Abstract
Integrin-linked kinase (ILK) is one of the few evolutionarily conserved focal adhesion proteins involved in diverse cell adhesion-dependent physiological and pathological responses. Despite more than a decade of studies and extensive literature, the kinase function of ILK is controversial. ILK contains a highly degraded kinase active site but it has been argued that ILK may be an unusual manganese (Mn)-dependent serine-threonine kinase that targets specific substrates such as glycogen synthase kinase-3β (GSK-3β). In this study, we have tackled this issue by a systematic bottom-up biochemical, proteomic, structural, and thermodynamic analysis of ILK. We show that recombinant ILK from either bacteria or mammalian cells exhibits no kinase activity on GSK-3β in the presence of either Mn2+ or the conventional kinase co-factor Mg2+. A comprehensive and unbiased whole cell-based kinase assay using entire mammalian CG-4 and C2C12 cell lysate did not identify any specific ILK substrates. High resolution crystallographic structure analysis further confirmed that the Mn-bound ILK adopts the same pseudo active site conformation as that of the Mg-bound ILK. More importantly, thermodynamic analysis revealed that the K220M mutation, previously thought to inactivate ILK by disrupting ATP binding, significantly impairs the structural integrity and stability of ILK, which provides a new basis for understanding how this mutation caused renal agenesis, a failure of fetal kidney development. Collectively, our data provide strong evidence that ILK lacks intrinsic kinase function. It is a bona fide pseudokinase that likely evolved from an ancestral catalytic counterpart to act as a distinct scaffold to mediate protein-protein interactions during focal adhesion assembly and many other cellular events.
Keywords: Cell Adhesion, Crystal Structure, Enzyme Catalysis, Integrin, Protein Kinases, Focal Adhesion, ILK
Introduction
The adhesion of cells to the extracellular matrix (ECM)3 is primarily mediated by heterodimeric integrin transmembrane receptors. Upon activation, the integrin extracellular domain adheres to the ECM by binding to numerous ligands such as fibronectin and fibrinogen. However, for cells to firmly adhere to the ECM, integrins must connect to the backbone of the cell, the actin cytoskeleton, via its cytoplasmic tails (CTs) (1–2). Such connection is mediated by the supramolecular focal adhesion (FAs) complex. The reassembly/disassembly of FAs can lead to dynamic cell adhesion processes such as cell migration and spreading, which are fundamental for a variety of physiological responses. Over the past decades, major effort has been made to search for proteins involved in FA assembly and regulation. Integrin-linked kinase (ILK) was discovered in 1996 as a binding partner of integrin β CTs (3) and was found to critically regulate FAs (4). Genetic and genomic analyses have established that ILK is one of the few essential and evolutionarily conserved proteins coupling integrins to actin (5–7), and its dysregulation has been linked to major human diseases such as heart failure (8–10) and cancer (11). ILK has a molecular mass of ∼51 kDa containing an ankyrin repeat domain at the N terminus (3, 12–13) and a kinase-like domain (KD) at the C terminus (3, 14). The latter led to the classification of ILK as a serine-threonine kinase upon its discovery (3). Since then, it has been widely thought that the kinase activity of ILK may be involved in integrin signaling and many other cellular activities (15). However, ILK has some significant variations in the putative catalytic site, suggesting that ILK may be a pseudokinase (16–18). Consistently, extensive genetic analyses demonstrated that the kinase activity of ILK is not required for normal tissue development and function (5–7, 19–21). By contrast, a number of biochemical and cell-based analyses did report that ILK was capable of directly phosphorylating diverse substrates including a generic substrate myelin basic protein and physiological targets including integrin β1 CT (3), myosin light chain kinase LC20 (22), β-parvin (23), cell survival kinase AKT/PKB (24), and glycogen synthase kinase-3β (GSK-3β) (24–26). Thus, the kinase function of ILK has remained as a controversial but important topic in integrin signaling and cell biology (15, 18, 27).
Approximately 10% of the human kinome encodes for proteins that lack one or more catalytic residues, suggesting a class of pseudokinases (16). While multiple laboratories have independently confirmed some representative pseudokinases such as STRADα (28), VRK3 (17), and ROP2 (29), a few previously proposed pseudokinases have been recently shown to still exhibit kinase activity possibly by some alternative structural mechanisms (30). For example, WNK kinase lacks a conserved catalytic lysine residue that is spatially compensated for by another nearby lysine that promotes catalysis (31). Therefore, combined structural and functional analyses should be performed to definitively define a pseudokinase. In the case of ILK, a high resolution structural analysis has recently shown that its catalytic site is highly degraded, which cannot be compensated for by any alternative residues (14). Biochemical characterizations further demonstrated that either bacteria-purified recombinant ILK or endogenous ILK was incapable of phosphorylating integrin β CTs, parvin, AKT, and the generic kinase substrate myelin basic protein, and it was suggested that the ILK purported activity might be due to heterogeneous components present in the partially purified ILK materials or indirect effects from cell-based experiments (14). Consensus seems to emerge from the perspective of both ILK function (18) and enzymology (30, 32) that ILK is a pseudokinase. Nevertheless, controversy continues with growing studies still indicating that ILK is a kinase (26, 33–36). In particular, a recent biochemical study (26) reported that ILK is a manganese (Mn)-dependent serine-threonine kinase that phosphorylates GSK-3β. This is completely inconsistent with the recent genetic and mutagenesis studies, which showed that ILK harboring a putative hyperactive mutation (S343D) or a putative kinase inactive mutation (K220A; K220M) had no effect on GSK-3β phosphorylation (21). It also contradicts the structural data that revealed a severely degraded catalytic core in ILK incapable of conferring the phosphor-transfer reaction, as is required for a bona fide GSK-3 kinase such as AKT (37).
To gain a clear insight into the function of ILK, we have undertaken a rigorous investigation of ILK by using a multidisciplinary approach. Here we show that either ILK expressed in bacteria or mammalian cells did not exhibit kinase activity on GSK-3β. Moreover, a comprehensive whole cell-based search did not identify any potential substrates for ILK. Using structural and thermodynamic approaches, we also found that the conserved ATP-binding lysine 220, which was previously thought to perform a catalytic function, plays a vital role in the structural integrity and stability of ILK, which explains why the mutation caused a defect in kidney development (21). Our results thus provide conclusive evidence that ILK is a bona fide pseudokinase and points to a new direction for elucidating the role of ILK in diverse physiological and pathological processes.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Anti-phospho-GSK-3β (Ser-9) and anti-AKT antibodies, and glutathione S-transferase (GST)-fused GSK-3 α/β (Ser-21/9) crosstide protein were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Anti-ILK and anti-GST antibodies and anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies were purchased from EMD Chemicals, Inc. (Gibbstown, NJ). Recombinant GST-fused active full-length AKT1 was purchased from SignalChem (Richmond, BC, Canada). Recombinant affinity purified Myc-tagged human full-length ILK was purchased from OriGene (Rockville, MD). Recombinant p38α kinase was purchased from Millipore (Billerica, MA). All restriction enzymes were purchased from New England Biolabs (Ipswich, MA).
Expression Plasmids and Recombinant Proteins
The bicistronic coexpression plasmid for the human ILK KD (residues 183–452)/α-parvin CH2 complex (residues 248–372) was generated using a pST39 vector (38) as previously described (14). Two surface-exposed cysteine residues of ILK KD (Cys-346 and Cys-422) were substituted by serine residues (Ser-346 and Ser-422) to increase the protein solubility for x-ray structural analysis. The His-tagged recombinant ILK KD/CH2 complex was expressed in Escherichia coli and the protein complex was purified from the bacterial cell lysate by Ni-affinity column, followed by HiLoad 16/60 Superdex75 gel filtration and Resource-S cation-exchange chromatography columns (all from GE Healthcare, Piscataway, NJ), as previously described (14). The ILK KD mutant (K220M or K220A)/CH2 complex was generated by site-directed mutagenesis using QuikChange Site-directed Mutagenesis kit (Stratagene, La Jolla, CA) with appropriate primer sets. The mutant protein was expressed in E. coli and purified as for the wild type. The N-terminal hexahistidine tag was removed by thrombin cleavage. The coexpression plasmid for full-length ILK (residues 1–452)/maltose-binding protein (MBP)-fused PINCH LIM1–2 (residues 1–127) complex was also generated as previously described (14). The ILK/MBP-PINCH LIM1–2 complex was expressed in E. coli, and the protein complex was purified by MBPTrap affinity column, followed by HiLoad 16/60 Superdex200 gel filtration chromatography column (both from GE Healthcare). The hexahistidine-tagged α-parvin CH2 protein (residues 248–372) was expressed and purified as previously described (39). The α-parvin CH2 gene was also subcloned into a pGEX4T1 vector, and the glutathione S-transferase (GST)-fused CH2 protein was expressed in E. coli, and purified by glutathione-Sepharose 4B resin (GE Healthcare) according to the manufacturer's protocol. The PINCH LIM1–2 (residues 1–127) was also subcloned into a pGEX4T1 vector, and the GST-fused PINCH LIM1–2 protein was expressed in E. coli and purified by glutathione-Sepharose 4B resin according to the manufacturer's protocol. All the DNA constructs were verified by sequencing analysis.
In Vitro Pull-down Assays
Each purified GST-fused α-parvin CH2 or GST (5 μg each) was immobilized on 40 μl of glutathione-Sepharose 4B (GE Healthcare) and equilibrated in pull-down binding buffer consisting of phosphate-buffered saline, 10% (v/v) glycerol, 0.1% (v/v) Nonidet P-40, and Complete EDTA-free Protease Inhibitor (Roche, Indianapolis, IN). The mammalian Myc-tagged ILK was added in each affinity bead by concentration-dependent addition (1 μg or 3 μg), and the reaction mixtures were incubated at 4 °C by a rotor for 2 h. The beads were extensively washed three times using binding buffer. The bound proteins were eluted in 30 μl of 20 mm reduced glutathione in binding buffer and resolved by SDS-PAGE. The samples were transferred onto a PVDF membrane (Millipore) for Western blot analysis. The bound proteins were probed with appropriate antibodies and detected using Pierce ECL Western blotting Substrate (Thermo SCIENTIFIC).
The Phosphorylation Assay of GSK-3β
Non-radioactive in vitro phosphorylation assay of GSK-3β was performed according to a previous report (26) by Western blot analysis with phospho-specific antibody. Briefly, the kinase assay was carried out in 50 μl of kinase reaction mixture consisting of 2 μg of GST-fused GSK-3 α/β crosstide, 1 mm ATP, 20 mm MgCl2 (or MnCl2), Complete EDTA-free Protease Inhibitor (Roche), 50 mm Hepes, pH 7.5, 85 mm KCl, 10 mm EGTA, 0.1% (v/v) Tween 80, 10 mm β-glycerophosphate, 1 mm Na3VO4, and 5 mm dithiothreitol. The bacterial or Myc-tagged mammalian ILK proteins were added in the reaction mixtures in a dose-dependent manner from 0.8 to 8 μg or from 0.1 to 2.5 μg, respectively. For the kinase inhibition studies by CH2, the quantity of mammalian ILK was set to 0.5 μg for each reaction. For the positive control for phosphorylation of GSK-3β, GST-fused active AKT protein (0.1 μg) was used. The reaction mixtures were incubated at 30 °C for 30 min and terminated by adding SDS sample buffer. The reaction mixtures were resolved by SDS-PAGE, transferred onto a PVDF membrane, and blocked by blocking buffer containing 5% (w/v) nonfat dry milk powder (Bio-Rad). Phosphorylated GSK-3β proteins were detected by anti-phospho-GSK-3β (Ser-9)-specific antibody according to the manufacturer's instruction. Each kinase assay was repeated at least two times.
In Vitro Radioactive Kinase Assays
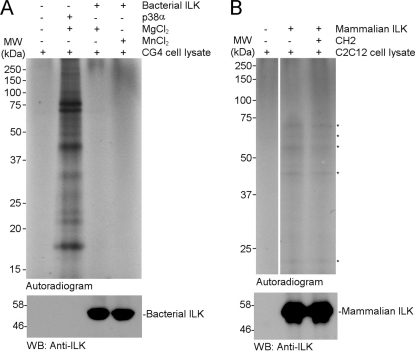
Kinase-inactive CG-4 (and C2C12) cell lysate for the kinase assays was prepared as follows. Briefly, cells were lysed in Nonidet P-40 buffer (50 mm Tris-HCl, pH 7.8, 150 mm NaCl, 1% (v/v) Nonidet P-40, 1 mm PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin). The cell lysate was treated at a concentration of 2 mg/ml with 20 mm 5′-4-fluorosulfonylbenzoyladenosine (FSBA) solubilized in DMSO for 1 h at 30 °C to inactivate endogenous kinases, and designated as kinase-inactive cell lysate. The lysate was then diluted 1:5 with Nonidet P-40 buffer without protease inhibitors and desalted using Millipore Amicon Ultra filtration columns with a 10 kDa molecular mass cutoff. Following concentration, the lysate concentration was adjusted to 4 mg/ml with Nonidet P-40 buffer and diluted 1:2 with 2× kinase assay buffer (40 mm MOPS pH 7.2, 50 mm β-glycerophosphate, 10 mm EGTA, 2 mm Na3VO4, 2 mm DTT, 50 mm MgCl2 (or MnCl2), 400 μm cold ATP, 2.5 μCi of [32P]ATP). Each kinase assay was performed in kinase assay buffer containing 100 μg of kinase-inactive lysate and each kinase, as indicated in the legend of Fig. 3. The kinase reaction mixtures were incubated at 30 °C for 3 h, terminated by addition of 5× SDS sample buffer, and analyzed by SDS-PAGE followed by autoradiography.
FIGURE 3.
Proteomic analysis of ILK kinase function. A, in vitro radioactive kinase assay with bacterial ILK. Endogenous kinase activity from CG4 cell lysate was blocked as described under “Experimental Procedures.” A kinase assay buffer containing [32P]ATP was added to 100 μg of kinase-inactive lysate, followed by the addition of a kinase (or buffer only without kinase for control). After SDS-PAGE, autoradiography was performed. Lane 1 from left, control (without bacterial ILK); lane 2, 500 ng of p38α; lane 3, 2 μg of bacterial ILK with Mg2+ as a cofactor; lane 4, 2 μg of bacterial ILK with Mn2+ as a cofactor. Similar results were obtained with C2C12 cell lysate (data not shown). B, in vitro radioactive kinase assay with mammalian ILK. C2C12 cell lysate treatment, kinase assay, and autoradiography were performed as above and described under “Experimental Procedures.” Lane 1 from left, control (without mammalian ILK); lane 2, 1 μg of mammalian ILK; lane 3, 1 μg of mammalian ILK in the presence of bacterially purified His-tagged parvin CH2 protein for kinase inhibition analysis (1 μg). The CH2 protein was preincubated with mammalian ILK for 10 min prior to performing the kinase assay. Notice that several 32P-incorporated lysate substrates (marked with asterisks) were detected by autoradiography; however, the band intensities are almost at the background level indicating some low level contamination by some unknown kinase as also found in Fig. 1 and supplemental Figs. S1 and S3. This is confirmed in lane 3 where this activity was not inhibited by CH2 that strongly binds to the p + 1 substrate site of ILK (14).
Quantitative ATP Binding Studies
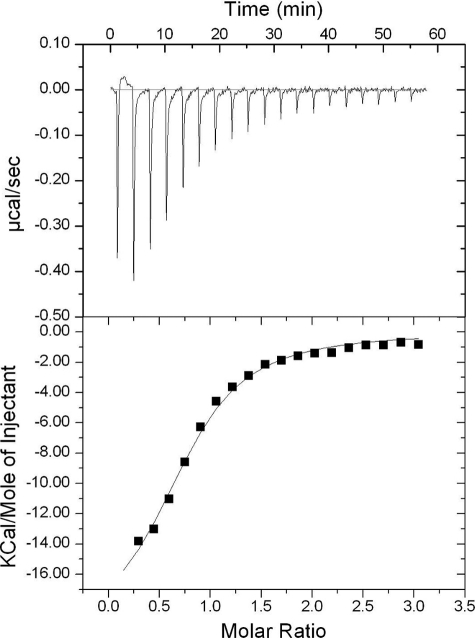
ATP-binding to ILK KD was measured by isothermal titration calorimetry with an iTC200 instrument from MicroCal (Northampton, MA). Typically, 300 μm of ATP solution containing 1.5 mm MgCl2 was titrated into 30 μm solution of the purified ILK KD/CH2 complex. The experiments were carried out at 25 °C in a buffer consisting of 10 mm Hepes, pH 7.5, 100 mm NaCl, 3% (v/v) glycerol, 0.03% (w/v) β-octyl glucoside, and 0.5 mm tris (2-carboxyethyl) phosphine hydrochloride. All solutions were degassed before being loaded into the cell and the titration syringe. The mixing speed was set at 800 rpm. The reference power was set to 6 μcal/s. The binding experiment was carried out in duplicate. The isothermal titration curves were recorded and analyzed using the ORIGIN software, as implemented in the iTC200 instrument, with a one site binding model.
Fluorescence Measurements
Fluorescence measurements were made using a QM-6SE (PTI, London, Ontario) spectrofluorometer. Sample temperature was controlled by a Peltier cell holder maintaining the heating rate of 60 °C/h. All intrinsic tryptophan residue fluorescence measurements were performed with excitation at 290 nm, emission at 335 nm and Glan polarizers at magic angle conditions.
Circular Dichroism
CD measurements were performed with a Jasco model J-715 (Jasco Inc., Easton, MD) spectropolarimeter equipped with a computer controlled Peltier sample holder. All measurements were taken in a 1 mm pathlength cell and corrected for solvent signal. The thermal stability of proteins was monitored at 222 nm by heating samples at a rate of 60 °C/h over the range 10–70 °C. The ellipticity was recorded at 0.5 °C intervals with time constant of 16 s.
Crystallization and Structure Refinement
Co-crystals of the ILK KD/CH2 in complex with manganese and ATP were grown at 4 °C by the hanging-drop vapor diffusion method in drops containing a 1:1 (v/v) ratio of protein solution (∼0.2 mm) and reservoir solution consisting of 50 mm Bis-Tris propane, pH 6.8, 12.5% (w/v) polyethylene glycol 5000 mono methyl ether, and 5% (v/v) 1-propyl alcohol. X-ray diffraction data were collected at 100 K using a Rigaku MicroMax-007HF microfocusing rotating anode generator (λ = 1.5418 Å), a Saturn 944+ CCD detector, and X-stream 2000 cooling system (The Case Pharmacology x-ray Facility, Cleveland, OH). The co-crystals of the MnATP bound form of the ILK KD/CH2 complex were isomorphous to those of the MgATP-bound form (14), and contained one ILK KD/CH2 complex bound to each manganese and ATP per asymmetric unit. The data were processed and scaled using the d*TREK (40). The atomic coordinates of the ILK KD/CH2 in complex with magnesium/ATP (Protein Data Bank code 3KMW) were used after omitting ATP, magnesium, and solvent molecules as the starting model for the refinement. The initial model was subjected to rigid-body refinement, followed by simulated annealing, positional and B-factor refinement, supplemented in CNS (41) and Refmac5 (42). Manganese and ATP were included into a region of 1Fo-Fc electron density maps contoured at 4σ observed in a cleft between N- and C-lobes of the ILK KD structure. Water molecules were added in the final rounds of the structure refinement by computing 1Fo-Fc density maps (>3σ) and 2Fo-Fc density maps (>1σ), where feasible hydrogen bonds were observed. Model building was carried out by COOT (43) and TURBO-FRODO (44). All the residues in the complex structure lie within allowed regions of the Ramachandran plot as defined by PROCHECK (45). The topology files for the ATP molecule were generated using PRODRG (46). Data collection and refinement statistics are summarized in Table 1.
TABLE 1.
Summary of data collection and refinement statistics of the ILK KD/α-parvin CH2 complex in the presence of MnATP
| Data collection | |
| Space group | P21 |
| Cell dimensions | |
| a, b, c (Å) | 44.02, 116.28, 47.28 |
| α, β, γ (°) | 90, 101.53, 90 |
| Resolution range (Å) | 43.13–1.80 (1.86–1.80)a |
| No. of total reflections | 209,047 |
| No. of unique reflections | 40,605 |
| I/σ(I) | 26.2 (8.0)a |
| Completeness (%) | 94.2 (76.2)a |
| Rmerge | 0.034 (0.136)a |
| Average redundancy | 5.15 (4.22)a |
| Refinement | |
| Resolution limit (Å) | 6.0–1.8 |
| No. of unique reflections | 39,349 |
| Rwork | 0.204 |
| Rfree | 0.237 |
| No. of non-hydrogen atoms | |
| Protein | 3,157 |
| ATP/Mn | 31/1 |
| Water | 328 |
| B-factors (Å2) | |
| Protein (overall) | 28.1 |
| ILK KD | 20.5 |
| CH2 | 35.7 |
| ATP/Mn | 19.3/20.4 |
| Water | 32.7 |
| RMSD | |
| Bond length (Å) | 0.017 |
| Bond angle (°) | 1.4 |
| Ramachandran plot, residues in | |
| Most favored regions | 321 (93.6%) |
| Additionally allowed regions | 22 (6.4%) |
| Generally allowed regions | 0 (0.0%) |
| Disallowed regions | 0 (0.0%) |
a Highest resolution shell is shown in parentheses.
Analysis of Structures
The superpositions of atomic coordinates were carried out by the secondary structure matching algorithm as implemented in COOT. The contacting residues between two sets of atoms were calculated by CNS (41). Molecular cartoon was drawn by PYMOL.
RESULTS
Recombinant ILK Generated from Either Bacteria or Mammalian Cells Cannot Phosphorylate GSK-3β
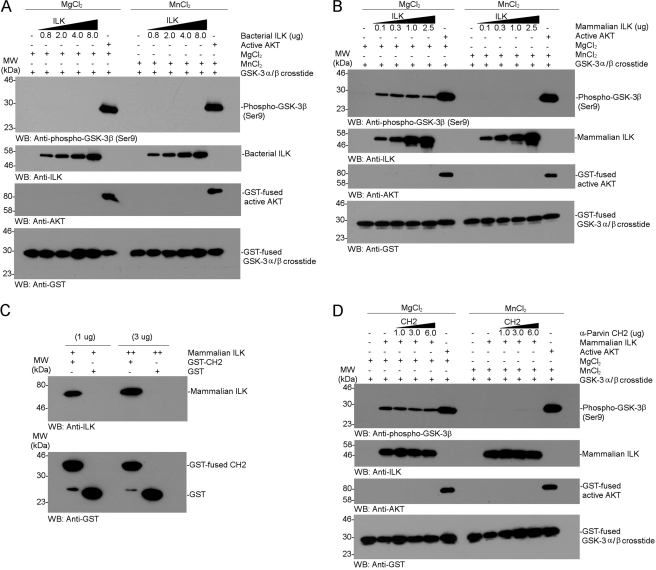
Previous kinase assays using bacterially expressed full-length ILK or partially purified endogenous ILK from chick did not show any activity on integrin β CTs, parvin, and AKT (14). However, Maydan et al. (26) suggested that recombinant ILK could specifically phosphorylate GSK-3 and the effect was more pronounced in the presence of Mn2+ than Mg2+. To assess this possibility, we first performed a kinase assay using bacterially expressed recombinant ILK and the same substrate, GST-fused GSK-3 α/β crosstide that was used by Maydan et al. (26). However, as shown in Fig. 1A, no kinase activity for GSK-3 α/β crosstide was observed in the presence of either Mn2+ or Mg2+. As a comparison, recombinant active AKT potently phosphorylated GSK-3 crosstide (Fig. 1A), consistent with the previously demonstrated and widely accepted phenomenon that GSK-3 is a substrate of AKT (47). Note that either Mg2+ or Mn2+ had no observable difference on the AKT-mediated phosphorylation of GSK-3 (Fig. 1A).
FIGURE 1.
Tests of GSK-3β phosphorylation by recombinant ILK expressed in bacteria or mammalian cells. A, non-radioactive in vitro GSK-3 kinase assay by a dose-dependent addition of bacterially expressed and purified full-length human ILK. The kinase reaction was carried out in the presence of either magnesium or manganese as cofactor. The phosphorylation of GSK-3 was examined by Western blot analysis with anti-phospho-specific antibody. GST-fused full-length active AKT was used as a positive control for the phosphorylation of GSK-3. B, in vitro GSK-3β kinase assay by a dose-dependent addition of Myc-tagged human ILK expressed in HEK293. Notice the very weak phosphorylation of GSK-3β in the presence of Mg2+ but not Mn2+, which is in contrast to data from Maydan et al. (26). C, in vitro protein-protein binding interaction between recombinant mammalian ILK and α-parvin CH2 by pull-down assay. The Myc-tagged ILK was potently bound to the GST-fused CH2 but not to the GST alone. D, kinase inhibition assay for the phosphorylation of GSK-3 by a dose-dependent addition of CH2. Notice that the phosphorylation of GSK-3β was not affected by CH2 at all, suggesting that the phosphorylation of GSK-3 was not mediated by Myc-tagged ILK but rather by some contaminating kinase (see Fig. 2).
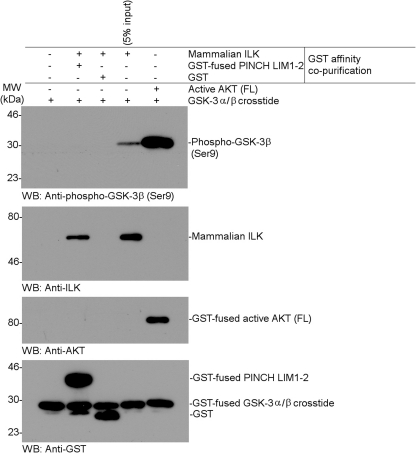
Because Maydan et al. (26) observed the kinase activity on GSK-3 using recombinant ILK from baculovirus-infected insect cells, we wondered whether the ILK function might depend on a eukaryotic expression system where ILK may be post-translationally modified. Therefore, we performed the same kinase assay using recombinant mammalian full-length ILK that is commercially available from OriGene, Inc. This ILK was Myc-tagged at the C terminus, expressed in a human embryonic kidney cell line (HEK293), and purified by affinity chromatography. Coomassie-stained SDS-gels of this ILK sample revealed multiple bands with two major bands at about equal intensities, one of which corresponds to ∼51 kDa ILK as confirmed by Western blot analysis (supplemental Fig. S1). As compared with control AKT, which potently phosphorylated GSK-3, the ILK sample very weakly phosphorylated GSK-3 (Fig. 1B). However, the effect was only observed in the presence of Mg2+ and not of Mn2+ (Fig. 1B), which is in contrast to what was reported by Maydan et al. (26) who showed that ILK phosphorylates GSK-3 in Mn-dependent manner. Moreover, the weak phosphorylation effect was not dependent on the ILK concentration, suggesting that it may be caused by some low level contaminating kinase in this partially purified sample (supplemental Fig. S1). To examine this possibility, we performed two independent experiments: 1) kinase inhibition assay and 2) a kinase assay using further affinity co-purified ILK. Previous structural studies have shown that α-parvin C-terminal calponin homology (CH2) domain tightly binds to ILK in a manner that is reminiscent to those of many protein kinase/substrate complexes (14). In particular, the p + 1 site critical for kinase substrate entry is completely blocked by CH2 (14). Therefore, if the phosphorylation of GSK-3 were exerted by ILK, the α-parvin CH2 domain would strongly inhibit the effect. Fig. 1C shows that while α-parvin CH2 directly bound to the Myc-tagged mammalian ILK, it had little effect on the weak phosphorylation of GSK-3 regardless of the CH2 concentration (Fig. 1D), suggesting that the phosphorylation of GSK-3β was not directly mediated by ILK (note that CH2 was in substantial excess of ILK based on the concentrations used in the assay). The above experiment suggests that the partially purified ILK sample might contain a contaminating serine/threonine kinase retained during the affinity purification of the Myc-tagged ILK from HEK293 cell lysate. We thus further purified the Myc-tagged ILK using GST-PINCH LIM1–2 that tightly binds to the ankyrin repeat domain of ILK (12–13). As expected, the Myc-tagged ILK, which was further affinity co-purified by GST-PINCH LIM1–2, no longer phosphorylated GSK-3 (Fig. 2). These experiments rule out the possibility that ILK expressed in either bacteria or a eukaryotic system can phosphorylate GSK-3.
FIGURE 2.
Test of GSK-3β phosphorylation by Myc-tagged ILK that was further purified by GST-PINCH LIM1–2 affinity chromatography. Each bacterially purified GST-fused PINCH LIM1–2 (residues 1–127) or GST (∼25 μg each) was immobilized on 40 μl of glutathione-Sepharose 4B (GE Healthcare) and equilibrated in pull-down binding buffer as described under “Experimental Procedures.” The mammalian Myc-tagged ILK (1.4 μg) was added in each affinity bead, and the reaction mixtures were incubated at 4 °C by a rotor for 2 h. The beads were extensively washed with binding buffer. The bound proteins were eluted in 60 μl of 20 mm reduced glutathione in binding buffer and designated as the GST affinity co-purified Myc-tagged ILK. Each 10 μl of eluent was analyzed by non-radioactive in vitro phosphorylation assay of GSK-3β using Western blot analysis with phosphospecific antibody, as described under “Experimental Procedures”.
Proteomic Whole Cell Search Did Not Identify Any Potential ILK Substrate
Although we showed that ILK is incapable of phosphorylating GSK-3 (Fig. 1) and other previously proposed substrates (14), one remaining possibility is that ILK may have an unusual and yet-unidentified substrate. To address this issue, we performed a kinase assay on CG-4 and C2C12 whole cell lysate using ILK expressed in both bacteria and HEK293 cells using a technique developed in the laboratory of R. Kothary.4 Protein from cells was extracted, and endogenous kinases in the cell lysate were inactivated to generate a pool of protein devoid of endogenous kinase activity (see experimental details under “Experimental Procedures”). A recombinant kinase can be added back into the kinase-inactivated lysate and a typical assay is performed to screen for substrates of the kinase of interest. This method allows for the identification/visualization of any potential ILK substrates in a proteomic screen fashion. Fig. 3 shows that as compared with the control of active p38α kinase that phosphorylates numerous substrates, ILK expressed in either bacteria (Fig. 3A) or HEK293 cells (Fig. 3B) has little effect on cell lysate. Some extremely faint bands appear in lysate labeled by impure Myc-tagged ILK (Fig. 3B), but these bands were clearly not from ILK since they were not affected at all by a kinase inhibition assay using the addition of excess α-parvin CH2. The direct application of this method on ILK provides unbiased biochemical evidence that ILK is a bona fide pseudokinase.
Structure of the ILK KD/α-Parvin CH2 Complex Bound to MnATP Reveals the Same Conformation as that Bound to MgATP
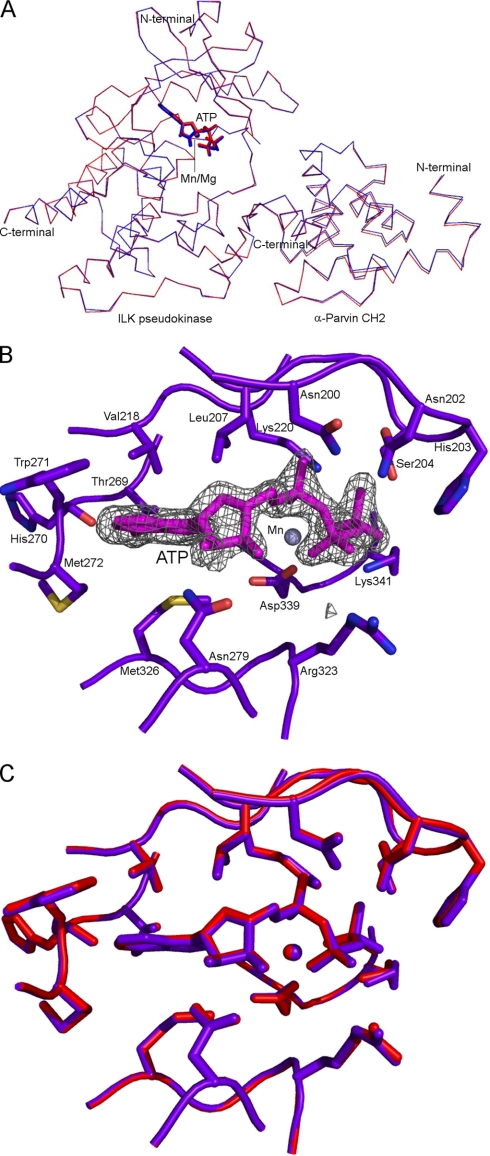
At the structural level, previous studies have shown that the ILK KD bound to α-parvin CH2 in the presence and absence of Mg-ATP reveals a pseudo-active site conformation that is unlikely to perform conventional catalysis. However, because Maydan et al. (26) indicated that ILK kinase activity might be Mn-dependent, we decided to examine if the structure of ILK KD/CH2 complex bound to Mn-ATP differs from that bound to Mg-ATP. We therefore crystallized and solved the 1.8-Å crystal structure of the ILK KD/CH2 complex in the presence of manganese (Mn) and ATP. The overall structure of the ILK KD (MnATP-bound form) is very similar to that of the previously determined MgATP-bound form with no significant root mean square deviation between the Cα atom pairs (Fig. 4A). One ATP molecule, which is typically hydrolyzed by either active or inactive kinases, is present in the nucleotide-binding cleft between the N- and C-terminal lobes (Fig. 4B). The conformation of ATP in the Mn-bound ILK shows no deviations from that in the previously determined Mg-bound form. The conformations of the ATP-binding residues in the Mn-bound ILK are all conserved as those in the Mg-bound form (Fig. 4C). One manganese ion appeared unambiguously as a relatively high peak (10σ) in the 1Fo-Fc electron density map. The position of the manganese ion in the Mn-bound form is almost identical to that of the magnesium ion in the Mg-bound form. The bound Mn ion is coordinated by the side chain of residues Asp-339 and two conserved water molecules, which are the same as the bound Mg ion (14). These structural data demonstrate that the Mn-bound ILK adopts the same structure as the Mg-bound form and that Mn binding does not convert ILK into a catalytically competent form. The structure is also fully consistent with the above biochemical and proteomic analyses that ILK lacks intrinsic kinase activity.
FIGURE 4.
Crystal structure analysis of the ILK KD bound to CH2 in the presence of manganese and ATP. A, an overlay of the crystal structures of the ILK KD/CH2 complexes between MnATP (colored in purple blue) and MgATP bound (colored in red) forms. B, close-up view of the MnATP-binding sites in the ILK KD. The ATP molecule and manganese ion are shown in stick (colored in magenta) and sphere models, respectively. The ATP molecule is superimposed with the 1Fo-Fc omit density map contoured at 4σ level. The contacting residues and atoms of the ILK KD to the ATP molecule were calculated using the maximum distance cutoff of 4 Å by CNS. C, superposition of the ATP-binding ILK residues between MnATP (colored in purple blue) and MgATP (colored in red) bound forms.
ATP Is Not Hydrolyzed When Bound to ILK with a Moderate Affinity
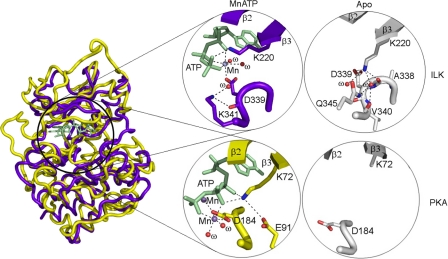
Despite being a pseudokinase, ILK still binds to ATP, which was also found for other pseudokinases such as STRADα (28). On the other hand, the fact that ATP was not hydrolyzed by ILK and other pseudokinases provides additional evidence that they lack kinase activity because ATP is typically hydrolyzed by either active or inactive kinases, the first step of the kinase reaction. To gain quantitative insight into the ATP-ILK interaction, we measured the ATP binding affinity for ILK by isothermal titration calorimetry (ITC), which revealed a moderate KD at 3.64 ± 0.49 μm (Fig. 5) similar to STRADα/ATP complex (28). ATP binding did not induce any gross conformational change of ILK except local rearrangement involving the side chains of Lys-220 and Lys-341 (Fig. 6). It remains to be determined if ATP has any non-catalytic role in regulating the scaffolding function of ILK. In the case of STRADα pseudokinase, it was found that ATP promotes the binding of STRADα to LKB kinase (28). However, ILK binds equally well to α-parvin CH2 in the absence and presence of ATP (14).
FIGURE 5.
Thermodynamic profile for the binding of the ILK KD/CH2 complex to ATP using ITC. Top panel shows raw data of injection profile after baseline correction. Bottom panel shows integration plots (heat released) for each injection, along with a solid line of non-linear least-squares fit for the data.
FIGURE 6.
Comparison of structures between ILK KD and PKA in the presence/absence of MnATP. Left, superposition of the crystal structures of the ILK KD (colored in purple blue) and PKA (colored in yellow). Right, close-up views of the MnATP-binding sites and the conserved ATP-binding lysine residues before and after ATP-binding.
The ATP Binding Lys-220 Is Required for the Structural Integrity of ILK Instead of Catalysis
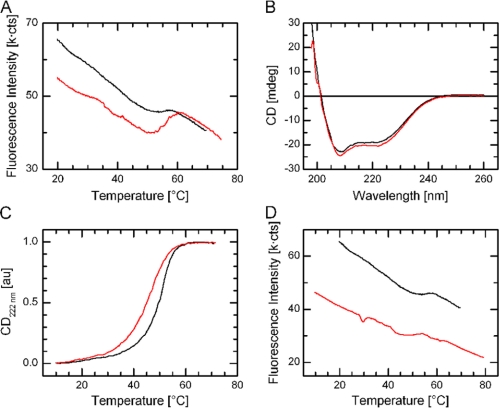
The ATP binding Lys-220 was previously thought to be important for ILK kinase activity (24, 26) but our studies totally disagree with these results. Curiously, the K220A or K220M mutation was linked to significant functional defects such as renal agenesis during kidney development (21, 48). What is then the interpretative basis for this defect? Careful structural examination reveals that K220 is structurally important even in the ATP-free form by forming a crucial hydrogen bonding network in the central core region of the ILK pseudokinase domain (Fig. 6). ILK K220 in ATP-free ILK is well ordered (supplemental Fig. S2A), which seems to be similar to the equivalent residue in other pseudokinases, such as Lys-203 in VRK3 (17) (supplemental Fig. S2B). This is in sharp contrast to the corresponding Lys in conventional kinases such as Lys-72 in PKA (49), which is often disordered and not required for structural stability (supplemental Fig. S2, C and D). It is thus conceivable that while ILK structural stability may not be affected before and after ATP binding due to the small native structural rearrangement (Fig. 7A), introduction of a hydrophobic residue such as Ala or Met into ILK Lys-220 would cause a dramatically unfavorable interaction with the nearby negatively charged residues (Fig. 6). This may in turn destabilize ILK and its interactions with target proteins, leading to the ILK-mediated developmental effect. To evaluate the possibility of K220A or K220M-induced ILK instability, we co-expressed ILK KD (either K220M or K220A) with CH2 and purified the mutant complexes from bacterial cultures. Whereas the K220A and K220M mutants can still form a complex with CH2 as judged from gel-filtration experiments, we found that the yields for both mutant complexes were extremely low. The yield of the ILK K220M/CH2 complex was better than that of K220A/CH2 complex, but still dramatically lower (0.43 mg from 24 liters of culture) than that of the WT complex (∼24 mg from 24 liters of culture). This finding is thus consistent with the structural importance of Lys-220, and its mutation likely disrupted ILK stability and dramatically decreased the yield of the ILK/CH2 complex. To quantitatively verify this, we further measured the thermodynamic properties of the ILK K220M mutant by circular dichroism (CD) and fluorescence spectroscopy that provide quantitative estimations of protein stability between the wild type and the K220M mutant forms. The CD measurements of the wild type as compared with the mutant proteins revealed no gross structural change (Fig. 7B). By contrast, CD denaturation studies revealed that there was significant decrease in the thermal melting temperature Tm of the mutant ILK (K220M) complex (Tm = 46.7 °C) versus that of the wild-type complex (Tm = 51.0 °C) (ΔTm = 4.3 °C), demonstrating that the K220M substitution substantially destabilized ILK (Fig. 7C), which is consistent with the extreme low yield of the ILK K220M/CH2 complex. Consistent with this, the fluorescence spectroscopy measurement also showed a similar Tm difference between the wild type and mutant proteins (Fig. 7D). Note that these quantitative thermodynamic data were measured in the form of ILK KD/CH2 complex due to the technical problem in expressing ILK alone (14). However, because Lys-220 is far away from the CH2 binding site and is not involved in regulating CH2 binding (14), the presence of CH2 does not complicate our data interpretation. At the least, our data demonstrate that the mutation dramatically reduced the overall stability of the ILK/parvin complex.
FIGURE 7.
Thermodynamic properties of wild type and the K220M mutant of the ILK KD bound to CH2. A, thermal denaturation curves of the ILK KD/CH2 complex between apo (colored in black) and ATP-bound (colored in red) forms by fluorescence analysis. B, an overlay of far-UV CD spectra of the recombinant ILK KD/CH2 complex between wild-type ILK (colored in black) and the K220M mutant (colored in red). C, an overlay of normalized thermal unfolding profiles of the recombinant ILK KD/CH2 complex between the wild type (colored in black) and the K220M mutant (colored in red). D, thermal denaturation curves of the recombinant ILK KD/CH2 complex between the wild type (colored in black) and the K220M mutant (colored in red) by tryptophan fluorescence analysis. C and D demonstrate that K220M is substantially less stable than the WT ILK.
DISCUSSION
In this study, we have undertaken a comprehensive biochemical, proteomic, structural, and thermodynamic analysis on the kinase function of ILK, a topic that has been under significant debate over the past decade. Our data strongly demonstrate that phosphorylation of GSK-3 is not directly mediated by ILK under a variety of conditions, with no preference for the non-physiological cofactor manganese as suggested by a recent biochemical study (26). Crystal structures of several AGC subfamily kinases that phosphorylate GSK-3 are available, and the active site and substrate binding residues in these AGC family kinases, such as AKT and PKA, are highly conserved and yet drastically different from the dramatically degraded active site of ILK KD (14), explaining fundamentally why ILK cannot directly phosphorylate GSK-3. The structure of Mn-bound ILK is indistinguishable from that of Mg-bound ILK, ruling out the possibility that ILK is a distinct Mn-specific kinase, which is also confirmed by our biochemical data. It is difficult to reconcile the discrepancy between our structural/biochemical findings and the biochemical studies by Maydan et al. (26) who showed that ILK has kinase activity and a preference for manganese when phosphorylating GSK-3. Because we used ILK expressed in both bacteria and a eukaryotic system, the discrepancy is apparently not due to the post-translational modification issue. One likely source of discrepancy is an unrelated contaminating kinase or a functionally related ILK binding kinase that was co-purified during the affinity purification. This scenario happened in the alternative biochemical assay we performed using GST affinity co-purified Myc-tagged ILK. Whereas the original ILK sample from OriGene (supplemental Fig. S1) showed a weak phosphorylation effect of GSK-3, further purification of ILK by GST-PINCH LIM1–2 completely eliminated this weak effect. Also, addition of α-parvin CH2 had no effect on the weak phosphorylation, further indicating that the phosphorylation is due to an uncharacterized kinase. We note that it is very difficult to pinpoint the exact contaminating kinase because a very tiny amount of kinase (even invisible by Western blotting, e.g. supplemental Fig. S3) can still exert an observable phosphorylation effect. Such low level contamination may be beyond detection by common techniques such as Western blot analysis and mass spectroscopy. In the study by Maydan et al. (26) a major component of ILK plus 95 minor components were detectable by mass spectroscopy including one arginine kinase. Perhaps some unknown kinases are undetectable by mass spectroscopy but still may be functional as demonstrated by our Western blot analysis (supplemental Fig. S3).
In addition to the tests on GSK-3 phosphorylation by ILK, we also show in a proteomic fashion that ILK cannot phosphorylate any potential substrate. These data, combined with our structural analysis, strongly demonstrate that ILK is a pseudokinase. Is it possible that ILK exerts the kinase activity in the presence of some protein regulators or co-factors? The lack of so many active site features in ILK does not seem to support this possibility (12). Furthermore, genetic studies (21) have shown clearly that putative ILK active or dead mutants had no effect on the putative ILK substrates GSK-3 or AKT activation, thus providing evidence that ILK kinase activity is not required in vivo. How would ILK then exert its cellular function as a pseudokinase? ILK has been shown to bind to many proteins including integrin β CTs, PINCH, parvin, and kindlin (18, 50). In particular, ILK forms a tight ternary complex with PINCH via its ankyrin repeat domain and parvin via its KD, respectively. This ternary complex is emerging as a major hub for shuffling protein traffic during FA assembly and ECM-integrin-actin signaling (18, 50). Thus, like several key cytoskeletal adaptors in regulating integrin-mediated cell adhesion and migration including talin, filamin, and kindlin, ILK simply acts as a scaffold to mediate distinct protein-protein interactions via its ankyrin repeat domain and KD. Disruption of ILK-mediated interactions may thus impair cytoskeleton function and the integrin-actin network, and possibly indirectly affect multiple signaling pathways such as AKT activation (18, 50). In this regard, it is of particular interest to note the non-catalytic role of the conserved ATP-binding lysine 220 residue in the ILK KD. Unlike the corresponding lysine that is disordered in ATP-free kinases, we found that Lys-220 plays a critical role in the structural integrity of ILK and its mutation to methionine (K220M) substantially impaired the stability of ILK. Thus, although Lys-220 is remote from the α-parvin binding interface, the decreased structural stability of the ILK mutant may in turn affect ILK binding to α-parvin as indicated by Lange et al. (21) and possibly other ILK-binding proteins. This finding provides a basis for understanding why the K220M mutation caused a significant phenotype, i.e. renal agenesis during kidney development (21). One can similarly explain two other phenotypic single amino acid substitution mutations identified in ILK KD, L308P that causes a lethal heart failure in zebrafish (8) and A262V that causes a severe dilated cardiomyopathy in humans (9). As shown in supplemental Fig. S4, residue L308 is positioned in the αE-helix and its side chain is buried inside a hydrophobic core formed by I244, V250, M305, L311, H318, I335, and M337. Therefore, the L308P substitution would clearly cause a disruption of the αE-helix, which in turn perturbs the structural integrity of the ILK KD, which was found to impair ILK binding to β-parvin (8), a homologue to α-parvin. Similarly, A262 is located in a loop in the N-lobe in the ILK KD near a bulky F190 and hydrophilic residue of C257 and T266 (supplemental Fig. S4). The A262V substitution would insert an extra methyl group to cause a steric clash with these surrounding residues, thus impairing the structural integrity of ILK KD and its target binding. Thus, it is the disruption of the structural integrity rather than a loss of kinase activity that causes the dysfunction of ILK and the related pathological disorders.
It is becoming increasingly clear that regulated protein-protein interactions mediated by scaffolding proteins play crucial roles in many signal transduction pathways. Assembly of signaling components mediated by scaffolding proteins can enhance spatial coordination to the signaling target for an efficient feedback mechanism (51). Given the significance of the ILK-mediated membrane-cytoskeleton linkage associated with signaling pathways in many biological processes, such as cardiovascular development, identification of the signaling molecules that directly interact with ILK and/or its binding partners will be necessary to provide new insights into the coordinated molecular and signaling mechanisms in ILK-mediated integrin biology.
Supplementary Material
Acknowledgments
We thank Drs. Xiongying Tu for the access to the Case Pharmacology x-ray facility, Song Tan for providing polycistronic coexpression vector, and Satya P. Yadav for help with isothermal titration calorimetry.
This work was supported by grants from the National Institute of Health (to J. Q.) and from Canadian Institutes of Health Research and the Multiple Sclerosis Society of Canada (to R. K.).
The atomic coordinates and structure factors (code 3REP) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
J. D. R. Knight, R. Tian, R. E. C. Lee, F. Wang, H. Zou, L. A. Megeney, D. Figeys, and R. Kothary, R. A Novel Whole-cell Lysate Kinase Assay Identifies Substrates of the p38 MAPK in Differentiating Myoblasts, submitted manuscript.
- ECM
- extracellular matrix
- CD
- circular dichroism
- CH2
- calponin homology 2
- CT
- cytoplasmic tail
- FA
- focal adhesion
- GSK-3
- glycogen synthase kinase-3
- GST
- glutathione S-transferase
- HEK293
- human embryonic kidney 293
- ILK
- integrin-linked kinase
- ITC
- isothermal titration calorimetry
- KD
- kinase-like domain
- LIM
- The Lin11-Isl-1-Mec-3
- MBP
- maltose-binding protein
- PINCH
- particularly interesting cysteine- and histidine-rich protein.
REFERENCES
- 1. Hynes R. O. (2002) Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 2. Qin J., Vinogradova O., Plow E. F. (2004) PLoS Biol. 2, e169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hannigan G. E., Leung-Hagesteijn C., Fitz-Gibbon L., Coppolino M. G., Radeva G., Filmus J., Bell J. C., Dedhar S. (1996) Nature 379, 91–96 [DOI] [PubMed] [Google Scholar]
- 4. Li F., Zhang Y., Wu C. (1999) J. Cell Sci. 112, 4589–4599 [DOI] [PubMed] [Google Scholar]
- 5. Zervas C. G., Gregory S. L., Brown N. H. (2001) J. Cell Biol. 152, 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mackinnon A. C., Qadota H., Norman K. R., Moerman D. G., Williams B. D. (2002) Curr. Biol. 12, 787–797 [DOI] [PubMed] [Google Scholar]
- 7. Sakai T., Li S., Docheva D., Grashoff C., Sakai K., Kostka G., Braun A., Pfeifer A., Yurchenco P. D., Fässler R. (2003) Genes Dev. 17, 926–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bendig G., Grimmler M., Huttner I. G., Wessels G., Dahme T., Just S., Trano N., Katus H. A., Fishman M. C., Rottbauer W. (2006) Genes Dev. 20, 2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knöll R., Postel R., Wang J., Krätzner R., Hennecke G., Vacaru A. M., Vakeel P., Schubert C., Murthy K., Rana B. K., Kube D., Knöll G., Schäfer K., Hayashi T., Holm T., Kimura A., Schork N., Toliat M. R., Nürnberg P., Schultheiss H. P., Schaper W., Schaper J., Bos E., Den Hertog J., van Eeden F. J., Peters P. J., Hasenfuss G., Chien K. R., Bakkers J. (2007) Circ. 116, 515–525 [DOI] [PubMed] [Google Scholar]
- 10. Ding L., Dong L., Chen X., Zhang L., Xu X., Ferro A., Xu B. (2009) Circ. 120, 764–773 [DOI] [PubMed] [Google Scholar]
- 11. Tan C., Cruet-Hennequart S., Troussard A., Fazli L., Costello P., Sutton K., Wheeler J., Gleave M., Sanghera J., Dedhar S. (2004) Cancer Cell 5, 79–90 [DOI] [PubMed] [Google Scholar]
- 12. Chiswell B. P., Zhang R., Murphy J. W., Boggon T. J., Calderwood D. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 20677–20682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y., Wang X., Hawkins C. A., Chen K., Vaynberg J., Mao X., Tu Y., Zuo X., Wang J., Wang Y. X., Wu C., Tjandra N., Qin J. (2009) J. Biol. Chem. 284, 5836–5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukuda K., Gupta S., Chen K., Wu C., Qin J. (2009) Mol. Cell 36, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McDonald P. C., Fielding A. B., Dedhar S. (2008) J. Cell Sci. 121, 3121–3132 [DOI] [PubMed] [Google Scholar]
- 16. Boudeau J., Miranda-Saavedra D., Barton G. J., Allesi D. R. (2006) Trends Cell Biol. 16, 466–472 [DOI] [PubMed] [Google Scholar]
- 17. Scheeff E. D., Eswaran J., Bunkoczi G., Knapp S., Manning G. (2009) Structure 17, 128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wickström S. A., Lange A., Montanez E., Fässler R. (2010) EMBO J. 29, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dai C., Stolz D. B., Bastacky S. I., St-Arnaud R., Wu C., Dedhar S., Liu Y. (2006) J. Am. Soc. Nephrol. 17, 2164–2175 [DOI] [PubMed] [Google Scholar]
- 20. Kanasaki K., Kanda Y., Palmsten K., Tanjore H., Lee S. B., Lebleu V. S., Gattone V. H., Jr., Kalluri R. (2008) Dev. Biol. 313, 584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lange A., Wickström S. A., Jakobson M., Zent R., Sainio K., Fässler R. (2009) Nature 461, 1002–1006 [DOI] [PubMed] [Google Scholar]
- 22. Deng J. T., Van Lierop J. E., Sutherland C., Walsh M. P. (2001) J. Biol. Chem. 276, 16365–16373 [DOI] [PubMed] [Google Scholar]
- 23. Yamaji S., Suzuki A., Sugiyama Y., Koide Y., Yoshida M., Kanamori H., Mohri H., Ohno S., Ishigatsubo Y. (2001) J. Cell Biol. 153, 1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Persad S., Attwell S., Gray V., Mawij N., Deng J. T., Leung D., Yan J., Sanghera J., Walsh M. P., Dedhar S. (2001) J. Biol. Chem. 276, 27462–27469 [DOI] [PubMed] [Google Scholar]
- 25. Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maydan M., McDonald P. C., Sanghera J., Yan J., Rallis C., Pinchin S., Hannigan G. E., Foster L. J., Ish-Horowicz D., Walsh M. P., Dedhar S. (2010) PLoS ONE 5, e12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Legate K. R., Montañez E., Kudlacek O., Fässler R. (2006) Nat. Rev. Mol. Cell Biol. 7, 20–31 [DOI] [PubMed] [Google Scholar]
- 28. Zeqiraj E., Filippi B. M., Goldie S., Navratilova I., Boudeau J., Deak M., Alessi D. R., van Aalten D. M. (2009) PLoS Biol. 7, e1000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Labesse G., Gelin M., Bessin Y., Lebrun M., Papoin J., Cerdan R., Arold S. T., Dubremetz J. F. (2009) Structure 17, 139–146 [DOI] [PubMed] [Google Scholar]
- 30. Zeqiraj E., van Aalten D. M. (2010) Curr. Opin. Struct. Biol. 20, 772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Min X., Lee B. H., Cobb M. H., Goldsmith E. J. (2004) Structure 12, 1303–1311 [DOI] [PubMed] [Google Scholar]
- 32. Taylor S. S., Kornev A. P. (2011) Trends Biochem. Sci. 36, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cabodi S., del Pilar Camacho-Leal M., Di Stefano P., Defilippi P. (2010) Nat. Rev. Cancer. 10, 858–870 [DOI] [PubMed] [Google Scholar]
- 34. Pontier S. M., Huck L., White D. E., Rayment J., Sanguin-Gendreau V., Hennessy B., Zuo D., St-Arnaud R., Mills G. B., Dedhar S., Marshall C. J., Muller W. J. (2010) Oncogene 29, 3374–3385 [DOI] [PubMed] [Google Scholar]
- 35. Perez V. A., Ali Z., Alastalo T. P., Ikeno F., Sawada H., Lai Y. J., Kleisli T., Spiekerkoetter E., Qu X., Rubinos L. H., Ashley E., Amieva M., Dedhar S., Rabinovitch M. (2011) J. Cell Biol. 192, 171–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Youn S. W., Lee S. W., Lee J., Jeong H. K., Suh J. W., Yoon C. H., Kang H. J., Kim H. Z., Koh G. Y., Oh B. H., Park Y. B., Kim H. S. (2011) Blood 117, 4376–4386 [DOI] [PubMed] [Google Scholar]
- 37. Yang J., Cron P., Good V. M., Thompson V., Hemmings B. A., Barford D. (2002) Nat. Struct. Biol. 9, 940–944 [DOI] [PubMed] [Google Scholar]
- 38. Tan S. (2001) Protein Expr. Purif. 21, 224–234 [DOI] [PubMed] [Google Scholar]
- 39. Wang X., Fukuda K., Byeon I. J., Velyvis A., Wu C., Gronenborn A., Qin J. (2008) J. Biol. Chem. 283, 21113–21119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pflugrath J. W. (1999) Acta Crystallogr. D Biol Crystallogr. 55, 1718–1725 [DOI] [PubMed] [Google Scholar]
- 41. Brünger A. T., Adams P. D., Clore G. M., Delano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 42. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 43. Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 44. Roussel A., Cambillau C. (1991) TURBO-FRODO in Silicon Graphics Geometry Partners Directory, Silicon Graphics, Mountain View, CA [Google Scholar]
- 45. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 46. Schüttelkopf A. W., van Aalten D. M. (2004) Acta Crystallogr. D Biol Crystallogr. 60, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 47. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 48. Smeeton J., Zhang X., Bulus N., Mernaugh G., Lange A., Karner C. M., Carroll T. J., Fässler R., Pozzi A., Rosenblum N. D., Zent R. (2010) Development 137, 3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Akamine P., Madhusudan, Wu J., Xuong N. H., Ten Eyck L. F., Taylor S. S. (2003) J. Mol. Biol. 327, 159–171 [DOI] [PubMed] [Google Scholar]
- 50. Wu C. (2005) Trends Cell Biol. 15, 460–466 [DOI] [PubMed] [Google Scholar]
- 51. Shaw A. S., Filbert E. L. (2009) Nat. Rev. Immunol. 9, 47–56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.