Abstract
Hepatitis C virus (HCV) infects more than three million new individuals worldwide each year. In a high percentage of patients, acute infections become chronic, eventually progressing to fibrosis, cirrhosis, and hepatocellular carcinoma. Given the lack of effective prophylactic or therapeutic vaccines, and the limited sustained virological response rates to current therapies, new approaches are needed to prevent, control, and clear HCV infection. Entry into the host cell, being the first step of the viral cycle, is a potential target for the design of new antiviral compounds. Despite the recent discovery of the tight junction-associated proteins claudin-1 and occludin as HCV co-receptors, which is an important step towards the understanding of HCV entry, the precise mechanisms are still largely unknown. In addition, increasing evidence indicates that tools that are broadly employed to study HCV infection do not accurately reflect the real process in terms of viral particle composition and host cell phenotype. Thus, systems that more closely mimic natural infection are urgently required to elucidate the mechanisms of HCV entry, which will in turn help to design antiviral strategies against this part of the infection process.
Keywords: Cellular polarization, Tight junctions, Lipoprotein metabolism, Hepatitis C virus
INTRODUCTION
Hepatitis C virus (HCV) is an enveloped, positive-polarity RNA virus that belongs to the Flaviviridae family and infects mainly hepatocytes[1]. The HCV genome encodes a polyprotein that is processed by host and viral proteases to yield ten mature products, which include three structural proteins [the capsid protein (core) and two envelope glycoproteins (E1 and E2)], the p7 protein, and the non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B). The HCV particle consists of a nucleocapsid surrounded by a lipid bilayer harboring the two envelope glycoproteins, which heterodimerize and play a major role in HCV entry[1]. Cellular infection begins with the attachment of the viral particle to the host cell and, after interacting with cell surface molecules, the virus is subjected to clathrin-mediated endocytosis and its envelope is fused with the endosomal membrane, releasing the viral genome into the cytosol[1]. These steps involve a set of attachment factors and cellular co-receptors, including highly sulfated heparan sulfate, the low-density lipoprotein receptor (LDL-R), the scavenger receptor class B type I (SR-BI), the tetraspanin CD81, and the tight junction (TJ) proteins, claudin-1 and occludin[2-4]. These molecules are not exclusively present in hepatocytes; therefore, HCV hepatotropism may be determined by other factors, such as the absence of inhibitory proteins[5].
In early reports, the soluble E2 envelope protein was employed as an approach to search for host cell HCV binding factors[6,7]. However, the conformation and function of the soluble glycoprotein may differ considerably from HCV envelope-anchored E2. In addition, envelope-anchored E2 is associated with E1[8], which may have additional implications in terms of receptor recognition and binding. The study of HCV entry was boosted by the use of HCV pseudotyped particles (HCVpp)[9], providing an infection system exclusively relying on the envelope glycoproteins. Since the establishment of a cell culture-derived HCV (HCVcc)[10-12], it has become the most powerful tool for studying HCV because it reflects the complete viral cycle. The use of both HCVpp and HCVcc has been of crucial importance in the discovery of new receptors, and has enabled high-throughput assays to test molecules for their ability to inhibit HCV entry.
INHIBITION OF HCV ENTRY AS A THERAPEUTIC ALTERNATIVE
HCV infection is the most frequent cause of liver failure worldwide[13,14]. Current therapies based on PEGylated interferon-alpha and ribavirin often fail to clear the infection and present a wide spectrum of systemic side effects[15]; therefore, alternative therapeutic options need to be developed. Among the different steps of HCV cycle, viral entry could be considered as a clinical target, especially in the context of orthotopic liver transplantation, where allograft reinfection occurs within hours after reperfusion and is followed by an accelerated chronic disease progression[16]. To date, several molecules have been found to inhibit HCV entry. Matsumura et al[17] showed that phosphorothioate oligonucleotides, previously described as HIV entry inhibitors[18], blocked HCV entry in vivo. Other inhibitors of HIV entry, such as cyanovirin-N, have also proved effective[19]. Furthermore, serum amyloid A, an acute phase protein mainly produced by the liver in response to different stimuli, including infections, has been demonstrated to inhibit HCV entry[20,21]. Moreover, the milk thistle (Silybum marianum)-derived silymarin and its purified flavonolignans have been recently shown to inhibit HCV infection both in vitro and in non-responder patients[22,23], blocking viral entry and transmission[24]. Therefore, increasing evidence suggests that blocking the entry step of HCV infection may be a good therapeutic alternative.
The genetic diversity of HCV contributes to its evasion from the host immune response[25], challenging the development of effective vaccines and virus-targeted inhibitors[26-28]. Nonetheless, this problem could be overcome by developing antiviral strategies aimed at blocking essential host factors for viral infection. To this end, multiple strategies have been pursued to inhibit HCV entry at different levels, including viral attachment, post-binding events, and fusion with the endosomal membrane[4,29]. One of these approaches consists of interfering with the interaction between the viral particle and cell surface co-receptors by the use of glycosaminoglycans, natural ligands, recombinant proteins, or blocking antibodies (Table 1). Notably, it has been demonstrated that antibodies against CD81 can prevent HCV infection of human liver-uPA-SCID mice[16], probably by inhibiting the E2-CD81 binding process[6,16,30]. As a more realistic and economical alternative, small molecules with similar properties could be used instead of blocking antibodies. In search of these compounds, several high-throughput screenings have been performed recently to identify molecules with the ability to inhibit HCV infection at the entry step[31-33]. Importantly, the possible cytopathic effects of these inhibitors should be assayed prior to starting clinical trials and considering them as potential therapeutic options. Chockalingam et al[32] developed a cell protection screen where cytotoxicity and inhibition of infection were evaluated simultaneously. As a more practical approach, Gastaminza et al[31] performed the screening with a set of drugs that had already been clinically approved.
Table 1.
Inhibition of infectivity by the blockade of hepatitis C virus co-receptors in different systems
|
Host |
Viral particle |
Ref. | ||||||
| Co-receptor | Blocking agent | Cell lines | PHH | In vivo | HCVpp | HCVcc | Serum | |
| Heparan sulfate | Heparin | x | Y | [87] | ||||
| x | Y | [30,88,89] | ||||||
| x1 | Y | [90] | ||||||
| LDL-R | Anti-LDL-R | x | N | [36] | ||||
| x | Y | [38] | ||||||
| Soluble LDL-R | x | Y | [38] | |||||
| LDLs/VLDLs | x | N | [9,37,91] | |||||
| x | N | [37] | ||||||
| x | Y | [38] | ||||||
| CD81 | Anti-CD81 | x | Y | [9,36,92] | ||||
| x | Y | [30,88,89] | ||||||
| x | Y | [9,92] | ||||||
| x | Y | [34] | ||||||
| x | Y | [34] | ||||||
| x | Y | [16] | ||||||
| CD81-LEL | x | Y | [9,36] | |||||
| x | Y | [34] | ||||||
| x | N | [34] | ||||||
| Knockdown | x | Y | [37] | |||||
| x | Y | [89] | ||||||
| x | Y | [34] | ||||||
| SR-BI | Anti-SR-BI | x | N | [36] | ||||
| x | Y | [35,93] | ||||||
| x | Y | [30,89] | ||||||
| BLT-4 | x | N/Y2 | [91,93] | |||||
| ITX 5061/7650 | x | Y | [94] | |||||
| x | Y | [94] | ||||||
| oxLDLs | x | Y | [37] | |||||
| x | Y | [37] | ||||||
| Knockdown | x | N/Y2 | [37] | |||||
| x | Y | [89] | ||||||
| Claudin-1 | Anti-claudin-1 | x | Y | [30,56] | ||||
| x | Y | [30,56] | ||||||
| x | Y | [56] | ||||||
| x | Y | [56] | ||||||
| Knockdown | x | Y | [42,43,95] | |||||
| x | Y | [42,43,95] | ||||||
| Occludin | Knockdown | x | Y | [43-45] | ||||
| x | Y | [43-45] | ||||||
x: Experimental system employed; Y: Inhibition of infection; N: No inhibition of infection;
HCV-core immortalized PHH;
Only in the presence of high density lipoproteins. HCVpp: Hepatitis C virus pseudotyped particles; HCVcc: Cell culture-derived hepatitis C virus.
The study of HCV infection and the search for inhibitory molecules are usually carried out with the use of HCVpp or HCVcc, and a highly permissive cell line, such as Huh7 and its derivatives. However, several conflicting results have arisen when attempting to validate the data in a more pathophysiologically relevant context (Table 1). A soluble form of CD81 was shown to prevent infection of Huh7.5 cells by HCVcc; however, it was not effective when primary human hepatocytes (PHH) were challenged with serum-derived virus[34]. Moreover, infection of hepatoma-derived cell lines with HCVpp and HCVcc does not seem to depend on LDL-R[35-37], whereas it has been demonstrated to participate in the infection of PHH with HCV from human plasma[38]. These facts stress the importance of being cautious with results obtained from HCV surrogates and cell lines, which should be validated in vivo whenever possible or at least in systems that more closely mimic real infection.
CELL POLARIZATION, TJ-ASSOCIATED PROTEINS, AND HCV ENTRY
In contrast to “simple” polarized cells, which present the typical epithelial columnar phenotype with individual basolateral and apical domains, hepatocytic polarity is very peculiar and complex[39]. The plasma membrane of polarized hepatocytes is divided into several basolateral and apical poles, the latter forming a continuous network of bile canaliculi (BC) into which bile is secreted[40]. BC are delimited by TJs, which maintain cell polarity by separating apical from basolateral domains and form the intercellular barrier between bile and blood[41]. Claudin-1 was the first TJ-associated protein to be described as a HCV co-receptor[42]. Soon after, occludin was also shown to participate in HCV entry[43-45]. Despite these discoveries clearly pointing to a role of TJs in HCV cell entry, recent works have reported conflicting data about how cell junctions and polarity influence HCV infection. Perturbation of cellular junctions by calcium depletion promotes opposing effects depending on the system employed, e.g. it decreases viral entry in Huh7 cells[46] but increases it in “simply” polarized Caco-2 cells[47]. Furthermore, junctional accumulation of claudin-1 has been shown to either improve[48] or hinder[49] infection of Huh7.5 and polarized HepG2 cells, respectively. Collectively, these data suggest that the HCV entry process may vary considerably depending on the polarization state of the target cells.
Several studies have questioned the importance of TJ integrity in the function of claudin-1 and occludin as HCV co-receptors. For example, HCV infection is not affected after knocking down other TJ-associated proteins, such as ZO-1 and JAM-A[45], and claudin-1’s association with CD81 at the basolateral membrane of HepG2 cells, but not at the TJ, defines HCV entry[50,51]. Furthermore, fluorescent HCV particle internalization generally occurs outside of cell-cell junctions[52], and VEGF induces a reduction of junctional occludin concomitant with an increase of HCV infectivity[53]. Moreover, claudin-1 and occludin mutants lacking domains that are important for their correct junctional localization and function are still capable of rendering cells susceptible to HCVpp entry[42,54,55]. Finally, HCV infection of HepG2 cells is negatively regulated by cell polarity[49,53], but is not affected by TNF-α- and IFN-γ-mediated TJ disruption[49], and claudin-1 blocking antibodies inhibit HCV infection without perturbing TJs[30,56]. Taken together, these data strongly suggest that the role of claudin-1 and occludin in viral entry is relevant, but not necessarily when these proteins are part of functional TJs, which may indeed be a barrier for HCV infection. Interestingly, it has recently been demonstrated that hepatitis A virus infects HepG2-derived cells from the basolateral domain and that TJ-dependent polarization restricts infection[57].
The mechanisms by which claudin-1 and occludin participate in HCV entry have not been clearly established. In both cases, an extracellular loop of the protein has been shown to be indispensable for infection[42,44,55,58]. Several reports have shown that occludin precipitates with HCV E2 in infected, transfected, or replicon-containing cells[43,55,59], but its direct interaction with viral particles or envelope glycoproteins has not been demonstrated. Additionally, it has been shown that occludin interacts with dynamin II, a well known regulator of endocytosis[55]. This observation, along with data obtained from cell-cell fusion experiments[45], suggests that occludin might participate in late steps of the HCV entry process. Interestingly, occludin endocytosis has been implicated in group B coxsackievirus infection, although not by directly interacting with the virus[60]. On the other hand, kinetic studies with blocking antibodies have shown that claudin-1 mediates an HCV entry step closely linked to CD81[30]. Indeed, it has been described that basolateral pools of claudin-1 are associated with CD81 in polarized HepG2 cells[50,51], and that disrupting this interaction, either by site directed mutagenesis or claudin-1 blocking antibodies, neutralizes HCV infection by reducing E2 association with the cell surface[30,51]. However, Cukierman et al[54] generated a mutant version of claudin-1 which, in spite of maintaining its interaction with CD81, no longer localized to cell-cell contacts and lost HCV receptor properties. This result suggests that, besides favoring E2 binding to the host cell, additional mechanisms involving claudin-1 participation in HCV entry may exist. Nevertheless, as these experiments were carried out in the non-hepatic, non-polarized HEK cell line, data should be carefully interpreted. This is a good example of how cell polarity may influence the results obtained, especially when studying features of TJ-associated proteins in a hepatocellular context.
LIPID METABOLISM AND HCV ENTRY
Cell polarization is crucial for the correct localization and function of TJ-associated proteins with HCV receptor activity, which could in turn be important for viral entry[41,61]. In addition, polarization may affect other steps of the HCV cycle, such as assembly and egress. Indeed, it has been shown that assembly of RNA enveloped viruses in MDCK cells is closely related to cell polarization[62]. It is also noteworthy that polarization is tightly linked to lipoprotein secretion[63,64], especially because some low density natural HCV particles have been found to be complexed with ApoB and/or ApoE-positive triglyceride-rich lipoproteins[65-67]. This association is believed to take place during viral egress[68] because HCVcc virions were found to be secreted in a manner that parallels the formation of VLDLs[69-71]. Thus, cell polarization may influence lipoprotein secretion, which is important for the generation of correctly assembled HCV progeny. Indeed, non-polarized Huh7-derived cells have been shown to be unable to secrete authentic, ApoB-containing VLDLs[72]. In addition, when HCVcc generated in these cells was subjected to isopycnic gradient ultracentrifugation, it was found to have higher average buoyant density than viral particles obtained from PHH[72]. Interestingly, the density profile of serum-derived viral particles obtained from HCVcc-infected animals was significantly lower than that of the initial HCVcc inoculum[73]. In both reports, specific infectivity of PHH and serum-derived HCV particles was shown to be greatly increased compared to standard HCVcc, and these properties were lost after passaging the virus again in Huh7.5 cells. These findings strongly suggest that HCVcc generated in Huh7-derived cells probably presents a defective lipoprotein association, which may in turn affect viral infection.
The lipoprotein composition and distribution within the viral particle may thus be important for the mechanisms underlying HCV entry[74]. Indeed, several reports have shown that lipoprotein lipase and hepatic triglyceride lipase alter both the physiological characteristics and the infectivity of HCV[75-77]. This notion is supported by the fact that LDL-R participates in the first steps of the entry process[38], and that viral particle density is inversely correlated to infectivity in vivo[66]. Additionally, the virus-host interaction could be affected in a context of a lipoprotein-defective viral particle because of changes in the exposure and accessibility of E2 to cellular co-receptors. In fact, this could explain the differences observed between HCVcc and serum-derived HCV infection in terms of inhibition by the soluble CD81 large extracellular loop[34]. Moreover, in contrast to serum-derived HCV[38], infection with HCVcc and HCVpp seems to be LDL-R independent[35-37], probably because of differences in lipoprotein composition.
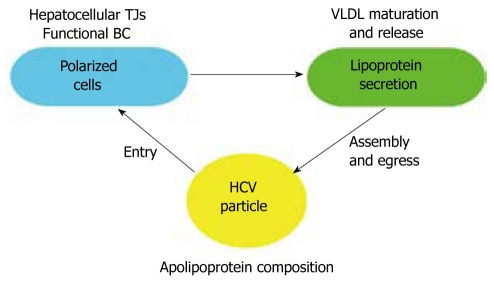
Therefore, it is necessary to study HCV entry in systems that more accurately mimic the viral cycle in a pathophysiological context, from viral particle generation to target cell infection. The interplay between HCV and hepatocytes seems to be closely related to their particular phenotype (Figure 1); therefore, data obtained from models that do not reflect hepatocellular polarization and lipoprotein secretion should be cautiously interpreted.
Figure 1.
Interplay among cell polarization, lipoprotein secretion, and hepatitis C virus particle assembly, release, and entry into host cells. The correct polarization of hepatocytes, implying the presence of functional bile canaliculi delimited by TJs, may be important for the proper maturation and secretion of lipoproteins. This process is tightly associated with the composition and assembly of hepatitis C virus (HCV) lipoviroparticles and their exit from infected cells. Finally, HCV entry may be affected by the lipoprotein composition of the viral particle, the hepatocellular polarization of target cells, and the localization of the TJ-associated proteins claudin-1 and occludin.
TOWARDS A MORE PHYSIOLOGICALLY RELEVANT MODEL
The use of HCVpp and HCVcc has meant a great advance in HCV research. Their adaptability to high-throughput analysis has enabled genome-wide screening for host proteins involved in the HCV cycle at different stages. In addition, they provide a valuable tool for testing the possible antiviral activity of large chemical libraries of compounds. However, it is important to bear in mind the limitations of these in vitro models, as both viral particles and target cells differ notably from serum-derived HCV and polarized hepatocytes, respectively. Thus, results obtained with HCVpp and HCVcc should be validated in systems that more closely mimic real HCV infection before establishing firm conclusions. To this end, significant work is being done by Molina and colleagues, who have already confirmed a role for LDL-R and CD81 in serum-derived HCV infection of PHH[34,38]. This model is considered an accurate in vitro system, as cells can be maintained in a differentiated phenotype to retain their polarity and drug metabolizing capacities. In addition, they can be infected with serum-derived HCV of any genotype and, in contrast to what is observed in hepatoma cell lines (e.g. Huh7 cells and derivatives), the innate immune response is fully preserved[78]. However, production of measurable titers of progeny virus in this system has not been achieved[79], indicating that the model fails to reflect the entire HCV cycle. Recently, it has been shown that HCVcc infection of PHH results in the robust production of infectious viral particles, which were in turn able to efficiently infect naïve PHH[72]. This primary cultured-derived HCV (termed HCVpc), compared to HCVcc, exhibited lower average buoyant density and higher specific infectivity, reminiscent of what is seen for virus recovered from the blood of animals infected with HCVcc[72,73]. Therefore, HCVpc infection of PHH emerges as a valuable tool for studying the complete HCV cycle in a more relevant context. Nevertheless, this system presents the drawbacks of working with PHH, such as restricted availability, the difficulty of studying long-term infections, and the heterogeneity of samples and results. Interestingly, Matrigel-embedded 3D cultures of Huh7 cells display a hepatocyte-like polarization and are susceptible to HCVcc infection. Progeny viruses generated by these cultures, similarly to HCVpc, also present a shift towards lower densities (our unpublished observations). This result suggests that if hepatocellular polarity is achieved, it is possible to generate viral particles that more closely mimic real HCV, even when cell lines are used as the source of virus.
Animal models are essential to validate in vitro data, because not only hepatocytes, but also the liver as a whole, may determine the mechanisms of HCV cell entry. Indeed, the liver sinusoidal endothelial cell-expressed protein, L-SIGN, has been shown to bind serum-derived HCV[80] and mediate trans-infection of Huh7 cells by HCVpp[81,82]. Additionally, co-culture of PHH with liver sinusoidal endothelial cells significantly increases the expression of the HCV co-receptor LDL-R[83]. Regarding in vivo obtained data, CD81 is the only co-receptor that has been demonstrated to participate in HCV infection using the human liver-uPA-SCID mouse model[16]. This system, albeit constituting a useful tool, is limited by the fact that the animals lack a functional immune system. This may be important, not only for the outcome of the infection, but also for the entry process itself, because DC-SIGN, expressed on dendritic cells, has been shown to capture and transmit HCVpp to Huh7 cells[81,82]. More recently, peripheral blood B cells have been shown to exert a similar function[84]. To date, chimpanzees are the only immunocompetent in vivo system for studying HCV infection, but their use is limited by ethical concerns, restricted availability, requirement of special facilities, and very high costs[85]. In search of a small, HCV susceptible and immunocompetent animal model, it has been proposed to combine human liver chimeric models with mice harboring a human hematolymphoid system[85], although this approach depends on the availability of human primary cells. Thus, the ideal model would be an immunocompetent mouse susceptible to HCV infection without the need of harboring human cells. Given that HCV species tropism is restricted to human and primates[58,85], an alternative strategy consists of using an HCV variant able to infect murine cells. Bitzegeio et al. adapted HCVcc to mouse CD81 and identified three envelope glycoprotein mutations which together enhanced infection of cells with mouse or other rodent receptors by approximately 100-fold[86], thus overcoming the species-specific restriction of HCV cell entry. Another possible approach would be to employ genetically engineered mice bearing the human entry factors that confer species specificity[44]. However, mouse hepatocytes fail to initiate viral replication[85]; therefore, these models would not be able to mimic the entire HCV cycle.
CONCLUSION
During HCV infection, hepatocytes almost exclusively constitute both the target and the virus-producer cells. Thus, it is mandatory to perform HCV studies in systems that closely mimic the complex nature of hepatocyte phenotype. These models should enable the generation of viral particles that resemble the ones found in vivo, and reproduce the hepatocyte physiology as accurately as possible. Only the combination of these two factors will provide the necessary information to establish firm conclusions. Nevertheless, the choice of employing HCVpp, HCVcc, HCVpc, or serum-derived HCV to infect cell lines, PHH, or animals should depend on the stage of the research process: the in vitro systems are more adequate for high throughput screenings and the in vivo models are essential for validating the data. Once the mechanisms of HCV entry are deciphered in detail, this step of the viral cycle could be an effective target for the development of antiviral compounds. These inhibitors should ideally be effective for a broad range of HCV genotypes and subtypes, and even for other viruses, such as HIV, that might share some entry mechanisms and co-infect some patients. Thus, blocking cellular factors might be a good therapeutic alternative in the fight against viral genetic variability. However, targeting host molecules could alter their physiological functions and result in harmful side effects. In addition, this approach does not rule out the possible emergence of viral variants that would be able to circumvent the specific effect of the entry inhibitor. Moreover, HCV cell-to-cell transmission may bypass the inhibition of cell-free virus entry and allow viral spread. Therefore, clinical strategies based on broad-spectrum compounds or the combination of different therapeutic molecules should be developed to simultaneously interfere with several steps of the viral cycle to efficiently control infection with the minimal side effects.
Footnotes
Supported by CIBERehd to Moreno-Otero R, López-Cabrera M and Majano PL; SAF2007-61201 (Ministerio de Educación y Ciencia) to López-Cabrera M; CP03/0020 (Instituto de Salud Carlos III), SAF2007-60677 (Ministerio de Educación y Ciencia) and PI10/00101 (Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, FEDER) to Majano PL. Benedicto I was financially supported by CIBERehd and Molina-Jiménez F by Instituto de Salud Carlos III and FIB Hospital de la Princesa
Peer reviewers: A Mithat Bozdayi, MD, PhD, Hepatology Institute, Department of Gastroenterology, Ankara Medical Faculty, Ankara University, 06100 Cebeci Ankara, Turkey; Takashi Kojima, DVM., PhD, Department of Pathology, Sapporo Medical University School of Medicine, S.1, W.17, Chuo-ku, Sapporo 060-8556, Japan
S- Editor Sun H L- Editor Stewart GJ E- Editor Ma WH
References
- 1.Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 2.Dubuisson J, Helle F, Cocquerel L. Early steps of the hepatitis C virus life cycle. Cell Microbiol. 2008;10:821–827. doi: 10.1111/j.1462-5822.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 3.Burlone ME, Budkowska A. Hepatitis C virus cell entry: role of lipoproteins and cellular receptors. J Gen Virol. 2009;90:1055–1070. doi: 10.1099/vir.0.008300-0. [DOI] [PubMed] [Google Scholar]
- 4.Zeisel MB, Barth H, Schuster C, Baumert TF. Hepatitis C virus entry: molecular mechanisms and targets for antiviral therapy. Front Biosci. 2009;14:3274–3285. doi: 10.2741/3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha-Perugini V, Montpellier C, Delgrange D, Wychowski C, Helle F, Pillez A, Drobecq H, Le Naour F, Charrin S, Levy S, et al. The CD81 partner EWI-2wint inhibits hepatitis C virus entry. PLoS One. 2008;3:e1866. doi: 10.1371/journal.pone.0001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 7.Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubuisson J. Hepatitis C virus proteins. World J Gastroenterol. 2007;13:2406–2415. doi: 10.3748/wjg.v13.i17.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633–642. [Google Scholar]
- 10.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami M, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 13.Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–2100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- 14.Pawlotsky JM. Pathophysiology of hepatitis C virus infection and related liver disease. Trends Microbiol. 2004;12:96–102. doi: 10.1016/j.tim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 16.Meuleman P, Hesselgesser J, Paulson M, Vanwolleghem T, Desombere I, Reiser H, Leroux-Roels G. Anti-CD81 antibodies can prevent a hepatitis C virus infection in vivo. Hepatology. 2008;48:1761–1768. doi: 10.1002/hep.22547. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura T, Hu Z, Kato T, Dreux M, Zhang YY, Imamura M, Hiraga N, Juteau JM, Cosset FL, Chayama K, et al. Amphipathic DNA polymers inhibit hepatitis C virus infection by blocking viral entry. Gastroenterology. 2009;137:673–681. doi: 10.1053/j.gastro.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaillant A, Juteau JM, Lu H, Liu S, Lackman-Smith C, Ptak R, Jiang S. Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation. Antimicrob Agents Chemother. 2006;50:1393–1401. doi: 10.1128/AAC.50.4.1393-1401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helle F, Wychowski C, Vu-Dac N, Gustafson KR, Voisset C, Dubuisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J Biol Chem. 2006;281:25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 20.Lavie M, Voisset C, Vu-Dac N, Zurawski V, Duverlie G, Wychowski C, Dubuisson J. Serum amyloid A has antiviral activity against hepatitis C virus by inhibiting virus entry in a cell culture system. Hepatology. 2006;44:1626–1634. doi: 10.1002/hep.21406. [DOI] [PubMed] [Google Scholar]
- 21.Cai Z, Cai L, Jiang J, Chang KS, van der Westhuyzen DR, Luo G. Human serum amyloid A protein inhibits hepatitis C virus entry into cells. J Virol. 2007;81:6128–6133. doi: 10.1128/JVI.02627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132:1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 23.Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, Schöniger-Hekele M, Holzmann H, Steindl-Munda P. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 24.Wagoner J, Negash A, Kane OJ, Martinez LE, Nahmias Y, Bourne N, Owen DM, Grove J, Brimacombe C, McKeating JA, et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology. 2010;51:1912–1921. doi: 10.1002/hep.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabo G, Dolganiuc A. HCV immunopathogenesis: virus-induced strategies against host immunity. Clin Liver Dis. 2006;10:753–771. doi: 10.1016/j.cld.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Stoll-Keller F, Barth H, Fafi-Kremer S, Zeisel MB, Baumert TF. Development of hepatitis C virus vaccines: challenges and progress. Expert Rev Vaccines. 2009;8:333–345. doi: 10.1586/14760584.8.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeisel MB, Baumert TF. HCV entry and neutralizing antibodies: lessons from viral variants. Future Microbiol. 2009;4:511–517. doi: 10.2217/fmb.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgel P, Schuster C, Zeisel MB, Stoll-Keller F, Berg T, Bahram S, Baumert TF. Virus-host interactions in hepatitis C virus infection: implications for molecular pathogenesis and antiviral strategies. Trends Mol Med. 2010;16:277–286. doi: 10.1016/j.molmed.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Barth H, Liang TJ, Baumert TF. Hepatitis C virus entry: molecular biology and clinical implications. Hepatology. 2006;44:527–535. doi: 10.1002/hep.21321. [DOI] [PubMed] [Google Scholar]
- 30.Krieger SE, Zeisel MB, Davis C, Thumann C, Harris HJ, Schnober EK, Mee C, Soulier E, Royer C, Lambotin M, et al. Inhibition of hepatitis C virus infection by anti-claudin-1 antibodies is mediated by neutralization of E2-CD81-claudin-1 associations. Hepatology. 2010;51:1144–1157. doi: 10.1002/hep.23445. [DOI] [PubMed] [Google Scholar]
- 31.Gastaminza P, Whitten-Bauer C, Chisari FV. Unbiased probing of the entire hepatitis C virus life cycle identifies clinical compounds that target multiple aspects of the infection. Proc Natl Acad Sci USA. 2010;107:291–296. doi: 10.1073/pnas.0912966107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chockalingam K, Simeon RL, Rice CM, Chen Z. A cell protection screen reveals potent inhibitors of multiple stages of the hepatitis C virus life cycle. Proc Natl Acad Sci USA. 2010;107:3764–3769. doi: 10.1073/pnas.0915117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldick CJ, Wichroski MJ, Pendri A, Walsh AW, Fang J, Mazzucco CE, Pokornowski KA, Rose RE, Eggers BJ, Hsu M, et al. A novel small molecule inhibitor of hepatitis C virus entry. PLoS Pathog. 2010;6:Epub ahead of print. doi: 10.1371/journal.ppat.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina S, Castet V, Pichard-Garcia L, Wychowski C, Meurs E, Pascussi JM, Sureau C, Fabre JM, Sacunha A, Larrey D, et al. Serum-derived hepatitis C virus infection of primary human hepatocytes is tetraspanin CD81 dependent. J Virol. 2008;82:569–574. doi: 10.1128/JVI.01443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, Pascale S, Scarselli E, Cortese R, Nicosia A, Cosset FL. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278:41624–41630. doi: 10.1074/jbc.M305289200. [DOI] [PubMed] [Google Scholar]
- 36.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci USA. 2003;100:7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Hahn T, Lindenbach BD, Boullier A, Quehenberger O, Paulson M, Rice CM, McKeating JA. Oxidized low-density lipoprotein inhibits hepatitis C virus cell entry in human hepatoma cells. Hepatology. 2006;43:932–942. doi: 10.1002/hep.21139. [DOI] [PubMed] [Google Scholar]
- 38.Molina S, Castet V, Fournier-Wirth C, Pichard-Garcia L, Avner R, Harats D, Roitelman J, Barbaras R, Graber P, Ghersa P, et al. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J Hepatol. 2007;46:411–419. doi: 10.1016/j.jhep.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Decaens C, Durand M, Grosse B, Cassio D. Which in vitro models could be best used to study hepatocyte polarity? Biol Cell. 2008;100:387–398. doi: 10.1042/BC20070127. [DOI] [PubMed] [Google Scholar]
- 40.Easter DW, Wade JB, Boyer JL. Structural integrity of hepatocyte tight junctions. J Cell Biol. 1983;96:745–749. doi: 10.1083/jcb.96.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee NP, Luk JM. Hepatic tight junctions: from viral entry to cancer metastasis. World J Gastroenterol. 2010;16:289–295. doi: 10.3748/wjg.v16.i3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wölk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Yang W, Shen L, Turner JR, Coyne CB, Wang T. Tight junction proteins claudin-1 and occludin control hepatitis C virus entry and are downregulated during infection to prevent superinfection. J Virol. 2009;83:2011–2014. doi: 10.1128/JVI.01888-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benedicto I, Molina-Jiménez F, Bartosch B, Cosset FL, Lavillette D, Prieto J, Moreno-Otero R, Valenzuela-Fernández A, Aldabe R, López-Cabrera M, et al. The tight junction-associated protein occludin is required for a postbinding step in hepatitis C virus entry and infection. J Virol. 2009;83:8012–8020. doi: 10.1128/JVI.00038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brazzoli M, Bianchi A, Filippini S, Weiner A, Zhu Q, Pizza M, Crotta S. CD81 is a central regulator of cellular events required for hepatitis C virus infection of human hepatocytes. J Virol. 2008;82:8316–8329. doi: 10.1128/JVI.00665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mee CJ, Grove J, Harris HJ, Hu K, Balfe P, McKeating JA. Effect of cell polarization on hepatitis C virus entry. J Virol. 2008;82:461–470. doi: 10.1128/JVI.01894-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarz AK, Grove J, Hu K, Mee CJ, Balfe P, McKeating JA. Hepatoma cell density promotes claudin-1 and scavenger receptor BI expression and hepatitis C virus internalization. J Virol. 2009;83:12407–12414. doi: 10.1128/JVI.01552-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mee CJ, Harris HJ, Farquhar MJ, Wilson G, Reynolds G, Davis C, van IJzendoorn SC, Balfe P, McKeating JA. Polarization restricts hepatitis C virus entry into HepG2 hepatoma cells. J Virol. 2009;83:6211–6221. doi: 10.1128/JVI.00246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris HJ, Farquhar MJ, Mee CJ, Davis C, Reynolds GM, Jennings A, Hu K, Yuan F, Deng H, Hubscher SG, et al. CD81 and claudin 1 coreceptor association: role in hepatitis C virus entry. J Virol. 2008;82:5007–5020. doi: 10.1128/JVI.02286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris HJ, Davis C, Mullins JG, Hu K, Goodall M, Farquhar MJ, Mee CJ, McCaffrey K, Young S, Drummer H, et al. Claudin association with CD81 defines hepatitis C virus entry. J Biol Chem. 2010;285:21092–21102. doi: 10.1074/jbc.M110.104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coller KE, Berger KL, Heaton NS, Cooper JD, Yoon R, Randall G. RNA interference and single particle tracking analysis of hepatitis C virus endocytosis. PLoS Pathog. 2009;5:e1000702. doi: 10.1371/journal.ppat.1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mee CJ, Farquhar MJ, Harris HJ, Hu K, Ramma W, Ahmed A, Maurel P, Bicknell R, Balfe P, McKeating JA. Hepatitis C virus infection reduces hepatocellular polarity in a vascular endothelial growth factor-dependent manner. Gastroenterology. 2010;138:1134–1142. doi: 10.1053/j.gastro.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cukierman L, Meertens L, Bertaux C, Kajumo F, Dragic T. Residues in a highly conserved claudin-1 motif are required for hepatitis C virus entry and mediate the formation of cell-cell contacts. J Virol. 2009;83:5477–5484. doi: 10.1128/JVI.02262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, Kuo W, Yang W, Liu W, Gibson GA, Dorko K, Watkins SC, Strom SC, Wang T. The second extracellular loop dictates Occludin-mediated HCV entry. Virology. 2010;407:160–170. doi: 10.1016/j.virol.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fofana I, Krieger SE, Grunert F, Glauben S, Xiao F, Fafi-Kremer S, Soulier E, Royer C, Thumann C, Mee CJ, et al. Monoclonal anti-claudin 1 antibodies prevent hepatitis C virus infection of primary human hepatocytes. Gastroenterology. 2010;139:953–964, 964.e1-e4. doi: 10.1053/j.gastro.2010.05.073. [DOI] [PubMed] [Google Scholar]
- 57.Snooks MJ, Bhat P, Mackenzie J, Counihan NA, Vaughan N, Anderson DA. Vectorial entry and release of hepatitis A virus in polarized human hepatocytes. J Virol. 2008;82:8733–8742. doi: 10.1128/JVI.00219-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michta ML, Hopcraft SE, Narbus CM, Kratovac Z, Israelow B, Sourisseau M, Evans MJ. Species-specific regions of occludin required by hepatitis C virus for cell entry. J Virol. 2010;84:11696–11708. doi: 10.1128/JVI.01555-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benedicto I, Molina-Jiménez F, Barreiro O, Maldonado-Rodríguez A, Prieto J, Moreno-Otero R, Aldabe R, López-Cabrera M, Majano PL. Hepatitis C virus envelope components alter localization of hepatocyte tight junction-associated proteins and promote occludin retention in the endoplasmic reticulum. Hepatology. 2008;48:1044–1053. doi: 10.1002/hep.22465. [DOI] [PubMed] [Google Scholar]
- 60.Coyne CB, Shen L, Turner JR, Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181–192. doi: 10.1016/j.chom.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perrault M, Pécheur EI. The hepatitis C virus and its hepatic environment: a toxic but finely tuned partnership. Biochem J. 2009;423:303–314. doi: 10.1042/BJ20091000. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Boulan E, Paskiet KT, Sabatini DD. Assembly of enveloped viruses in Madin-Darby canine kidney cells: polarized budding from single attached cells and from clusters of cells in suspension. J Cell Biol. 1983;96:866–874. doi: 10.1083/jcb.96.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Traber MG, Kayden HJ, Rindler MJ. Polarized secretion of newly synthesized lipoproteins by the Caco-2 human intestinal cell line. J Lipid Res. 1987;28:1350–1363. [PubMed] [Google Scholar]
- 64.Ratcliffe DR, Iqbal J, Hussain MM, Cramer EB. Fibrillar collagen type I stimulation of apolipoprotein B secretion in Caco-2 cells is mediated by beta1 integrin. Biochim Biophys Acta. 2009;1791:1144–1154. doi: 10.1016/j.bbalip.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.André P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Bréchot C, Paranhos-Baccalà G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.André P, Perlemuter G, Budkowska A, Bréchot C, Lotteau V. Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis. 2005;25:93–104. doi: 10.1055/s-2005-864785. [DOI] [PubMed] [Google Scholar]
- 67.Diaz O, Delers F, Maynard M, Demignot S, Zoulim F, Chambaz J, Trépo C, Lotteau V, André P. Preferential association of Hepatitis C virus with apolipoprotein B48-containing lipoproteins. J Gen Virol. 2006;87:2983–2991. doi: 10.1099/vir.0.82033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gastaminza P, Cheng G, Wieland S, Zhong J, Liao W, Chisari FV. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol. 2008;82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang KS, Jiang J, Cai Z, Luo G. Human apolipoprotein e is required for infectivity and production of hepatitis C virus in cell culture. J Virol. 2007;81:13783–13793. doi: 10.1128/JVI.01091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gastaminza P, Kapadia SB, Chisari FV. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J Virol. 2006;80:11074–11081. doi: 10.1128/JVI.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meunier JC, Russell RS, Engle RE, Faulk KN, Purcell RH, Emerson SU. Apolipoprotein c1 association with hepatitis C virus. J Virol. 2008;82:9647–9656. doi: 10.1128/JVI.00914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Podevin P, Carpentier A, Pène V, Aoudjehane L, Carrière M, Zaïdi S, Hernandez C, Calle V, Méritet JF, Scatton O, et al. Production of infectious hepatitis C virus in primary cultures of human adult hepatocytes. Gastroenterology. 2010;139:1355–1364. doi: 10.1053/j.gastro.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 73.Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA, Lanford RE, Feinstone SM, Major ME, Leroux-Roels G, et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci USA. 2006;103:3805–3809. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Popescu CI, Dubuisson J. Role of lipid metabolism in hepatitis C virus assembly and entry. Biol Cell. 2009;102:63–74. doi: 10.1042/BC20090125. [DOI] [PubMed] [Google Scholar]
- 75.Thomssen R, Bonk S. Virolytic action of lipoprotein lipase on hepatitis C virus in human sera. Med Microbiol Immunol. 2002;191:17–24. doi: 10.1007/s00430-001-0106-x. [DOI] [PubMed] [Google Scholar]
- 76.Andréo U, Maillard P, Kalinina O, Walic M, Meurs E, Martinot M, Marcellin P, Budkowska A. Lipoprotein lipase mediates hepatitis C virus (HCV) cell entry and inhibits HCV infection. Cell Microbiol. 2007;9:2445–2456. doi: 10.1111/j.1462-5822.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu Y, Hishiki T, Sugiyama K, Ogawa K, Funami K, Kato A, Ohsaki Y, Fujimoto T, Takaku H, Shimotohno K. Lipoprotein lipase and hepatic triglyceride lipase reduce the infectivity of hepatitis C virus (HCV) through their catalytic activities on HCV-associated lipoproteins. Virology. 2010;407:152–159. doi: 10.1016/j.virol.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Gondeau C, Pichard-Garcia L, Maurel P. Cellular models for the screening and development of anti-hepatitis C virus agents. Pharmacol Ther. 2009;124:1–22. doi: 10.1016/j.pharmthera.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 79.Buck M. Direct infection and replication of naturally occurring hepatitis C virus genotypes 1, 2, 3 and 4 in normal human hepatocyte cultures. PLoS One. 2008;3:e2660. doi: 10.1371/journal.pone.0002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gardner JP, Durso RJ, Arrigale RR, Donovan GP, Maddon PJ, Dragic T, Olson WC. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc Natl Acad Sci USA. 2003;100:4498–4503. doi: 10.1073/pnas.0831128100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lozach PY, Amara A, Bartosch B, Virelizier JL, Arenzana-Seisdedos F, Cosset FL, Altmeyer R. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J Biol Chem. 2004;279:32035–32045. doi: 10.1074/jbc.M402296200. [DOI] [PubMed] [Google Scholar]
- 82.Cormier EG, Durso RJ, Tsamis F, Boussemart L, Manix C, Olson WC, Gardner JP, Dragic T. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc Natl Acad Sci USA. 2004;101:14067–14072. doi: 10.1073/pnas.0405695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nahmias Y, Casali M, Barbe L, Berthiaume F, Yarmush ML. Liver endothelial cells promote LDL-R expression and the uptake of HCV-like particles in primary rat and human hepatocytes. Hepatology. 2006;43:257–265. doi: 10.1002/hep.21016. [DOI] [PubMed] [Google Scholar]
- 84.Stamataki Z, Shannon-Lowe C, Shaw J, Mutimer D, Rickinson AB, Gordon J, Adams DH, Balfe P, McKeating JA. Hepatitis C virus association with peripheral blood B lymphocytes potentiates viral infection of liver-derived hepatoma cells. Blood. 2009;113:585–593. doi: 10.1182/blood-2008-05-158824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ploss A, Rice CM. Towards a small animal model for hepatitis C. EMBO Rep. 2009;10:1220–1227. doi: 10.1038/embor.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bitzegeio J, Bankwitz D, Hueging K, Haid S, Brohm C, Zeisel MB, Herrmann E, Iken M, Ott M, Baumert TF, et al. Adaptation of hepatitis C virus to mouse CD81 permits infection of mouse cells in the absence of human entry factors. PLoS Pathog. 2010;6:e1000978. doi: 10.1371/journal.ppat.1000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barth H, Schnober EK, Zhang F, Linhardt RJ, Depla E, Boson B, Cosset FL, Patel AH, Blum HE, Baumert TF. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J Virol. 2006;80:10579–10590. doi: 10.1128/JVI.00941-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koutsoudakis G, Kaul A, Steinmann E, Kallis S, Lohmann V, Pietschmann T, Bartenschlager R. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J Virol. 2006;80:5308–5320. doi: 10.1128/JVI.02460-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeisel MB, Koutsoudakis G, Schnober EK, Haberstroh A, Blum HE, Cosset FL, Wakita T, Jaeck D, Doffoel M, Royer C, et al. Scavenger receptor class B type I is a key host factor for hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology. 2007;46:1722–1731. doi: 10.1002/hep.21994. [DOI] [PubMed] [Google Scholar]
- 90.Basu A, Kanda T, Beyene A, Saito K, Meyer K, Ray R. Sulfated homologues of heparin inhibit hepatitis C virus entry into mammalian cells. J Virol. 2007;81:3933–3941. doi: 10.1128/JVI.02622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Voisset C, Callens N, Blanchard E, Op De Beeck A, Dubuisson J, Vu-Dac N. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J Biol Chem. 2005;280:7793–7799. doi: 10.1074/jbc.M411600200. [DOI] [PubMed] [Google Scholar]
- 92.Cormier EG, Tsamis F, Kajumo F, Durso RJ, Gardner JP, Dragic T. CD81 is an entry coreceptor for hepatitis C virus. Proc Natl Acad Sci USA. 2004;101:7270–7274. doi: 10.1073/pnas.0402253101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartosch B, Verney G, Dreux M, Donot P, Morice Y, Penin F, Pawlotsky JM, Lavillette D, Cosset FL. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79:8217–8229. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Syder AJ, Lee H, Zeisel MB, Grove J, Soulier E, Macdonald J, Chow S, Chang J, Baumert TF, McKeating JA, et al. Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J Hepatol. 2011;54:48–55. doi: 10.1016/j.jhep.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 95.Meertens L, Bertaux C, Cukierman L, Cormier E, Lavillette D, Cosset FL, Dragic T. The tight junction proteins claudin-1, -6, and -9 are entry cofactors for hepatitis C virus. J Virol. 2008;82:3555–3560. doi: 10.1128/JVI.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]