Abstract
An increasing number of studies demonstrate the important role of several susceptibility genes for schizophrenia, such as neuregulin-1 and DISC1, in early postnatal and adult neurogenesis. Its significance for the pathophysiology of the disease, including its relation to neurotransmitter systems implicated in schizophrenia (like the dopamine system), remains, however, unknown. Here, we review molecular and cellular components of the dopamine system associated with postnatal neurogenesis and plasticity, both in rodents and in primates, and discuss their possible implication in schizophrenia. We focus mainly on the islands of Calleja, complex aggregations of granule cells in the ventral striatum, generated early postnatally in the subventricular zone. In contrast to the involution of the primate olfactory bulb, the islands of Calleja attain their maximal development in humans, an evolution paralleled by a larger ventral subventricular zone and more connections with other structures, including temporal cortical areas. The islands of Calleja express high levels of neuronal nitric oxide (NO) synthase and D3 dopamine receptors and are densely interconnected by dopaminergic projections with the ventral tegmental area. D3 receptors modulate subventricular zone neurogenesis and dopamine release. Their genetic deletion induces striatal hyperdopaminergia. We review data indicating a high plasticity of postnatal islands of Calleja, potentially facilitating susceptibility to schizophrenia-related risk factors. In this context, we propose a new pathophysiological model, where altered neurogenesis of the islands of Calleja may contribute to dysfunction of the dopamine and NO systems and psychosis through convergence of genetic and environmental disease-associated factors.
Keywords: subventricular zone, islands of Calleja, D3 dopamine receptors, clozapine
Introduction
Schizophrenia is a chronic, highly debilitating neuropsychiatric disorder with presumed neurodevelopmental background1 and teenage/young adult onset of symptoms. Therefore, pathologic changes during postnatal/juvenile development may be critical in triggering the delayed manifestation of schizophrenia. Susceptibility genes for schizophrenia, like neuregulin-1 and Disrupted-in-Schizophrenia 1 (DISC1), control postnatal and adult neurogenesis in the subventricular zone and dentate gyrus.2–5 However, it remains unclear if their potent effect on neurogenesis induces neurotransmitter (eg, dopamine) dysregulations associated with schizophrenia. Moreover, schizophrenia is a complex disease with disturbances located mainly in frontal brain areas that are not classical sites of neurogenesis (the prefrontal cortex, striatum, and nucleus accumbens). Focusing exclusively on the olfactory bulb, with low translational significance for humans, or on the dentate gyrus with sparse dopaminergic innervation6 may be insufficient to understand schizophrenia-associated dopaminergic alterations.
Secondary Neurogenesis And Migration Patterns From The Subventricular Zone
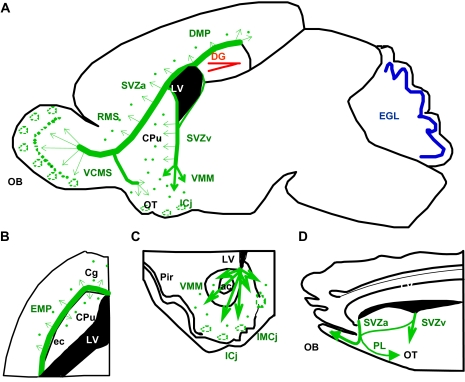
The subventricular zone is the main site of neurogenesis in early postnatal and adult life.7 Initially, it was thought that neuroblasts from the early postnatal subventricular zone migrate exclusively along the rostral migratory stream to the olfactory bulb.8,9 However, recent fate-mapping studies identified additional migratory routes (figures 1A–C). Accordingly, neuroblasts also disperse extensively into subcortical regions, in the so-called ventral migratory mass, generating, eg, the granule cells of the islands of Calleja.10 In fact, neuroblasts from the early postnatal subventricular zone migrate along all elongations of the callosal system into adjacent regions (the medial prefrontal cortex, striatum, and nucleus accumbens) and differentiate into specific GABAergic interneurons, similar to other granule cells.11,12
Fig. 1.
Sites of Secondary Neurogenesis And Migration Pathways in The Postnatal And Adult Mammalian Brain. (A–C) Schematic representation of the pattern of secondary neurogenesis in the early postnatal rodent brain in sagittal (A), horizontal (B), and coronal sections (C). At early postnatal stages, migration of neuroblasts from the subventricular zone occurs not only to the olfactory bulb, in the rostral migratory stream, but also along the external capsule, forming the external migratory pathway, the alveus of the hippocampus, forming the dorsal migratory pathway, and dispersing into the striatum in the ventral migratory mass. The ventrocaudal migratory stream detaches from the rostral migratory stream caudally into the olfactory tubercle. (D) Migratory pathways of neuroblasts from the subventricular zone of adult squirrel monkeys represented in a sagittal section show, despite the involution of the olfactory bulb, striking similarities with the migratory pattern in the postnatal rodent brain. Neuroblasts and migratory streams originating in the subventricular zone are represented in green, neurogenesis in the dentate gyrus in red, and in the cerebellar external granular layer in blue in the color version of the figure online (see references10–12; see references in figure 1D; see Supplementary Material.6,7 ac, anterior commisure; Cg, cingulate cortex; CPu, caudate-putamen; DMP, dorsal migratory pathway; DG, dentate gyrus; ec, external capsule; EGL, external granular layer; EMS, external migratory stream; ICj, islands of Calleja; IMCj, insula magna of Calleja; LV, lateral ventricle; Pir, piriform cortex; PL, posterior limb of the RMS; SVZa, anterior part of the subventricular zone; SVZv, ventral part of the subventricular zone; VCMS, ventrocaudal migratory stream.
This widespread early postnatal migratory pattern becomes restricted mainly to the rostral migratory stream in the adult.13 However, newborn neurons with similar morphology can be detected in the same limbic areas (including the islands of Calleja) in adult rats.14,15 Strikingly, in adult primates, neuronal migratory pathways from the subventricular zone, especially into subcortical areas, are similar to the migratory streams in young rodents, suggesting continuous neurogenesis in these areas (figure 1D; see Supplementary Material section).
An Example of Secondary Neurogenesis: The Islands of Calleja
The islands of Calleja represent accumulations of granule neurons in the ventral striatum, most likely GABAergic neurons, located at the pial border of the basal forebrain, with the major island at the medial border of the nucleus accumbens (figures 1A and 1C),16,17 representing in fact a single continuous complex structure18 of cells interconnected via gap junctions into a “functional syncitium.”19
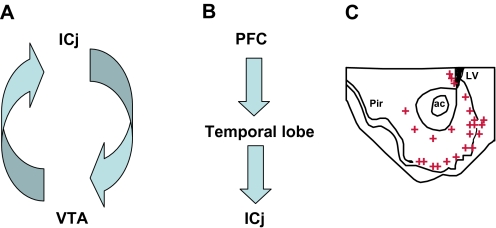
The rodent islands of Calleja receive dense dopaminergic projections from the ventral tegmental area and the substantia nigra, topographically strictly separated in a medial vs a lateral division.20 Their relation with mesencephalic dopaminergic neurons is bidirectional20 (figure 2A). In primates, the input from the olfactory bulb to the islands of Calleja may be replaced by nonolfactory projections,21 with the rostral temporal lobe,22 allowing interconnections with the prefrontal cortex,23 with potential relevance for schizophrenia (figure 2B).
Fig. 2.
Connections of the Islands of Calleja in Rodents (A) and Nonhuman Primates (B). Whereas the islands of Calleja (ICj) are connected in rodents bidirectionally with the ventral tegmental area (VTA) (A), the ICj in monkeys receive a direct projection from the temporal cortex, interconnected with other cortical areas such as the prefrontal cortex (PFC) (B). (C) Expression Pattern of D3 Receptors in The Frontal Brain. The highest expression occurs in the ICj and the subventricular zone, with a lower expression level in the shell of the nucleus accumbens. D3-expressing neurons are indicated by crosses. ac, anterior commisure; LV, lateral ventricle; Pir, piriform cortex.
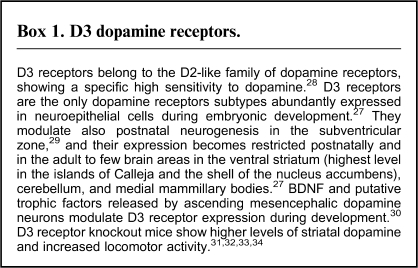
The islands of Calleja express the highest level of dopamine D3 receptors, both in rodents24 and in humans,25 where it is found also in the ventral putamen, caudate, and globus pallidum.26 D3 receptors show a specific expression pattern, mainly restricted to few limbic areas (figure 2C),24 with abundant expression in the ventricular/subventricular zone (see Figure 3).27 The system of reciprocal dopaminergic connections with the ventral tegmental area, with D3 receptors located postsynaptically and presynaptically (the latter with putative autoreceptor function),28,35 appears as a highly sensitive regulator of the extracellular dopamine level.
Fig. 3.
D3 Dopamine Receptors.
Besides the modulatory role on the dopamine system, other functions of the islands of Calleja are less understood. Similar to other granule cells, they contain also high levels of neuronal nitric oxide synthase (nNOS)36,37 and have been proposed to modulate the blood flow in the entire ventral striatum via NO production.38 Moreover, they exhibit high levels of gonadotropic hormones and receptors and have been suggested as a regulatory system integrating reproductive behavior into cortical functions.39
Implications for Schizophrenia
Gene-Environment Interactions With Possible Action on The Islands of Calleja
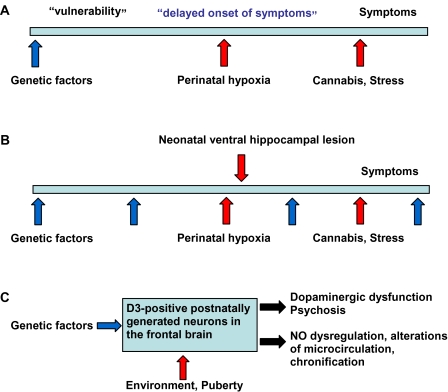
What could be the significance of an extended period of neurogenesis at postnatal and possibly adult stages for schizophrenia? Several environmental risk factors associated with schizophrenia act during early postnatal/juvenile life: perinatal hypoxia, cannabis consumption, and stress.40 Even in the case of early embryonic triggers, structural changes affecting the dopamine system may occur during adolescence.41 A possible role of secondary neurogenesis in these changes has not been reported. Granule cells generated by secondary neurogenesis are particularly susceptible at postnatal/juvenile stages to noxious events affecting various steps of their development (proliferation, migration, differentiation, and integration into local circuits).42,43 The classic view of the “two-hit” hypothesis of schizophrenia44 postulates that genetic factors determine early in embryonic development a “vulnerability” that remains silent until adulthood, when it becomes clinically manifest due to detrimental environmental factors (figure 4A). Susceptibility genes for schizophrenia, like neuregulin-1 and DISC1, controlling postnatal/adult neurogenesis may exert their influence continuously during life (figure 4B). We hypothesize that, by acting on the islands of Calleja, genetic and environmental factors may contribute to dysregulations of the dopamine system, subsequent psychotic symptoms, and, over long-term periods, possible neurodegenerative changes via nNOS-mediated disturbances in local vasculature (figure 4C).
Fig. 4.
Proposed Model for The Gene-Environment Interaction in Schizophrenia. (A, B) Schematic representation of the interaction between genetic factors (darker arrows, shown in blue in online figure) and environmental risk factors (lighter arrows, shown in red in online figure) in schizophrenia. (A) The classic “two-hit” hypothesis postulates that gene defects act in early embryonic development, inducing a “vulnerability” that turns into psychosis in the adult due to environmental triggers. (B) Susceptibility genes for schizophrenia exert their influence also after birth, by regulating postnatal and adult neurogenesis, together with environmental factors. (C) Postnatally generated neurons in the frontal brain, dispersed or grouped in structures like the islands of Calleja, may represent the link between genetic and environmental factors and postnatal neurogenesis, triggering both acute psychotic and chronic neurodegenerative manifestations of the disease.
Potential Pathophysiological Mechanisms
According to our model, postnatal dysfunctional neurogenesis/dysgenesis of D3-expressing islands of Calleja may alter the system of reciprocal dopaminergic connections with the ventral tegmental area. A disturbance of D3 receptors may remove dopamine neurons from feedback control by dopamine, via postsynaptic and/or D3 receptors mesencephalic autoreceptors.35,45 Both pharmacological evidence46 and analysis of D3 knockout mice31,32 support a role of D3 receptors in controlling dopamine release, showing elevated dopamine levels (twice as high as in wild-type mice) in the nucleus accumbens and striatum of D3 knockout mice. The degree of hyperdopaminergia in D3 knockout mice may be even higher but is reduced by neuroadaptive mechanisms in these mice.45 Interestingly, hyperdopaminergia in D3 knockout mice occurs also in the dorsal striatum, a region devoid of D3 receptors.32
The hyperdopaminergia in D3 knockout mice, although less accentuated than in mice lacking the dopamine transporter (5-fold increase compared with wild-type mice),47 supports a significant modulator role of D3 on dopamine release. Correspondingly, behavioral changes related to striatal hyperdopaminergia, like hyperlocomotion33 and increased locomotor response to amphetamines,34 are less evident (in some studies even absent48,49) in D3 receptor knockout mice, compared with the extreme hyperactivity of dopamine transporter knockout mice.47 Even if alterations of a D3 receptor deletion are not very accentuated, they may provide a “baseline” subclinical pathology, which may become clinically manifest in conjunction with additional triggers, like stress.
Interestingly, hyperdopaminergic tone in dopamine transporter knockout mice disturbs induction of prefrontal cortex long-term potentiation, possibly resulting in impaired executive functions.50 Considering the low expression of dopamine transporter in the prefrontal cortex,51 the spill over of excessive striatal dopamine may cause these deficiencies seen in these mice.50 It is unknown if similar alterations are caused by D3 receptor deficiency.
Not only dopaminergic pathways may be affected by postnatal/juvenile triggers. Considering the high expression of nNOS by the islands of Calleja and their proposed modulator role on blood flow in the ventral striatum,38 disorders of their neurogenesis may induce local hypoperfusion. Such a scenario opens the possibility that postnatal alterations of a plastic structure induce long-term deficiencies in the ventral striatum, contributing to chronic symptoms of schizophrenia. Of note, early postnatal pharmacological inhibition of nNOS induces long-term social deficits, reversed by clozapine.52 Results in mice lacking nNOS are contradictory, reporting either decreased53 or increased social interaction.54
Perinatal Hypoxia And Alterations in Subventricular Zone Neurogenesis
Schizophrenia-associated environmental risk factors, acting on subventricular zone neurogenesis, may accentuate preexisting genetically induced dopaminergic dysregulations (figure 4C). Perinatal ischemia, eg, strongly affects subventricular zone neurogenesis, resulting in increased reactive cell proliferation and ectopic intrastriatal migration.21 Even prolonged periods of early postnatal hypoxia, causing extensive neuronal damage, are followed by a robust increase in subventricular zone cell proliferation.55 However, this regeneration is dependent on factors sustaining neurogenesis (like specific growth factors) and is severely disrupted in their absence.56 Possible consequences of this regeneration for structures formed at those stages, like the islands of Calleja, and consequently for the dopamine system have not been investigated. Importantly, in humans, decreased brain-derived neurotrophic factor (BDNF) levels at birth correlate with the risk of developing schizophrenia later.57 This result appears important for the model presented here because BDNF modulates both neurogenesis and expression of D3 receptors.30 Therefore, low levels of BDNF may impair regenerative posthypoxic neurogenesis and accentuate its effect, toward reaching the level of manifest symptoms. The role of other risk factors (stress, cannabis consume, gonadotropic hormones at puberty) in our model needs still to be determined.
Dopamine Dysregulation in The Neonatal Ventral Hippocampal Lesion Model
The pathways described here may correlate also with changes seen in the neonatal ventral hippocampal lesion model.58 In this model, a lesion in the ventral hippocampus at postnatal day 7 (P7) induces hyperdopaminergic behavior, starting at P35 and being evident at P56.59 Interestingly, among dopamine receptors, only the D3 subtype shows a strong progressive downregulation of expression in this model, starting at P41.60 These results appear relevant for our model, considering the late neurogenesis/maturation and plasticity of D3-positive neurons. The mechanisms underlying these changes in this model are unknown. One possibility is that deafferentiation from the ventral hippocampus to the ventral striatum may disturb the integration and functionality of these late-generated neurons. Future studies need to determine the mechanisms underlying this selective effect on D3-expressing neurons.
The Islands of Calleja as Site of Antipsychotic Action
Our hypothesis may have implications not only regarding the pathogenesis but also the treatment of schizophrenia. While D3 receptors may have a role in plasticity in the nervous system, they are not considered to be a major or common target of antipsychotic action. For example, D3 receptors are not generally occupied by all antipsychotics, eg, haloperidol and remoxipride have very low affinities for D3, unlike D2 receptors, which are consistently occupied by 50–80% at clinical doses.61 Interestingly, however, clozapine, unlike other antipsychotics, activates directly the islands of Calleja at high doses, as revealed by brain mapping using the immediate early gene c-fos in rodents62 and primates.63 The involvement of D3 receptors or other receptors in mediating this effect is unclear.64–67 This specific effect raises the possibility that the unique role of clozapine in the therapy of negative and cognitive symptoms may occur by reversing defects in neurogenesis/plasticity in these structures.
Conclusions and Perspectives
Our model offers a dynamic view of pathogenic events in schizophrenia, opening the possibility for early therapeutic interventions. We wish to underline that our model may not be the only one contributing to dopamine dysregulation, being complementary to other mechanisms inducing dopamine alterations in the striatum of DISC1-mutant mice,68 in frontal cortex neurons after pre/perinatal knockdown of DISC169 or after transient neonatal exposure to neuregulin-1.70 We also acknowledge that several other neurodevelopmental mechanisms, eg, impacting on long- and short-range connectivity may be simultaneously active consequent to genetic risk for schizophrenia. Future studies should test both in animal models and in patients the molecular and cellular alterations proposed here. Regarding mechanisms relevant to schizophrenia, experiments in rodents may include analysis of the alterations at cellular level and of the systems presented here (dopamine, NO) in genetic models or in spatially restricted (eg, subventricular zone) ablations of susceptibility genes in interaction with environmental factors (like perinatal hypoxia). In patients, future experiments may include postmortem analysis of this structure and of relevant molecules (like D3 receptors and nNOS). All these investigations may contribute to a better understanding of the specific structures and molecular pathways implicated in schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Deutsche Forschungsgemeinschaft (GA-427/11-1) to P.G and D.I.
Supplementary Material
Acknowledgments
The authors declare no conflict of interest.
References
- 1.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 2.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghashghaei HT, Weber J, Pevny L, et al. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci U S A. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao Y, Ge X, Frank CL, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan X, Chang JH, Ge S, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verney C, Baulac M, Berger B, Alvarez C, Vigny A, Helle KB. Morphological evidence for a dopaminergic terminal field in the hippocampal formation of young and adult rat. Neuroscience. 1985;14:1039–1052. doi: 10.1016/0306-4522(85)90275-1. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki SO, Goldman JE. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J Neurosci. 2003;23:4240–4250. doi: 10.1523/JNEUROSCI.23-10-04240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Marchis S, Fasolo A, Puche AC. Subventricular zone-derived neuronal progenitors migrate into the subcortical forebrain of postnatal mice. J Comp Neurol. 2004;476:290–300. doi: 10.1002/cne.20217. [DOI] [PubMed] [Google Scholar]
- 11.Dayer AG, Jenny B, Potter G, et al. Recruiting new neurons from the subventricular zone to the rat postnatal cortex: an organotypic slice culture model. Eur J Neurosci. 2008;27:1051–1060. doi: 10.1111/j.1460-9568.2008.06091.x. [DOI] [PubMed] [Google Scholar]
- 12.Inta D, Alfonso J, von Engelhardt J, et al. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci U S A. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. doi: 10.1007/BF00239197. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro LA, Ng K, Zhou QY, Ribak CE. Subventricular zone-derived, newly generated neurons populate several olfactory and limbic forebrain regions. Epilepsy Behav. 2009;14:74–80. doi: 10.1016/j.yebeh.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer G, Gonzalez-Hernandez T, Carrillo-Padilla F, Ferres-Torres R. Aggregations of granule cells in the basal forebrain (islands of Calleja): Golgi and cytoarchitectonic study in different mammals, including man. J Comp Neurol. 1989;284:405–428. doi: 10.1002/cne.902840308. [DOI] [PubMed] [Google Scholar]
- 17.Fallon JH, Riley JN, Sipe JC, Moore RY. The islands of Calleja: organization and connections. J Comp Neurol. 1978;181:375–395. doi: 10.1002/cne.901810209. [DOI] [PubMed] [Google Scholar]
- 18.de Vente J, Hani L, Steinbusch HE, Steinbusch HW. The three dimensional structure of the islands of Calleja: a single heterogenous cell complex. Neuroreport. 2001;12:565–568. doi: 10.1097/00001756-200103050-00026. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell JV, Horne AL. Evidence for enhancement of gap junctional coupling between rat island of Calleja granule cells in vitro by the activation of dopamine D3 receptors. J Physiol. 1998;506:175–194. doi: 10.1111/j.1469-7793.1998.175bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallon JH, Loughlin SE, Ribak CE. The islands of Calleja complex of rat basal forebrain. III. Histochemical evidence for a striatopallidal system. J Comp Neurol. 1983;218:91–120. doi: 10.1002/cne.902180106. [DOI] [PubMed] [Google Scholar]
- 21.Herrick CJ. The functions of the olfactory parts of the cerebral cortex. Proc Natl Acad Sci U S A. 1933;19:7–14. doi: 10.1073/pnas.19.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Hoesen GW, Mesulam MM, Haaxma R. Temporal cortical projections to the olfactory tubercle in the rhesus monkey. Brain Res. 1976;109:375–381. doi: 10.1016/0006-8993(76)90537-0. [DOI] [PubMed] [Google Scholar]
- 23.Pandya DN, Dye P, Butters N. Efferent cortico-cortical projections of the prefrontal cortex in the rhesus monkey. Brain Res. 1971;31:35–46. doi: 10.1016/0006-8993(71)90632-9. [DOI] [PubMed] [Google Scholar]
- 24.Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- 26.Seeman P, Wilson A, Gmeiner P, Kapur S. Dopamine D2 and D3 receptors in human putamen, caudate nucleus, and globus pallidus. Synapse. 2006;60:205–211. doi: 10.1002/syn.20298. [DOI] [PubMed] [Google Scholar]
- 27.Diaz J, Ridray S, Mignon V, Griffon N, Schwartz JC, Sokoloff P. Selective expression of dopamine D3 receptor mRNA in proliferative zones during embryonic development of the rat brain. J Neurosci. 1997;17:4282–4292. doi: 10.1523/JNEUROSCI.17-11-04282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levesque D, Diaz J, Pilon C, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N, N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y, Wang WZ, et al. Dopamine stimulation of postnatal murine subventricular zone neurogenesis via the D3 receptor. J Neurochem. 2010;114:750–760. doi: 10.1111/j.1471-4159.2010.06799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- 31.Koeltzow TE, Xu M, Cooper DC, et al. Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice. J Neurosci. 1998;18:2231–12228. doi: 10.1523/JNEUROSCI.18-06-02231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph JD, Wang YM, Miles PR, et al. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors. Neuroscience. 2002;112:39–49. doi: 10.1016/s0306-4522(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 33.Accili D, Fishburn CS, Drago J, et al. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci U S A. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNamara RK, Logue A, Stanford K, Xu M, Zhang J, Richtand NM. Dose-response analysis of locomotor activity and stereotypy in dopamine D3 receptor mutant mice following acute amphetamine. Synapse. 2006;60:399–405. doi: 10.1002/syn.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz J, Pilon C, Le Foll B, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao VL, Butterworth RF. Regional distribution of binding sites for the nitric oxide synthase inhibitor L-[3H]nitroarginine in rat brain. Neurochem Res. 1996;21:355–359. doi: 10.1007/BF02531652. [DOI] [PubMed] [Google Scholar]
- 37.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 38.Meyer G, Gonzalez-Hernandez T, Galindo-Mireles D, Carrillo-Padilla F, Ferres-Torres R. NADPH-d activity in the islands of Calleja: a regulatory system of blood flow to the ventral striatum/pallidum? Neuroreport. 1994;5:1281–1284. doi: 10.1097/00001756-199406020-00032. [DOI] [PubMed] [Google Scholar]
- 39.Stevens JR. Schizophrenia: reproductive hormones and the brain. Am J Psychiatry. 2002;159:713–719. doi: 10.1176/appi.ajp.159.5.713. [DOI] [PubMed] [Google Scholar]
- 40.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “Just the Facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100:4–19. doi: 10.1016/j.schres.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 41.Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci. 2010;30:1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noguchi KK, Walls KC, Wozniak DF, Olney JW, Roth KA, Farber NB. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ. 2008;15:1582–1592. doi: 10.1038/cdd.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauser KF, Khurdayan VK, Goody RJ, Nath A, Saria A, Pauly JR. Selective vulnerability of cerebellar granule neuroblasts and their progeny to drugs with abuse liability. Cerebellum. 2003;2:184–195. doi: 10.1080/14734220310016132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- 45.Le Foll B, Diaz J, Sokoloff P. Neuroadaptations to hyperdopaminergia in dopamine D3 receptor-deficient mice. Life Sci. 2005;76:1281–1296. doi: 10.1016/j.lfs.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Gainetdinov RR, Sotnikova TD, Grekhova TV, Rayevsky KS. In vivo evidence for preferential role of dopamine D3 receptor in the presynaptic regulation of dopamine release but not synthesis. Eur J Pharmacol. 1996;308:261–269. doi: 10.1016/0014-2999(96)00300-7. [DOI] [PubMed] [Google Scholar]
- 47.Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung MY, Skryabin BV, Arai M, et al. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- 49.Chourbaji S, Brandwein C, Vogt MA, et al. Dopamine receptor 3 (D3) knockout mice show regular emotional behaviour. Pharmacol Res. 2008;58:302–307. doi: 10.1016/j.phrs.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Xu TX, Sotnikova TD, Liang C, et al. Hyperdopaminergic tone erodes prefrontal long-term potential via a D2 receptor-operated protein phosphatase gate. J Neurosci. 2009;29:14086–14099. doi: 10.1523/JNEUROSCI.0974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freed C, Revay R, Vaughan RA, et al. Dopamine transporter immunoreactivity in rat brain. J Comp Neurol. 1995;359:340–349. doi: 10.1002/cne.903590211. [DOI] [PubMed] [Google Scholar]
- 52.Black MD, Simmonds J, Senyah Y, Wettstein JG. Neonatal nitric oxide synthase inhibition: social interaction deficits in adulthood and reversal by antipsychotic drugs. Neuropharmacology. 2002;42:414–420. doi: 10.1016/s0028-3908(01)00180-0. [DOI] [PubMed] [Google Scholar]
- 53.Trainor BC, Workman JL, Jessen R, Nelson RJ. Impaired nitric oxide synthase signaling dissociates social investigation and aggression. Behav Neurosci. 2007;121:362–369. doi: 10.1037/0735-7044.121.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanda K, Nishi A, Matsuo N, et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fagel DM, Ganat Y, Silbereis J, et al. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2006;199:77–91. doi: 10.1016/j.expneurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Fagel DM, Ganat Y, Cheng E, et al. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–1211. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannon TD, Yolken R, Buka S, Torrey EF. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol Psychiatry. 2008;64:797–802. doi: 10.1016/j.biopsych.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 60.Flores G, Barbeau D, Quirion R, Srivastava LK. Decreased binding of dopamine D3 receptors in limbic subregions after neonatal bilateral lesion of rat hippocampus. J Neurosci. 1996;16:2020–2026. doi: 10.1523/JNEUROSCI.16-06-02020.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- 62.Guo N, Vincent SR, Fibiger HC. Phenotypic characterization of neuroleptic-sensitive neurons in the forebrain: contrasting targets of haloperidol and clozapine. Neuropsychopharmacology. 1998;19:133–145. doi: 10.1016/S0893-133X(97)00202-9. [DOI] [PubMed] [Google Scholar]
- 63.Wirtshafter D, Asin KE. Effects of haloperidol and clozapine on Fos expression in the primate striatum. Neuroreport. 2003;14:2429–2432. doi: 10.1097/00001756-200312190-00028. [DOI] [PubMed] [Google Scholar]
- 64.Hurley MJ, Stubbs CM, Jenner P, Marsden CD. Dopamine D3 receptors are not involved in the induction of c-fos mRNA by neuroleptic drugs: comparison of the dopamine D3 receptor antagonist GR103691 with typical and atypical neuroleptics. Eur J Pharmacol. 1996;318:283–293. doi: 10.1016/s0014-2999(96)00798-4. [DOI] [PubMed] [Google Scholar]
- 65.Kovacs KJ, Csejtei M, Laszlovszky I. Double activity imaging reveals distinct cellular targets of haloperidol, clozapine and dopamine D(3) receptor selective RGH-1756. Neuropharmacology. 2001;40:383–393. doi: 10.1016/s0028-3908(00)00163-5. [DOI] [PubMed] [Google Scholar]
- 66.Southam E, Lloyd A, Jennings CA, et al. Effect of the selective dopamine D3 receptor antagonist SB-277011-A on regional c-Fos-like expression in rat forebrain. Brain Res. 2007;1149:50–57. doi: 10.1016/j.brainres.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 67.Robertson GS, Lee CJ, Sridhar K, et al. Clozapine-, but not haloperidol-, induced increases in deltaFosB-like immunoreactivity are completely blocked in the striatum of mice lacking D3 dopamine receptors. Eur J Neurosci. 2004;20:3189–3194. doi: 10.1111/j.1460-9568.2004.03774.x. [DOI] [PubMed] [Google Scholar]
- 68.Lipina TV, Niwa M, Jaaro-Peled H, et al. Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia. Genes Brain Behav. 2010;9:777–789. doi: 10.1111/j.1601-183X.2010.00615.x. [DOI] [PubMed] [Google Scholar]
- 69.Niwa M, Kamiya A, Murai R, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kato T, Abe Y, Sotoyama H, et al. Transient exposure of neonatal mice to neuregulin-1 results in hyperdopaminergic states in adulthood: implication in neurodevelopmental hypothesis for schizophrenia. Mol Psychiatry. doi: 10.1038/mp.2010.10. doi:10.1038/mp.2010.10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.