Abstract
Frontal hypoactivation has consistently been demonstrated in schizophrenia patients. We hypothesized that this well-known deficit is asymmetrical, ie, centered over left frontal locations and, in-line with Crow's theory, associated with both loss of linguistic asymmetry and correlated with positive symptoms. Electroencephalography delta band was used as a quantitative index of cortical inhibition in 17 paranoid schizophrenia patients with prevailing positive symptoms and 17 matched control subjects. Delta amplitude was measured by 38 electrodes, while participants performed 3 linguistic tasks, visuoperceptual, rhyming, and semantic judgment. Compared with control subjects, patients did not show overall delta band differences, revealing no detrimental effects of pharmacological treatment. In healthy participants, analysis of 4 quadrants/regions of interest revealed higher delta amplitude in right vs left anterior sites, indicating significant left anterior disinhibition during linguistic processing. Instead, patients showed bilateral delta band distribution and, compared with control subjects, significant greater delta amplitude (ie, brain inhibition) in linguistic left anterior centers. Patients’ left hypofrontality was functionally related to their lack of hemispheric specialization for language and was positively correlated with higher levels of delusions (P1) and conceptual disorganization (P2) Positive and Negative Syndrome Scale subscales. Results suggest, in schizophrenia patients, a functional deficit of Broca's area, a region playing a fundamental hierarchical role between and within hemispheres by integrating many basic processes in linguistic and conceptual organization. The significant correlation between lack of anterior asymmetry and increased positive symptoms is in-line with Crow's hypothesis postulating the etiological role of disrupted linguistic frontal asymmetry on the onset of the key symptoms of schizophrenia.
Keywords: EEG rhythm, psychosis, delusions, lateralization, electroencephalography
Introduction
Much literature has investigated abnormal brain organization and functioning in schizophrenia patients.1–6 Evidence from functional neuroimaging techniques shows that the most important and reliable finding is clear-cut hypofrontality, ie, significant dysfunction in the activation of the prefrontal cortex, typically associated with deficits in attention, action planning, and working memory.7–12 Schizophrenia patients’ hypofrontality has also consistently been found with electrophysiological techniques analyzing slow electroencephalography (EEG) activity.13–18 Among other low-frequency EEG rhythms, the delta band (0.1–3.9 Hz) usually prevails in human infants during the first 2 years of life and gradually decreases with age.19–22 In healthy adults, the delta band is typically observed in the deepest stages of sleep (also called slow wave sleep23–25) and, when it appears in the waking brain, is considered a marker of brain damage or a pathological condition. Most EEG studies found increased levels of delta in frontal sites during a resting state, in schizophrenia patients compared with control subjects, independent of closed/open eye condition. In addition, results by Fehr et al13 revealed higher focal slow waves in left frontal regions and in bilateral temporal and posterior areas of patients. Pascual-Marqui et al17 also showed the larger spectral amplitude of the delta band in patients’ frontal areas, mainly in the left hemisphere: The authors interpreted this phenomenon, exhibited in a resting condition, as a marker of a functional deficit of cerebral coordination and therefore as a state of functional disconnection. Another study by Tauscher et al18 using EEG coherence analysis showed significantly lower delta band coherence in left frontal electrodes (ie, Fp1–F7) in schizophrenia patients compared with healthy control subjects. Interestingly, the extent of delta band coherence, obtained in a resting condition, was inversely correlated with the Positive and Negative Syndrome Scale (PANSS) positive syndrome subscale, suggesting a direct link between the severity of positive symptoms and patients’ hypofrontality. Positive symptoms were also significantly correlated with increased delta and theta activity in frontal, parietal, and right hemispheric regions by Fehr et al13
From a more general point of view, there is evidence that slow wave activity, particularly in delta band range, marks pathological brain abnormality resulting from neurological damage, such as cerebral infarct, contusion, local infection, tumor, or subdural hematoma.26–32 Additional evidence of the link between slow delta activity and brain damage comes from studies of neurological or psychiatric patients affected by Alzheimer disease, mild cognitive impairment, aphasia, dyslexia, schizophrenia, or depression,32–38 in which slow wave activity is related to the extent of cognitive impairment and brain damage and increased delta activity is considered a clear-cut marker of altered brain functioning.33,35,36 According to quoted literature, delta EEG activity measured in healthy sleeping subjects was correlated with a significant positron emission tomography (PET) metabolic inhibition in sleep-relevant brain regions.23 Thus, a large literature from different fields and subject samples provides converging evidence that increased delta EEG power is a quantitative and reliable index of neural inhibition. In 2 previous studies, we used a paradigm validated for language39–41 in order to investigate delta spectral activity elicited by various linguistic processes in both dyslexic children and nonfluent aphasic patients.35,36 In these studies, we demonstrated that delta activity, given its role as a physiological index of functional inhibition, is not only especially suited for measuring functional impairment in language lateralization in children with learning disabilities and in brain-damaged patients but may also represent a useful and sensitive tool to assess reorganization and recovery in impaired neural networks. The same paradigm was used to assess Crow's42,43 hypothesis on schizophrenia disorder: We found evidence of a significant lack of left hemispheric dominance for language in schizophrenia patients, in both automatic recognition potential44 and in contingent negative variation.45 On the basis of previous studies on language lateralization44,45 and past evidence of patients’ hypofrontality during resting conditions,13,16,17 we expected that the main deficit observed in patients’ frontal sites would mainly depend on a primary deficit in functional linguistic integration hierarchically organized by Broca's area. This linguistic region is traditionally known to be specialized in phonological processing, articulation, and linguistic production in contrast with left posterior regions specialized in comprehension, lexical and semantic processing, and language output monitoring (see Indefrey and Levelt46 for a review). However, recent views highlighted an important hierarchical role of Broca's area over left posterior linguistic regions, both in overall linguistic reorganization after brain damage in aphasics47,48 and in linguistic domains other than phonological ones, such as in semantics and syntax.49,50 The interhemispheric and intrahemispheric hierarchical role of Broca's area would explain the lack of linguistic integration and the metalinguistic disorganization observed in schizophrenia. According to Crow,42,43 the deficit in linguistic dominance, in turn, leads to lack of hemispheric integration, confusion between inner and external voices (hallucinations), and thought disorders and delusions. Therefore, compared with control subjects, schizophrenia patients are expected to exhibit greater delta activity over anterior left cortical sites, ie, in regions currently considered essential, in addition to phonological segregation of words, also for the organization of the whole linguistic network. The present experiment is innovative with respect to past similar investigations44,45 for 2 aspects. First, the use of delta EEG band, an index of large inhibited dysfunctional cortical regions, provides complementary information with respect to evoked potentials, a physiological measure marking specific cortical networks actively engaged by tasks. Second, the finding of a link between key psychiatric indices (positive symptoms) and frontal linguistic asymmetry would more strongly support Crow's etiological hypothesis42,43 on the main mechanism postulated at the origin of the schizophrenic disorder: the disruption of the typical linguistic hemispheric asymmetry measured in healthy individuals. Thus, in-line with past evidence linking behavior and brain asymmetry with other methods and nonlinguistic tasks,13,18 we expected to find, in schizophrenia patients, a significant correlation between laterality indices obtained for every task and a subset of positive symptoms (ie, delusions, conceptual disorganization, and hallucinations) measured by the PANSS.51
Methods and Materials
Participants
The psychiatric group consisted of 17 schizophrenia inpatients (4 women, 13 men; mean age ± SD: 39.7 ± 11.1 y; mean years of education ± SD: 10.2 ± 2.7 y) recruited from the Ospedale Psichiatrico Giudiziario (Forensic Psychiatric Hospital) of Castiglione delle Stiviere (Mantova, Italy) according to the following criteria: All patients were right-handed according to the Edinburgh Handedness Inventory52; they had been diagnosed as schizophrenic during the acute phase, on the basis of positive or negative symptoms exhibited for more than 6 months according to Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition Revised) criteria; and at the time of the present study, all patients were in a chronic state, as attested by an average time from onset of 14.5 years (SD = ±8.6). The diagnosis, ascertained by the psychiatrists of the ward by administering Structured Clinical Interview for DSM disorders, classified 1 patient as disorganized (International Classification of Diseases, Tenth Revision [ICD-10], F20.1), 2 with paranoid/residual (ICD-10 F20.0/F20.5), and 14 with paranoid schizophrenia (ICD-10 F20.0). In addition, prior to the experimental session, schizophrenia patients were screened to ascertain the severity of symptoms according to the Italian version of PANSS. The sample was characterized by relatively high levels of blunted affect (construct N1; mean ± SD: 4.3 ± 0.9), emotional withdrawal (construct N2; mean ± SD: 4.5 ± 1.0), and passive/apathetic social withdrawal (construct N4; mean ± SD: 4.3 ± 1.2) as negative symptoms and relatively high ratings of delusions (construct P1; mean ± SD: 4.6 ± 1.6) and conceptual disorganization (construct P2; mean ± SD: 3.6 ± 1.3) as positive symptoms. Six patients were treated with typical antipsychotic drugs (ie, chlorpromazine, clotiapine, clucopenthixol, haloperidol, and methotrimeprazine), 6 patients with atypical antipsychotic drugs (ie, aripiprazole, clozapine, olanzapine, quetiapine, and risperidone), and 5 patients with both typical and atypical antipsychotic drugs.
The control group consisted of 17 right-handed healthy volunteers (6 women, 11 men; not significant) matched for age (mean ± SD: 41.8 ± 20.2 y; t32 = 0.37, not significant) and educational level (mean ± SD: 11.9 ± 2.9 y; t32 = 1.75, not significant) to the patient group.
Both healthy adults and patients gave their informed consent to participation in this study, which was approved by the local ethics committee and performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Stimuli, Tasks, and Procedure
Stimuli consisted of bi- or trisyllabic Italian content words selected from a frequency dictionary of 5000 written Italian words.53 Words were presented in pairs on a 17″ computer monitor one at a time with an interstimulus interval of 2 seconds: The first word (W1) remained on the screen for 1 second and the second word (W2 or target) until the subject responded by pressing a keyboard button, in no case longer than 5 seconds.41 Word pairs were administered in 3 separate blocks, which corresponded to 3 linguistic tasks: Thus, the same words were presented as W1 but in different randomized order across tasks. Upon W2-target presentation, participants had to decide whether word pairs rhymed (phonological task), whether target word W2 was of the same category as W1 (semantic task), and whether word pairs were written in the same upper or lower case (orthographic control task; for further details, see Spironelli and Angrilli41). For motor responses, subjects used their left index or middle finger to press the keyboard buttons corresponding to match-mismatch conditions. Each task included 80 trials/word pairs, 50% matches being randomly interspersed with 50% mismatch trials; task order was randomly varied across participants.
Data Recording and Analysis
Electrophysiological activity was continuously recorded in DC mode by 38 tin electrodes, 31 placed on an elastic cap (ElectroCap, Eaton, Ohio) according to the International 10-20 system54 and the other 7 applied below each eye (Io1, Io2), on the 2 external canthi (F9, F10), nasion (Nz), and mastoids (M1, M2). All cortical sites were online referred to Cz and off-line rereferenced to the average reference. Data were stored in NeuroScan software, version 4.1. Amplitude resolution was 0.1 μV; bandwidth ranged from DC to 100 Hz (6 dB per octave). Sampling rate was set at 500 Hz, and impedance was kept below 5 kΩ. Behavioral measures collected from each participant included error rates (ERs) and response times (RTs) to the second stimulus, and mean performance was compared between groups and among tasks.
EEG data were divided into four 1024-millisecond time intervals (given the constraint of Brain Electrical Source Analysis [BESA] software [5.1 version] to use 2n samples, we needed to force the width of each interval to 512 samples, corresponding to a 1024-ms interval). Thus, fast Fourier transform (FFT) was performed after windowing the signal with a cosine-tapered window and included 512 samples/lines corresponding to 0.977-Hz resolution. Each interval represented a different processing phase required by tasks: 1024 milliseconds before W1 onset (baseline interval), 1024 milliseconds after W1 onset (W1 interval), 1000–1024 milliseconds after W1 onset (initial interstimulus interval [iISI]), and 1976–3000 milliseconds after W1 onset (terminal interstimulus interval, tISI), with W1-iISI and iISI-tISI intervals slightly overlapping (24 and 48 ms, respectively, ie, less than matching previous studies35,55,56), the first interval of stimulus processing (W1) was clearly related to word reading, the second (iISI) referred to cognitive operations associated with the stimulus encoding in verbal working memory,57–60 and the third (tISI) reflected the late processing of word features necessary for comparison with the following stimulus.40,41,60,61 As eye movement artifacts may affect delta band amplitude, particularly over frontal locations, each trial epoch was corrected for blinks and eye movements according to Ille et al62 by means of BESA software. Artifact rejection was automatically applied to all epochs, with amplitude and derivative thresholds (150 μV and 100 μV/ms, respectively). Remaining epochs were then visually inspected for any residual artifacts. A total of 7.3% of trials was rejected by control subjects and 17.8% by schizophrenia patients, evenly distributed across tasks. For each participant, FFT was performed on all artifact-free epochs that were averaged for each interval and task. The last step consisted of normalizing delta band amplitude (nominally 0.5–4 Hz, effective range: 0.977–3.908 Hz) for all recorded locations by computing the percentage of delta amplitude for each electrode in the 0.977–100 Hz spectral range. (More exactly, percentage of delta amplitude was obtained by the sum of all values within the delta spectral band divided by the sum of all values within the a priori selected overall spectral range [0.97–100 Hz].) The normalization procedure allowed us to compare subjects with large differences in spectral energy and to measure the relative contribution (percentage) of delta spectral amplitude in comparison with other EEG bands. After transformation, EEG data underwent analysis of variance (ANOVA) to compare, across time intervals, the average delta amplitude measured by 4 groups of electrodes, representing different regions of interest.35,55,56 Thus, 4 clusters, each including the average activity of 4 electrodes, were selected: anterior left (F7, FT7, F3, FC3), anterior right (F8, FT8, F4, FC4), posterior left (P3, P7, TP7, T7), and posterior right (P4, P8, TP8, T8).
With regard to behavioral measures (mean ERs and RTs), ANOVAs included the between-subject factor group (2 levels: control subjects vs patients) and within-subject factor task (3 levels: orthographic vs phonological vs semantic task).
ANOVA was carried out on EEG data with the following variables: group (2 levels: control subjects vs patients), task (3 levels: orthographic vs phonological vs semantic), interval (4 levels: baseline vs W1 vs iISI vs tISI), region (2 levels: anterior vs posterior), and laterality (2 levels: left vs right hemisphere). The Greenhouse-Geisser (GG) correction was applied where sphericity assumption was violated; in this case, uncorrected df, ϵ values, and corrected probability levels are listed. Post hoc comparisons were computed with Newman-Keuls tests, at P <.05.
In addition, for the patient group only, Pearson correlation analysis was carried out between selected PANSS scores and laterality indices obtained during task processing in order to ascertain whether specific positive PANSS symptoms—delusions (P1), conceptual disorganization (P2), and hallucinatory behavior (P3)—represented a behavioral correlate significantly linked with delta cortical distribution. The laterality index was computed as the difference of the mean activity of left (electrodes: F7, FT7, F3, FC3) minus right (electrodes: F8, FT8, F4, FC4) anterior clusters; similar lateralization scores were also computed for posterior clusters (left [electrodes: P3, P7, TP7, T7] minus right [electrodes: P4, P8, TP8, T8] posterior quadrants). The laterality index was positive when patients had a higher delta percentage in the left hemisphere and negative when they had a higher delta percentage in the right hemisphere. Therefore, positive correlations marked those patients with higher scores on PANSS constructs, revealing more severe symptoms, and a higher delta percentage in the left hemisphere, corresponding to greater inhibition of left vs right locations.
Results
Behavioral Data
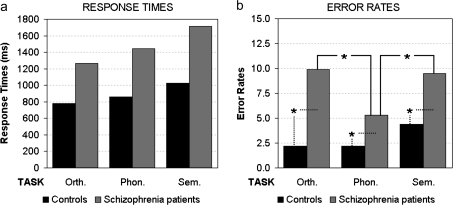
RTs showed the significant main effect of group (F1,32 = 21.36, P < .001), patients being slower than control subjects (1476 [SD: ±542 ms] vs 888 ms [SD: ±232 ms], respectively). The main effect of task (F2,64 = 35.22, P < .001, GG ϵ = 0.99) revealed longer RTs for the semantic (1371 ms [SD: ±556 ms]) than the phonological task (1152 ms [SD: ±499 ms]; P < .001), which in turn induced longer RTs than the orthographic task (1022 ms [SD: ±416 ms]; P < .001).
Analysis of ERs showed the significant main effect of group (F1,32 = 23.90, P < .001), patients’ ERs being higher than those of control subjects (8.2% [SD: ±5.9%] vs 2.9% [SD: ±2.3%], respectively), and task (F2,64 = 7.21, P < .01, GG ϵ = 0.67), ERs being higher for both semantic (6.9% [SD: ±5.0%]) and orthographic tasks (6.0% [SD: ±6.0%]) than the phonological task (3.7% [SD: ±4.0%], P < .001 and P < .01, respectively). However, the 2-way group-by-task interaction (F2,64 = 3.52, P = .05, GG ϵ = 0.67) revealed significant post hoc differences only for patients (figure 1b).
Fig. 1.
Response Time Analysis (a) Showed Significant Main Effects of Group and Task Factors. Error rate analysis (b) revealed 2-way group-by-task interaction. Asterisks indicate significant post hoc comparisons.
Control subjects showed the same percentage of errors in all tasks (less than 5%), but schizophrenia patients exhibited significantly higher ERs in both orthographic and semantic tasks than in the phonological task (P < .001). In addition, patients made more errors than healthy control subjects in all tasks (P < .001 for orthographic and semantic tasks, P < .05 for phonological).
Electrophysiological Data
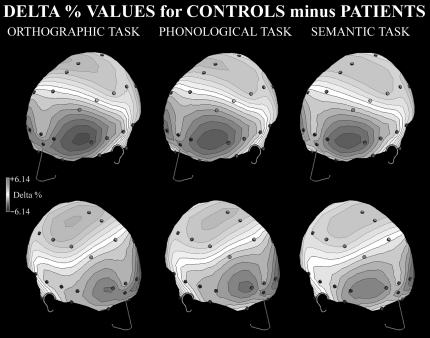
Figure 2 shows the difference in delta percentage between control subjects and schizophrenia patients for each task: higher levels of delta band in patients vs control subjects are shown in blue and lower levels in red.
Fig. 2.
Spline Maps of Differences Between Delta Percentage Values for Control Subjects and Patients. Dark levels, patients' greater delta activity; clear levels, control subjects' higher delta activity. Panels: left, orthographic task; central, phonological task; and right, semantic task.
Qualitative analysis of spline maps suggests that, compared with control subjects, schizophrenia patients have greater levels of delta rhythm in anterior regions, particularly in the left hemisphere (figure 2). Conversely, control subjects showed higher percentages of delta band in the posterior regions of both hemispheres, without task differences.
ANOVA on normalized delta band showed the significant main effect of interval (F3,96 = 63.76, P < .001, GG ϵ = 0.66): Independent of group, a higher delta level was found in W1 (9.15% [SD: ±3.83%]) compared with iISI (8.60% [SD: ±3.56%], P < .001) and between these 2 intervals and both baseline (8.22% [SD: ±3.42%]) and tISI (8.14% [SD: ±3.41%], all P <.001). However, the 2-way group-by-interval interaction (F3,96 = 10.53, P < .001, GG ϵ = 0.66) revealed that this pattern marked delta distribution in control subjects, whereas patients had greater delta amplitude in both W1 and iISIs compared with baseline and tISI (all P <.001).
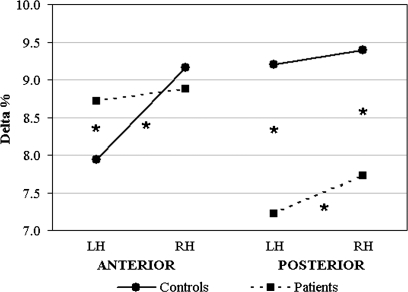
The main effect of laterality was also significant (F1,32 = 7.55, P < .01), revealing greater delta distribution on right (8.79% [SD: ±3.56%]) vs left hemisphere (8.27% [SD: ±3.58%]). However, the 3-way group-by-region-by-laterality interaction (F1,32 = 9.32, P < .01) showed that control subjects, but not patients, had smaller delta amplitude in left than right anterior sites (P < .001), regardless of task. In addition, schizophrenics had higher delta levels in left anterior locations compared with control subjects (P < .01), whereas no group differences were found in right anterior sites (figure 3, left panel). Conversely, in posterior locations, patients showed greater delta distribution in right vs left hemisphere (P < .05). In addition, in posterior locations, control subjects had higher delta amplitude in both hemispheres compared with schizophrenics (all P <.001; figure 3, right panel).
Fig. 3.
Delta Band Analysis: Significant 3-Way Group-by-Region-by-Laterality Interaction. Control subjects’ and patients' delta distributions (black and dotted lines, respectively) are shown for anterior (left panel) and posterior brain regions (right panel). Asterisks indicate significant post hoc comparisons.
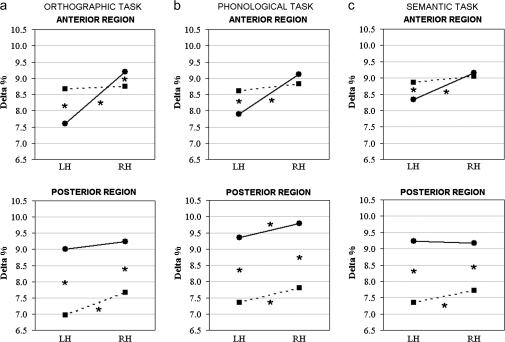
The 4-way group-by-task-by-region-by-laterality interaction (F2,64 = 4.31, P < .05, GG ϵ = 0.90] revealed specific patterns of delta amplitude in schizophrenia patients and control subjects. During the phonological task (figure 4b), in anterior regions, control subjects showed significant higher right vs left delta levels (P < .001), whereas patients showed bilateral delta distribution (figure 4b). In contrast, both groups had greater delta amplitude in right vs left posterior locations (all P < .01). Concerning between-group differences, schizophrenics had greater delta levels than control subjects in anterior left locations (P < .001), and control subjects had higher delta amplitude in both posterior sites (P < .001, figure 4b).
Fig. 4.
Delta Band Analysis: Significant 4-Way Group-by-Task-by-Region-by-Laterality Interaction. (a) Orthographic, (b) phonological, and (c) semantic tasks in control subjects (black line) and schizophrenia patients (dotted black line) in anterior (left) and posterior sites (right).
A different pattern of activation was found during both semantic (figure 4c) and orthographic tasks (figure 4a): Healthy control subjects had significant greater right vs left delta amplitude in anterior sites (all P <.001) and bilateral delta distribution in posterior locations, whereas patients showed bilateral delta levels in anterior clusters and significant greater delta amplitude at right compared with left posterior sites (P < .05 and P < .001, respectively). Concerning between-group differences, as for the phonological task, schizophrenics exhibited greater delta distribution than control subjects in left anterior locations (P < .01 and P < .001 for semantic and orthographic tasks, respectively) and in both posterior clusters (all P < .001; figures 4a and 4c). In addition, control subjects had higher delta amplitude in anterior right sites, specifically during the orthographic task (P < .05).
From a different point of view, control subjects showed significant delta lateralization in anterior locations, regardless of task (figure 4, top row): However, a significant lower delta percentage was found in left sites for orthographic and phonological vs semantic tasks (all P <.001). Instead, in posterior sites, specifically during phonological processing, control subjects had significant higher delta levels in right locations compared with delta amplitude measured in the right clusters of both orthographic and semantic tasks (all P <.001; figure 4, bottom row). Conversely, patients exhibited significant less delta distribution in posterior left vs right locations, regardless of task (figure 4, bottom row): Moreover, significant lower delta levels were found in left sites for orthographic compared with phonological and semantic tasks (all P <.01).
Pearson Correlations
This analysis, made only on patients’ data, provided essential information for interpreting schizophrenics’ delta distribution within the linguistic domain. Pearson correlations were computed between the scores achieved by patients on delusions (P1), conceptual disorganization (P2) and hallucinatory behavior (P3), and laterality indices (computed as the difference of left minus right delta amplitude) obtained from delta distributions and collapsed across all intervals in all 3 tasks. As mentioned above, positive correlations indicated that greater delta rhythm in the left vs right hemisphere was correlated with more severe symptoms.
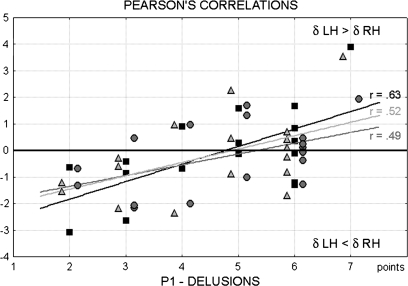
An interesting and clear-cut finding concerned the first construct (P1) of the positive subscale of the PANSS, delusions: Three significant positive correlations were found between the scores obtained for the P1 construct and the laterality indices of anterior clusters in orthographic, phonological, and semantic tasks (r12 = 0.63, P < .01; r12 = 0.49, P < .05; and r12 = 0.52, P < .05, respectively). The greater the delta amplitude in left anterior regions (regardless of task), the higher the score reached in the delusion domain (figure 5).
Fig. 5.
Pearson Correlations Between First Construct (P1) of Positive Subscale of the Positive and Negative Syndrome Scale (Delusions) and Delta Anterior Laterality (Left Minus Right Delta Amplitude) in Orthographic (Black Squares), Phonological (Dark Gray Circles), and Semantic Tasks (Light Gray Triangles).
Significant positive correlations were also found between scores on the conceptual disorganization (P2) construct and laterality indices measured at the anterior clusters of orthographic (r12 = 0.50, P < .05), phonological (r12 = 0.51, P < .05), and semantic tasks (r12 = 0.56, P < .05). The greater the delta level in left anterior regions, the higher the score of the conceptual disorganization domain. No significant correlations were found for the P3 construct, corresponding to hallucinatory behavior. (In order to ascertain the specificity of symptoms/lateralization associations, we have also correlated laterality indices and negative PANSS symptoms: No significant correlations were found.)
Discussion
The present study examined the lateralization of delta band across different linguistic tasks in schizophrenia patients and matched control subjects, using a validated linguistic paradigm.35,36 Most of the literature showed converging and unambiguous evidence that delta band is an index of functional and structural cortical inhibition. At the neurophysiological level, delta activity is generated by those neurons suffering for disconnection/deafferentiation or which underwent to partial damage around a brain lesion (death neurons in the core of the lesion are electrically silent); therefore, the disruption of the original extended neural network leads to synchronized, high-amplitude, slow electrical activity.33,34,36 At the functional level, this is contrary of high-frequency rhythms, such as gamma band, in which neurons from intact neural networks, when engaged in high-level cognitive processing, fire synchronously at very high frequency and with low amplitudes (see, for review, Hermann et al63 and Kaiser et al64). All neurons generating delta rhythm are recruited in low-frequency, nonspecific activity and cannot be involved in specific high-frequency processes. Therefore, delta band is a quantitative index of the amount of neurons not engaged in specific cognitive processes and indicates neural inhibition.27,32 In addition to neurological studies, this interpretation has been further demonstrated by the significant negative correlation found between local delta activity and regional metabolism as measured by PET in healthy sleeping subjects.23 In the present experiment, delta band was used to measure functional alterations in language lateralization. As a first important consideration, the main effect of group factor was not significant: Patients had no overall greater delta amplitude with respect to healthy control subjects. This result suggests that patients had no structural or pharmacological dependent impairments. Past studies have found significant greater levels of delta amplitude in schizophrenia patients compared with healthy control subjects13,16,17 as in other psychiatric patients (eg, Mientus et al16), but all past studies compared groups in a resting condition. Moreover, delta amplitude was rarely normalized to allow better between-group comparisons. The main result of the present study is the significant higher delta percentage found in the anterior left regions of patients compared with control subjects (figures 3 and 4, upper panels). Control subjects had a coherent pattern of lateralization, delta activity being higher in right than left anterior sites, whereas schizophrenics showed relatively greater delta amplitude in left anterior locations. Thus, control subjects showed a common pattern of lateralization across tasks, with an overall greater disinhibition of left frontal centers in all linguistic conditions. This asymmetry was mainly frontal and is related to the rationale of our paradigm that stresses on working memory—a strategic choice useful for measuring hypofrontality in schizophrenia. The only task difference, in control subjects, was found at posterior sites, the rhyming judgment showing greater left than right disinhibition. The phonological activity induced by this task needs the activation of both anterior and posterior linguistic centers by specifically involving, with respect to the other 2 tasks, the verbal working memory. It should be noted that evoked potentials, using the same paradigm, are more sensitive to task/linguistic manipulation41,65. This is because evoked potentials are elicited by synchronous but relatively smaller percentage of neurons and networks actively engaged by the task. Indeed, in a previous study based on slow cortical potentials,45 we have showed that the typical linguistic networks recruited by phonological task were activated in control subjects’ left frontal sites but were bilateral in schizophrenic patients. Instead, delta EEG band is an index sensitive to low-frequency synchronous neurons not recruited by the specific task, which are functionally damaged or lost their connectivity with linguistic important cortical regions.34,36 Therefore, it measures the contribution of large amounts of inhibited neurons: This feature makes delta band a complementary method especially suited for studying functional deficits in psychiatric or neurological patients.
Results of the present experiment do not contrast with other current and apparently incongruous empirical findings on schizophrenia. Part of the current literature is focused on temporal lobe anomalies that, in some studies, have been associated to auditory hallucinations.66,67 In-line with those findings, in the present experiment, an altered activity—consisting in both smaller delta amplitude (ie, greater disinhibition) than control subjects in all posterior sites and in a reversed delta lateralization—was found over temporal regions. Patients’ left posterior regions revealed the smallest delta amplitude (figure 3), ie, the greatest disinhibition, whereas the left frontal ones showed the greatest inhibition. This altered pattern indicates a loss of connectivity in patients’ left anterior centers (larger delta) and an increased unspecific activation of left posterior sites. Therefore, left temporal areas, when disconnected and no longer hierarchically controlled by left frontal regions, may undergo uncontrolled activation of isolated linguistic networks, and, in the end, this could lead to hallucinations. This interpretation is consistent with past experiments showing a reduction of hallucination symptoms after repeated transcranial magnetic stimulation of left temporoparietal cortex.66,67 However, the observed altered posterior activation in our sample did not lead to significant hallucinations probably because of an efficient pharmacological treatment: This can also explain the lack of significant correlation between hallucinatory behavior (ie, the P3 construct) and posterior delta asymmetry. In addition, for the above-mentioned reasons, delta EEG band might be less suited to highlight an excess of activation of left temporal lobe neurons associated to hallucinations. This is a matter for future studies with evoked potentials.
Within left posterior sites, lower delta levels were found in orthographic rather than in both phonological and semantic tasks: This result may reflect patients’ difficulty in performing the dual task like the orthographic one. Although simple visuoperceptual matching was required (upper vs lower case recognition), words also automatically activate their linguistic representation and the corresponding networks within the left hemisphere. Thus, patients may have greater difficulties than control subjects in inhibiting automatic linguistic processes for correct execution of visual matching judgments. In support of this interpretation, significantly higher ERs were found in patients during the orthographic task, in comparison with their performances on both the phonological task and control subjects’ performance on the orthographic one (figure 1b).
Matching studies that found significant hypofrontality in schizophrenia during a resting condition,13–17 our patients also showed reduced frontal lobe activity. However, unlike previous studies carried out on patients in a resting state, we found significant hypoactivity in left anterior regions, ie, in the cluster of electrodes selectively involved in linguistic processing. Indeed, our patients showed a consistent inhibition of left frontal regions compared with those of control subjects and the lack of the left frontal asymmetry expected for linguistic tasks. According to recent views on the role of Broca's area, there is increasing evidence that this region, in addition to its acknowledged role in articulation and phonological encoding,40,46,68 is also dedicated to hierarchical and metalinguistic organization of high-level linguistic processes (see, for review, Bookheimer49 and Hagoort50), and therefore, also within the left hemisphere, this area would play a dominant role in leading high-order linguistic organization. In support of this, empirical data from recovered aphasic patients with evident lesions in Broca's area showed that plastic reorganization of both phonological and semantic processes occurred in the residual left prefrontal areas spared by the lesion, rather than in intact posterior linguistic regions.47,48 A recent investigation on functional resting state connectivity confirmed the unification model in which the 3 main subdivisions of Broca's area—pars triangularis, pars opercularis, and pars orbitalis—are functionally connected with middle frontal, parietal, and temporal regions and take account for its relevant role in phonological, syntactic, and semantic processing.69 The larger left frontal delta increase observed in our schizophrenia patients indicates that a relatively high proportion of neurons in this region is not recruited (ie, functionally inhibited), especially when the integration of basic cognitive processes is involved (most of which are organized and integrated in language). This impairment would lead to the loss of integration between left and right frontal regions and, along the anteroposterior asymmetry, between left anterior and left posterior linguistic regions. Our data and interpretations are in agreement with studies of Hoffman et al70, who postulated a disconnection between left anterior-posterior linguistic networks and suggested that the leading deficit in schizophrenia involves verbal working memory rather than attentional-perceptual cognitive processes.
To discover the etiological aspects of a psychiatric disorder, it is important to investigate the link between behavior and brain activity. The correlation of these 2 domains allows us to disentangle different hypotheses and to make stronger assumptions on a specific theory. Matching Crow's42,43 hypothesis, we expected a significant correlation between delta band asymmetry and schizophrenics’ positive symptoms. In agreement with our hypotheses, the delta frontal asymmetry was positively correlated with the highest scores in 2 positive symptom domains, ie, P1 and P2 constructs of the PANSS: Patients with higher delta levels in left anterior sites showed a greater extent of delusions and conceptual disorganization (averaged across all tasks correlation was 0.55 and 0.52, respectively). The loss of frontal asymmetry may be the result of 2 interacting mechanisms, the lack of left hemisphere specialization for language and the lack of inhibition of homologous regions in the right hemisphere. Such a result is in-line with prior research on another sample of patients carried out with different methods (slow evoked potentials45). In addition to past research, in the present study, delta EEG band was able to highlight an important functional link between left frontal inhibition and key positive symptoms of schizophrenia. Furthermore, in schizophrenic patients, the inhibition of left anterior linguistic centers and the relative disinhibition of the left posterior ones, with respect to control subjects, point to an altered hierarchy and connectivity also between anterior and posterior linguistic networks. Given the central role of left inferior frontal gyrus, and particularly Broca's area, in unification processes that are necessary49,50,69 to organize not only all linguistic functions but also hierarchically structured behaviors,71 it is coherently possible to suppose that the pervasive thought and behavioral disorders observed in schizophrenia are causally related to decreased activity, loss of connectivity, and dominance of such critical area. This important result does not exclude that another key symptom of the disorder, the auditory hallucinations—possibly generated by uncontrolled disconnected activation of left posterior sites (see above)—might be better detected with other indices or more targeted samples of unmedicated patients.66,67,72
Funding
Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 2006110284_001); University of Padova (project no. CPDA047438 to A.A.).
Acknowledgments
CPDA047438 No author reports any biomedical financial interest or potential conflict of interest.
References
- 1.Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 2.Petty RG. Structural asymmetries of the human brain and their disturbance in schizophrenia. Schizophr Bull. 1999;25:121–139. doi: 10.1093/oxfordjournals.schbul.a033360. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger DR, Eagan MF, Bertolino A, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 4.McCarley RW, Salisbury DF, Hirayasu Y, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- 5.Cullen TJ, Walker MA, Eastwood SL, Esiri MM, Harrison PJ, Crow TJ. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br J Psychiatry. 2006;188:26–31. doi: 10.1192/bjp.bp.104.008169. [DOI] [PubMed] [Google Scholar]
- 6.Esiri MM, Crow TJ. Neuropathology of psychiatric disorders. In: Louis DN, Love S, Ellison DW, editors. Greenfield's Neuropathology. 8th ed. London, UK: Arnold; 2008. pp. 431–470. [Google Scholar]
- 7.Andreasen NC, Rezai K, Allinger R, et al. Hypofrontality in neuroleptic-naïve patients and in patients with chronic schizophrenia: assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry. 1992;49:943–958. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg TE, Gold JM. Neurocognitive deficits in schizophrenia. In: Hirsch SR, Weinberger DR, editors. Schizophrenia. Oxford, UK: Blackwell Science; 1995. pp. 146–162. [Google Scholar]
- 9.Goldman-Rakic PS, Friedman HR. The circuitry of working memory revealed by anatomic and metabolic imaging. In: Levin HS, Eisenberg HA, Benton AL, editors. Frontal Lobe Function and Dysfunction. New York, NY: Oxford University Press; 1991. pp. 1025–1038. [Google Scholar]
- 10.Ingvar DH, Franzén G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr Scand. 1974;50:425–462. doi: 10.1111/j.1600-0447.1974.tb09707.x. [DOI] [PubMed] [Google Scholar]
- 11.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 12.Wienberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci. 1996;351:1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- 13.Fehr T, Kissler J, Moratti S, Wienbruch C, Rockstroh B, Elbert T. Source distribution of neuromagnetic slow waves and MEG-delta activity in schizophrenia patients. Biol Psychiatry. 2001;50:108–116. doi: 10.1016/s0006-3223(01)01122-2. [DOI] [PubMed] [Google Scholar]
- 14.Guich SM, Buchsbaum MS, Burgwald L, et al. Effect of attention on frontal distribution of delta activity and cerebral metabolic rate in schizophrenia. Schizophr Res. 1989;2:439–448. doi: 10.1016/0920-9964(89)90012-1. [DOI] [PubMed] [Google Scholar]
- 15.John ER, Prichep LS, Alper KR, et al. Quantitative electrophysiological characteristics and subtyping of schizophrenia. Biol Psychiatry. 1994;36:801–826. doi: 10.1016/0006-3223(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 16.Mientus S, Gallinat J, Wuebben Y, et al. Cortical hypoactivation during resting EEG in schizophrenics but not in depressives and schizotypal subjects as revealed by low resolution electromagnetic tomography (LORETA) Psychiatry Res. 2002;116:95–111. doi: 10.1016/s0925-4927(02)00043-4. [DOI] [PubMed] [Google Scholar]
- 17.Pascual-Marqui RD, Lehmann D, Koenig T, et al. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naïve, first-episode, productive schizophrenia. Psychiatry Res. 1999;90:169–179. doi: 10.1016/s0925-4927(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 18.Tauscher J, Fischer P, Neumeister A, Rappelsberger P, Kasper S. Low frontal electroencephalographic coherence in neuroleptic-free schizophrenic patients. Biol Psychiatry. 1998;44:438–447. doi: 10.1016/s0006-3223(97)00428-9. [DOI] [PubMed] [Google Scholar]
- 19.Gasser T, Verleger R, Bacher P, Sroka L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr Clin Neurophysiol. 1988;69:91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- 20.Harmony T, Marosi E, Díaz de León AE, Becker J, Fernández T. Effect of sex, psychosocial disadvantages and biological risk factors on EEG maturation. Electroencephalogr Clin Neurophysiol. 1990;75:482–491. doi: 10.1016/0013-4694(90)90135-7. [DOI] [PubMed] [Google Scholar]
- 21.John ER, Alan H, Prichep L, Trepetin M, Brown D, Kaye H. Developmental equations for the EEG. Science. 1980;210:1255–1258. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- 22.Matoušek M, Petersén I. Frequency analysis of the EEG in normal children and in normal adolescents. In: Kellaway P, Petersén I, editors. Automation of Clinical Electroencephalography. New York, NY: Raven Press; 1973. pp. 75–102. [Google Scholar]
- 23.Hofle N, Paus T, Reutens D, et al. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J Neurosci. 1997;17:4800–4808. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steriade M. Brain electrical activity and sensory processing during waking and sleeping. In: Kryger MH, Roth T, Dement WC, editors. Sleep Medicine. Philadelphia, PA: Saunders Company; 2000. pp. 93–111. [Google Scholar]
- 25.Steriade M, McCarley RW. Brain Stem Control of Wakefulness and Sleep. New York, NY: Plenum Press; 1990. [Google Scholar]
- 26.De Jongh A, de Munck J, Baayen J, Jonkman E, Heethaar R, van Dijk B. The localization of spontaneous brain activity: first results in patients with cerebral tumors. Clin Neurophysiol. 2001;112:378–385. doi: 10.1016/s1388-2457(00)00526-5. [DOI] [PubMed] [Google Scholar]
- 27.De Jongh A, Baayen J, de Munck J, Heethaar R, Vandertop WP, Stam CJ. The influence of brain tumor treatment on pathological delta activity in MEG. Neuroimage. 2003;20:2291–2301. doi: 10.1016/j.neuroimage.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Gloor P, Ball G, Schaul N. Brain lesions that produce delta waves in the EEG. Neurology. 1977;27:326–333. doi: 10.1212/wnl.27.4.326. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka A, Kimura M, Yoshinaga S, Tomonaga M, Mizoguchi T. Quantitative electroencephalographic correlates of cerebral blood flow in patients with chronic subdural hematomas. Surg Neurol. 1998;50:235–240. doi: 10.1016/s0090-3019(97)90063-x. [DOI] [PubMed] [Google Scholar]
- 30.Vieth J, Kober H, Kamada K, Ganslandt O. Normal and abnormal MEG activity in border zones of brain lesions. In: Koga Y, Nagata K, Hirata K, editors. Brain Topography Today. Amsterdam, The Netherlands: Elsevier; 1998. pp. 39–46. [Google Scholar]
- 31.Vieth J, Kober H, Ganslandt O, Möller M, Kamada K. The clinical use of MEG activity associated with brain lesions. In: Nenonen J, Ilmoniemi R, Katila T, editors. Biomag 2000. Espoo, Finland: Helsinki University of Technology; 2001. pp. 387–394. [Google Scholar]
- 32.Babiloni C, Frisoni G, Steriade M, et al. Frontal white matter volume and delta EEG sources negatively correlate in awake subjects with mild cognitive impairment and Alzheimer's disease. Clin Neurophysiol. 2006;117:1113–1129. doi: 10.1016/j.clinph.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Hensel S, Rockstroh B, Berg P, Elbert T, Schönle W. Left-hemispheric abnormal EEG activity in relation to impairment and recovery in aphasic patients. Psychophysiology. 2004;41:394–400. doi: 10.1111/j.1469-8986.2004.00164x. [DOI] [PubMed] [Google Scholar]
- 34.Meinzer M, Elbert T, Wienbruch C, Djundja D, Barthel G, Rockstroh B. Intensive language training enhances brain plasticity in chronic aphasia. BMC Biol. 2004;2:20. doi: 10.1186/1741-7007-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penolazzi B, Spironelli C, Angrilli A. Delta EEG activity as a marker of dysfunctional linguistic processing in developmental dyslexia. Psychophysiology. 2008;45:1025–1033. doi: 10.1111/j.1469-8986.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 36.Spironelli C, Angrilli A. EEG delta band as a marker of brain damage in aphasic patients after recovery of language. Neuropsychologia. 2009;47:988–994. doi: 10.1016/j.neuropsychologia.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Szelies B, Mielke R, Kessler J, Heiss W-D. Prognostic relevance of quantitative topographical EEG in patients with poststroke aphasia. Brain Lang. 2002;82:87–94. doi: 10.1016/s0093-934x(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 38.Wienbruch C, Moratti S, Elbert T, et al. Source distribution of neuromagnetic slow wave activity in schizophrenic and depressive patients. Clin Neurophysiol. 2003;114:2052–2060. doi: 10.1016/s1388-2457(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 39.Spironelli C, Angrilli A. Developmental aspects of automatic word processing: language lateralization of early ERP components in children, young adults and middle-aged subjects. Biol Psychol. 2009;80:35–45. doi: 10.1016/j.biopsycho.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Angrilli A, Dobel C, Rockstroh B, Stegagno L, Elbert T. EEG brain mapping of phonological and semantic tasks in Italian and German languages. Clin Neurophysiol. 2000;111:706–716. doi: 10.1016/s1388-2457(99)00308-9. [DOI] [PubMed] [Google Scholar]
- 41.Spironelli C, Angrilli A. Language lateralization in phonological, semantic and orthographic tasks: a slow evoked potential study. Behav Brain Res. 2006;175:296–304. doi: 10.1016/j.bbr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20:339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- 43.Crow TJ. Schizophrenia as the price that Homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Rev. 2000;31:118–129. doi: 10.1016/s0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 44.Spironelli C, Angrilli A, Stegagno L. Failure of language lateralization in schizophrenia patients: an ERP study on early linguistic components. J Psychiatry Neurosci. 2008;33:235–243. [PMC free article] [PubMed] [Google Scholar]
- 45.Angrilli A, Spironelli C, Elbert T, Marano G, Stegagno L. Schizophrenia as failure of left hemispheric dominance for the phonological component of language. PLoS One. 2009;4:e4507. doi: 10.1371/journal.pone.0004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Angrilli A, Elbert T, Cusumano S, Stegagno L, Rockstroh B. Temporal dynamics of linguistic processes are reorganized in aphasics’ cortex: an EEG mapping study. Neuroimage. 2003;20:657–666. doi: 10.1016/S1053-8119(03)00395-1. [DOI] [PubMed] [Google Scholar]
- 48.Spironelli C, Angrilli A, Pertile M. Language plasticity in aphasics after recovery: evidence from slow evoked potentials. Neuroimage. 2008;40:912–922. doi: 10.1016/j.neuroimage.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- 50.Hagoort P. On Broca, brain, and binding: a new framework. Trends Cogn Sci. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS): development and standardization. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 52.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 53.Bortolini V, Tagliavini C, Zampolli A. Lessico di frequenza della lingua italiana contemporanea [Lexical frequency in current Italian] Milano, Italy: Aldo Garzanti Editore; 1972. [Google Scholar]
- 54.Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol. 2001;112:713–719. doi: 10.1016/s1388-2457(00)00527-7. [DOI] [PubMed] [Google Scholar]
- 55.Spironelli C, Penolazzi B, Vio C, Angrilli A. Inverted EEG theta lateralization in dyslexic children during phonological processing. Neuropsychologia. 2006;44:2814–2821. doi: 10.1016/j.neuropsychologia.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Spironelli C, Penolazzi B, Angrilli A. Dysfunctional hemispheric asymmetry of theta and beta EEG activity during linguistic tasks in developmental dyslexia. Biol Psychol. 2008;77:123–131. doi: 10.1016/j.biopsycho.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Birbaumer N, Elbert T, Canavan A, Rockstroh B. Slow potentials of the cerebral cortex and behaviour. Physiol Rev. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- 58.Rösler F, Heil M, Röder B. Slow negative brain potentials as reflections of specific modular resources of cognition. Biol Psychol. 1997;45:109–142. doi: 10.1016/s0301-0511(96)05225-8. [DOI] [PubMed] [Google Scholar]
- 59.Ruchkin DS, Johnson R, Maheffey D, Sutton S. Toward a functional categorization of slow waves. Psychophysiology. 1988;25:339–353. doi: 10.1111/j.1469-8986.1988.tb01253.x. [DOI] [PubMed] [Google Scholar]
- 60.Ruchkin DS, Johnson RJ, Grafman J, Canoune H, Ritter W. Multiple visuospatial working memory buffers: evidence from spatiotemporal patterns of brain activity. Neuropsychologia. 1997;35:195–209. doi: 10.1016/s0028-3932(96)00068-1. [DOI] [PubMed] [Google Scholar]
- 61.Rockstroh B, Elbert T, Canavan A, Lutzenberger W, Birbaumer N. Slow Cortical Potentials and Behaviour. München, Germany: Urban und Schwarzenberg; 1989. [Google Scholar]
- 62.Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J Clin Neurophysiol. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Herrmann CS, Munk MHJ, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Kaiser J, Lutzenberger W. Induced gamma-band activity and human brain function. Neuroscientist. 2004;9:475–484. doi: 10.1177/1073858403259137. [DOI] [PubMed] [Google Scholar]
- 65.Spironelli C, Angrilli A. Influence of phonological, semantic and orthographic tasks on the early linguistic components N150 and N350. Int J Psychophysiol. 2007;64:190–198. doi: 10.1016/j.ijpsycho.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Hoffman RE, Hawkins KA, Gueorguieva R, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60:49–56. doi: 10.1001/archpsyc.60.1.49. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman RE, Hampson M, Wu K, et al. Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cereb Cortex. 2007;17:2733–2743. doi: 10.1093/cercor/bhl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 69.Xiang H-D, Fonteijn HM, Norris DG, Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cereb Cortex. 2010;20:549–560. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]
- 70.Hoffman RE, Rapaport J, Mazure CM, Quinlan DM. Selective speech perception alterations in schizophrenic patients reporting hallucinated “voices”. Am J Psychiatry. 1999;156:393–399. doi: 10.1176/ajp.156.3.393. [DOI] [PubMed] [Google Scholar]
- 71.Koechlin E, Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 72.Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Felber S, Fleischhacker WW. Language lateralization in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. Psychiatry Res. 2006;146:185–190. doi: 10.1016/j.pscychresns.2005.11.003. [DOI] [PubMed] [Google Scholar]