Abstract
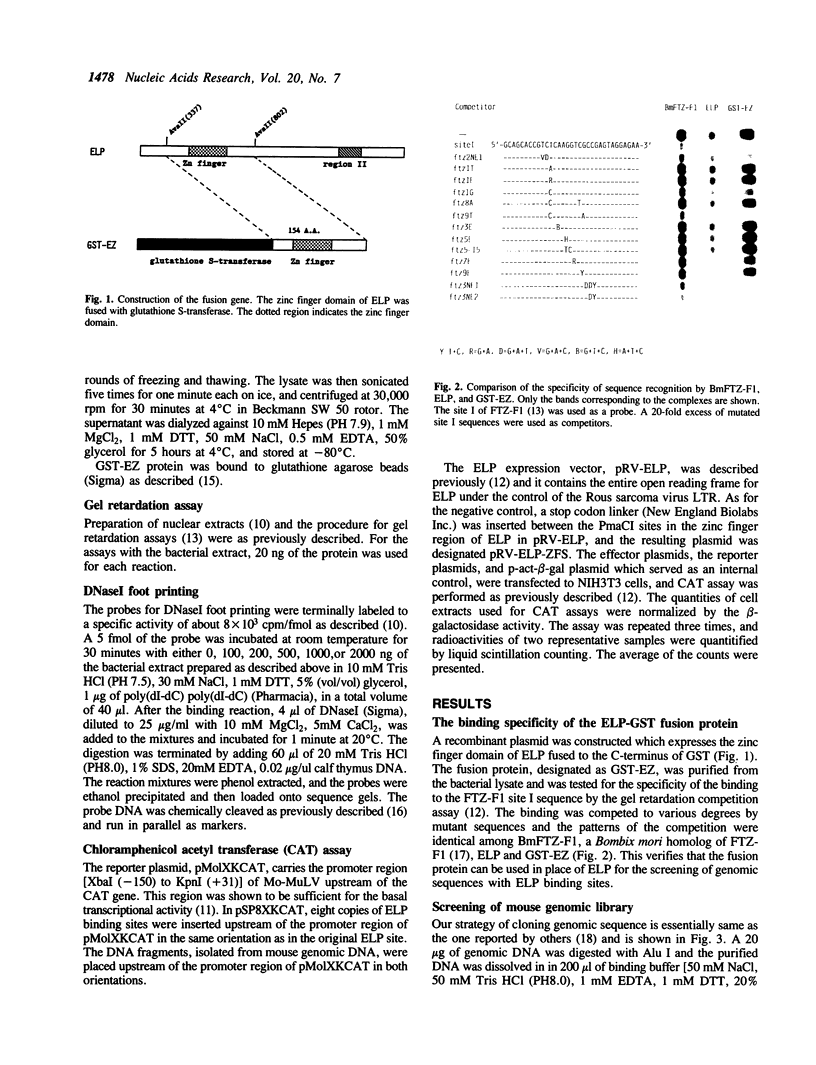
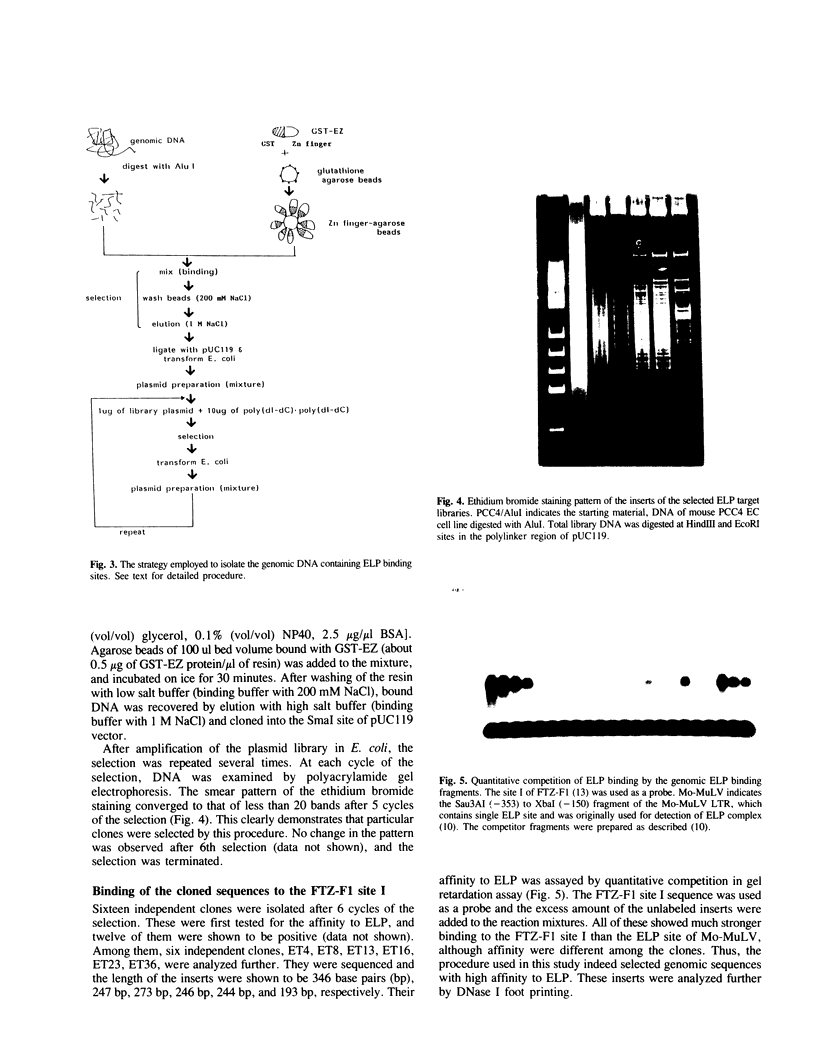
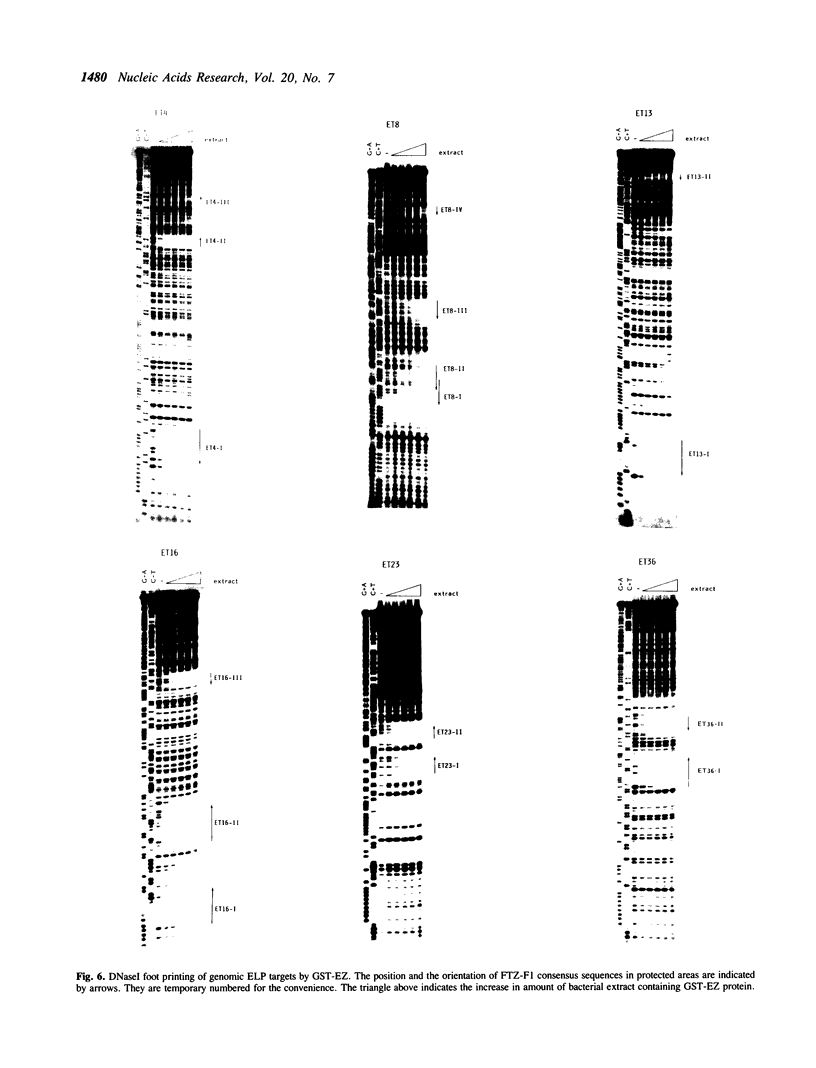
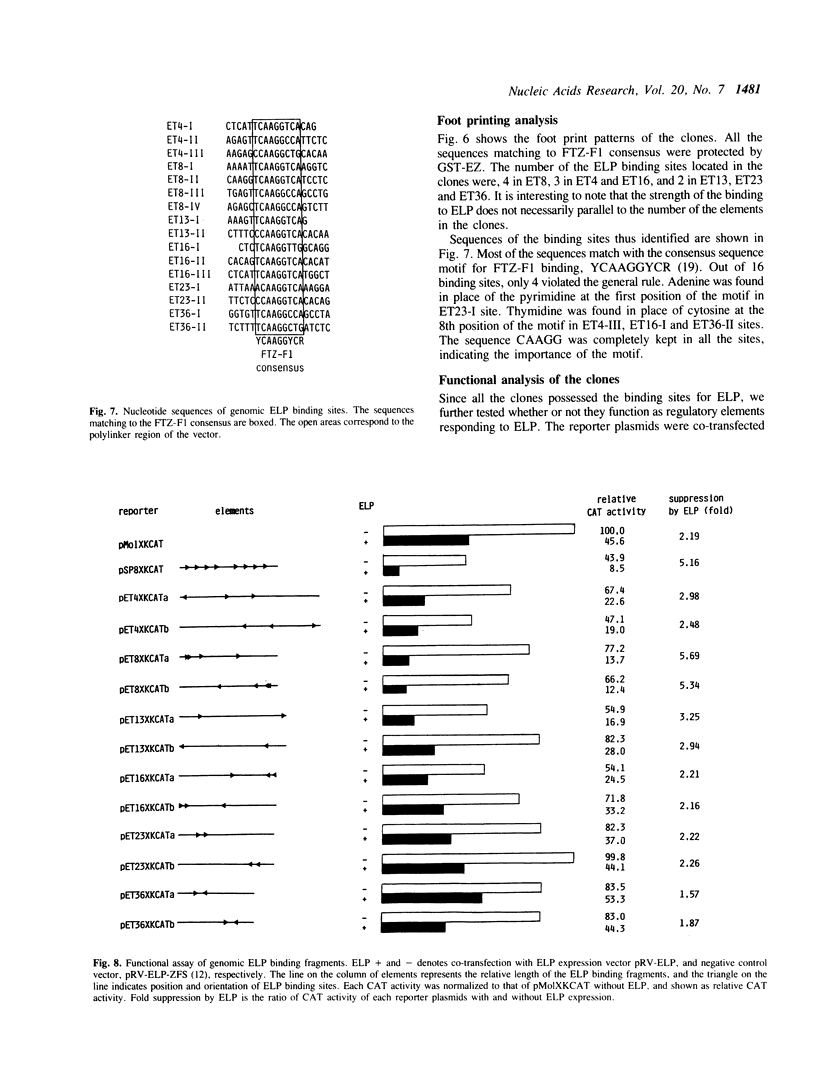
ELP, the embryonal LTR binding protein, is a member of the nuclear receptor superfamily and a mouse homologue of Drosophila FTZ-F1. ELP is expressed specifically in undifferentiated mouse embryonal carcinoma cells and participates in suppression of the Moloney murine leukemia virus genome. The zinc finger domain of the protein was fused with glutathione S-transferase and was successfully used for isolating genomic targets. Sixteen genomic fragments were isolated and twelve of them strongly interacted with ELP. Six of the ELP binding fragments were analyzed further. All of these contained the multiple binding sites for ELP, which matched well with the consensus binding sequence for FTZ-F1, YCAAGGYCR. Among these, three fragments functioned as negative regulatory elements in response to ELP, when placed upstream to the promoter region of the Moloney leukemia virus. These results indicate that ELP may function as a negative transcription factor for a variety of cellular sequences, in addition to suppressing expression of Moloney leukemia virus in early embryonal cells. It was also shown that the procedure employed here works well for isolation of genomic targets of transcription factors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedenkapp H., Borgmeyer U., Sippel A. E., Klempnauer K. H. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988 Oct 27;335(6193):835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- Eul J., Meyer M. E., Tora L., Bocquel M. T., Quirin-Stricker C., Chambon P., Gronemeyer H. Expression of active hormone and DNA-binding domains of the chicken progesterone receptor in E. coli. EMBO J. 1989 Jan;8(1):83–90. doi: 10.1002/j.1460-2075.1989.tb03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainsod A., Margalit Y., Haffner R., Gruenbaum Y. Non-immunological precipitation of protein-DNA complexes using glutathione-S-transferase fusion proteins. Nucleic Acids Res. 1991 Jul 25;19(14):4005–4005. doi: 10.1093/nar/19.14.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. P., Luisi B. F., Korszun Z. R., Basavappa R., Sigler P. B., Yamamoto K. R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988 Aug 11;334(6182):543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- Gould A. P., Brookman J. J., Strutt D. I., White R. A. Targets of homeotic gene control in Drosophila. Nature. 1990 Nov 22;348(6299):308–312. doi: 10.1038/348308a0. [DOI] [PubMed] [Google Scholar]
- Inoue S., Kondo S., Hashimoto M., Kondo T., Muramatsu M. Isolation of estrogen receptor-binding sites in human genomic DNA. Nucleic Acids Res. 1991 Aug 11;19(15):4091–4096. doi: 10.1093/nar/19.15.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Selection of sequences recognized by a DNA binding protein using a preparative southwestern blot. Nucleic Acids Res. 1991 Sep 11;19(17):4675–4680. doi: 10.1093/nar/19.17.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler K. W., Vogelstein B. Whole genome PCR: application to the identification of sequences bound by gene regulatory proteins. Nucleic Acids Res. 1989 May 25;17(10):3645–3653. doi: 10.1093/nar/17.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorgna G., Ueda H., Clos J., Wu C. FTZ-F1, a steroid hormone receptor-like protein implicated in the activation of fushi tarazu. Science. 1991 May 10;252(5007):848–851. doi: 10.1126/science.1709303. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Oliphant A. R., Brandl C. J., Struhl K. Defining the sequence specificity of DNA-binding proteins by selecting binding sites from random-sequence oligonucleotides: analysis of yeast GCN4 protein. Mol Cell Biol. 1989 Jul;9(7):2944–2949. doi: 10.1128/mcb.9.7.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sompayrac L., Danna K. J. Method to identify genomic targets of DNA binding proteins. Proc Natl Acad Sci U S A. 1990 May;87(9):3274–3278. doi: 10.1073/pnas.87.9.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiesen H. J., Bach C. Target Detection Assay (TDA): a versatile procedure to determine DNA binding sites as demonstrated on SP1 protein. Nucleic Acids Res. 1990 Jun 11;18(11):3203–3209. doi: 10.1093/nar/18.11.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Niwa O., Yokoro K. Analysis of the binding proteins and activity of the long terminal repeat of Moloney murine leukemia virus during differentiation of mouse embryonal carcinoma cells. J Virol. 1991 Jun;65(6):2979–2986. doi: 10.1128/jvi.65.6.2979-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Niwa O., Yokoro K. Mechanism of suppression of the long terminal repeat of Moloney leukemia virus in mouse embryonal carcinoma cells. Mol Cell Biol. 1989 Nov;9(11):4670–4676. doi: 10.1128/mcb.9.11.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Ueda H., Hirose S., Niwa O. Embryonal long terminal repeat-binding protein is a murine homolog of FTZ-F1, a member of the steroid receptor superfamily. Mol Cell Biol. 1992 Mar;12(3):1286–1291. doi: 10.1128/mcb.12.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Hirose S. Defining the sequence recognized with BmFTZ-F1, a sequence specific DNA binding factor in the silkworm, Bombyx mori, as revealed by direct sequencing of bound oligonucleotides and gel mobility shift competition analysis. Nucleic Acids Res. 1991 Jul 11;19(13):3689–3693. doi: 10.1093/nar/19.13.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Hirose S. Identification and purification of a Bombyx mori homologue of FTZ-F1. Nucleic Acids Res. 1990 Dec 25;18(24):7229–7234. doi: 10.1093/nar/18.24.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Sonoda S., Brown J. L., Scott M. P., Wu C. A sequence-specific DNA-binding protein that activates fushi tarazu segmentation gene expression. Genes Dev. 1990 Apr;4(4):624–635. doi: 10.1101/gad.4.4.624. [DOI] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]