM α-actin and SM22α promoter loci was also decreased around 50% (Fig. 4C). These effects were selective in that we observed no change in these histone marks and SRF binding within the c-fos gene promoter locus (supplemental Fig. S2A). Consistent with these results in A404 cells, we also demonstrated that WDR5+/− ES-derived EBs showed reduced H3K4me1 and H3K4me3 as compared with wild-type ES-derived EBs at day 15 within SMC-marker gene promoter loci (Fig. 4D) but not the c-fos promoter (supplemental Fig. S2B), the time period when we also observed impaired induction of SMC genes in our earlier studies in this model system (Fig. 1C). Taken together, these results indicate that knockdown of WDR5 can decrease H3K4me and the binding of the SMC lineage-specific transcription factors SRF/myocardin.
WDR5 Interacts with the Homeodomain Protein Pitx2 and Regulates SMC-marker Gene Expression in a Pitx2-dependent Manner
The preceding results provide evidence that WDR5 is required for maximum induction of SMC-marker genes during the early stages of SMC differentiation both in vitro and in vivo. Moreover, results of mass spectrometric proteomic analyses identified a number of WDR5 interactive proteins previously implicated in control of SMC differentiation. However, none of these proteins are obvious candidates for regulating selective recruitment of WDR5 and its associated HKMTs to SMC gene loci during the early stages of differentiation of multipotential progenitor cells into SMC lineages. As such, a critical unresolved question was how the widely expressed protein WDR5 was recruited to SMC lineage-specific gene loci.
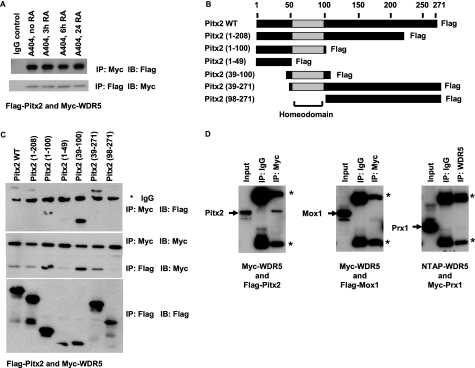
Pitx2 is a bicoid-type homeodomain protein that was previously shown by our laboratory to be required for initial SMC differentiation in vitro and in vivo (21). Of particular relevance to the present studies, Pitx2 was expressed in undifferentiated A404 cells and was further induced after RA treatment with levels peaking before the induction of SMC-marker genes. In addition, siRNA-induced suppression of Pitx2 inhibited the induction of SMC-marker gene with the greatest effects occurring during the early stages of SMC differentiation as reported previously and was further confirmed here (supplemental Fig. S3). We thus hypothesized that Pitx2 may interact with WDR5 and associated HKMTs and mediate H3K4 methylation of SMC gene loci including SMC-marker genes such as SM α-actin and SM-MHC. As an initial test of this hypothesis, we performed co-immunoprecipitation assays to determine whether WDR5 and Pitx2 interact within A404 cells and if this is altered in response to RA induced SMC differentiation of these cells. Of major interest, we observed significant binding of Pitx2 to WDR5 in A404 cells in the absence of RA treatment, although the interaction persisted after RA induced differentiation of cells into SMC (Fig. 5A). Indeed, this observation may explain why Pitx2 was not identified in our mass spectrometry analyses as WDR5 constitutively interacted with Pitx2 and this was not changed after RA-induced SMC differentiation. To further investigate these interactions, we used FLAG-tagged Pitx2 constructs that contained different deletions (Fig. 5B). Deletion of the homeodomain of Pitx2a resulted in near complete abolition of WDR5 binding to Pitx2. In contrast, deletion of either the N terminus or C terminus did not affect the WDR5 interaction (Fig. 5C). These results indicate that the homeodomain of Pitx2 is required for the interaction between Pitx2 and WDR5. Of interest, we observed no interaction between WDR5 and two other homeodomain proteins Prx1 and Mox1 known to be expressed in SMCs (Fig. 5D). These findings are consistent with our previous observation that Pitx2, but not the related homeodomain proteins Prx1 and Mox1, is required for the initial induction of SMC-marker genes during early stages of SMC development in vitro and in vivo (21). Moreover, results indicate that the interaction between Pitx2 and WDR5 is highly selective and requires the homeodomain.
FIGURE 5.
WDR5 bound with the homeodomain protein Pitx2. A, coimmunoprecipitation assays were performed in A404 cells cotransfected with Myc-WDR5 expression plasmid and wild-type FLAG-Pitx2 expression plasmid at different time points of RA treatment. IP, antibody that was used for immunoprecipitation; IB, antibody that was used for Western blot. B, shown is a schematic representation of Pitx2 and its deletion mutants. C, coimmunoprecipitation assays were performed in COS cells cotransfected with Myc-WDR5 expression plasmid and wild-type FLAG-Pitx2 expression plasmid or deletion mutants. The asterisk indicated antibody heavy chain. D, immunoprecipitated proteins from COS cells cotransfected with WDR5 expression plasmid and three homeodomain protein expression plasmids were analyzed by Western blot. They are: Myc-WDR5 and FLAG-Pitx2, Myc-WDR5 and FLAG-Mox1, and NTAT-WDR5 and Myc-Prx1 expression plasmids. Asterisks indicate antibody heavy/light chain.
WDR5 Is Recruited to SMC-marker Gene Loci in a Pitx2-dependent Manner
Thus far we have shown that Pitx2 and WDR5 interact within SMC progenitor cells and that siRNA-induced suppression of either Pitx2 (21) or WDR5 markedly inhibits SMC-marker gene expression. To determine whether WDR5 and Pitx2 are recruited to SMC-marker genes, we used ChIP analysis to measure the binding of these factors. Because of the unavailability of ChIP-grade WDR5 antibody, stable A404 cell lines containing either the Myc-tagged WDR5 expression plasmid or the empty vector were generated. These stable cell lines have a similar differentiation potential as well as comparable WDR5 expression as the original A404 cell line (Fig. 6A). The expression of Myc-WDR5 in stable cell lines was confirmed by Western blot assays (supplemental Fig. S4A). The ChIP assays using Pitx2 and Myc antibodies found that Pitx2 was bound to the SM α-actin and SM22α promoters but not with the c-fos gene promoter (supplemental Fig. S4B) in both stable cell lines, whereas WDR5 was only bound to those promoters in WDR5 stable cell line but not in the control cell line (Fig. 6B). We next performed sequential ChIP assays to test whether Pitx2 and WDR5 bound simultaneously to the same chromatic fragments. The chromatin was immunoprecipitated first with Pitx2 antibody and second with Myc-antibody against Myc-WDR5. Results showed significant enrichment of WDR5 in chromatin fragments containing Pitx2 within SMC-marker gene promoter loci (Fig. 6C). Similarly, the reciprocal sequential ChIP showed that Pitx2 was enriched in WDR5 chromatin fragments (Fig. 6C). These results indicate that Pitx2 and WDR5 can co-occupy the same physical locus and regulate SMC-marker gene transcription.
FIGURE 6.
WDR5 bound to SMC-marker gene promoter loci. A, stable cell lines containing either empty or Myc-WDR5 expression plasmid had comparable differentiation potential to the original A404 cell line. Control stable, stable A404 cell line containing empty pcDNA3.1 myc/his vector. B, WDR5 and Pitx2 bound to the SM α-actin and SM22α gene promoters as determined by ChIP assays. Stable cell lines containing either empty or Myc-WDR5 expression plasmids were processed to ChIP assays using Myc antibody and Pitx2 antibody. C, WDR5 and Pitx2 bound to the same physical chromatin within SM α-actin promoter. Sequential ChIP (1) assays were performed using Pitx2 antibody and Myc antibody in those stable cell lines as described in A.
To further test if WDR5 is recruited to the SMC-specific promoters through a Pitx2-dependent mechanism, we performed the following experiments. First, we determined the effects of Pitx2 knockdown on SMC gene expression as well as H3K4me levels within the SM α-actin and SM22α promoters by ChIP assays. Results showed that Pitx2 knockdown decreased SMC-marker gene expression (supplemental Fig. S3), consistent with our previously reported results (21). We extended those results in the present studies by showing that siRNA-induced knockdown of Pitx2 also decreased SRF binding and reduced H3K4me1 as well as H3K4me3 levels within the SM α-actin promoter (Fig. 7A). More interestingly, knockdown of Pitx2 in the A404 stable cell line stably transfected with Myc-tagged WDR5 expression plasmid greatly decreased the WDR5 binding within SM α-actin promoter (Fig. 7B). As such, knockdown of Pitx2 had virtually identical effects on H3K4 methylation and activation of SMC-marker genes as did knockdown of WDR5 in A404 SMC progenitor cells. Second, we tested if Pitx2 binding to the SM α-actin promoter was required for H3K4 methylation of this promoter during differentiation of A404 progenitor cells into SMC. For these studies, we generated stable A404 cell lines containing wild-type or TAATCT (ATTA) cis element-mutated SM α-actin promoter-enhancer transgenes (Fig. 7C) and then performed ChIP assays to assess changes in H3K4me in each respective transgene as compared with the endogenous SM α-actin gene locus. The ATTA cis element has been reported to be the binding site of Pitx2 (21). As shown in Fig. 7D, the wild-type and ATTA mutant stable cell lines had comparable capacity in terms of induction of endogenous SM α-actin expression, suggesting that the transgene does not influence the endogenous cell activity. However, the ATTA mutation nearly abolished RA-induced transgene promoter activity (supplemental Fig. S5). Notably, although the endogenous SM α-actin gene displayed enrichment of SRF as well as H3K4me modifications that were virtually identical between wild-type and mutant stable cell lines, mutation of ATTA markedly decreased H3K4me1 and H3K4me3 levels (Fig. 7E) as well as the SRF binding within the transgene promoter locus. These results indicate that an intact Pitx2 homeodomain cis-element is required for 1) mediating H3K4me modifications and SRF binding within the SM α-actin promoter locus and 2) for RA-induced increases in expression of this gene.
FIGURE 7.
Mutation of the Pitx2 binding site within the SM α-actin promoter nearly abolished RA-induced activation of the gene and decreased the H3K4me1 and H3K4me3 levels. A, siRNA induced suppression of Pitx2 in A404 cells decreased SRF binding and the level of H3K4me1 and H3K4me3 within the SM α-actin promoter. B, siRNA-induced knockdown of Pitx2 decreased WDR5 binding in stable cell line expressing Myc-WDR5 as described in Fig. 5B. C, the genotype of stable cell lines containing either wild-type or ATTA mutant SM α-actin promoter-enhancer-luciferase transgene was examined by semiquantitative PCR using luciferase primers. D, the induction of the endogenous SM α-actin gene in these two stable cell lines (as described in C was comparable with original A404 as determined by real-time RT-PCR. E, ATTA mutation decreased the H3K4me1 and H3K4me3 levels within the transgene promoter by ChIP assays. The enrichment of SRF and H3K4me1, H3K4me2, and H3K4me3 was determined by ChIP assays using primers specific for the transgene and the endogenous gene. Values represent the mean ± S.E. of three independent experiments. WT, stable cell line containing the wild-type SM α-actin promoter-enhancer-luciferase transgene; ATTA_Mut, stable cell line containing the TAATCT mutant SM α-actin promoter-enhancer-luciferase transgene.
Taken together, the preceding results support a model wherein the sequence-specific transcription factor Pitx2 mediates the binding of WDR5 and its associated HKMT complexes to SMC-marker gene promoters during the initial stages of differentiation of SMC from embryonic progenitor cells.
DISCUSSION
Locus- and Cell-selective Recruitment of WDR5 Is Mediated by the Sequence-specific Homeodomain Transcription Factor Pitx2 during Differentiation of SMC Lineage
Results of the present studies provide evidence for a novel mechanism for cell-selective recruitment of WDR5, a core factor of the MLL family of H3K4 HKMT, during SMC lineage determination from multipotent embryonic cells during early development. Of major interest, results support a model in which acquisition of a SMC-specific pattern of H3K4me of SMC-marker gene loci is initiated by binding of the mesodermally restricted homeodomain protein Pitx2 to these gene loci. Indeed, there are several lines of evidence that Pitx2 is a key rate-limiting factor in differentiation of stem cells or progenitor cells into differentiated SMC. First, we previously demonstrated that overexpression of Pitx2 in SMC progenitor cells induced SMC-marker gene expression (21), and Pitx2 gene knock-out mice showed markedly impaired differentiation of vascular SMC. Second, as reported by Bernstein et al. (1) the transcription start site of the Pitx2 gene is marked with bivalent histone modifications consisting of active H3K4me2 and repressive H3 lysine 27 methylation in pluripotent ES cells, which indicates that the Pitx2 gene is poised to be activated in ES cells and may be involved in controlling early developmental events including cell lineage determination. Indeed, the Pitx2 gene is also expressed in SMC progenitor A404 cells, which is consistent with our model that it initiates the binding of WDR5 and H3K4 HKMT complexes to SMC-marker gene loci during early SMC differentiation (21). Third, morpholino-induced suppression of WDR5 in X. laevis embryos (Fig. 2) was associated with a variety of selective SMC developmental defects including a lack of gut coiling and defects in gut patterning similar to defects observed in the Pitx2 null mice (8, 39). Fourth, results of the present studies show that Pitx2 and WDR5 interact and that binding of Pitx2 to the homeodomain binding site within the SM α-actin gene was required for maximal induction of this gene during differentiation of A404 cells into SMC lineages as well as for histone patterning. Fifth, although our data show that Pitx2 was required for H3K4me of SMC-marker gene promoters and recruitment of WDR5 to SMC promoters during SMC lineage determination, results of our proteomic mass spectrometric analyses also identified a number of additional WDR5 interactive proteins in differentiated SMCs that may cooperate with Pitx2 in modulating SMC gene expression. Taken together results support a novel mechanism as to how the ubiquitously expressed HKMT co-factor WDR5 can mediate cell lineage-specific epigenetic patterning during development. Moreover, such epigenetic patterning can regulate binding of downstream lineage-specific transcription factors and thereby regulate cellular differentiation.
Histone H3 Lysine 4 Methylation Regulates SMC Gene Transcription
Our results also provide evidence that regulation of H3K4me is at least one of the important mechanisms by which WDR5 regulates SMC gene expression. H3K4me has been linked to transcriptional activation in a variety of species. Increasing evidence suggests that H3K4me acts through the recruitment of effector proteins, which in turn execute specific downstream functions on the chromatin template. For example, CHD1 binds with H3K4me through the chromodomain and links H3K4me with SAGA- and SLIK-dependent acetylation (40, 41). An ISWI-containing ATP-dependent chromatin-remodeling complex mediates a direct association with H3K4me through a subunit called bromodomain and plant homeodomain finger transcription factor (42). These results suggest that H3K4me is directly coupled to further chromatin modification mechanisms necessary to execute specific biological functions. Indeed, genome-wide ChIP-sequence assays demonstrate that H3K4me3 is required for subsequent acetylation and gene activation. Knockdown of WDR5, which down-regulates H3K4me levels, significantly decreases histone acetylation upon histone deacetylase inhibitor treatment (43). Moreover, emerging evidence shows that H3K4me plays an important role in initiating or priming lineage-specific genes for activation during cell lineage determination by interacting with tissue-specific or selective transcription factors. For example, H3K4me2 can interact with Pax7 within the chromatin to initiate myogenesis (11). Previous results from our laboratory also showed that the H3K4me2 can interact with SMC-selective transcription factor myocardin to induce SMC differentiation (19). However, a critical distinguishing feature of our previous studies is that we showed that H3K4me2 enrichment of SMC gene loci was upstream of CArG-SRF-myocardin binding, which is required for transcriptional activation of virtually all SMC-marker genes. As such, results suggest that H3K4me serves as an upstream signal for gene activation during SMC lineage determination and that this process is initiated via Pitx2 binding to its cis element and subsequent recruitment of WDR5 and its associated MLLs. Because several MLL family members might be involved in the modification of H3K4me mediated by WDR5, further investigation of which specific enzymes contribute to the H3K4me modification in SMCs will be important for future study.
Our current results show that the level of H3K4me1 and H3K4me3, but not of H3K4me2, is significantly decreased within SMC-marker gene promoter by knockdown of WDR5 during the differentiation of SMC from progenitor cells or pluripotent ES cells. The transition between H3K4me1, H3K4me2, and H3K4me3 states is a regulated event, and it has been reported that different methylation states are associated with distinct regulatory outcomes (44, 45). In vitro binding experiments by Wysocka et al. (8) have shown that WDR5 binds with either unmodified or dimethylated H3 lysine 4 histone tails. They also showed that WDR5 is important for the H3K4me2 to H3K4me3 conversion by the H3K4 HKMTs, indicating that it participates in both reading and writing the H3K4 methyl mark. However, if and how WDR5 contributes to the modification of H3K4me2 is unknown. One possibility is that H3K4me2 acts as a reservoir for H3K4me1 and H3K4me3 and thus keeps quite stable within gene promoters. The other possibility is that other H3K4 HKMTs contribute to the H3K4me2 modification, and knockdown WDR5 does not influence the level of H3K4me2. In mammals, there are at least 10 known or predicted H3K4 HKMTs. Except MLL family members, there are other H3K4 HKMTs including ASH1, SET7/9, SMYD3, and Meisets (46) that do not contain WDR5 as a core factor and might contribute to the H3K4me2 modification within the SMC-marker gene promoter loci.
Evidence That WDR5 Mediates Chromatin Remodeling and Transcriptional Activation during Cell Lineage Determination
Results of the present studies showed that WDR5 interacted with ATAC2 (Fig. 4), which is a weaker histone acetyltransferases protein contained within human histone deacetylase complexes. Despite many recent advances in the understanding of WDR5 and its role within MLLs, evidence is emerging that WDR5 also functions as a subunit in other nuclear complexes. For example, Thompson et al. (47) found that WDR5 is contained within a human chromodomain helicase DNA-binding protein 8 (CHD8)-containing ATP-dependent chromatin remodeling complex. Moreover, Herring and co-workers (48) reported that CHD8 interacted with SRF and modulated the function of SRF-dependent SMC-marker genes. Of interest, we report herein that WDR5 is contained within human ATAC2 histone acetyltransferase complexes, results consistent with two previous reports (34, 35). However, the function of WDR5 within these complexes is unknown. It is interesting to speculate that the interaction of WDR5 with ATAC2 might further stabilize its association with selective gene loci as well as contribute to the propagation of activating histone modifications including H4 acetylation and H3K4me3 (49–52). Alternatively, WDR5 may serve to directly target chromatin-modifying and chromatin-remodeling complexes (such as CHD8) to chromatin substrates through its association with sequence-specific and/or lineage-specific transcription factors.
In summary, our data support a model in which acquisition of a SMC-specific pattern of H3K4me of SMC-marker gene loci during early development of SMC lineage is initiated by binding of the mesodermally restricted homeodomain protein Pitx2 to these gene loci and subsequent Pitx2-dependent binding of WDR5. WDR5 in turn mediates binding of H3K4 HKMT, which will modify the tail of histone H3 lysine 4. Whereas these events alone are not sufficient for transcriptional activation of SMC-marker genes, we postulate that they make these gene loci permissive for binding of SRF and potent SMC-selective SRF co-activators such as myocardin, which mediate additional histone modifications including histone hyperacetylation and profound and coordinate activation of the SMC differentiation program. In addition, of broad significance, our studies provide a novel mechanism to explain how histone-modifying enzymes and their co-factors, despite being globally expressed in virtually all cell types, can mediate not only locus-selective but also cell-selective epigenetic patterning critical for cell lineage determination and coordinate activation of cell selective genes during development.
Supplementary Material
Acknowledgments
We thank Mary E. McCanna and Rupande Tripathi for technical assistance. We are indebted to Nicholas E. Sherman for mass spectrometry analysis in the W. M. Keck Biomedical Mass Spectrometry Core at University of Virginia.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 HL19242, R01 HL57353, and R01 HL 38854 (to G. K. O.). This work was also supported by American Hearth Association Grant 09POST2250363 (to Q. G.). Work in the laboratory of P. T. and N. T. was supported by the CNRS, the University of Bordeaux 2, and the Association Francaise contre les Myopathies.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- ESC
- embryonic stem cell
- H3K4me
- histone H3 lysine 4 methylation
- MLL
- mixed lineage leukemia
- HKMT
- H3K4 methyltransferase
- SMC
- smooth muscle cell
- EB
- embryoid body
- SRF
- serum response factor
- SM-MHC
- smooth muscle myosin heavy chain
- bFGF
- basic FGF
- RA
- retinoic acid.
REFERENCES
- 1. Bernstein B. E., Mikkelsen T. S., Xie X., Kamal M., Huebert D. J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., Jaenisch R., Wagschal A., Feil R., Schreiber S. L., Lander E. S. (2006) Cell 125, 315–326 [DOI] [PubMed] [Google Scholar]
- 2. Mikkelsen T. S., Ku M., Jaffe D. B., Issac B., Lieberman E., Giannoukos G., Alvarez P., Brockman W., Kim T. K., Koche R. P., Lee W., Mendenhall E., O'Donovan A., Presser A., Russ C., Xie X., Meissner A., Wernig M., Jaenisch R., Nusbaum C., Lander E. S., Bernstein B. E. (2007) Nature 448, 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan G., Tian S., Nie J., Yang C., Ruotti V., Wei H., Jonsdottir G. A., Stewart R., Thomson J. A. (2007) Cell Stem Cell 1, 299–312 [DOI] [PubMed] [Google Scholar]
- 4. Zhao X. D., Han X., Chew J. L., Liu J., Chiu K. P., Choo A., Orlov Y. L., Sung W. K., Shahab A., Kuznetsov V. A., Bourque G., Oh S., Ruan Y., Ng H. H., Wei C. L. (2007) Cell Stem Cell 1, 286–298 [DOI] [PubMed] [Google Scholar]
- 5. Lee J. H., Tate C. M., You J. S., Skalnik D. G. (2007) J. Biol. Chem. 282, 13419–13428 [DOI] [PubMed] [Google Scholar]
- 6. Lee J. H., Skalnik D. G. (2005) J. Biol. Chem. 280, 41725–41731 [DOI] [PubMed] [Google Scholar]
- 7. Milne T. A., Hughes C. M., Lloyd R., Yang Z., Rozenblatt-Rosen O., Dou Y., Schnepp R. W., Krankel C., Livolsi V. A., Gibbs D., Hua X., Roeder R. G., Meyerson M., Hess J. L. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wysocka J., Swigut T., Milne T. A., Dou Y., Zhang X., Burlingame A. L., Roeder R. G., Brivanlou A. H., Allis C. D. (2005) Cell 121, 859–872 [DOI] [PubMed] [Google Scholar]
- 9. Steward M. M., Lee J. S., O'Donovan A., Wyatt M., Bernstein B. E., Shilatifard A. (2006) Nat. Struct. Mol. Biol. 13, 852–854 [DOI] [PubMed] [Google Scholar]
- 10. Trievel R. C., Shilatifard A. (2009) Nat. Struct. Mol. Biol. 16, 678–680 [DOI] [PubMed] [Google Scholar]
- 11. McKinnell I. W., Ishibashi J., Le, Grand F., Punch V. G., Addicks G. C., Greenblatt J. F., Dilworth F. J., Rudnicki M. A. (2008) Nat. Cell Biol. 10, 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rampalli S., Li L., Mak E., Ge K., Brand M., Tapscott S. J., Dilworth F. J. (2007) Nat. Struct. Mol. Biol. 14, 1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demers C., Chaturvedi C. P., Ranish J. A., Juban G., Lai P., Morle F., Aebersold R., Dilworth F. J., Groudine M., Brand M. (2007) Mol. Cell 27, 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mo R., Rao S. M., Zhu Y. J. (2006) J. Biol. Chem. 281, 15714–15720 [DOI] [PubMed] [Google Scholar]
- 15. Sierra J., Yoshida T., Joazeiro C. A., Jones K. A. (2006) Genes Dev. 20, 586–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Owens G. K. (1995) Physiol. Rev. 75, 487–517 [DOI] [PubMed] [Google Scholar]
- 17. Owens G. K., Kumar M. S., Wamhoff B. R. (2004) Physiol. Rev. 84, 767–801 [DOI] [PubMed] [Google Scholar]
- 18. Manabe I., Owens G. K. (2001) Circ. Res. 88, 1127–1134 [DOI] [PubMed] [Google Scholar]
- 19. McDonald O. G., Wamhoff B. R., Hoofnagle M. H., Owens G. K. (2006) J. Clin. Invest. 116, 36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDonald O. G., Owens G. K. (2007) Circ. Res. 100, 1428–1441 [DOI] [PubMed] [Google Scholar]
- 21. Shang Y., Yoshida T., Amendt B. A., Martin J. F., Owens G. K. (2008) J. Cell Biol. 181, 461–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mortensen R. M., Conner D. A., Chao S., Geisterfer-Lowrance A. A., Seidman J. G. (1992) Mol. Cell. Biol. 12, 2391–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sinha S., Hoofnagle M. H., Kingston P. A., McCanna M. E., Owens G. K. (2004) Am. J. Physiol. Cell Physiol. 287, C1560–C1568 [DOI] [PubMed] [Google Scholar]
- 24. Thyagarajan B., Olivares E. C., Hollis R. P., Ginsburg D. S., Calos M. P. (2001) Mol. Cell. Biol. 21, 3926–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshida T., Gan Q., Shang Y., Owens G. K. (2007) Am. J. Physiol. Cell Physiol. 292, C886–C895 [DOI] [PubMed] [Google Scholar]
- 26. Yoshida T., Gan Q., Owens G. K. (2008) Am. J. Physiol. Cell Physiol. 295, C1175–C1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gan Q., Yoshida T., Li J., Owens G. K. (2007) Circ. Res. 101, 883–892 [DOI] [PubMed] [Google Scholar]
- 28. Barillot W., Tréguer K., Faucheux C., Fédou S., Thézé N., Thiébaud P. (2008) Dev. Dyn. 237, 3373–3386 [DOI] [PubMed] [Google Scholar]
- 29. McBurney M. W. (1993) Int. J. Dev. Biol. 37, 135–140 [PubMed] [Google Scholar]
- 30. Sinha S., Wamhoff B. R., Hoofnagle M. H., Thomas J., Neppl R. L., Deering T., Helmke B. P., Bowles D. K., Somlyo A. V., Owens G. K. (2006) Stem Cells 24, 1678–1688 [DOI] [PubMed] [Google Scholar]
- 31. Stoller J. Z., Huang L., Tan C. C., Huang F., Zhou D. D., Yang J., Gelb B. D., Epstein J. A. (2010) Exp. Biol. Med. (Maywood) 235, 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weiskirchen R., Gressner A. M. (2000) Biochem. Biophys. Res. Commun. 274, 655–663 [DOI] [PubMed] [Google Scholar]
- 33. Chang D. F., Belaguli N. S., Chang J., Schwartz R. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suganuma T., Gutiérrez J. L., Li B., Florens L., Swanson S. K., Washburn M. P., Abmayr S. M., Workman J. L. (2008) Nat. Struct. Mol. Biol. 15, 364–372 [DOI] [PubMed] [Google Scholar]
- 35. Guelman S., Kozuka K., Mao Y., Pham V., Solloway M. J., Wang J., Wu J., Lill J. R., Zha J. (2009) Mol. Cell. Biol. 29, 1176–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wysocka J., Myers M. P., Laherty C. D., Eisenman R. N., Herr W. (2003) Genes Dev. 17, 896–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dou Y., Milne T. A., Ruthenburg A. J., Lee S., Lee J. W., Verdine G. L., Allis C. D., Roeder R. G. (2006) Nat. Struct. Mol. Biol. 13, 713–719 [DOI] [PubMed] [Google Scholar]
- 38. Patel A., Dharmarajan V., Vought V. E., Cosgrove M. S. (2009) J. Biol. Chem. 284, 24242–24256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campione M., Steinbeisser H., Schweickert A., Deissler K., van Bebber F., Lowe L. A., Nowotschin S., Viebahn C., Haffter P., Kuehn M. R., Blum M. (1999) Development 126, 1225–1234 [DOI] [PubMed] [Google Scholar]
- 40. Pray-Grant M. G., Daniel J. A., Schieltz D., Yates J. R., 3rd, Grant P. A. (2005) Nature 433, 434–438 [DOI] [PubMed] [Google Scholar]
- 41. Sims R. J., 3rd, Chen C. F., Santos-Rosa H., Kouzarides T., Patel S. S., Reinberg D. (2005) J. Biol. Chem. 280, 41789–41792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wysocka J., Swigut T., Xiao H., Milne T. A., Kwon S. Y., Landry J., Kauer M., Tackett A. J., Chait B. T., Badenhorst P., Wu C., Allis C. D. (2006) Nature 442, 86–90 [DOI] [PubMed] [Google Scholar]
- 43. Wang Z., Zang C., Cui K., Schones D. E., Barski A., Peng W., Zhao K. (2009) Cell 138, 1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huebert D. J., Kamal M., O'Donovan A., Bernstein B. E. (2006) Methods 40, 365–369 [DOI] [PubMed] [Google Scholar]
- 45. Bernstein B. E., Kamal M., Lindblad-Toh K., Bekiranov S., Bailey D. K., Huebert D. J., McMahon S., Karlsson E. K., Kulbokas E. J., 3rd, Gingeras T. R., Schreiber S. L., Lander E. S. (2005) Cell 120, 169–181 [DOI] [PubMed] [Google Scholar]
- 46. Ruthenburg A. J., Allis C. D., Wysocka J. (2007) Mol. Cell 25, 15–30 [DOI] [PubMed] [Google Scholar]
- 47. Thompson B. A., Tremblay V., Lin G., Bochar D. A. (2008) Mol. Cell. Biol. 28, 3894–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rodenberg J. M., Hoggatt A. M., Chen M., Touw K., Jones R., Herring B. P. (2010) Am. J. Physiol. Cell Physiol. 299, C1058–C1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nie Z., Yan Z., Chen E. H., Sechi S., Ling C., Zhou S., Xue Y., Yang D., Murray D., Kanakubo E., Cleary M. L., Wang W. (2003) Mol. Cell. Biol. 23, 2942–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakamura T., Mori T., Tada S., Krajewski W., Rozovskaia T., Wassell R., Dubois G., Mazo A., Croce C. M., Canaani E. (2002) Mol. Cell 10, 1119–1128 [DOI] [PubMed] [Google Scholar]
- 51. Bultman S. J., Gebuhr T. C., Pan H., Svoboda P., Schultz R. M., Magnuson T. (2006) Genes Dev. 20, 1744–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dou Y., Milne T. A., Tackett A. J., Smith E. R., Fukuda A., Wysocka J., Allis C. D., Chait B. T., Hess J. L., Roeder R. G. (2005) Cell 121, 873–885 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.