Abstract
A general method has been developed for arylation of readily available 1H-perfluoroalkanes. The method employs aryl iodide and 1H-perfluoroalkane reagents, DMPU solvent, TMP2Zn base, and a copper chloride/phenanthroline catalyst. Preliminary mechanistic studies are reported.
Many pharmaceuticals and agrochemicals contain aryl-trifluoromethyl or aryl-polyfluoroalkyl linkages.1 Consequently, introduction of fluoroalkyl substituents into aromatic systems has attracted intense interest. Trichloromethyl groups and other functionalities can be converted to trifluoromethyl moieties by treatment with a fluorinating reagent.2 A halide, or, rarely, a hydrogen on an aromatic ring can be replaced with a trifluoromethyl group under transition metal catalysis. Examples of such reactions include palladium-catalyzed trifluoromethylation of aryl chlorides3 and ortho-trifluoromethylation of 2-phenylpyridines.4 More commonly, however, copper is employed for polyfluoroalkylation of aryl iodides. Typically, trifluoromethyltrialkylsilane reagents are used in combination with a stoichiometric copper source.5 A recent pioneering report describes reactions catalytic in copper. However, only electron-deficient aryl iodides react in high yields.6 Cross-coupling of aryl iodides and perfluoroalkyl iodides by employing 1–3 equiv copper metal has also been reported.7 Arene reactions with RFI proceed by radical mechanisms and often result in isomer mixtures.8
In most of the above cases, RFSiR3 reagents have been employed. However, only trifluoromethyl-, pentafluoroethyl-, and heptafluoropropyltrialkylsilanes are commercially available. Thus, a widely available perfluoroalkyl source should be sought to develop a generally useful synthetic methodology. We report here a method for copper-catalyzed 1H-perfluoroalkane arylation by aryl iodides.
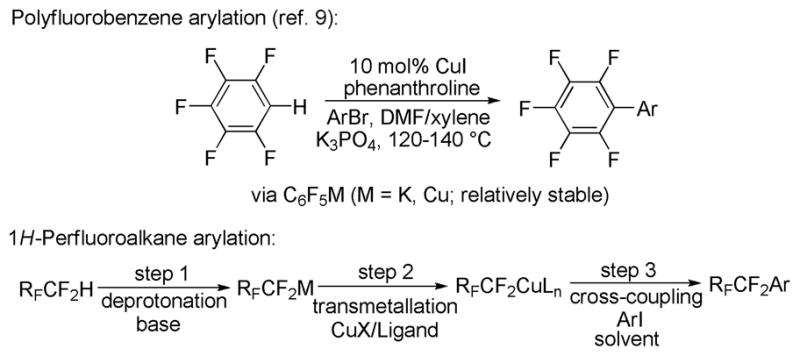
Based on previous work on copper-catalyzed arylation of polyfluoroarenes,9 we considered the arylation of 1H-perfluoroalkanes (Scheme 1). Lowest homologues of 1H- perfluoroalkanes are among the cheapest sources of RF groups. Several issues had to be addressed to develop a viable method (Scheme 1). First, stability of the perfluoroalkyl metal reagent generated in the deprotonation step needs to be considered. In contrast to pentafluoroarylmetals,10 most perfluoroalkyl metals are unstable.11 Only mercury, cadmium, bismuth, thallium, and zinc perfluoroalkyls are relatively stable.11,12 A viable methodology will not use highly toxic Cd, Hg, or Tl reagents; thus, Bi or Zn bases must be employed. Second, base type needs to be determined. Trifluoromethane possesses pKa of about 31 requiring an amide base for deprotonation.13 Bismuth amides are photolytically and thermally unstable.14 Consequently, a zinc amide base should be employed. Amide moiety should be hindered to prevent copper-catalyzed amination of aryl iodide.15 These considerations led to selection of zinc bis-2,2,6,6-tetramethylpiperidide (TMP2Zn) base.16
Scheme 1.
Reaction Development Considerations
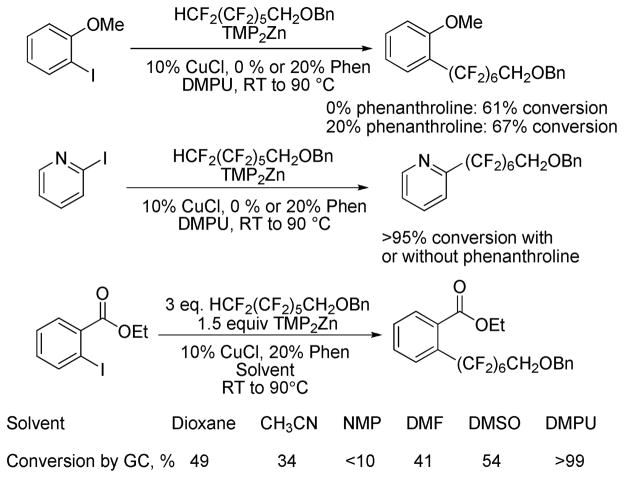
The reaction was optimized with respect to ligand and solvent (Scheme 2). For perfluoroalkylation of electron-rich 2-methoxyiodobenzene, phenanthroline ligand additive afforded an increased conversion. However, high conversion to the product was observed for 2-iodopyridine perfluoroalkylation both in the presence and absence of phenanthroline. Presumably, phenanthroline ligand stabilizes perfluoroalkyl copper species.6 Consequently, for functionalization of more reactive aryl iodides phenanthroline may be omitted. Solvent optimization showed that best results are obtained in DMPU which was used in all further reactions.
Scheme 2.
Reaction Optimization
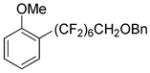
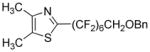
Perfluoroalkylation scope with respect to aryl iodides is presented in Table 1. We were pleased to discover that benzylated α,α,ω-trihydroperfluoroheptanol was arylated by a number of aryl iodides under the optimized reaction conditions. Electron-rich 2-iodoanisole and 4-iodotoluene are reactive affording coupling products in moderate yields (entries 1 and 2). Reactions with electron-poor ArI are higher yielding (entries 3–5, 7, 11). Functional groups such as trifluoromethoxy (entry 3), nitrile (entry 4), bromide (entry 7), and ester (entry 11) are tolerated. Iodinated heterocycles such as 2-iodopyridine, 2-iodo-4,5-dimethylthiazole, and 8-iodocaffeine react to give products in good to excellent yields (entries 8–10). 2,6-Disubstituted electron-rich aryl iodides do not afford the coupling products. Instead, iodide moiety is reduced. Unactivated aryl bromides are unreactive. Thus, reaction of 4-bromobiphenyl with benzylated α,α,ω-trihydroperfluoroheptanol under standard reaction conditions afforded <5% conversion to coupling product.
Table 1.
Perfluoroalkylation Scope with Respect to ArIa
|
| ||||
|---|---|---|---|---|
| entry | aryl iodide | product | yield, % | |
| 1 | 2-MeOC6H4I |
|
51 | |
| 2 | 4-CH3C6H4I |
|
51 | |
| 3 | 3-CF3OC6H4I |
|
55 | |
| 4b | 4-NCC6H4I |
|
83 | |
| 5 | 3-CF3C6H4I |
|
61 | |
| 6 | 4-C6H5C6H4I |
|
62 | |
| 7 | 4-BrC6H4I |
|
53 | |
| 8 | 2-Iodopyridine |
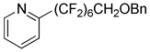
|
85 | |
| 9b | 2-Iodo-4,5- dimethylthiazole |
|
63 | |
| 10b | 8-Iodocaffeine |
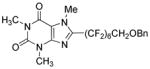
|
94 | |
| 11b | Ethyl-2- iodobenzoate |
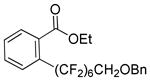
|
92 | |
TMP2Zn (0.5 mmol), RFH (0.5 mmol), DMPU, then ArI (1.5 mmol), phenanthroline (0.1 mmol) and CuCl (0.05 mmol), 90 °C.
TMP2Zn (0.75 mmol), RFH (1.5 mmol), DMPU, then ArI (0.5 mmol), phenanthroline (0.1 mmol) and CuCl (0.05 mmol).
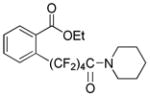
The reaction scope with respect to 1H-perfluoroalkanes is presented in Table 2. The most difficult coupling partner is trifluoromethane (entry 1). Trifluoromethyl copper decomposes generating pentafluoroethylcopper unless it is stabilized by HMPA.17 About 10% of pentafluoroethylated substrate was observed in the crude reaction mixture and purification by HPLC was required to obtain pure ethyl 2-(trifluoromethyl)benzoate. Reactions with other 1H-perfluoroalkanes, such as C2F5H, CF3CF2CF2H, and 1H- perfluorohexane are high-yielding (entries 2–5). Substrates possessing two –CF2H moieties can be either monoarylated (entries 6 and 7) or diarylated (entry 8) depending on the reaction stoichiometry. Some functionality such as chloro and amide (entries 9 and 10) is tolerated. 2H-Heptafluoropropane is unreactive.18
Table 2.
Perfluoroalkylation Scope with Respect to RFHa
 | |||
|---|---|---|---|
| entry | 1H-polyfluoroalkane | product | yield |
| 1b | CF3H |
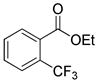
|
51 |
| 2 | C2F5H |
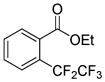
|
96 |
| 3 | CF3CF2CF2H |
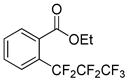
|
83 |
| 4 | CF3(CF2)4CF2H |
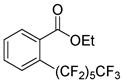
|
87 |
| 5 | CF3(CF2)8CF2H |
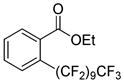
|
81 |
| 6 | H(CF2)6H |
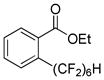
|
79 |
| 7 | H(CF2)8H |
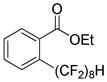
|
84 |
| 8c | H(CF2)8H |
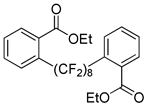
|
63 |
| 9 | H(CF2)4Cl |
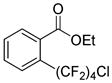
|
94 |
| 10d | H(CF2)4CONC5H10 |
|
62 |
TMP2Zn (0.75 mmol), RFH (1.5–5 mmol), DMPU, ArI (0.5 mmol), phenanthroline (0.1 mmol), CuCl (0.05 mmol), 90 °C.
Phenanthroline (1 mmol).
TMP2Zn (1 mmol), RFH (0.5 mmol), DMPU, ArI (4 mmol), phenanthroline (0.1 mmol) and CuCl (0.05 mmol).
TMP2Zn (0.5 mmol), RFH (0.5 mmol), DMPU, ArI (1.5 mmol), phenanthroline (0.1 mmol) and CuCl (0.05 mmol).
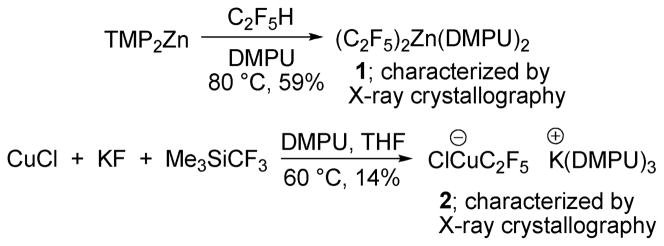
Preliminary mechanistic studies have been performed. The intermediate bis(perfluoroethyl)zinc species 1 was prepared by the reaction of TMP2Zn with pentafluoroethane (Scheme 3). The complex was characterized by 1H and 19F NMR, X-ray crystallography, and elemental analysis. Additionally, anionic copper complex 2 was prepared in low yield by reaction of CuCl, KF, and TMSCF3 (Scheme 3). Complex 2 exists as a temperature and moisture sensitive colorless solid that slowly decomposes at RT under argon atmosphere over the course of several hours, but is stable for at least 4 weeks at −35 °C under inert atmosphere. It was characterized by 1H and 19F NMR as well as X-ray crystallographic analysis.
Scheme 3.
Reaction Intermediate Synthesis
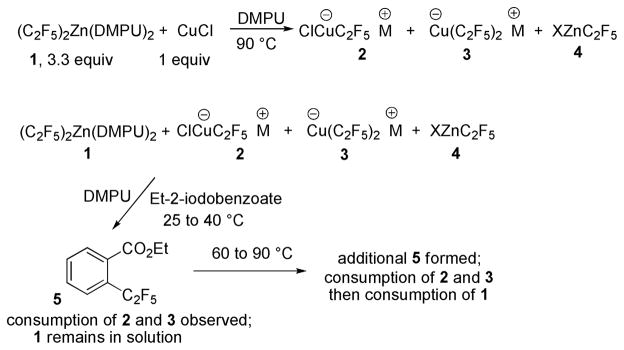
Several 19F NMR experiments were carried out to determine the identity of the species present in reaction mixture and their reactivity. Mixing CuCl and excess 1 in DMPU solvent affords negligible amounts of zinc to copper transmetallation products at 45 °C. However, the reaction at 90 °C affords several species that were tentatively identified by comparison with NMR of authentic 2 and reported spectral data for 3 (Scheme 4).19 Furthermore, a preformed mixture of 2 and 3 in the presence of excess 1 was subjected to the reaction with ethyl-2-iodobenzoate at 25 °C, 40 °C, 60 °C, and 90 °C. At 25 °C and 40 °C, consumption of 2 and 3 is observed and 5 is formed; however, 1 does not undergo transmetallation with copper halide. Further heating at 60 °C is required for transmetallation to occur. Heating to 90 °C leads to fast consumption of aryl iodide and 1 followed by reappearance of 2 and 3. These experiments show that transmetallation appears to be the turnover-limiting for pentafluoroethylation of ethyl 2-iodobenzoate.
Scheme 4.
NMR Experiments
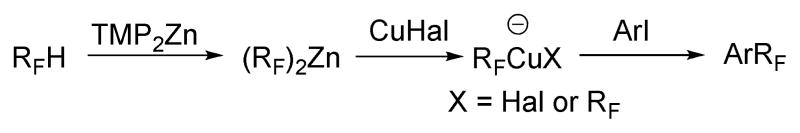
The general reaction mechanism is presented in Scheme 5. Deprotonation of 1H-perfluoroalkanes with TMP2Zn affords bis(perfluoroalkyl)zinc species. Subsequent transmetallation with copper halide produces a mixture of anionic Cu species that react with aryl iodide, either directly or via a neutral perfluoroalkyl compound,5f to give the coupling product.
Scheme 5.
Reaction Mechanism
In conclusion, we have developed a general method for arylation of readily available 1H-perfluoroalkanes. The method employs aryl iodide and 1H-perfluoroalkane reagents, DMPU solvent, TMP2Zn base, and a copper chloride/phenanthroline catalyst.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (Grant No. E-1571), National Institute of General Medical Sciences (Grant No. R01GM077635), A. P. Sloan Foundation, Camille and Henry Dreyfus Foundation, and Norman Hackerman Advanced Research Program for supporting this research. We thank Dr. James Korp for collecting and solving the X-ray structures.
Footnotes
SUPPORTING INFORMATION AVAILABLE Experimental procedures, characterization data for new compounds, and X-ray crystallography data for 1 and 2. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Jeschke P. ChemBioChem. 2004;5:570. doi: 10.1002/cbic.200300833. [DOI] [PubMed] [Google Scholar]; (b) Purser S, Moore PR, Swallow S, Gouverneur V. Chem Soc Rev. 2008;37:320. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]; (c) Schlosser M. Angew Chem, Int Ed. 2006;45:5432. doi: 10.1002/anie.200600449. [DOI] [PubMed] [Google Scholar]
- 2.(a) Dmowski W, Wielgat J. J Fluorine Chem. 1987;37:429. [Google Scholar]; (b) Bloodworth AJ, Bowyer KJ, Mitchell JC. Tetrahedron Lett. 1987;28:5347. [Google Scholar]; (c) Hudlicky M. Organic Reactions. 1988;35:513. [Google Scholar]
- 3.Cho EJ, Senecal TD, Kinzel T, Zhang Y, Watson DA, Buchwald SL. Science. 2010;328:1679. doi: 10.1126/science.1190524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Wang X, Truesdale L, Yu JQ. J Am Chem Soc. 2010;132:3648. doi: 10.1021/ja909522s. [DOI] [PubMed] [Google Scholar]; (b) Ball ND, Kampf JW, Sanford MS. J Am Chem Soc. 2010;132:2878. doi: 10.1021/ja100955x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton DJ, Lu L. Top Current Chem. 1997;193:45.Dubinina GG, Furutachi H, Vicic DA. J Am Chem Soc. 2008;130:8600. doi: 10.1021/ja802946s.Urata H, Fuchikami T. Tetrahedron Lett. 1991;32:91.Folléas B, Marek I, Normant JF, Saint-Jalmes L. Tetrahedron. 2000;56:275.Pd: Grushin VV, Marshall WJ. J Am Chem Soc. 2006;128:4632. doi: 10.1021/ja0602389.Ni: Dubinina GG, Brennessel WW, Miller JL, Vicic DA. Organometallics. 2008;27:3933.
- 6.Oishi M, Kondo H, Amii H. Chem Commun. 2009:1909. doi: 10.1039/b823249k. [DOI] [PubMed] [Google Scholar]
- 7.(a) Xiao JC, Ye C, Shreeve JM. Org Lett. 2005;7:1963. doi: 10.1021/ol050426o. [DOI] [PubMed] [Google Scholar]; (b) Mcloughlin VCR, Thrower J. Tetrahedron. 1969;25:5921. [Google Scholar]; (c) Croxtall B, Fawcett J, Hope EG, Stuart AM. J Chem Soc, Dalton Trans. 2002:491. [Google Scholar]
- 8.Review: Furin GG. Russ Chem Rev. 2000;69:491.
- 9.(a) Do HQ, Daugulis O. J Am Chem Soc. 2008;130:1128. doi: 10.1021/ja077862l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Do HQ, Khan RMK, Daugulis O. J Am Chem Soc. 2008;130:15185. doi: 10.1021/ja805688p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairncross A, Sheppard WA. J Am Chem Soc. 1968;90:2186.Coe PL, Stephens R, Tatlow JC. J Chem Soc. 1962:3227.However, C6F5Li can be hazardous: Kinsella E, Massey AG. Chem Ind (London) 1971;36:1017B.
- 11.Burton DJ, Yang ZY. Tetrahedron. 1992;48:189. [Google Scholar]
- 12.(a) Naumann D, Tyrra W. J Organomet Chem. 1987;334:323. [Google Scholar]; (b) Nair HK, Morrison JA. Inorg Chem. 1989;28:2816. [Google Scholar]
- 13.(a) Andreades S. J Am Chem Soc. 1964;86:2003. [Google Scholar]; (b) Chabinyc ML, Brauman JI. J Am Chem Soc. 1998;120:10863. [Google Scholar]
- 14.Vehkamäki M, Hatanpää T, Ritala M, Leskelä M. J Mater Chem. 2004;14:3191. [Google Scholar]
- 15.Shafir A, Buchwald SL. J Am Chem Soc. 2006;128:8742. doi: 10.1021/ja063063b. [DOI] [PubMed] [Google Scholar]
- 16.(a) Rees WS, Jr, Just O, Schumann H, Weimann R. Polyhedron. 1998;17:1001. [Google Scholar]; (b) Hlavinka ML, Hagadorn JR. Organometallics. 2007;26:4105. [Google Scholar]
- 17.Wiemers DM, Burton DJ. J Am Chem Soc. 1986;108:832. [Google Scholar]
- 18.Nair HK, Burton DJ. J Fluorine Chem. 1992;56:341. [Google Scholar]
- 19.(a) Naumann D, Schorn C, Tyrra W. Z Anorg Allg Chem. 1999;625:827. [Google Scholar]; (b) Lange H, Naumann DJ. Fluorine Chem. 1984;26:435. [Google Scholar]; (c) Naumann D, Roy T, Caeners B, Hütten D, Tebbe K-F, Gilles T. Z Anorg Allg Chem. 2000;626:999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.