Abstract
Gerodermia osteodysplastica (GO) is an autosomal recessive disorder characterized by wrinkly skin and osteoporosis. Here we demonstrate that GO is caused by loss-of-function mutations in SCYL1BP1, which is expressed at high levels in skin and osteoblasts. The protein localizes to the Golgi apparatus and interacts with Rab6, identifying SCYL1BP1 as a novel golgin. These results associate abnormalities of the secretory pathway with age-related changes in connective tissues.
Characteristic changes associated with human aging are a lax and wrinkly skin as well as a reduction of bone mass. Although the heritability of peak bone mass has been estimated to range between 60 and 70%, only a few genetic determinants have been identified so far. Skin wrinkling is a feature of several uncommon monogenic disorders so far mainly assigned to mutations in extracellular matrix components (Supplementary References). In this study we investigated the molecular cause of gerodermia osteodysplastica (also: geroderma osteodysplasticum, GO; OMIM 231070), a rare autosomal recessive disorder characterized by lax, wrinkled skin, joint laxity, and a typical face with a prematurely aged appearance. Skeletal signs include severe osteoporosis leading to frequent fractures, malar and mandibular hypoplasia, and a variable degree of growth retardation1,2. According to the current definition, GO can be regarded as a segmental progeroid disorder involving bone and skin3.
Our study involved patients from thirteen families all showing the typical GO phenotype (Fig. 1a, Supplementary Methods, and Supplementary Fig. 1). Bone mineral density was significantly reduced in all patients, frequently resulting in fractures of long bones or vertebrae (Supplementary Fig. 1). We performed linkage analysis initially in four Mennonite pedigrees with a total of twelve affected individuals from Germany, Mexico, and Canada without known relationship to each other (Supplementary Methods). The anabaptist community of the Mennonites was founded in the 16th century in Friesland in the north of the Netherlands, and after periods of prosecution, a large part of the community migrated to Northern and Middle America. Many of them kept a common, specific language, which is an ancient German dialect also known as Mennonite Low German.
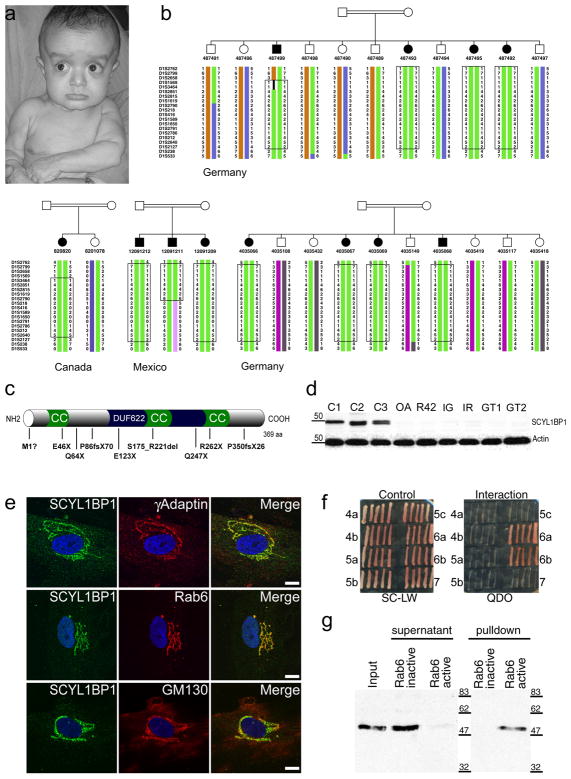
Figure 1. Clinical features of gerodermia osteodysplastica (GO), identification of mutations in SCYL1BP1, and characterization of SCYL1BP1 as a golgin.
(a) 9-month old patient. Note the typical facial appearance with sagging cheeks and pronounced wrinkling at the chest and dorsum of the hands giving him a prematurely aged appearance. (b) Haplotype analysis in four Mennonite pedigrees in the candidate region on chromosome 1q24. The shared haplotype defined a 5.1-cM candidate interval and indicated homozygosity by descent and a common founder in the patients from Germany, Canada, and Mexico. The boxes mark the respective homozygous intervals. (c) Schematic representation of the SCYL1BP1 structure showing coiled-coil domains (CC), the uncharacterized domain DUF622, and the nine different mutations found in GO families. (d) GO mutations lead to a complete loss of the protein as shown in a Western blot analysis of patient fibroblast lysates. C1-C3, control fibroblasts. (e) Co-staining of SCYL1BP1 with various Golgi marker proteins in control cells. Signals of γ-adaptin were in the same compartment but did not overlap completely. Co-localization with Rab6 pointed to the localization in the trans-Golgi network. Co-staining with Golgi matrix protein GM130 excluded a localization in the cis-Golgi compartment. Scale bar 10 μm. (f) Yeast two-hybrid screening against a library of ARF, Arl, and Rab small GTPases. Growth on selective medium indicated interaction with Rab6a and Rab6b. (g) Confirmation of the Rab6 interaction by pull-down experiments. A GST-Rab6 fusion protein was used to pull down SCYL1BP1 from HeLa lysates without or in the presence of GTP. Active GTP-bound Rab6 pulled down SCYL1BP1 efficiently while the GDP-locked T27N mutant was unable to interact.
A genome-wide scan identified a homozygous region on chromosome 1q24 with a combined multipoint lod score of 12.0 (Supplementary Fig. 2). The shared homozygous haplotype in all affected persons from the Mennonite pedigrees was consistent with a founder effect and defined a candidate region between markers D1S1569 and D1S218 (Fig. 1b), which is 5.1 cM in length corresponding to 5.7 Mb containing 102 genes. In a systematic sequencing approach we detected a homozygous nonsense mutation, p.E123X, in the gene SCYL1BP1 (FLJ11752, NTKLBP1) in all affected individuals from all four Mennonite families (Table 1, Supplementary Methods, and Supplementary Table 1). We were not able not trace back the Canadian and Mexican patients to their European ancestors. our results also demonstrate the importance of religious beliefs and a common, specific language for the preservation of an isolated population over several generations and even large distances, and the power of homozygosity mapping in such populations.
Table 1. Mutations in SCYL1BP1 identified in 13 families with GO.
The numbering of cDNA positions and deduced protein changes is based on a transcript that starts 25 triplets later than predicted in GenBank (accession no. NM_152281).
| Patient | Origin | Mutation | Exon | Protein | Genotype |
|---|---|---|---|---|---|
| OA | Oman | c.-1_1GA>CT | 1 | p.Met1? | homozygous |
| P1 | Pakistan | c.-1_1GA>CT | 1 | p.Met1? | homozygous |
| P2 | Pakistan | c.-1_1GA>CT | 1 | p.Met1? | homozygous |
| GT1 | Germany | c.136G>T | 2 | p.Glu46X | heterozygous |
| LS | Libya | c.190C>T | 2 | p.Gln64X | homozygous |
| IG | Italy | c.257delC | 2 | p.Pro86ArgfsX70 | homozygous |
| GT2b | Germany | c.367G>T | 2 | p.Glu123X | homozygous |
| GD1b | Germany | c.367G>T | 2 | p.Glu123X | homozygous |
| CD1b | Canada | c.367G>T | 2 | p.Glu123X | homozygous |
| MPb | Mexico | c.367G>T | 2 | p.Glu123X | homozygous |
| UR42 | USA | c.662+5G>C | 4 | p.Ser175_Arg221del | heterozygous |
| IR | Italy | c.739C>T | 5 | p.Gln247X | homozygous |
| UR42 | USA | c.784C>T | 5 | p.Arg262X | heterozygous |
| UT | USA | c.784C>T | 5 | p.Arg262X | homozygous |
| GT1 | Germany | c.1050_1053delTCTT | 5 | p.Phe350LeufsX26a | heterozygous |
predicted peptide is five residues longer than SCYL1BP1
Mennonite pedigrees
In nine additional GO patients from Germany, Italy, Oman, Pakistan, Libya, Mexico, and the U.S. we found eight other mutations, including five nonsense mutations, two frameshift mutations, one splice site mutation, and one mutation of the deduced methionine start codon (Fig. 1c and Table 1). Obligatory carriers were all heterozygous for the respective mutation, and the mutations were not found on 100 chromosomes from ethnically matched control persons. A reading frame of 394 codons has been predicted for SCYL1BP1 (GenBank accession no. NM_152281). However, the sequence of SCYL1BP1 is highly conserved among species, and a comparison with orthologous sequences makes a later translation start much more likely resulting in a 369-codon reading frame. This translational start is further corroborated by the prediction of translation using the algorithm NetStart4.
SCYL1BP1 encodes the soluble protein SCY1-like 1 binding protein 1 (SCYL1BP1; NTKL-binding protein 1). The most notable features of its structure are a large number of charged amino acids, many predicted phosphorylation sites, and two or three predicted coiled-coil domains (Fig. 1c). Using antibodies against SCYL1BP1 we identified a band of 47–50 kDa in Western blot analysis (Supplementary Methods). In all available patient fibroblasts, we were not able to detect significant changes in SCYL1BP1 mRNA quantities by qRT-PCR, however, SCYL1BP1 protein was completely absent in these cases, including the case OA with the mutation p.M1? (Fig. 1d), pointing to loss-of-function mutations through instability of the protein.
Staining with markers for cellular compartments revealed a clear localization of SCYL1BP1 to the Golgi apparatus (Fig. 1e and Supplementary Methods). No significant alterations in Golgi morphology or in the distribution of different Golgi marker proteins were detected in patient fibroblasts (Supplementary Fig. 3). Scyl1bp1 showed high expression in osteoblasts and in the skin (Supplementary Fig. 4), which correlates with the main sites of disease manifestation. Protein expression was shown to increase during osteoblast differentiation. Expression was also present in osteoclasts albeit at lower levels.
Since SCYL1BP1 is a soluble protein with coiled-coil domains known to mediate protein-protein interaction, we sought for binding partners in a library of mammalian small G-proteins of the Rab, ARF, and Arl families that are known as important determinants of intracellular membrane traffic5 using a yeast two-hybrid interaction screen. A specific interaction of SCYL1BP1 with Rab6 was identified (Fig. 1f). Pull-down experiments confirmed this interaction. The interaction was additionally shown to be dependent on the activation state of Rab6 thus further corroborating its specificity (Fig. 1g). In contrast, an interaction with Rab1 was not detected, confirming the specificity of the yeast two-hybrid screening (Supplementary Fig. 5). Golgi localization, the presence of coiled-coil domains, and the interaction with a Rab protein are the hallmarks of the golgin protein family6. Therefore, SCYL1BP1 can be classified as a golgin. To our knowledge, this is the first example of a golgin causing a hereditary human disorder.
Mouse Scyl1bp1 was identified initially as a binding partner of the N-terminal kinase-like protein (Scyl1) in a yeast two-hybrid analysis7. Scyl1 was shown to be mutated in the mdf mouse mutant that shows spinocerebellar neurodegeneration8. Human SCYL1 was found to bind to adaptor proteins of clathrin-coated vesicles9, and a recent search for binding partners of Scyl1 by mass spectrometry identified an interaction with components of coatomer complex I (COPI) coats10. This observation fits very well with our finding that SCYL1BP1 is associated with the Golgi apparatus and involved in the secretory pathway.
The three Rab6 isoforms are central components of cellular membrane trafficking mainly residing in the trans-Golgi network5. One important function of Rab6 is the recruitment of motor proteins that promote vesicle movement. Rab6 is known to be involved in the retrograde transport from endosomes to the Golgi apparatus and plays a crucial role in the secretory pathway from the Golgi to the plasma membrane11,12. Interestingly, a functional interaction of Rab6 with the conserved oligomeric Golgi complex (COG) has been demonstrated recently13. This is intriguing since patients with mutations in COG7, a subunit of this complex, show glycosylation defects and wrinkled skin14. Impaired glycosylation, wrinkly skin, and bone defects are also features observed in autosomal recessive cutis laxa type 2 (ARCL2; OMIM 219200). ARCL2 was recently shown to be due to mutations in the vacuolar-type H+-ATPase subunit gene ATP6V0A215. Given the phenotypic similarities between GO and ARCL2, we analyzed the glycosylation pattern of serum transferrin from GO patients but did not detect any glycosylation abnormalities2.
Our results show that two common aging phenomena, skin wrinkling and osteoporosis, can be associated with mutations in a novel golgin, SCYL1BP1. Future studies are needed to investigate the pathophysiological similarities between GO and overlapping disorders to identify a putative common mechanism regulating connective tissue homeostasis during aging.
Supplementary Material
Acknowledgments
We are grateful to all patients and their family members for participating in this study. Thank you to Jane Evans, Bernie N. Chodirker and Klaus Wrogemann, University of Manitoba, Canada, for their support. We wish to thank Françoise André, Björn Fischer, Susanne Kolberg, and Nadine Wittstruck for excellent technical assistance. This work was funded by grants from the German Federal Ministry of Education and Research and the Ministry for Innovation, Science, Research, and Technology of the Land Nordrhein Westfalen to H.C.H. and by funds from the Deutsche Forschungsgemeinschaft (collaborative research centre 577) to U.K.. The sample IG was obtained from the “Cell Line and DNA Bank from Patients affected by Genetic Diseases” collection (http://www.gaslini.org/labdppm.htm) supported by Italian Telethon grants. We thank Hanswalter Zentgraf for the generation of monoclonal antibodies.
Footnotes
Statement
The study has been approved by the local institutional review board and followed the Declaration of Helsinki protocols. Samples were taken after obtaining written informed consent. The permission to publish photos was given.
References
- 1.Hunter AG. J Med Genet. 1988;25:854–857. doi: 10.1136/jmg.25.12.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajab A, et al. Am J Med Genet A. 2008;146:965–976. doi: 10.1002/ajmg.a.32143. [DOI] [PubMed] [Google Scholar]
- 3.Martin GM. Cell. 2005;120:523–532. doi: 10.1016/j.cell.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen AG, Nielsen H. Proc Int Conf Intell Syst Mol Biol. 1997;5:226–233. [PubMed] [Google Scholar]
- 5.Short B, Haas A, Barr FA. Biochim Biophys Acta. 2005;1744:383–395. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Barr FA, Short B. Curr Opin Cell Biol. 2003;15:405–413. doi: 10.1016/s0955-0674(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 7.Di Y, et al. J Hum Genet. 2003;48:315–321. doi: 10.1007/s10038-003-0031-5. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt WM, et al. EMBO Rep. 2007;8:691–697. doi: 10.1038/sj.embor.7401001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmid EM, et al. PLoS Biol. 2006;4:e262. doi: 10.1371/journal.pbio.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burman JL, et al. J Biol Chem. 2008 doi: 10.1074/jbc.M801869200. in press. [DOI] [PubMed] [Google Scholar]
- 11.Grigoriev I, et al. Dev Cell. 2007;13:305–314. doi: 10.1016/j.devcel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Mallard F, et al. J Cell Biol. 2002;156:653–664. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, et al. Mol Biol Cell. 2007;18:4129–4142. doi: 10.1091/mbc.E07-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, et al. Nat Med. 2004;10:518–523. doi: 10.1038/nm1041. [DOI] [PubMed] [Google Scholar]
- 15.Kornak U, et al. Nat Genet. 2008;40:32–34. doi: 10.1038/ng.2007.45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.