Abstract
Objectives
To determine whether healthy aging is associated with increased sleepiness, and whether healthy older adults experience more sleepiness when acutely sleep deprived.
Design
A 5-day inpatient circadian rhythm-sleep study consisting of 3 baseline nights, followed by an extended 26-hour wake episode in constant conditions.
Setting
Intensive Physiological Monitoring Unit, General Clinical Research Center, Brigham and Women’s Hospital.
Participants
37 healthy participants without medical, psychological, and sleep disorders: 26 young (7 women, 19 men; mean age 21.9 ± 3.3 years, range 18–29) and 11 “young-old” adults (3 women, 8 men; mean age 68.1 ± 3.6 years, range 65–76).
Intervention
An extended 26-hour wake episode in constant conditions.
Measurements
Electro-encephalographic-verified wakefulness, slow eye movements, sustained attention, subjective sleepiness.
Results
Across the first 16 hours corresponding to the usual waking day, both groups rated themselves as alert, had similar levels of vigilance, and little evidence of sleepiness. As the wake episode continued, the older subjects were less impaired, showing faster reaction times, fewer performance lapses and attentional failures, and less frequent unintentional sleep episodes.
Conclusion
This small study suggests that excessive sleepiness is not normal in healthy “young-old” adults. Symptoms of excessive sleepiness in this population, including reliance on caffeine to maintain alertness, should be evaluated and treated. Further study is needed to determine whether increased daytime sleepiness in middle-old (75–84 years) and old-old (85+) adults is normal, or is instead associated with sleep restriction, undiagnosed sleep disorders, medication side effects, mood disorders, and/or other medical disorders that disrupt sleep.
Keywords: vigilance, circadian rhythm, neurobehavioral performance
INTRODUCTION/BACKGROUND
Healthy aging is associated with a decline in sleep quality and duration, including decreases in sleep depth, as measured by arousal threshold; sleep intensity, as measured by electroencephalographic (EEG) slow wave activity (SWA); sleep continuity, as measured by awakenings during the night; and sleep duration (1). These age-related changes in sleep occur even in the absence of clinically significant sleep disorders, such as sleep-disordered breathing or periodic limb movement disorder.
Perhaps because of this deterioration of sleep quality and quantity, a similar deterioration in waking alertness and performance is often accepted as a parallel consequence of aging. Given the restorative role attributed to EEG SWA during sleep, the age-related decline in SWA might be expected to lead to an age-related decline in the quality of wakefulness. While this is consistent with data from many survey studies that find increased levels of daytime sleepiness among older adults (2–4), often such daytime sleepiness is associated with chronic medical conditions, undiagnosed sleep disorders, and/or medication use (2;5;6). Some reports have suggested that the response of some older people to acute sleep deprivation can be comparable to or even better than that of younger adults. However, those studies (7;8) excluded older adults unless their sleep quality was very high [higher than typical for their age group (1)] or did not control for differences in sleep timing or duration between young and older subjects (9).
To explore whether increased sleepiness and a decline in the ability to cope with sleep loss are indeed consequences of aging, we conducted a study in which we compared both subjective and objective measures of sleepiness, vigilance, and performance in healthy “young-old” (range 65–76 years) and young (range 18–29 years) adults during a 26-hour extended waking vigil.
METHODS
Subjects
Included were data from 37 participants who took part in 9-day inpatient circadian rhythm studies in our laboratory (see Table 1). Hormonal data from ten of the older participants were published in an unrelated report (10).
Table 1.
Demographic and Baseline Night Sleep Characteristics of Older and Young Subjects. Mean (± SD).
| Older subjects | Young subjects | Pvalue | |
|---|---|---|---|
| Age | 68.1 ± 3.6 | 21.9 ± 3.3 | |
| Gender | 3 women, 8 men | 7 women, 19 men | |
| Sleep efficiency (%) | 82.0 ± 6.6 | 93.6 ± 3.5 | <.01 |
| Duration of slow wave sleep (Minutes) | 10.13 ± 7.34 | 17.25 ± 6.95 | <.02 |
| Awakenings (Number) | 30.3 ± 6.99 | 4.54 ± 4.24 | <.01 |
| Awakenings (Duration, minutes) | 2.42 ± 0.76 | 2.04 ± 2.67 | .53 |
Sleep efficiency in young adults is typically >90% under these laboratory conditions, while in healthy young-old adults it typically averages between 75–85%, and is often even lower in many older adults or those taking medications, with medical conditions, or with undiagnosed sleep disorders [1;2;9;22]. The amount of slow wave sleep in non-sleep-deprived young adults is typically about 15–25% of sleep time (~60–90 minutes of an 8-hour sleep episode), and this declines with age such that young-old adults often average as little as 5% slow wave sleep (10–25 minutes of an 8-hour sleep episode) [1;2;9]. The number of awakenings during sleep is typically quite low in young adults and increases with age [1], although the exact number depends on the criteria used to define an awakening.
Subjects were not taking medications, reported no chronic medical problems, and were in good medical and psychological health [determined by serum chemistry, complete blood count, urinalysis, physical examination, electrocardiogram, chest radiograph (older subjects only), psychological questionnaires, and interview with a clinical psychologist]. All were without significant sleep complaint, and older subjects underwent an overnight sleep screen to exclude those with clinically-significant sleep disorders. Only those who denied a recent history of regular night work and transmeridian travel (>1 time zone) were included, and each subject maintained a regular, self-selected sleep schedule (8 hours per night) for at least a week prior to study.
Study Protocol
Each study began with three baseline days, with 8 hours time in bed at night, scheduled at each subject’s habitual times. Upon waking after the third night, subjects began a constant routine (CR) procedure, during which they remained in bed and awake throughout their usual waking day, followed by at least 10 additional hours.
Constant Routine (CR)
The CR consisted of a 26-hour vigil of monitored wakefulness (11) during which subjects were restricted to semi-recumbent bed rest in constant dim light, and were given hourly snacks to spread their caloric and fluid intake across day and night.
Throughout each CR, subjective alertness was assessed twice per hour with a Karolinska Sleepiness Scale [KSS (12)], which required the subject to select a number spanning the range from “very alert” [=1] to “very sleepy” [=9]; subjective estimates from this task oscillate with circadian phase (13) and are sensitive to acute sleep deprivation (14).
Sustained attention was assessed every two hours using the Psychomotor Vigilance Task [PVT (15)], a test of reaction time (RT) in which the subject is asked to maintain the fastest possible RTs to a simple visual stimulus for 10 minutes, with a random inter-stimulus interval (~100 trials per administration). Performance can be assessed in several ways, including average RT and number of lapses of attention (defined as RT >500 milliseconds). This test is sensitive to both circadian phase (13;16) and sleep loss (13;16;17), and does not show long-lasting training effects.
Throughout the 26-hour vigil, the EEG and electrooculogram (EOG) were recorded continuously (18). These data were used to monitor inadvertent sleep episodes and attentional failures, defined by intrusions of slow rolling eye movements (SEM); SEM vary with circadian phase and increase with acute sleep loss in young adults (13;18).
Data Analysis
Baseline night sleep data were visually scored in 30-second epochs according to established criteria (19), and sleep efficiency [the percentage of time between lights off and lights on that the subject was in any stage of sleep], sleep depth [the total amount of time spent in Stage 3 and Stage 4 sleep], and sleep continuity [the number of awakenings after sleep onset, defined as any epoch or series of epochs during which the subject was awake for >15 seconds] were calculated for each subject and then compared between the two groups.
Each KSS rating was assigned an elapsed time with respect to scheduled wake time, an hourly mean was calculated for each subject, and the hourly data were averaged across subjects in the young and older groups. Summary data from each PVT included mean RT and number of lapses of attention (RT >500 milliseconds). The PVT data were assigned an elapsed time with respect to scheduled wake time and were then averaged across subjects in the young and older groups. The RT data were not normally distributed and were transformed (1/RT) for analysis. The lapse data were analyzed assuming a Poisson distribution.
EEG/EOG data were unavailable for part or all of the 26-hour vigil for 7 of the young and 1 of the older subjects, and only data from subjects with a complete recording were included in the EEG/EOG analysis. The EEG signals were visually scored in 30-second epochs for inadvertent sleep (19), and then the EOG signals for all waking epochs were scored for SEM. Each epoch of EEG/EOG data was assigned an elapsed time with respect to scheduled wake time, and the data were then averaged across subjects per hour in the young and older groups. These data were analyzed assuming a Poisson distribution, and were also converted to a binary format (1=hourly bin with at least one SEM, 0=no eye movement) for further analysis.
Statistical Methods
Data are presented as mean ± SD unless otherwise noted. Sleep data from the baseline night were compared between the older and young groups using two-sample Student t-test, adjusting for unequal variances if necessary. Comparisons between age groups for the pooled 16-hour data corresponding to the usual waking day were performed in the same manner. Data from the 26-hour vigil were tested for normality using the Shapiro-Wilke test. Analysis of wake-dependent changes across the 26-hour vigil, and overall group differences across the 26–hour vigil, were performed with a general linear model with factors ‘elapsed time awake’ and ‘age group’. KSS and PVT data were also analyzed using mixed model analysis, because significant individual differences in response to sleep deprivation have been reported in these types of data (20). In this model, both the intercept (mean level) and the slope (change across time) were random effects between subjects. The probability for having an accidental sleep episode during the 26-hour vigil was compared between the age groups using survival analysis.
Ethical Approval
The studies were reviewed and approved by the Partners Human Research Committee, and conformed to the principles outlined in the Declaration of Helsinki. Each participant gave written informed consent prior to study.
RESULTS
Baseline Night Sleep Quality
The sleep efficiency of the older subjects was lower than that of the young subjects on the night before the 26-hour vigil (see Table 1). Older subjects spent fewer minutes in the deepest stages of nonREM sleep and had more awakenings during the night, although the duration of awakenings did not differ between the groups (see Table 1).
Subjective Alertness
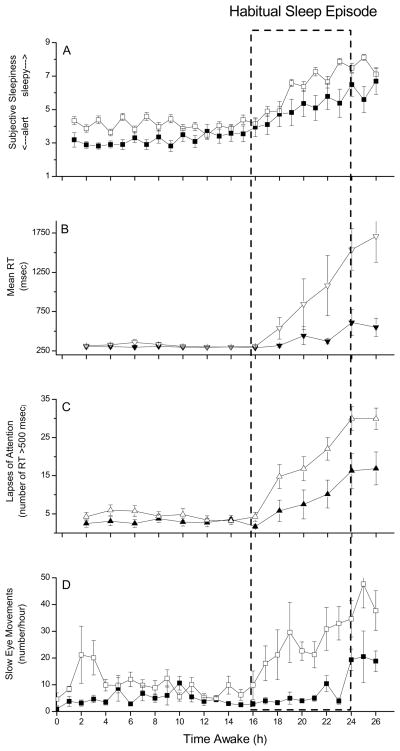
Across the 26-hour vigil, the older subjects rated themselves as more alert than the young subjects on the KSS [4.16 ± 1.37 vs. 5.69 ± 0.76, P < .01]. As the duration of waking was extended into the usual nighttime hours, the self-ratings of both groups indicated they felt progressively sleepier [estimate of increase in sleepiness rating per hour = 0.15 ± 0.03, P < .01; Figure 1A], and there were age group differences in subjective alertness [estimate of group difference = 0.59 ± 0.31, P = .06]. Mixed model analysis supported these results [elapsed time: P < .01; age group: P < .04] with no significant interaction [elapsed time * age group: P = .6], suggesting that sleepiness in both groups similarly increased across the 26 hours.
Figure 1.
Subjective Sleepiness, Reaction Time, Lapses of Attention, and Attentional Failures Across 26 Hours of Wakefulness in Young and Young-Old Subjects. Group average (± SEM) data are plotted with respect to time since scheduled awakening for the older (filled symbols) and young (open symbols) subjects. Dashed box indicates time of usual sleep episode.
Subjective sleepiness ratings from the Karolinska Sleepiness Scale (KSS; scale range from 1=very alert to 9=very sleepy) are presented in Panel A.
Mean reaction time (RT, in milliseconds) from each 10-minute Psychomotor Vigilance Task (PVT) are presented in Panel B. As indicated in the first 16 hours of data, “normal” mean RT using this test in well-rested individuals is ~250 milliseconds and shows little variability [17;18].
The total number of lapses of attention (reaction times >500 milliseconds) from each 10-minute PVT are presented in Panel C. Well-rested individuals will typically have very few (<5 per test administration) lapses of attention under these conditions [17;18].
Attentional failures, defined as intrusions of slow eye movements (SEM) from continuous electro-oculographic recordings during EEG-verified wakefulness, were summed hourly and are presented in Panel D. Well-rested individuals will typically have very few SEM under these conditions [12;18].
Sustained Attention
Average RT on the PVT was faster in the older subjects across the 26-hour vigil [362.02 ± 232.81 milliseconds vs. 628.63 ± 997.34 milliseconds, P < .01; see Figure 1B]. General linear model analysis revealed that as waking was extended, RT slowed [estimate of reduction in RT per hour = 23.09 ± 121.95 milliseconds, P < .01; Figure 1B], and there was an interaction between elapsed time and age group [estimate of interaction = 23.53 ± 97.08, P < .01] suggesting that the slowing of RT with time awake differed between the age groups. That analysis did not show age as a significant independent effect on RT [estimate of group difference = 4.66 ± 7.30, P = .12], likely due to the large individual variability in the response to time awake. However, mixed model analysis, which takes into account individual differences, indicated that there were significant effects of both elapsed time [P < .01] and age group [P = .03] on RT, as well as a significant interaction [elapsed time * age group: P < .01].
The slowing of RT as the vigil progressed was in part due to an increase in the number of lapses of attention [estimate of increase in the number of lapses per hour = 0.11 ± 0.02, P < .01; Figure 1C]. Overall, the older subjects had fewer lapses than the young subjects [6.18 ± 9.33 vs. 11.63 ± 14.4, P < .01]. While general linear model analysis did not indicate a difference between age groups in the number of lapses [estimated effect of age group = 0.49 ± 0.52, P = .35] nor a significant interaction between time awake and age [estimated interaction effect = 0.01 ± 0.02, P = .76], mixed model analysis indicated a significant effect of elapsed time [P < .01] and a significant interaction between elapsed time and age [P < .01].
Attentional Failures
There were few attentional failures (as indexed by SEM) in either age group across the first 16 hours awake, although the older subjects had even fewer than the young subjects [4.86 ± 4.56 vs. 10.34 ± 15.36, P < .01]. As waking was extended, the number of attentional failures in both groups increased, with the older subjects showing fewer attentional failures during the 10 hours of extended wakefulness [10.75 ± 13.25 vs. 30.43 ± 32.1, P < .01; Figure 1D]. General linear model analysis indicated an increase in attentional failures with elapsed time [estimate of increase in the number of attentional failures per hour = 0.06 ± 0.02, P < .01], with young subjects having more attentional failures overall [estimate of group difference = 0.82 ± 0.33, P < .02], and no interaction between elapsed time and age [P = .82]. Analysis of these data in binary format was consistent with this [estimate of effect of elapsed time = 0.07 ± 0.01, P < .01; estimate of group difference = 1.39 ± 0.33, P < .01; estimate of interaction, P = .36].
Inadvertent Sleep Episodes
None of the 10 older subjects had any epoch of EEG recording that met the criteria for sleep (19) throughout the 26 hours, whereas 11 of the 19 young subjects (58%) for whom we had complete recordings fell asleep (Log-Rank Test = 8.87, P < .01). Of those young subjects who fell asleep, most slept for short durations (< 10 minutes total). While most of the inadvertent sleep was Stage 1, the two participants with the greatest amount of sleep (21 and 26 minutes total) also had epochs of Stage 2.
DISCUSSION
We found that healthy young-old subjects were better able than younger subjects to maintain alertness and performance and to sustain wakefulness during 26 hours of acute sleep deprivation. Young adults were at greater risk of falling asleep, exhibited greater slowing in their response times, and rated themselves as sleepier throughout the 26-hour vigil than did the older participants. These findings challenge the conventional belief that older people are sleepier than young adults (4;5;21), and demonstrate that healthy young-old people are less likely to succumb to the adverse effects of sleep loss on sustained performance than are younger adults. We found that during normal daytime hours the attention and subjective alertness of these healthy older adults were not impaired when compared with that of the young adults. As the duration of waking extended beyond the typical 16-hour day, the older subjects were able to remain awake while more than half of the young adults were unable to do so. During the nighttime hours the older participants rated themselves as more alert, demonstrated faster reaction times, and had fewer attentional failures. This occurred despite the fact that the sleep quality and quantity of the older subjects on the night before the acute sleep deprivation was significantly worse compared with the young adults, as is typical of even such healthy young-old individuals.
These data are consistent with aspects of prior reports comparing the response of young and older adults to acute sleep deprivation or to chronic partial sleep deprivation. Bonnet & Rosa (7) compared performance at night before and after two nights of sleep deprivation. They found few differences in reaction time between young and older subjects on the first night of sleep deprivation, but on the second night the older subjects were better able to maintain their performance. However, in that study, older subjects had to have a sleep efficiency above 85% to be included [better than average for older subjects (1)], and all subjects were allowed to drink coffee each morning and to leave the laboratory for several hours following the first night of sleep deprivation, potentially confounding the results. In a more recent study (8), another group subjected young and older men to 40 hours of sleep deprivation and assessed their performance on the Psychomotor Vigilance Task. They found that the older subjects were better able to maintain their performance when sleep deprived, although their older subjects had slower reaction times during daytime hours than their young subjects. In that study, older subjects had to demonstrate sleep efficiency better than 80% and an apnea-hypopnea index less than 5 in order to be included, making them non-representative of most older adults (1;22). In another study that included 36 hours of sleep deprivation (23), young subjects had significantly more inadvertent sleep episodes than older subjects, as we found in our study. Our study measured all of these factors (subjective sleepiness, inadvertent sleep, and reaction time) simultaneously, and monitored both young and older subjects when they slept before the vigil as well as throughout the sleep deprivation. Furthermore, our older subjects, while healthy and without significant sleep disorders, were not selected on the basis of their baseline sleep quality, as was the case in some of the prior studies. Our study conditions thus allowed for control of many of the factors that had confounded these prior studies. Our conclusions are also consistent with data from several studies comparing the response of young and older subjects to chronic partial sleep deprivation (24;25)
There are several mechanisms that could account for our finding that healthy young-old adults exhibit greater endurance to acute sleep loss than do younger adults. The most straightforward would be that sleep need declines with age and that slow wave activity, sleep continuity, and sleep duration decline as a result of this decreased need for sleep. An age-related reduction in the rate at which sleep need builds up during wakefulness could then account for our results. An alternative hypothesis is that sleep ability, rather than sleep need, changes with age. Animal data indicate that adenosine levels in the basal forebrain, a putative marker of homeostatic sleep drive, increase with age (26), suggesting that sleep pressure should be greater with age. If this is so, why did our older subjects show a reduced sleep tendency? One explanation is that the ventrolateral preoptic nucleus (VLPO) of the hypothalamus, which functions as a neurobiological switch to enable the transition from wakefulness to sleep (27;28), exhibits an age-related decline in cell number in humans (29). If a fixed number of VLPO cells must be activated to initiate the transition from wakefulness into sleep, then an age-related decline in cell number could make that transition more difficult even in the presence of an elevated sleep drive. In fact, partial lesions of the VLPO reduce total sleep time and sleep consolidation in young animals whose sleep need has presumably not been altered (30). Of course, both mechanisms could be operating, i.e., decreased sleep need and decreased ability to initiate and maintain sleep with advancing age. Additional studies in mammalian models are needed to determine the exact mechanisms underlying our findings, and studies in both healthy and “typical” older humans, including middle-old and old-old individuals, in which other measures of performance and mental functions are collected under conditions of acute and chronic sleep loss are also needed.
Regardless of the mechanism, our data are consistent with the observation that more than half of sleep-related motor vehicle crashes happen in drivers under 30 years of age compared with only 5% of such accidents in drivers over age 65 (31), and that younger drivers are five times more likely to report dozing behind the wheel than older drivers (3). Yet there is widespread acceptance of the notions that older people are sleepy and that daytime sleepiness is a normal consequence of aging. Given that healthy older people are not sleepier, why is this belief so widespread among the general public and many physicians?
First, the sleep of even very healthy young-old adults (such as those in this study) is disrupted when compared with the sleep of younger adults. When the sleep of young adults is experimentally disrupted to mimic the sleep of a typical older adult (32), those young adults become sleepier during the day. Many older adults, unlike the healthy participants in our study, take medications or have medical conditions (arthritis and other chronic pain conditions; chronic respiratory conditions; heartburn or reflux disease) that disrupt sleep, leading to daytime sleepiness. Furthermore, many older individuals have undiagnosed sleep-disordered breathing (22) which can disrupt sleep leading to daytime sleepiness. At present, an estimated 80–90% of patients in the US with such sleep disorders are undiagnosed or untreated (31).
Our findings highlight that sleepiness is not an inevitable consequence of aging, and suggest instead that daytime sleepiness in older people may be a consequence of medical conditions, medications, and undiagnosed sleep disorders associated with aging. Older adults presenting to their physician with the symptoms of (i.e., falling asleep during the daytime) or a complaint of daytime sleepiness should be diagnosed and treated for the underlying cause of that sleepiness.
Acknowledgments
We wish to thank the study participants; the subject recruiters; technicians of the DSM Chronobiology Core for staffing the extended wake episodes; the Brigham and Women’s Hospital General Clinical Research Center staff; E.J. Silva, M.J. Duverne-Joseph, and A.M. Guzik for assistance with the data processing; J.M. Ronda for I.S. assistance with data collection and analysis; and Dr. J. Ellis. We also acknowledge the contribution of Dr. Sat Bir S. Khalsa, who was Project Leader during the data collection segment of approximately half of the studies of young subjects.
Funding: The studies were supported by grants R01 AG06072 and R01 MH45130 from the National Institutes of Health (NIH) and were conducted in the Brigham and Women’s Hospital General Clinical Research Center supported by NIH grant M01 RR02635. Support for analysis of the data was also provided by NIH grant P01 AG09975.
Footnotes
Paper presentations relevant to this work: Willson HJ, Ellis J, Guzik AM, Czeisler CA, Duffy JF. Healthy older adults have fewer attentional failures than young adults during extended wakefulness. Sleep 2007; 30:A114 (abstract presented at Sleep 2007 meeting, Minneapolis, MN, June 2007).
CONFLICT OF INTEREST
Dr. Duffy, Dr. Wang, and Ms. Willson report no conflicts of interest. The studies reported here were funded by NIH research grants, and did not test the effects of any drug or medical device. As such, there are no actual conflicts. However, Dr. Czeisler lists here all his potential conflicts: he has received consulting fees from or served as a paid member of scientific advisory boards for: Actelion, Ltd.; Cephalon, Inc.; Delta Airlines; Eli Lilly and Co.; Garda Siochana Inspectorate (Dublin, Ireland); Johnson & Johnson; Koninklijke Philips Electronics, N.V.; Portland Trail Blazers; Sanofi-Aventis, Inc.; Sleep Multimedia, Inc.; Respironics, Inc; Sepracor, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc.; Zeo Inc. (formerly Axon Labs, Inc.). He owns an equity interest in Zeo Inc. (formerly Axon Labs, Inc.); Lifetrac, Inc.; Somnus Therapeutics, Inc.; and Vanda Pharmaceuticals, Inc. He has received lecture fees from the Cephalon, Inc. and Sanofi-Aventis, Inc.
Dr. Czeisler has also had clinical trial research contracts from Cephalon, Inc., and Merck; an investigator-initiated research grant from Cephalon, Inc.; and his research laboratory at the Brigham and Women’s Hospital has received unrestricted research and education funds and/or support for research expenses from Cephalon, Inc. and ResMed.
The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which Dr. Czeisler directs, has received unrestricted research and educational gifts and endowment funds from: : Aetna US Healthcare, Alertness Solutions, Inc., Axon Sleep Labs, Inc., Boehringer Ingelheim Pharmaceuticals, Inc., Bristol-Myers Squibb, Catalyst Group, Cephalon, Inc., Clarus Ventures, Comfortaire Corporation, Committee for Interns and Residents, Farrell Family Foundation, George H. Kidder, Esq., Gerald McGinnis, GlaxoSmithKline, Herbert Lee, Innovative Brands Group, Jazz Pharmaceuticals, Jordan’s Furniture, King Koil Sleep Products, Division of Blue Bell Mattress, Land and Sky, Merck & Co., Inc., MPM Capital, Nature’s Rest, Neurocrine Biosciences, Inc., Orphan Medical/Jazz Pharmaceuticals, Park Place Corporation, Peter C. Farrell, Ph.D., Pfizer, Inc., Purdue Pharma L.P., ResMed, Inc., Respironics, Inc., Sanofi-Aventis, Inc., Sanofi-Synthelabo, Sealy Mattress Company, Sealy, Inc., Sepracor, Inc., Simmons Co., Sleep HealthCenters LLC, Spring Aire, Spring Air Mattress Co., Takeda Pharmaceuticals, Tempur-Pedic, Tempur-Pedic Medical Division, Total Sleep Holdings, Vanda Pharmaceuticals, Inc., and the Zeno Group, together with gifts from many individuals and organizations through an annual benefit dinner. The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon, Inc., Takeda Pharmaceuticals, Sanofi-Aventis, Inc. and Sepracor, Inc.
Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms.
References
- 1.Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier Saunders; 2005. pp. 24–38. [Google Scholar]
- 2.Prinz PN, Vitiello MV, Raskind MA, et al. Geriatrics: Sleep disorders and aging. N Engl J Med. 1990;323:520–526. doi: 10.1056/NEJM199008233230805. [DOI] [PubMed] [Google Scholar]
- 3.Executive Summary of the 2003 “Sleep in America” Poll. Washington, DC: National Sleep Foundation; 2003. [Google Scholar]
- 4.Feinsilver SH. Sleep in the elderly. What is normal? Clin Geriatr Med. 2003;19:177–188. doi: 10.1016/s0749-0690(02)00064-2. [DOI] [PubMed] [Google Scholar]
- 5.Whitney CW, Enright PL, Newman AB, et al. Correlates of daytime sleepiness in 4578 elderly persons: The Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet MH, Rosa RR. Sleep and performance in young adults and older normals and insomniacs during acute sleep loss and recovery. Biol Psychol. 1987;25:153–172. doi: 10.1016/0301-0511(87)90035-4. [DOI] [PubMed] [Google Scholar]
- 8.Adam M, Rétey JV, Khatami R, et al. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep. 2006;29:55–57. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- 9.Brendel DH, Reynolds CF, III, Jennings JR, et al. Sleep stage physiology, mood, and vigilance responses to total sleep deprivation in healthy 80- year-olds and 20-years-olds. Psychophysiology. 1990;27:677–685. doi: 10.1111/j.1469-8986.1990.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy JF, Dijk DJ. Getting through to circadian oscillators: Why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 12.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt JK, Cajochen C, Ritz-De Cecco A, et al. Low-dose, repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–381. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 14.Gillberg M, Kecklund G, Åkerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–241. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 15.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods, Instruments & Computers. 1985;17:652–655. [Google Scholar]
- 16.Dinges DF, Orne MT, Whitehouse WG, et al. Temporal placement of a nap for alertness: Contributions of circadian phase and prior wakefulness. Sleep. 1987;10:313–329. [PubMed] [Google Scholar]
- 17.Dinges DF, Kribbs NB. Performing while sleepy: Effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. Chichester, UK: John Wiley and Sons, Ltd; 1991. pp. 97–128. [Google Scholar]
- 18.Cajochen C, Khalsa SBS, Wyatt JK, et al. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol Regul Integr Comp Physiol. 1999;277:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, D.C: U.S. Government Printing Office; 1968. [Google Scholar]
- 20.Van Dongen HPA, Olofsen E, Dinges DF, et al. Mixed-model regression analysis and dealing with interindividual differences. Methods Enzymol. 2004;384:139–171. doi: 10.1016/S0076-6879(04)84010-2. [DOI] [PubMed] [Google Scholar]
- 21.Philip P, Taillard J, Sagaspe P, et al. Age, performance and sleep deprivation. J Sleep Res. 2004;13:105–110. doi: 10.1111/j.1365-2869.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 22.Pavlova MK, Duffy JF, Shea SA. Polysomnographic respiratory abnormalities in asymptomatic individuals. Sleep. 2008;31:241–248. doi: 10.1093/sleep/31.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buysse DJ, Monk TH, Reynolds CF, III, et al. Patterns of sleep episodes in young and elderly adults during a 36-hour constant routine. Sleep. 1993;16:632–637. [PubMed] [Google Scholar]
- 24.Stenuit P, Kerkhofs M. Age modulates the effects of sleep restriction in women. Sleep. 2005;28:1283–1288. doi: 10.1093/sleep/28.10.1283. [DOI] [PubMed] [Google Scholar]
- 25.Bliese PD, Wesensten NJ, Balkin TJ. Age and individual variability in performance during sleep restriction. J Sleep Res. 2006;15:376–385. doi: 10.1111/j.1365-2869.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 26.Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, et al. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–370. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Gaus SE, Strecker RE, Tate BA, et al. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–294. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 28.Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: Sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21:482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- 29.Hofman MA, Swaab DF. The sexually dimorphic nucleus of the preoptic area in the human brain: A comparative morphometric study. J Anat. 1989;164:55–72. [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J, Greco MA, Shiromani P, et al. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colten HR, Alteveogt BM, editors. Institute of Medicine. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, D.C: National Academies Press; 2006. [PubMed] [Google Scholar]
- 32.Bonnet MH. The effect of sleep fragmentation on sleep and performance in younger and older subjects. Neurobiol Aging. 1989;10:21–25. doi: 10.1016/s0197-4580(89)80006-5. [DOI] [PubMed] [Google Scholar]