Abstract
Background
TNFRSF13B, which encodes transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), is mutated in 10% of patients with common variable immunodeficiency. One of the 2 most common TACI mutations in common variable immunodeficiency, C104R, abolishes ligand binding and is found predominantly in the heterozygous state. The murine TACI mutant C76R is the equivalent of the human TACI mutant C104R.
Objective
We sought to define the consequence of the C76R mutation on TACI function in mice that express both wild-type TACI and the murine C76R mutant.
Methods
Transgenic mice that express murine TACI C76R, the counterpart of human TACI C104R, on the TACI+/− B6/129 background (C76R/TACI+/− mice) were constructed. Serum immunoglobulins and antibody responses to the type II T-independent antigen trinitrophenylated (TNP)-Ficoll were determined by means of ELISA. B-cell proliferation in response to a proliferation-inducing ligand was determined based on tritiated thymidine incorporation into DNA. IgG1 secretion by B cells in response to a proliferation-inducing ligand plus IL-4 was determined by means of ELISA.
Results
C76R/TACI+/− mice had significantly impaired antibody responses to the type II T-independent antigen TNP-Ficoll compared with TACI+/+ B6/129 control animals, and their B cells were impaired in their capacity to proliferate and secrete IgG1 in response to TACI ligation. Unexpectedly, TACI+/− mice had similarly impaired B-cell function as C76R/TACI+/− littermates. Impaired TACI function caused by haploinsufficiency was confirmed in TACI+/− mice on the C57BL/6 background.
Conclusion
These results suggest that the human TACI mutant C104R might impair TACI function in heterozygotes through haploinsufficiency.
Keywords: Transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), common variable immunodeficiency, a proliferation-inducing ligand (APRIL), haploinsufficiency, murine model
Common variable immunodeficiency (CVID) is one of the most prevalent primary immunodeficiency syndromes, affecting approximately 1 in 25,000 white persons.1 It presents as recurrent infections, primarily of the respiratory tract, and is associated with autoimmune manifestations in up to 20% of patients and increased susceptibility of lymphoproliferative disorders.2,3 Patients with CVID have significant reductions in IgG, IgA, and/or IgM levels with impaired antibody responses to immunizations or natural antigen exposure.
TNFRSF13B, the gene encoding transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), is mutated in 5% to 10% of patients with CVID.4,5 TACI is a member of the TNF receptor family expressed predominantly on B cells; its primary ligands are B-cell activating factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL),6 which are expressed by multiple cells, including dendritic cells, macrophages, and neutrophils.7 The extracellular region of TACI contains 2 cysteine-rich domains (CRDs). The first CRD is necessary for ligand-independent assembly of TACI into multi-meric complexes8; the second CRD is required for binding to APRIL and BAFF. Ligand binding causes clustering of the intra-cellular domains of TACI, recruitment of signaling molecules that include calcium modulator and cyclophilin ligand and tumor necrosis factor receptor-associated factor proteins, and activation of the transcription factors nuclear factor of activated T cells and nuclear factor κB.8-12 TACI is important in immunoglobulin class switching, immunoglobulin production, and regulation of B-cell homeostasis.9,13-15 TACI-deficient mice have low levels of serum immunoglobulins and impaired antibody response to the type II T-independent antigens Pneumovax and trinitrophenylated (TNP)-Ficoll.14,16 With age, TACI−/− mice have fatal disorders characterized by autoimmune nephritis and lymphoproliferation leading to lymphoma in 15%.9
Two coding variants, C104R and A181E, account for more than 90% of TACI mutations found in patients with CVID. The C104R mutation is located in the second CRD and abolishes ligand binding and, consequently, signaling.4,5 The A181E mutation, which is in the transmembrane domain, does not affect ligand binding but severely impairs ligand signaling and TACI function in vitro and in vivo.16 The vast majority of patients with the C104R and A181E mutations are heterozygotes, yet B cells from these patients have impaired responses to TACI ligation in vitro.4,5 The mechanisms by which these mutations in the heterozygous state affect TACI function are not known. A previous study with 293T cell transfectants demonstrated that the C104R mutant is expressed normally at the cell surface, does not interfere with the expression of wild-type (WT) TACI, and preassembles with WT TACI but does not interfere with its ability to bind ligand.8
To evaluate the effect of C104R mutation on the function of WT TACI in B cells, we generated transgenic mice that express C76R TACI, the murine equivalent of the human C104R mutant, on a TACI+/− background. The results suggest that TACI function in human C104R heterozygotes is impaired through haploinsufficiency.
METHODS
Generation of TACI−/− mice that express TACI transgenes
PCR-generated TACI WT and mutated gene products were cloned in the XhoI site of the pBSVE6BK vector containing an Eμ enhancer and immunoglobulin heavy chain (IgVH) promoter.17,18Not1-Pvu1–digested fragments of the pBSVE6BK vector containing TACI-cloned products were injected into pronuclei of fertilized oocytes from C57BL/6/SJL mice. Founders were identified by means of PCR analysis of genomic DNA and then crossed with TACI−/− mice on the C57BL/6/129Sv background.14 All mice were bred and housed in a specific pathogen-free animal facility. All experimental procedures performed on the animals were approved by the Animal Care and Use Committee of Children's Hospital, Boston, Massachusetts.
Flow cytometry
Single-cell suspensions were stained with fluorochrome-conjugated antibodies in PBS containing 1% BSA and Fc-block (BD PharMingen, San Jose, Calif), washed, and analyzed on a FACSCanto cytometer (Becton Dickinson, Mountain View, Calif). Conjugated murine mAbs used in these studies were as follows: fluorescein isothiocyanate–labeled anti-B220 (RA3-6B2, BD Phar-Mingen), phycoerythrin-labeled anti-mouse TACI (R&D Systems, Minneapolis, Minn), and allophycocyanin-labeled anti-CD4 (GK1.5; eBioscience, San Diego, Calif).
Immunizations
Mice 8 to 12 weeks of age were used for immunization experiments. TNP-Ficoll was used for type II T-independent immunizations. Mice received a single intraperitoneal injection of TNP-Ficoll (TNP[83]-AECM-Ficoll; Biosearch Technologies, Novato, Calif) at the indicated doses. On days 0 and 14, serum was collected for measurement of TNP-specific immunoglobulin production.
Total serum immunoglobulins and TNP-specific antibody measurements
Total serum immunoglobulins were assayed by means of ELISA, as previously described.19 To measure anti-TNP–specific antibody levels, high-capacity ELISA plates (Maxsorb; Nunc, Ruskilde, Denmark) were coated with 10 μg/mLTNP-conjugated BSA (TNP40-BSA, Biosearch Technologies), and sera at 1:300, 1:900, and 1:2,700 dilutions in 1% BSA were analyzed. Alkaline phosphatase–conjugated isotype-specific antibodies (Southern Biotechnology Associates, Birmingham, Ala) were used as revealing antibodies.
Naive B-cell purification, in vitro immunoglobulin synthesis, and proliferation of B cells
B cells were purified from splenic cell suspensions by means of negative selection with CD43-conjugated magnetic beads from Miltenyi Biotec (Bergisch Gladbach, Germany). Naive B cells were purified from splenic cell suspensions by means of negative selection, as described previously.16 Purified B cells (>95% B220+ by means of FACS analysis) were suspended in RPMI containing 10% FCS, L-glutamine, and 50 μmol/L β-mercaptoethanol (complete medium). B cells (106 cells/mL) were cultured in complete medium alone, ZZ-APRIL (25 nmol/L) with or without IL-4 (50 ng/mL, R&D Systems), or anti-mouse CD40 (100 ng/mL, HM40-3, BD PharMingen) plus IL-4. ZZ-APRIL is a trimeric form of human APRIL that binds murine TACI.6 Cultures were pulsed after 72 hours with 1 μCi tritiated thymidine, harvested 16 to 20 hours later, and scintillation counted to measure proliferation. Supernatants were collected after 6 days of culture and assayed for IgG1 by means of ELISA.
Data analysis
All flow cytometric data were analyzed with FlowJo software (Version 8.8.6; Treestar, Inc, Ashland, Ore) and fluorescence plotted on biexponential axes. Statistical analysis was performed with Prism 4.0a software (GraphPad Software, Inc, La Jolla, Calif).
RESULTS
Generation and phenotypic analysis of lymphocytes in TACI−/− mice reconstituted with C76R TACI transgene
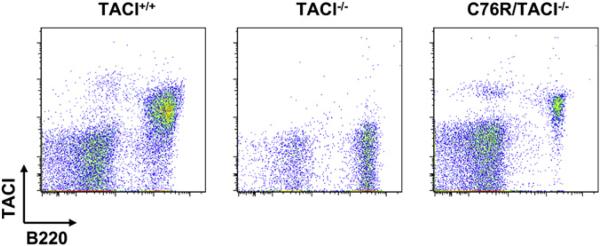
Mutation of the C104 residue in human TACI and the C76 residue in murine TACI abolishes ligand binding.4,5,8 To assess whether C76R, the murine equivalent of the human C104R mutant, dominantly interferes with TACI function in B cells, we constructed transgenic mice that express this mutant on the TACI+/− background. We used a C76R murine TACI construct driven by the Eμ enhancer VH promoter, as shown in Fig 1, A, to express the mutant selectively in B cells. The transgene was first placed on the TACI−/− background to generate C76R/TACI−/− mice to verify the expression of the mutant protein on the B-cell surface. Splenic B cells from these mice selectively expressed the TACI mutant on their surface (see Fig E1 in this article's Online Repository at www.jacionline.org). Because, as expected, TACI-dependent functions were abolished in C76R/TACI−/− mice, data on these mice are not presented here.
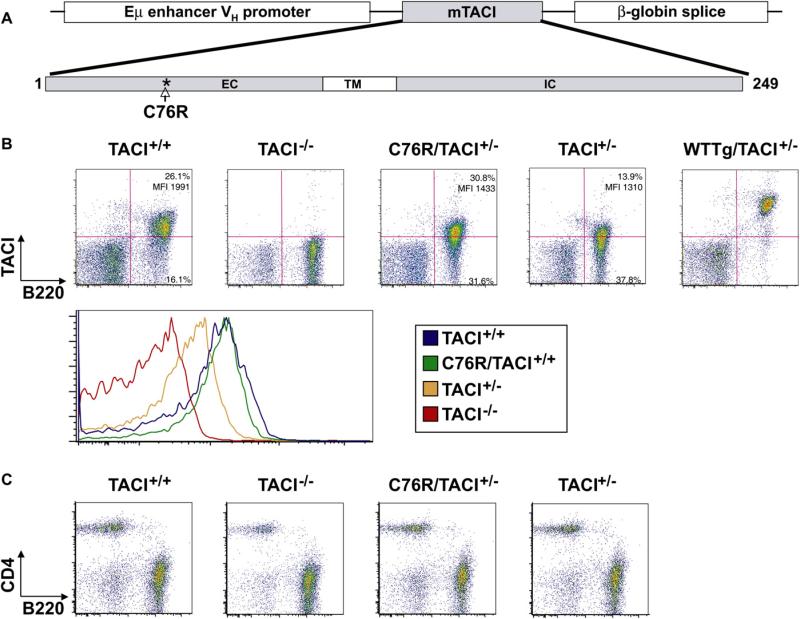
FIG 1.
Characterization of C76R/TACI+/− transgenic mice. A, Schematic representation of the murine C76R TACI (mTACI) transgene. EC, extracellular; IC, intracellular; TM, transmembrane. B and C, Representative FACS analysis of splenocytes from TACI+/+, TACI−/−, C76R/TACI+/−, and TACI+/− mice for B220 and TACI surface expression. Relevant quadrant percentages and MFIs are provided. TACI expression on B220+ B cells (Fig 1, B) and CD4 and B220 expression (Fig 1, C) are shown. Fig 1, B, also shows TACI surface expression on WT Tg/TACI+/− mice, which were not further studied because the percentage of their B cells that expressed TACI and the intensity of TACI expression on these B cells were significantly higher than in TACI+/+ mice and C76R/TACI+/− mice.
FIG E1.
TACI surface expression of C76R transgenic mice. A representative biexponential FACS plot of TACI expression on C76R/TACI−/− mice is shown. Single-cell suspensions were stained with fluorochromeconjugated antibodies in PBS containing 1% BSA and Fc-block (BD PharMingen), washed, and analyzed on a FACSCanto cytometer (Becton Dickinson). Conjugated anti-mouse mAbs used were fluorescein isothiocyanate–labeled anti-B220 (RA3-6B2, BD PharMingen) and phycoerythrin-labeled anti-mouse TACI (R&D Systems).
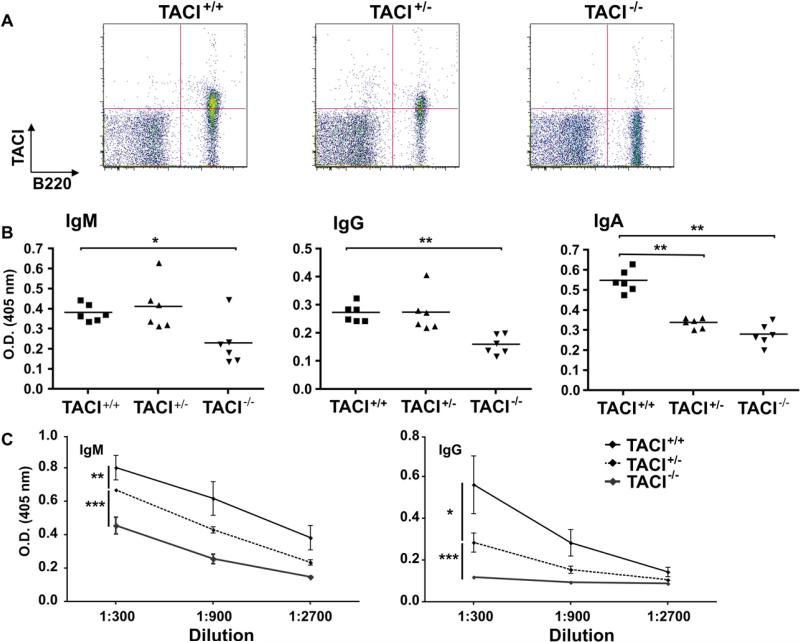
C76R/TACI−/− mice were crossed with TACI+/+ mice to generate C76R/TACI+/− mice and TACI+/− littermates that were transgene negative. Fig 1, B, shows that the C76R/TACI+/− mice we studied had comparable percentages of TACI+ splenic B cells as TACI+/+ mice (52.6% ± 7.8% vs 52.5% ± 6.2%, n = 6, P > .05). The staining of splenocytes from TACI−/− mice was used to gate on TACI+ cells. The intensity of TACI expression on TACI+ B cells, as determined based on mean fluorescence intensity (MFI), was comparable in C76R/TACI+/− and TACI+/+ mice (1,468 ± 186 vs 1,661 ± 210, n = 6, P >.05). Analysis of histograms confirmed comparable TACI expression by B220+ splenocytes from C76R/TACI+/− mice and TACI+/+ mice (Fig 1, B). In contrast to B cells from TACI+/+ and C76R/TACI+/− mice, virtually all B cells from a line of TACI+/− mice that carried a WT TACI transgene that we previously generated were found to express TACI (93.3% ± 1.3%, n = 4), and the intensity of TACI expression on their B cells (10,874 ± 2,568 MFI, n = 4) was significantly higher than that of TACI+/+ mice and C76R/TACI+/− mice (Fig 1, B). We therefore restricted our comparison of C76R/TACI+/− mice to TACI+/+ mice because they had comparable percentages of B cells that expressed comparable amounts of TACI. The percentage of splenic B cells that express TACI was significantly lower in TACI+/− mice compared with that seen in TACI+/+ mice (36.6% ± 13.9% vs 52.5% ± 6.2%, n = 6 each, P = .019). The MFI of TACI expression on TACI+ B cells was also significantly lower in TACI+/− mice than in TACI+/+ mice (1,250 ± 49 vs 1,661 ± 210, n = 6, P = .002). Analysis of histograms confirmed decreased TACI expression in TACI+/− mice compared with that seen in TACI+/+ control mice (Fig 1, B). These results suggest that TACI haploinsufficiency results in decreased surface expression of TACI on B cells.
The thymus in C76R/TACI+/− mice was normal in size, architecture, cellularity, and relative distribution of CD4+ and CD8+ cells (data not shown). Bone marrow from C76R/TACI+/− mice had normal cellularity and a normal percentage and expression profile of B220+CD43+ pro-B cells, B220loIgM− pro- and pre-B cells, and B220loIgM+ immature B cells (data not shown). FACS analysis of splenocytes revealed that the distribution of B220+ and CD4+ cells was comparable in C76R/TACI+/− mice and TACI+/− littermates (Fig 1, C). The percentage of B220+ cells was 52.6% ± 6.7% for TACI+/+ mice, 57.3% ± 5.3% for TACI+/− mice, and 56.6% ± 2.3% for C76R/TACI+/− mice (n = 3). As previously reported,14,15 the percentage of splenic B220+ cells in TACI−/− mice (71.5% ± 11.0%, n = 3) was significantly increased compared with that seen in TACI+/+ mice. The distribution of transitional T1 and T2 cells, marginal zone B cells, and follicular B cells in the spleen was comparable between C76R/TACI+/− mice and TACI+/− littermates, with no statistically significant differences among the means of 3 independent experiments (data not shown). This suggests that introduction of the C76R transgene did not interfere with B-cell development.
C76R/TACI+/− mice have impaired responses to type II T-independent antigen
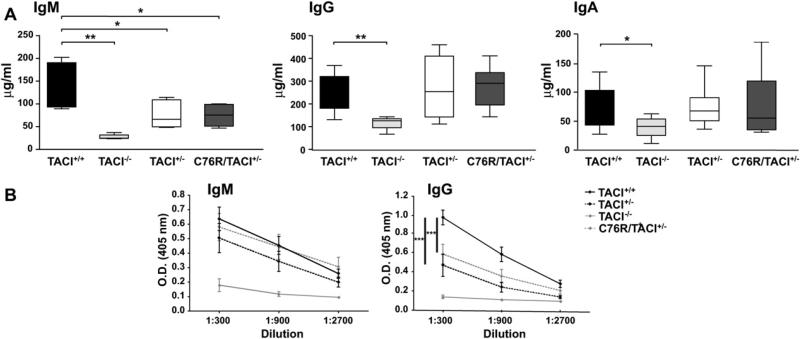
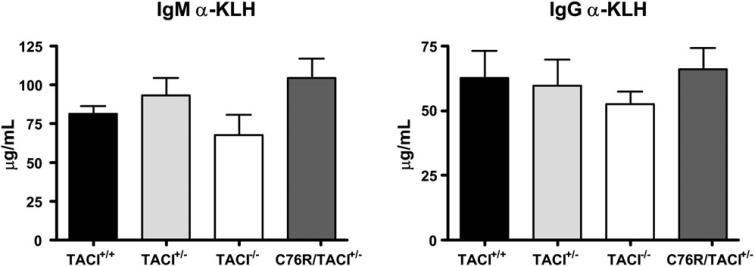
As previously reported,14 TACI−/− mice had significantly lower serum IgM and IgA levels than TACI+/+ control animals (Fig 2, A) and deficient IgM and IgG responses to immunization with the type II T-independent antigen TNP-Ficoll (Fig 2, B). In addition, as we previously reported,16 TACI−/− mice reared in our facility had significantly lower serum IgG levels as well. Serum levels of IgM, but not IgG or IgA, were significantly decreased in C76R/TACI+/− mice and their TACI+/− littermates compared with those seen in TACI+/+ control animals (Fig 2, A). C76R/TACI+/− mice had a significantly lower IgG, but not IgM, antibody response to TNP-Ficoll than TACI+/+ control animals (Fig 2, B). TACI+/− mice had significantly lower IgG, but not IgM, antibody responses to TNP-Ficoll than TACI+/+ control animals as well. The IgG responses to TNP-Ficoll of C76R/TACI+/− mice and their TACI+/− littermates were comparable and intermediate between those of TACI+/+ and TACI−/− control animals. The impaired antibody response of C76R/TACI+/− mice and TACI+/− littermates to TNP-Ficoll was not due to a global B-cell dysfunction because these mice had normal IgM and IgG responses to the T-dependent antigen keyhole limpet hemocyanin (KLH) (see Fig E2 in this article's Online Repository at www.jacionline.org). These results suggest that the heterozygous C76R mutation impairs TACI-dependent B-cell function in vivo because of haploinsufficiency.
FIG 2.
Serum immunoglobulins and antibody responses to TNP-Ficoll in C76R/TACI+/− transgenic mice. A, Serum IgM, IgG, and IgA levels from nonimmunized 8- to 12-week-old TACI+/+, TACI−/−, TACI+/−, and C76R/TACI+/− mice. The median (center line), 25th to 75th percentiles (box), and lowest and highest values (bars) are shown for each group. B, IgM and IgG anti-TNP antibody responses to TNP-Ficoll. Bars represent SEMs. The Mann-Whitney test was used in Fig 2, A, and 2-way ANOVA was used in Fig 2, B, to calculate significance (n = 10-12 mice per group). *P < .05, **P < .01, and ***P < .001.
FIG E2.
Antibody responses to KLH in C76R/TACI+/− transgenic mice. Mice (n = 4 per group) were immunized on day 0 with 200 μg of KLH administered intraperitoneally and 200 μg of KLH administered subcutaneously, boosted on day 14 with 25 μg of KLH, and bled on day 21. Plates were coated with KLH (10 μg/mL) in sodium carbonate buffer (pH 9.0) to measure anti-KLH–specific antibody. The wells were blocked with 2% BSA for 2 hours and incubated with diluted sera overnight at 4°C. Alkaline phosphatase–conjugated isotype-specific antibodies (BD PharMingen) were used as revealing antibodies. None of the differences between groups were significant, as analyzed with the Mann-Whitney test.
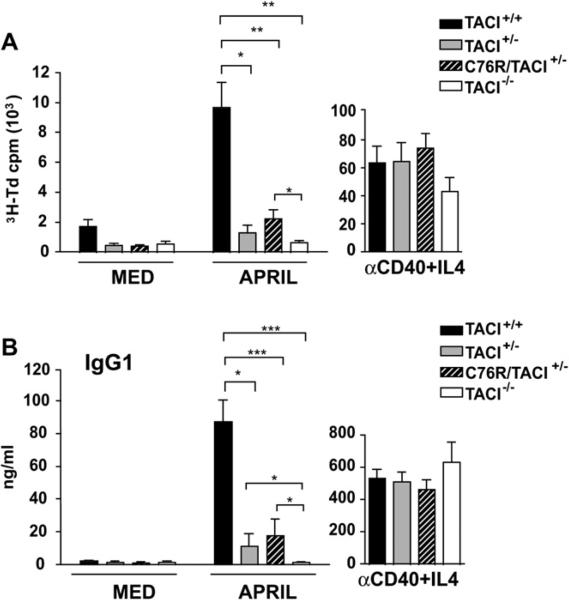
Proliferation and in vitro immunoglobulin production in response to APRIL are impaired in C76R/TACI+/− mice
APRIL induces TACI-dependent proliferation and production of IgG1 in murine splenic B cells.13,16 We examined the capacity of negatively selected naive B cells from C76R/TACI+/− mice to proliferate and secrete IgG1 in response to stimulation with APRIL. As previously reported,16 B cells from TACI+/+ mice, but not TACI−/− mice, proliferate in response to APRIL (Fig 3, A). B cells from C76R/TACI+/− mice proliferate significantly less to APRIL than B cells from TACI+/+ mice. The proliferation of TACI+/− B cells to APRIL was significantly lower than that of TACI+/+ B cells but was comparable with that of B cells from C76R/TACI+/− littermates. B cells from all 4 strains of mice studied proliferate comparably in response to anti-CD40 plus IL-4 (Fig 3, A).
FIG 3.
Proliferation and immunoglobulin synthesis in vitro by naive B cells from C76R/TACI+/− transgenic mice. Proliferation (A) and IgG1 synthesis (B) in response to APRIL are shown. B cells were stimulated with anti-CD40 plus IL-4 as a control for proliferation and IgG1 production (n = 5-9 mice per group). The Mann-Whitney test was used to calculate significance. MED, Medium alone. *P < .05, **P < .01, and ***P < .001.
APRIL stimulation caused naive B cells from TACI+/+ mice, but not TACI−/− mice, to secrete IgG1 (Fig 3, B). B cells from C76R/TACI+/− mice secreted significantly less IgG1 in response to APRIL than did B cells from TACI+/+ mice. TACI+/− B cells secreted significantly lower amounts of IgG1 in response to APRIL than did TACI+/+ B cells but comparable amounts of IgG1 as did B cells from C76R/TACI+/− littermates. IgG1 secretion in response to anti-CD40 plus IL-4 was comparable in all 4 strains.
These results suggest that the heterozygous C76R mutation impairs TACI-dependent B-cell function in vitro and that this impairment might be due to haploinsufficiency.
TACI+/− mice on a C57BL/6 background exhibit haploinsufficiency of TACI function in vivo and in vitro
The above experiments used mice with the mixed C57BL/6/129 background. TACI−/− mice that had been backcrossed into the C57BL/6 background for 11 generations were crossed with C57BL/6 WT mice to generate TACI+/− mice on the homogeneous C57BL/6 background to confirm that TACI haploinsufficiency impairs the B-cell response. Fig 4, A, shows that TACI+/− mice on the C57BL/6 background have significantly lower percentages of TACI-expressing B cells compared with TACI+/+ control animals (44.8% ± 3.9% vs 61.7% ± 8.6%, n = 5 each, P = .005). Furthermore, the MFI of TACI expression on TACI+/− B cells was significantly lower in TACI+/− mice compared with that seen in TACI+/+ mice (1,464 ± 22 vs 1,818 ± 115, n = 5 each, P = .0002). These results confirm that haploinsufficiency results in decreased surface expression of TACI on B cells.
FIG 4.
Decreased serum IgA levels and impaired antibody responses to TNP-Ficoll in TACI+/− mice on the C57BL/6 background. A, TACI and B220 expression on splenocytes from TACI+/+, TACI+/−, and TACI−/− mice on the C57BL/6 background. B, Serum IgM, IgG, and IgA levels from nonimmunized 8- to 12-week-old mice. C, IgM and IgG anti-TNP antibody responses to TNP-Ficoll. The results in Fig 4, B and C, are presented and analyzed as in Fig 2 (n = 10-12 mice per group).
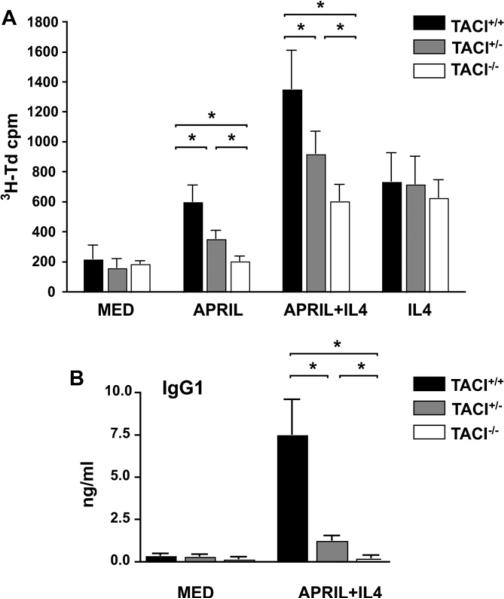
As previously reported,16 TACI−/− mice on a C57BL/6 background had significantly decreased serum IgG levels (Fig 4, B). They also had decreased serum IgM and IgA levels (Fig 4, B) and poor IgM and IgG antibody responses to TNP-Ficoll (Fig 4, C). TACI+/- mice on a C57BL/6 background had normal serum levels of IgM and IgG but significantly decreased levels of serum IgA (Fig 4, B). More importantly, TACI+/− mice on a C57BL/6 background had significantly lower IgM and IgG responses to TNP-Ficoll than TACI+/+ control animals (Fig 4, C).
B cells from TACI+/+ mice on the C57BL/6 background proliferated in response to APRIL and proliferated and secreted IgG1 in response to APRIL plus IL-4, albeit to quite a lesser extent than B cells from TACI+/+ mice on the B6/129 background (Fig 5). B cells from TACI+/− mice on the C57BL/6 background proliferated significantly less to APRIL and APRIL plus IL-4 and secreted significantly less IgG1 in response to APRIL plus IL-4 compared with B cells from TACI+/+ mice on the same background (Fig 5). As expected, B cells from TACI−/− mice on the C57BL/6 background did not proliferate to APRIL, and addition of APRIL did not enhance their proliferation to IL-4 alone. They also did not secrete IgG1 in response to APRIL plus IL-4. B cells from all 3 strains of mice proliferated comparably in response to IL-4 and anti-CD40 plus IL-4 (data not shown). These results indicate that haploinsufficiency affects TACI-dependent B-cell function in vivo and in vitro.
FIG 5.
Proliferation and immunoglobulin synthesis in vitro by purified B cells from TACI+/− mice on the C57BL/6 background. Proliferation (A) and IgG1 synthesis (B) in response to APRIL are shown (n = 5-9 mice per group). Results were analyzed as in Fig 3. MED, medium alone. *P < .05, **P < .01, and ***P < .001.
DISCUSSION
Our data demonstrate that the murine C76R TACI mutation, which abolishes ligand binding and corresponds to the human C104R TACI mutation associated with CVID, causes a significant impairment of TACI function in vivo and in vitro because of haploinsufficiency.
We examined the effect of the C76R mutation on the function of B cells that expressed 1 WT TACI allele by expressing the C76R mutant transgene in B cells of TACI+/− mice. Surface expression of the mutant was directly verified by placing the trans-gene on a TACI−/− background (see Fig E1), which is consistent with the observation that TACI is expressed on the surface of 293T cell transfectants and that TACI is expressed on B cells from subjects homozygous for C104R, the human counterpart of C76R.20 More importantly, B cells from the C76R/TACI+/− transgenic mice that we studied expressed comparable levels of TACI on their surface as B cells from TACI+/+ mice (Fig 1, B and C). The fact that the presence of the transgene normalized TACI expression in B cells from TACI+/− mice suggests that both mutant and WT TACI were expressed at comparable levels in C76R/TACI+/− B cells. This mimics closely the situation in which one of the 2 TACI alleles carries the C76R mutation and provides a good model to study the effect of heterozygous C76R TACI mutation on B-cell function, a scenario relevant to subjects heterozygous for the C104R TACI mutation.
C76R/TACI+/− transgenic mice had normal B-cell development and, like TACI+/− mice, did not exhibit B-cell lymphoproliferation or splenomegaly. This is in contrast to TACI−/− mice, which demonstrate increased numbers of circulating and splenic B cells and splenomegaly caused by increased B-cell proliferation.9,15 One possible explanation of B-cell expansion in TACI−/− mice is that TACI normally competes for BAFF binding with BAFF-R and that increased availability of BAFF to engage BAFF-R drives B-cell expansion in TACI−/− mice. This is supported by our previous finding that B-cell expansion and splenomegaly are no more observed in TACI−/− mice, which express in their B cells a mutant A144E TACI transgene that binds ligand normally but does not signal.16 However, there are also data to suggest that TACI normally delivers an apoptotic signal and that loss of this signal contributes to B-cell expansion in TACI−/− mice.9
C76R/TACI+/− transgenic mice had significantly impaired IgM and IgG antibody responses to the type II T-independent antigen TNP-Ficoll compared with TACI+/+ control animals (Fig 2, B). Furthermore, antibody responses to TNP-Ficoll were intermediate between those of TACI+/+ and TACI−/− mice and comparable with those of TACI+/− littermates. These results indicate that the mutant transgene did not exert a dominant-negative effect on the function of the WT allele in vivo and that the lower antibody response of C76R/TACI+/− mice to type II T-independent antigen was due to haploinsufficiency. The in vitro response of B cells from C76R/TACI+/− mice to TACI ligation also revealed strong evidence for haploinsufficiency. Proliferation and secretion of IgG1 in response to TACI ligation by APRIL were impaired to a comparable degree in B cells from C76R/TACI+/− mice and their TACI+/− littermates relative to the response of B cells from TACI+/+ control animals (Fig 3). Because we compared C76R/TACI+/− mice and TACI+/− littermates on the mixed C57BL/6/129/Sv background of the mixed background, we used 10 to 12 mice per group in the vivo experiments and 5 to 9 mice per group in the in vitro experiments. Thus we are reasonably confident that the B-cell responses of C76R/TACI+/− and TACI+/− mice are comparable. We had previously noted that TACI−/− mice reared in our facility had significantly lower serum IgG, IgA, and IgM levels than TACI+/+ littermate control animals16; however, we did not detect a significant decrease in the serum levels of these immunoglobulins in C76R/TACI+/− mice or in their TACI+/− littermates, except for lower serum IgM levels in TACI+/− mice (Fig 2, A).
Decreased surface expression of TACI and impairment of TACI-dependent B-cell responses caused by haploinsufficiency was confirmed in TACI+/− mice bred for 11 generations on the C57BL/6 background. The percentage of TACI-expressing B cells, the level of TACI expression on TACI-expressing B cells, antibody responses to immunization with TNP-Ficoll (Fig 4, A and C), and in vitro B-cell proliferation and secretion of IgG1 (Fig 5) in response to stimulation with APRIL were all significantly lower in TACI+/− mice on the C57BL/6 background than in TACI+/+ control animals on the same background. The lower percentage of TACI-expressing B cells and the lower expression of TACI on these cells might have contributed to the haploinsufficiency of the B-cell response in TACI+/− mice. Although serum levels of IgM, IgA, and IgG were significantly lower in TACI−/− mice than in TACI+/+ control animals on the C57BL/6 background (Fig 4, B), only serum IgA levels were significantly lower in TACI+/− mice on the C57BL/6 background than in TACI+/+ control animals on the same background. The low serum IgA and IgM antibody response to TNP-Ficoll in TACI+/− mice on the C57BL/6 background, but not on a mixed C57BL/6/129/Sv background, and the low serum IgM level in TACI+/− mice on a mixed C57BL/6/129/Sv background, but not on the C57BL/6 background, are likely explained by genetic differences between the 2 backgrounds. It should be noted that the antibody responses of TACI+/− mice on the C57BL/6/129/Sv background were reported originally as normal. The fact that we found that these mice have an impaired antibody response to TNP-Ficoll and ZZ-APRIL in vitro might be due to different environmental effects related to the different animal facilities and to the fact that the genetic makeup of mice on a mixed background is variable.
We had previously postulated that the C104R mutant might act as a dominant-negative mutant based on results of BAFF-driven fold induction of nuclear factor κB luciferase reporter gene activity in 293T cells cotransfected with WT TACI, C104R TACI, or both.8 This in vitro assay has limitations because it uses a transient transfection strategy for expression of TACI in a nonlymphoid cell line, which might not directly reflect the absolute or relative amounts of assembled WT and mutant TACI in vivo in B cells that express WT and mutant TACI. The in vivo data presented in this article strongly suggest that the C104R TACI mutation in the heterozygous state results in significant impairment of TACI function caused by haploinsufficiency. However, we have examined only a limited number of TACI-dependent functions. It is conceivable that for some effects of the C76R transgene, the underlying cause is haploinsufficiency, whereas for others, it might be a dominant-negative effect. Because the heterozygous C104R mutation is found in 0.5% of healthy subjects,21-23 it is clear that this mutation by itself does not result in CVID. This is consistent with our finding that serum IgG levels are normal in C76R/TACI+/− mice. B cells from the majority of patients with CVID, including those with TACI mutations, have impaired response to Toll-like receptor (TLR) 9 ligation by CpG.24,25 Because TACI ligation synergizes with TLR4, TLR9, and CD40 ligation in driving B-cell differentiation and plasma cell generation (our unpublished data),26,27 it is possible that TACI haploinsufficiency might aggravate the effect of impaired signaling through TLR, CD40, and/or other pathways on B-cell differentiation in patients with CVID.
Clinical implications.
Heterozygous mutation in C104R, which destroys ligand binding, might result in an impaired B-cell response to T-independent antigens because of haploinsufficiency.
Acknowledgments
We thank the members of the Geha laboratory, Drs H. Oettgen, J. Manis, and L. Notarangelo, for useful discussions.
Supported by National Institute of Health grants AI-031541 and T32-AI-007512; the American Academy of Allergy, Asthma & Immunology GSK Award (J.J.L.); and the Austrian Science Fund J2744-B12 (I.R.).
Abbreviations used
- APRIL
A proliferation-inducing ligand
- BAFF
B-cell activating factor of the TNF family
- CRD
Cysteine-rich domain
- CVID
Common variable immunodeficiency
- KLH
Keyhole limpet hemocyanin
- MFI
Mean fluorescence intensity
- TACI
Transmembrane activator and calcium modulator and cyclophilin ligand interactor
- TLR
Toll-like receptor
- TNP
Trinitrophenylated
- WT
Wild-type
Footnotes
Disclosure of potential conflict of interest: S. R. Dillon is an employee and stockholder of ZymoGenetics, Inc. R. Bram has received payment from St Jude Children's Hospital and ZymoGenetics for patents related to the TACI gene. R. S. Geha has received research support from the National Institutes of Health. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.International Union of Immunological Societies Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. Clin Exp Immunol. 1999;118(suppl 1):1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 3.Di Renzo M, Pasqui AL, Auteri A. Common variable immunodeficiency: a review. Clin Exp Med. 2004;3:211–7. doi: 10.1007/s10238-004-0027-2. [DOI] [PubMed] [Google Scholar]
- 4.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–34. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 5.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 6.Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5:235–46. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- 7.Mackay F, Schneider P, Rennert P, Browning J. BAFF and APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 8.Garibyan L, Lobito AA, Siegel RM, Call ME, Wucherpfennig KW, Geha RS. Dominant-negative effect of the heterozygous C104R TACI mutation in common variable immunodeficiency (CVID). J Clin Invest. 2007;117:1550–7. doi: 10.1172/JCI31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–88. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 10.von Bulow GU, Bram RJ. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–41. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- 11.Marsters SA, Yan M, Pitti MR, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF Homologues BLys and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol. 2000;10:785–8. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 12.Yan M, Marsters SA, Grewal IS, Wang H, Ashkenazi A, Dixit VM. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat Immunol. 2000;1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 13.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 15.Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. 2001;2:638–43. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- 16.Lee JJ, Rauter I, Garibyan L, Ozcan E, Sannikova T, Dillon SR, et al. The murine equivalent of the A181E TACI mutation associated with common variable immunodeficiency severely impairs B-cell function. Blood. 2009;114:2254–62. doi: 10.1182/blood-2008-11-189720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jabara H, Laouini D, Tsitsikov E, Mizoguchi E, Bhan A, Castigli E, et al. The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity. 2002;17:265–76. doi: 10.1016/s1074-7613(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 18.Shaw AC, Swat W, Ferrini R, Davidson L, Alt FW. Activated Ras signals developmental progression of recombinase-activating gene (RAG)-deficient pro-B lymphocytes. J Exp Med. 1999;189:123–9. doi: 10.1084/jem.189.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spergel J, Mizoguchi E, Brewer J, Martin T, Bhan A, Geha R. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to metacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salzer U, Bacchelli C, Buckridge S, Pan-Hammarstrom Q, Jennings S, Lougaris V, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113:1967–76. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castigli E, Wilson S, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet. 2007;39:430–1. doi: 10.1038/ng0407-430. [DOI] [PubMed] [Google Scholar]
- 22.Pan-Hammarstrom Q, Salzer U, Du L, Bjorkander J, Cunningham-Rundles C, Nelson DL, et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet. 2007;39:429–30. doi: 10.1038/ng0407-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Radigan L, Salzer U, Behrens TW, Grimbacher B, Diaz G, et al. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. J Allergy Clin Immunol. 2007;120:1178–85. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham-Rundles C, Radigan L, Knight AK, Zhang L, Bauer L, Nakazawa A. TLR9 activation is defective in common variable immune deficiency. J Immunol. 2006;176:1978–87. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]
- 25.Yu JE, Knight AK, Radigan L, Marron TU, Zhang L, Sanchez-Ramon S, et al. Toll-like receptor 7 and 9 defects in common variable immunodeficiency. J Allergy Clin Immunol. 2009;124:349–56. e1–3. doi: 10.1016/j.jaci.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozcan E, Garibyan L, Lee JJ, Lam K-P, Bram RJ, Geha RS. Transmembrane activator, calcium modulator, and cyclophilin ligand indicator drives plasma cell differentiation of LPS-activated B cells. J Allergy Clin Immunol. 2009;123:1277–86. e5. doi: 10.1016/j.jaci.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol. 2007;120:885–91. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]