Abstract
Although schizophrenia is characterized by gray matter (GM) abnormalities, particularly in the prefrontal and temporal cortices, it is unclear whether cerebral cortical GM is abnormal in individuals at ultra-high-risk (UHR) for psychosis. We addressed this issue by studying cortical thickness in this group with magnetic resonance imaging (MRI). We measured cortical thickness of 29 individuals with no family history of psychosis at UHR, 31 patients with schizophrenia, and 29 healthy matched control subjects using automated surface-based analysis of structural MRI data. Hemispheric mean and regional cortical thickness were significantly different according to the stage of the disease. Significant cortical differences across these 3 groups were found in the distributed area of cerebral cortices. UHR group showed significant cortical thinning in the prefrontal cortex, anterior cingulate cortex, inferior parietal cortex, parahippocampal cortex, and superior temporal gyrus compared with healthy control subjects. Significant cortical thinning in schizophrenia group relative to UHR group was found in all the regions described above in addition with posterior cingulate cortex, insular cortex, and precentral cortex. These changes were more pronounced in the schizophrenia group compared with the control subjects. These findings suggest that UHR is associated with cortical thinning in regions that correspond to the structural abnormalities found in schizophrenia. These structural abnormalities might reflect functional decline at the prodromal stage of schizophrenia, and there may be progressive thinning of GM cortex over time.
Keywords: MRI, gray matter, cortical thinning, surface-based analysis
Introduction
Schizophrenia is a chronic and debilitating brain disorder, with lifetime prevalence in approximately 1% of the population. It causes significant disruption to daily activity and social functioning and may thus result in significantly decreased quality of life from its earliest stages through its chronic course.1 The detection of reliable early indicators of vulnerability and the development of early interventions following the onset of psychosis have become major areas of cutting-edge research and have contributed to the understanding of the pathophysiological mechanisms of schizophrenia. In this regard, researchers recently have developed an interest in a high-risk approach, which explores possible vulnerability indicators for psychosis in prodromal individuals identified by clinical state–based criteria, as being ultra-high-risk (UHR) or clinical high risk for schizophrenia.2 Within this approach, researchers have reported neurocognitive deficits including attention and working memory dysfunction3,4 and neurophysiological deficits, such as impaired mismatch negativity (MMN) and P300 event-related potential,5,6 which were associated with psychotic symptoms as potential vulnerability markers. Recently, magnetic resonance imaging (MRI) has been used to investigate cortical atrophy in vivo and has provided evidence of progressive structural brain alterations in individuals with either schizophrenia or UHR.7–9 This suggests that morphological brain abnormalities are potential vulnerability markers for developing schizophrenia, albeit with unclear results.10
Morphological postmortem studies in schizophrenia have reported increased neuronal packing density with a corresponding reduction in cortical thickness in the prefrontal cortex (PFC)11 and anterior cingulate cortex (ACC)12 and increased microglia density in both frontal and temporal cortical regions.13 However, postmortem studies are limited by small sample sizes and the labor intensiveness of the measurement techniques. Recently, structural MRI studies have been applied to identify regional changes in cortical gray matter (GM) in schizophrenia and have produced a wealth of evidence for abnormalities of brain structure.9,14 The most consistent results from accumulated data were lower total GM volume and GM reductions in the superior temporal gyrus (STG), medial temporal gyrus, and PFC with promising findings in ACC in schizophrenia subjects relative to healthy control (HC) subjects.9 Similar abnormalities have been repeatedly described in the early stages of psychosis, ie, first-episode schizophrenia, albeit with negative findings.15 These findings indicate that some brain regions experience GM atrophy associated with the early stages of illness and raise questions about the regional pattern and timing (prior to or after onset of psychosis) of these changes. A few studies have attempted to address these questions by investigating brain changes in UHR using a manual region of interest (ROI)–based or voxel-based morphometry (VBM) method. Previous studies employing either ROI or VBM methods have reported differences in the hippocampus, STG, insula, and amygdala in UHR subjects compared with HC subjects7 and revealed reduced GM volume in the STG and insula in individuals who later developed psychosis (converters) compared with nonconverters,7,16,17 although some studies showed no difference between UHR subjects and HC subjects.18 Although recently accumulating data have frequently shown brain abnormalities in UHR subjects, the results are somewhat equivocal. The discrepancies in these findings may partly arise from differences in sample characteristics, symptom severity, and analysis approaches. For instance, the ROI method is specific to particular brain regions, and the VBM method exhibits limited accuracy in cortical morphology, particularly in sulcal regions that have been obscured by the partial volume effect, and its results are sensitive to the size of smoothing. More recently, cortical thickness analyses from MRI data have been developed, such as surface-based methods, estimated from the nodes of a 3-dimensional (3-D) polygonal mesh,19,20 or voxel-based cortical thickness methods, calculated at every volumetric point within the cortex.21 Cortical thickness in different regions reflects the size, density, and arrangement of cells. Microscopic changes of cortical thickness may indicate alterations in dendrites or dendritic spines and changes in myelination within specific brain systems.22,23 Thus, this method more closely reflects the underlying anatomical reality than previous GM density or volume estimating methods and provides a quantitative index of cytoarchitectural abnormalities24 and disturbances in normal brain development.25
The aim of the present study was to investigate whether there were structural cortical changes in UHR subjects who had no family history of psychosis, compared with HC subjects across the entire brain using automated surface-based method. The surface-based method was used because of its well-established geometric/topologic accuracy and mesh characteristics.26 We also tried to determine if the severity and distribution of cortical atrophy differed among UHR, schizophrenia, and HC groups, which may help us to understand how UHR might evolve into psychosis from a neurodevelopmental perspective. We hypothesized that cortical thinning would appear in the regions predicted by previous studies, ie, PFC, STG, and ACC.10,27 We expected milder changes of cortical thinning in UHR group than schizophrenia group, compared with HC group, and that these changes might interact with effects of age and clinical features.
Methods
Subjects
UHR subjects and schizophrenia patients were recruited within a prospective, longitudinal project to investigate people at high risk for schizophrenia from the Seoul Youth Clinic. The UHR group (n = 29) was defined according to the Comprehensive Assessment of At-Risk Mental States (CAARMS) criteria.28 However, individuals were excluded if they had a first- to third-degree relatives with a psychotic disorder to investigate the effect of clinical state in the prodromal phase of psychosis. Twenty-eight of the UHR subjects met attenuated psychotic symptoms criteria, showing positive psychotic symptoms of subthreshold intensity or frequency; the remaining 1 subject met brief limited intermittent psychotic symptoms criteria, showing frank psychotic symptoms for less than 1 week. Among the 29 UHR subjects, 11 individuals had met the criteria for major depressive disorder, 1 had dysthymia, and 2 had anxiety disorder not otherwise specified. The remaining 15 subjects had no concurrent psychiatric diagnosis. Eight individuals in the UHR group were receiving low-dose treatment with atypical antipsychotics, one with atypical antipsychotics and selective serotonin reuptake inhibitor (SSRI), and one with SSRI and lithium at baseline. Study participants overlapped with subjects included in our previous investigations of UHR subjects8 but were not identical because some UHR subjects who had been included in our previous study had a family history of psychosis and were excluded from the current study to minimize any hereditary factors. The schizophrenia group consisted of 31 patients who fulfilled Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria for schizophrenia, as diagnosed by the Structured Clinical Interview for DSM-IV.29 All patients were receiving drug treatment at the time of investigation. The 29 age- and IQ-matched HC subjects, without a lifetime history of any psychiatric disorder or treatment, were recruited from an Internet advertisement. HC subjects were screened using the Structured Clinical Interview for DSM-IV Axis I Disorders Non-Patient Edition30 with an additional exclusion criterion of any first- to third-degree biological relatives with a psychiatric disorder. The demographic characteristics of the subjects in each group are presented in table 1. Participants were excluded if they had a history of substance abuse or dependence, neurological disease, head injury or medical illness with documented cognitive sequelae, or intellectual disability (IQ < 70). All procedures were performed in accordance with the current version of the Declaration of Helsinki. The Institutional Review Board of the Seoul National University Hospital approved all work. All subjects were provided with written informed consent including parental consent for those who were less than 18 years old.
Table 1.
Demographic and Clinical Variables and Mean Cortical Thickness for Each Hemisphere of the Subjects
| UHR Subjects (n = 29) | Schizophrenia Subjects (n = 31) | HC Subjects (n = 29) | Analysis |
||
| F, χ2, or T | P | ||||
| Age (y) | 22.24 ± 4.33 | 24.26 ± 4.24 | 23.24 ± 2.71 | 2.063a | 0.133 |
| Gender (male/female) | 15/14 | 17/14 | 15/14 | 0.079b | 0.961 |
| Handedness (right/left) | 28/1 | 30/1 | 28/1 | 0.001b | 1.000 |
| Education (y) | 13.28 ± 2.43 | 13.74 ± 2.18 | 14.48 ± 1.21 | 2.649a | 0.076 |
| IQ | 107.82 ± 15.17 | 102.29 ± 11.16 | 110.24 ± 14.67 | 2.664a | 0.076 |
| Parental SES | 2.75 ± 1.04 | 2.44 ± 0.92 | 2.47 ± 0.84 | 0.836a | 0.438 |
| Duration (y) | 4.55 ± 3.02 | ||||
| CAARMS total score | 37.41 ± 15.17 | ||||
| PANSS total score | 53.66 ± 11.69 | 57.72 ± 13.60 | −1.222c | 0.227 | |
| PANSS positive | 12.72 ± 3.40 | 13.10 ± 3.24 | −0.435c | 0.666 | |
| PANSS negative | 11.72 ± 3.53 | 16.55 ± 5.60 | −3.927c | <0.001 | |
| PANSS general | 29.17 ± 7.22 | 28.07 ± 6.99 | 0.591c | 0.557 | |
| Intracranial volume | 1165.76 ± 87.58 | 1105.15 ± 116.89 | 1134.38 ± 97.48 | 2.657a | 0.076 |
| Mean cortical thickness (mm) | |||||
| Left hemisphere | 3.68 ± 0.12 | 3.52 ± 0.16 | 3.72 ± 0.11 | 20.689d | <0.001, HC = UHR > schizophrenia |
| Right hemisphere | 3.67 ± 0.12 | 3.53 ± 0.17 | 3.72 ± 0.13 | 13.754d | <0.001, HC = UHR > schizophrenia |
Note: UHR, ultra-high-risk; HC, healthy control; SES, Hollingshead socioeconomic status (highest = 1, lowest = 5); CAARMS, Comprehensive Assessment of At-Risk Mental States; PANSS, Positive and Negative Syndrome Scale. Data given as mean ± SD.
Analysis of variance.
χ2 test.
Independent t test.
Analysis of covariance.
Clinical Interviews and Assessments
At the study intake, in addition to CAARMS, the Positive and Negative Syndrome Scale (PANSS)31 was administered to both UHR and schizophrenia groups to quantify the burden of psychotic symptoms of these subjects. All participants were assessed with the Hollingshead Scale for parental socioeconomic status (SES). The Korean version of the Wechsler Adult Intelligence Scale was administered to all subjects to estimate their IQ. The family interview for genetic studies32 was used to document any family history of psychotic disorders. UHR subjects enrolled in this study were monitored longitudinally by experienced psychiatrists to detect the conversion to psychosis on at least a monthly basis. Conversion to psychosis was determined using the criteria developed by the Personal Assessment and Crisis Evaluation clinic: Acute psychosis was defined as the presence of at least one symptom, such as hallucinations, delusions, or formal thought disorder, at least several times a week and persisting for longer than 1 week.33 Eight of the UHR subjects (27.59%) made the transition to psychosis: 5 with schizophrenia (3 paranoid types and 2 undifferentiated types) and 3 with bipolar disorder with psychotic features. The mean interval between the acquisition of MR images and the onset of psychosis for them was 11 ± 8.33 months.
MRI Acquisition
All structural MRI scans were acquired in axial plane using a 1.5-T scanner (Avanto, Siemens, Erlangen, Germany) and T1-weighted 3-D magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence. Parameters were as follows: echo time/repetition time = 4.76/1160 milliseconds, flip angle = 15°, field of view = 230 mm, voxel size = 0.45 × 0.45 × 0.9 mm. From a visual inspection, all scans were judged to be excellent without obvious motion artifacts, signal loss, or gross pathology when evaluated by a neuroradiologist (C.H.C.).
Image Processing and Measurement of Cortical Thickness
The raw native MR images were corrected for intensity nonuniformity resulting from inhomogeneities in the magnetic field using the N3 algorithm.34 Spatial normalization to a stereotaxic space was performed using a 9-parameter linear registration.35 The registered and corrected volumes were classified as white matter, GM, cerebrospinal fluid, and background using an advanced neural net classifier.36 The surfaces of the inner and outer cortex were automatically fitted using the Constrained Laplacian-based Automated Segmentation with Proximities algorithm.37 The reconstructed hemispheric cortical surfaces consisted of 81 920 meshes of discrete triangular elements. Inner and outer surfaces had the same vertex number (40 962), and there was a close correspondence between the counterpart vertices of the inner and outer cortical surfaces. The cortical thickness was defined as the Euclidean distance between these linked vertices.20 To compare the thicknesses of corresponding regions of the surface model between the groups at a vertex level, the thickness values were spatially normalized using surface-based 2-dimensional (2-D) nonlinear registration.38 The sphere-to-sphere warping algorithm was used for 2-D surface registration. To match patterns of sulcal folding, we used the crown distance transform feature. Vertices of each subject were nonlinearly registered to an average template on the sphere by matching crowns of gyri between subjects using the crown distance transform feature.38 Sulcal variability was reduced in all areas of the cortex using optimal parameter values, which was proved by entropy measures.39 Using the transformation, thickness information on vertices was transformed to an average template. For global analysis, averaged values of the thickness of the whole surface in each hemisphere were analyzed.
Statistical Analysis
Diffusion smoothing with a full-width half-maximum of 20 mm was used to blur each cortical thickness map, which increased the signal-to-noise ratio and statistical power.40,41 Global and regional differences of cortical thickness among the 3 groups were analyzed using an analysis of covariance (ANCOVA) with age, gender, and intracranial volume (ICV) as covariates. The ICV was measured by summing all voxel volumes within the brain mask including GM, white matter, and cerebrospinal fluid. The brain mask was generated using the FSL BET algorithm.42 Significant differences among 3 groups were reported when they passed a whole-brain false discovery rate (FDR) correction with an FDR-corrected P < .01, and their surface sizes exceeded 20 mm2.43 Global and regional differences in the cortical thicknesses between 2 groups (ie, UHR group vs HC group, schizophrenia group vs HC group, and schizophrenia group vs UHR group) were also analyzed using an independent t test. The statistical analysis of regional cortical thickness was performed on a vertex-by-vertex basis, and the results of these tests are mapped onto the surface model where significant results are indexed in color. We conducted permutation tests with P value at .01 to confirm that the significant changes between 2 groups were not purely by chance for multiple comparisons as follows.44 Subjects were randomly assigned to groups across 10 000 new randomized analyses at each vertex, and the number of significant results (ie, GM thickness at any vertex that significantly differed between groups at the threshold of P = .01) that occurred in the real test for group differences was compared with the null distribution of significant results that occurred by chance.
After identifying the regions showing differences in cortical thickness among these 3 groups and between the UHR group and control subjects as ROIs, we computed mean cortical thickness within each ROI. Then, a correlation was performed to establish relationships between mean cortical thickness at each ROI and clinical variables (age, CAARMS score, PANSS score). In addition, 2-tailed t tests were performed on z-transformed correlation coefficients of each variable and mean cortical thickness within each ROI between groups.45
Results
Demographic Characteristics and Clinical Symptoms
The demographic and clinical characteristics of the 3 groups are shown in the table 1.The age, gender ratio, handedness, level of education, IQ, and parental SES were not significantly different among the 3 groups. The UHR group scored significantly less on negative symptoms, measured by PANSS, than the schizophrenia group (UHR group, 11.72 ± 3.53; schizophrenia group, 16.55 ± 5.60; P < .001).
Comparison of the Hemispheric Mean Cortical Thickness
ANCOVA revealed that the mean cortical thickness in each hemisphere differed among the UHR, schizophrenia, and HC groups (left hemisphere, F2,83 = 20.689, P < .001; right hemisphere, F2,83 = 13.754, P < .001). Comparisons between 2 groups showed that the mean cortical thickness in schizophrenia group differed from that in the HC and UHR groups, but there was no difference between the UHR and HC groups (figure 1, table 1).
Fig. 1.
Mean Cortical Thickness in 3 Groups for Each Hemisphere. The plots show gradual decreases in the mean cortical thickness according to the psychotic stages. UHR, ultra-high-risk (n = 29); schizophrenia (n = 31); HC subjects, healthy control subjects (n = 29).
Cortical Thickness in UHR vs Schizophrenia vs HC Groups
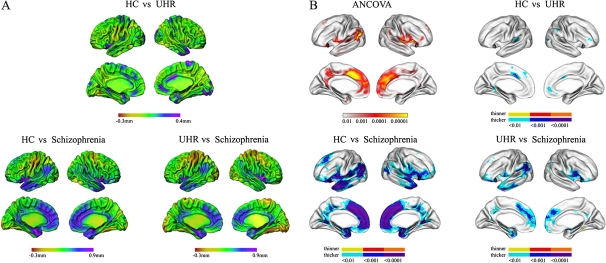
Figure 2 presents the differences in the mean cortical thickness at each vertex between groups (figure 2A) and statistically significant differences in cortical thickness between 2 groups (figure 2B). There were significant group differences across the 3 groups in the bilateral insula and medial frontal cortex extending from the ACC and left STG, superior frontal cortex, posterior cingulate cortex (PCC), inferior temporal cortex, parahippocampal cortex, in addition to the right STG, inferior frontal cortex, precentral cortex, postcentral cortex, middle temporal cortex, inferior parietal cortex, uncus, and PCC. Relative to HC group, the UHR group showed cortical thinning in the left STG, right lingual cortex, right inferior frontal and parietal cortex, right middle temporal cortex, as well as the bilateral ACC, parahippocampal cortex, and medial frontal cortex. To further illustrate the magnitude of these effects, mean cortical thicknesses in these regions for all 3 groups are presented in figure 3. In each of these areas, gradual decreases in mean cortical thickness could be found, according to psychotic stages, ie, HC group > UHR group > schizophrenia group. All these regions showed statistically significant differences among the 3 groups. Relative to the UHR group, the schizophrenia group had significant cortical thinning in the left STG, superior frontal cortex, parahippocampal cortex, and inferior temporal cortex and in the right insular cortex, uncus, posterior cingulate cortex, precentral cortex, and middle temporal cortex, as well as in the bilateral medial frontal cortex extending from the ACC and the inferior parietal cortex. Comparison between the schizophrenia and HC groups revealed that schizophrenia patients showed cortical thinning in the left superior frontal cortex, inferior temporal cortex, and precuneus, in addition to the right parahippocampal cortex, inferior parietal cortex, lingual cortex, and precentral cortex, as well as in the bilateral insular cortex, inferior frontal cortex, STG, PCC, and ACC.
Fig. 2.
Regional Maps of the Differences in the Mean Cortical Thickness (A) and Statistical Maps Between Groups: HC Subjects vs UHR Subjects, HC Subjects vs Schizophrenia Subjects, and UHR Subjects vs Schizophrenia Subjects (B). UHR, ultra-high-risk (n = 29); schizophrenia (n = 31); HC subjects, healthy control subjects (n = 29).
Fig. 3.
Brain Regions with Significant Reduced Cortical Thickness in UHR Subjects Compared with HC Subjects. In the left hemisphere, the cortical thickness was decreased in superior temporal gyrus (L1), anterior cingulate cortex (ACC, L2), parahippocampal cortex (L3), and medial superior frontal cortex (L4). In the right hemisphere, the cortical thickness was reduced in dorsal ACC (R1), rostral ACC (R2), inferior frontal cortex (R3), and inferior parietal cortex (R4). All these regions showed statistically significant differences among 3 groups. In the boxplot, the x-axis shows brain regions with significant reduction of cortical thickness, and the y-axis indicates cortical thickness (millimeters). The horizontal thick line inside each box indicates the median value. The upper and lower boundaries of each box mean lower quartile and upper quartile values, respectively. The whiskers represent smallest and largest nonoutlier observations. The circle depicts a mild outlier, and asterisk indicates an extreme outlier. Scatterplots and age correlation slopes for mean cortical thickness (millimeters) with increasing age within regions for UHR and HC subjects. The x-axis indicates age (y), and the y-axis indicates cortical thickness (millimeters). Significant correlations between age and mean cortical thickness within each region are marked (asterisk). UHR, ultra-high-risk (n = 29); schizophrenia (n = 31); HC subjects, healthy control subjects (n = 29); ACC, anterior cingulate cortex.
Correlation Analysis
For the UHR group, mean cortical thickness in the regions of left ACC, left medial superior frontal cortex, right ACC, and right inferior frontal and parietal cortex was correlated with their age, showing a significantly steeper downward-directed age correlation slope in the UHR group compared with HC group (figure 3). The other variables were not correlated with GM thickness in the UHR group.
Discussion
To our knowledge, this is the first study to investigate structural cortical differences between UHR group without a family history of psychosis, schizophrenia group, and HC group in terms of cortical thickness in a cross-sectional manner. The major findings of the present study were reduced cortical thickness in the ACC, PFC, STG, and inferior parietal regions in the UHR group compared with HC group; these regions were more extended in schizophrenia group. Age in UHR individuals was negatively correlated with cortical thickness in these regions. ANCOVA among the 3 groups revealed significant differences in mean cortical thickness for each hemisphere and regional cortical thickness differences in distributed regions.
Mounting evidence suggests that, in general, schizophrenia subjects have structural GM deficits in widespread regions, particularly in the frontal and temporal cortices, as a psychopathological feature, and frontotemporal dysconnectivity. The occurrence of these deficits may be linked to functional decline at the prodromal stage of schizophrenia. As expected, our UHR group showed cortical thinning in several frontal cortices and in the STG. The pattern of cortical thinning found in this study was in line with previous MRI studies in schizophrenia and UHR groups, compared with HC group.7,10,46,47 Many previous studies have repeatedly reported morphologic abnormalities of the STG and adjacent temporal cortex, particularly the primary auditory cortex and planum temporale in the left hemisphere,9,48 and its functional deficits include auditory hallucinations or thought disorders in schizophrenia.49,50 Volume reductions in the left posterior STG in schizophrenia were highly correlated with the amplitude reduction of MMN51 and the reduction of the magnetic counterpart of MMN (MMNm).52 Disturbances in MMN may index structural abnormalities of the left posterior STG in schizophrenia. In our previous work, the UHR group showed a negative correlation between left MMNm dipole moment and clinical symptoms, in addition to a smaller right MMNm dipole moment than in HC group.6 These results may implicate structural deficits of the STG in UHR individuals. VBM studies demonstrated that UHR individuals had smaller left STGs than HC individuals.7,53 In addition, Takahashi et al17 have recently reported a progressive decrease of the STG in UHR subjects, using detailed ROI analyses on longitudinal MRI data. Our findings and other work suggest that temporal lobe abnormalities may precede the onset of psychosis and progressively worsen over time. In line with this assumption and consistent with previous studies, our schizophrenia subjects had widespread reductions in the STG compared with HC subjects.
In addition to the STG atrophy, our UHR group showed cortical thinning in the frontal lobe including the PFC, medial frontal cortex, and ACC. First, decreased cortical thickness of the PFC in UHR subjects, compared with HC subjects, has been found consistently by other groups.16,54 Impairments in the PFC may be expected to lead to cognitive dysfunction such as in attention and working memory in UHR individuals.3,4 Some neuroimaging studies have found prefrontal hypofunction in UHR groups, which is implicated in the pathophysiology of schizophrenia.55,56 Second, the medial frontal cortex implicated in the default mode network has a role in social cognition.57 Thus, a decreased medial frontal cortex corresponds with our previous assumption, derived from a psychological study using a theory-of-mind task, showing decreased social cognition and implicating a deficit in the medial frontal cortex.58 Third, the UHR group exhibited significantly reduced cortical thickness in the ACC. The ACC is related to impaired cognition (self-monitoring) and disorganization in patients with schizophrenia.59,60 In the present study, decreased GM of ACC in the UHR group is consistent with previous MRI studies.16 In addition, some VBM studies have reported decreased ACC GM in converters, relative to nonconverters.7,16 In comparison analysis between converters (n = 8) and nonconverters (n = 22) in the present study (not described), it was observed that converters showed reduced cortical thickness in right ACC in addition to left lingual cortex, right superior temporal cortex, and bilateral inferior temporal cortex (at a more lenient threshold of P < .05; see Supplementary figure S1). However, these findings should be considered preliminary given the small sample size and more lenient threshold. Recently, Fornito et al27 have indicated that abnormalities of the ACC precede psychosis onset by applying a cortical surface-based protocol for parcellating the ACC. Abnormalities of these midline brain structures including the medial frontal cortex and ACC are compatible with prior suggestions about neurodevelopmental anomalies in UHR subjects.61 Abnormalities of cavum septi pellucidi and the ACC folding have been reported in UHR subjects, which reflect early neurodevelopmental anomalies,8,62 as were previously observed in schizophrenia patients.63,64 It suggests that early neurodevelopmental anomalies may lead to disturbances on the subsequent brain maturation and produce further late neurodevelopmental changes during the initial stages of a psychotic illness.65 Longitudinal MRI studies of UHR subjects have reported progressive GM loss before and after psychosis onset in the medial temporal and prefrontal regions using voxel-based approach.16,66
In the UHR group, we found negative correlations between age and cortical thickness in some regions, particularly the right PFC (Brodmann area [BA] 10), right inferior parietal cortex, and bilateral ACC. Several longitudinal MR studies have reported progressive loss of GM volume with increasing age, in addition to duration and severity of illness, in first-episode and chronic schizophrenia,67–69 although these findings are inconsistent.70 Studies of normal brain maturation have consistently reported GM volume reductions in frontal and parietal cortices in adolescence.71 O'Donnell et al72 exhibited a significant inverse correlation between age and cortical thickness in the frontopolar cortex (BA 10) through late childhood and adolescence. Thompson et al73 described the trajectory of GM loss in a longitudinal structural study of early-onset schizophrenia, which started in the parietal cortex and then progressed forward to temporal and frontal cortices, reflecting an exaggeration of the normal cortical developmental processes. In this context, structural changes in the UHR group may be interpreted as an abnormal acceleration of normal cortical developmental processes. Further study is needed to verify this interpretation.
Based on dynamic neuroanatomical changes across clinical conditions, structural neuroimaging studies have recently tried to identify UHR individuals or schizophrenia individuals and predict conversion using neuroanatomical pattern classification. Koutsouleris et al74 have distinguished UHR group from HC group by depending on structural between-group differences with respect to GM volume. Cortical thickness analysis using principal component analysis discriminated between schizophrenia and HC groups.24 However, a recent study has reported that cortical thickness asymmetry analysis, which evaluated the cortical asymmetry of corresponding regions between left and right hemispheres, contributes to detection of UHR and first-episode psychosis but not cortical thickness analysis.75 The discrepancies between these results may result from differences in analysis approaches and sample characteristics.
There are notable differences in sample characteristics between our dataset and previously published research. The patients with schizophrenia who participated in this study were in maintenance therapy periods after the recovery from their first psychotic episodes, and the clinical status of the patients was relatively stable. It was thought that chronic patients with schizophrenia were less suitable for comparison of brain structures between UHR and schizophrenia groups because there may be a potential for underlying structural brain abnormalities occurred from the chronic symptoms. Thus, the chronic schizophrenia patients were not appropriate for the purpose of the study that was to investigate dynamic brain changes preceding onset of psychosis. Our UHR individuals had no family history of psychosis, while schizophrenia has a strong genetic component.76 It is thus hard to generalize our results to schizophrenia. A potential explanation for structural differences we found is that these differences may be associated with environmental or epigenetic factors rather than genetic factors. Genetic factors may not be necessary to produce structural changes in UHR subjects. Among the 8 converters, 5 individuals converted to schizophrenia and others converted to other forms of psychiatric disorders. Therefore, our results may be related to psychosis rather than schizophrenia.
Several limitations in the present study should be taken into account. First, all schizophrenia patients and 10 UHR subjects were taking atypical antipsychotics, raising the possibility of a medication confound. Recent studies suggest that antipsychotic treatment may contribute to the changes of cortical thickness,77,78 although some studies have not found these effects.46,47 However, in this study, the UHR subjects took psychotropic medications only after the complete clinical evaluations, and the time intervals between the beginning of medications and the MRI scans were relatively short (mean = 4.64 ± 4.48 d). Additional analysis revealed no significant differences in cortical thickness between 10 medicated and 19 unmedicated subjects in the UHR group. Comparisons between the 19 unmedicated UHR subjects and HC subjects remained significant in terms of differences in ROIs. However, we still cannot rule out the confounding effects in comparisons between schizophrenia and other groups. Further research is needed to examine more precisely how the effects of treatment affect these changes in cortical thickness. Second, the present study was not able to directly compare longitudinal brain changes in converters with those in nonconverters. So, it is not clear whether the structural changes found led to the progressive effects of the disease or other factors. However, a previous study using a similar methodology lacked a HC group and so did not address the deviation from normal brain changes.54 The present study contained HC, schizophrenia, and UHR groups without a family history of psychosis, and the sample size for each group was modest as these subjects were relatively difficult to recruit.
In summary, we demonstrated a pattern of cortical thinning in the UHR group compared with HC group, particularly in the ACC, PFC, STG, and inferior parietal regions, and these changes were further extended for schizophrenia by applying surface-based method. These structural abnormalities could be potential candidates for vulnerability markers for the development of schizophrenia. Further longitudinal investigation of these groups will clarify the issue of progressive brain changes related to the illness process.
Supplementary Material
Supplementary figure S1 is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Korea Science and Engineering Foundation funded by the Ministry of Education, Science and Technology (World Class University program R32-10142).
Supplementary Material
Acknowledgments
The authors thank all patients for participating in the study. We are also grateful to a number of individuals who provide valuable contributions to the study, including Na Young Shin, Soo Jin Kwon, and Go-Eun Jang, the clinical and nursing staff of the Clinical Cognitive Neuroscience Center. The authors also gratefully acknowledge the technical support of Yong-Sik Jung, Kiho Im, and Sun Hyung Kim.
References
- 1.Shim G, Kang DH, Chung YS, Yoo SY, Shin NY, Kwon JS. Social functioning deficits in young people at risk for schizophrenia. Aust N Z J Psychiatry. 2008;42:678–685. doi: 10.1080/00048670802203459. [DOI] [PubMed] [Google Scholar]
- 2.Cannon TD. Clinical and genetic high-risk strategies in understanding vulnerability to psychosis. Schizophr Res. 2005;79:35–44. doi: 10.1016/j.schres.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Brewer WJ, Wood SJ, Phillips LJ, et al. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006;32:538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lencz T, Smith CW, McLaughlin D, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Bramon E, Shaikh M, Broome M, et al. Abnormal P300 in people with high risk of developing psychosis. NeuroImage. 2008;41:553–560. doi: 10.1016/j.neuroimage.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Shin KS, Kim JS, Kang DH, et al. Pre-attentive auditory processing in ultra-high-risk for schizophrenia with magnetoencephalography. Biol Psychiatry. 2009;65:1071–1078. doi: 10.1016/j.biopsych.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Borgwardt SJ, Riecher-Rössler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Choi JS, Kang DH, Park JY, et al. Cavum septum pellucidum in subjects at ultra-high risk for psychosis: compared with first-degree relatives of patients with schizophrenia and healthy volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1326–1330. doi: 10.1016/j.pnpbp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood SJ, Pantelis C, Velakoulis D, Yücel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull. 2008;34:322–329. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- 12.Bouras C, Kövari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol. 2001;102:373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- 13.Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- 14.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 15.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 16.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 18.Ziermans TB, Durston S, Sprong M, et al. No evidence for structural brain changes in young adolescents at ultra high risk for psychosis. Schizophr Res. 2009;112:1–6. doi: 10.1016/j.schres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. NeuroImage. 2001;13:375–380. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- 20.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Hutton C, De Vita E, Ashburner J, Deichmann R, Turner R. Voxel-based cortical thickness measurements in MRI. NeuroImage. 2008;40:1701–1710. doi: 10.1016/j.neuroimage.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benes FM, Francine M. Why does psychosis develop during adolescence and early adulthood? Curr Opin Psychiatry. 2003;16:317–319. [Google Scholar]
- 23.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 24.Yoon U, Lee JM, Im K, et al. Pattern classification using principal components of cortical thickness and its discriminative pattern in schizophrenia. NeuroImage. 2007;34:1405–1415. doi: 10.1016/j.neuroimage.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Arnold SE. Neurodevelopmental abnormalities in schizophrenia: insights from neuropathology. Dev Psychopathol. 1999;11:439–456. doi: 10.1017/s095457949900214x. [DOI] [PubMed] [Google Scholar]
- 26.Lee JK, Lee JM, Kim JS, Kim IY, Evans AC, Kim SI. A novel quantitative cross-validation of different cortical surface reconstruction algorithms using MRI phantom. NeuroImage. 2006;31:572–584. doi: 10.1016/j.neuroimage.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Fornito A, Yung AR, Wood SJ, et al. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 2008;64:758–765. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Patient Edition (SCID-P) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Non-Patient Edition (SCID-NP) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 31.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 32.Gershon ES, Guroff JJ. Information from relatives. Diagnosis of affective disorders. Arch Gen Psychiatry. 1984;41:173–180. doi: 10.1001/archpsyc.1984.01790130069010. [DOI] [PubMed] [Google Scholar]
- 33.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 34.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 35.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 36.Zijdenbos AP, Forghani R, Evans AC. Automatic quantification of MS lesions in 3D MRI brain data sets: validation of INSECT. Lect Notes Comput Sci. 1998;1496:439–448. [Google Scholar]
- 37.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 38.Robbins S. Anatomical standardization of the human brain in euclidean 3-space and on the cortical 2-manifold [PhD thesis] School of Computer Science, McGill University, Montreal, Quebec, Canada; 2003. [Google Scholar]
- 39.Robbins S, Evans AC, Collins DL, Whitesides S. Tuning and comparing spatial normalization methods. Med Image Anal. 2004;8:311–323. doi: 10.1016/j.media.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Chung MK, Worsley KJ, Robbins S, et al. Deformation-based surface morphometry applied to gray matter deformation. NeuroImage. 2003;18:198–213. doi: 10.1016/s1053-8119(02)00017-4. [DOI] [PubMed] [Google Scholar]
- 41.Chung MK, Robbins SM, Dalton KM, Davidson RJ, Alexander AL, Evans AC. Cortical thickness analysis in autism with heat kernel smoothing. NeuroImage. 2005;25:1256–1265. doi: 10.1016/j.neuroimage.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 42.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 44.Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- 45.Nesvåg R, Lawyer G, Varnäs K, et al. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 47.Narr KL, Bilder RM, Toga AW, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- 48.Kwon JS, McCarley RW, Hirayasu Y, et al. Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry. 1999;56:142–148. doi: 10.1001/archpsyc.56.2.142. [DOI] [PubMed] [Google Scholar]
- 49.Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- 50.Shenton ME, Kikinis R, Jolesz FA, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 51.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamasue H, Yamada H, Yumoto M, et al. Abnormal association between reduced magnetic mismatch field to speech sounds and smaller left planum temporale volume in schizophrenia. NeuroImage. 2004;22:720–727. doi: 10.1016/j.neuroimage.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 53.Meisenzahl EM, Koutsouleris N, Gaser C, et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res. 2008;102:150–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 54.Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood SJ, Berger G, Velakoulis D, et al. Proton magnetic resonance spectroscopy in first episode psychosis and ultra high-risk individuals. Schizophr Bull. 2003;29:831–843. doi: 10.1093/oxfordjournals.schbul.a007049. [DOI] [PubMed] [Google Scholar]
- 57.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 58.Chung YS, Kang DH, Shin NY, Yoo SY, Kwon JS. Deficit of theory of mind in individuals at ultra-high-risk for schizophrenia. Schizophr Res. 2008;99:111–118. doi: 10.1016/j.schres.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Assaf M, Rivkin PR, Kuzu CH, et al. Abnormal object recall and anterior cingulate overactivation correlate with formal thought disorder in schizophrenia. Biol Psychiatry. 2005;59:452–459. doi: 10.1016/j.biopsych.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 60.Kim JJ, Lee YJ, Lee DS, Lee MC, Kwon JS. Regional prefrontal abnormalities during working memory in schizophrenia: a topography-based functional activation study. Psychiatr Invest. 2004;1:37–43. [Google Scholar]
- 61.Pantelis C, Velakoulis D, Wood SJ, et al. Neuroimaging and emerging psychotic disorders: the Melbourne ultra-high risk studies. Int Rev Psychiatry. 2007;19:371–381. doi: 10.1080/09540260701512079. [DOI] [PubMed] [Google Scholar]
- 62.Yücel M, Wood SJ, Phillips LJ, et al. Morphology of the anterior cingulate cortex in young men at ultra-high risk of developing a psychotic illness. Br J Psychiatry. 2003;182:518–524. doi: 10.1192/bjp.182.6.518. [DOI] [PubMed] [Google Scholar]
- 63.Kwon JS, Shenton ME, Hirayasu Y, et al. MRI study of cavum septi pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. Am J Psychiatry. 1998;155:509–515. doi: 10.1176/ajp.155.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yücel M, Stuart GW, Maruff P, et al. Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol Psychiatry. 2002;52:15–23. doi: 10.1016/s0006-3223(02)01312-4. [DOI] [PubMed] [Google Scholar]
- 65.Pantelis C, Yücel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 66.Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 68.Farrow TFD, Whitford TJ, Williams LM, Gomes L, Harris AWF. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58:713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 69.Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura M, Salisbury DF, Hirayasu Y, et al. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62:773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 72.O'Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. NeuroImage. 2005;24:948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 73.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koutsouleris N, Meisenzahl EM, Davatzikos C, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66:700–712. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haller S, Borgwardt SJ, Schindler C, Aston J, Radue EW, Riecher-Rössler A. Can cortical thickness asymmetry analysis contribute to detection of at-risk mental state and first-episode psychosis? A pilot study. Radiology. 2009;250:212–221. doi: 10.1148/radiol.2501072153. [DOI] [PubMed] [Google Scholar]
- 76.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 77.Smieskova R, Fusar-Poli P, Allen P, et al. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?–a systematic review. Curr Pharm Des. 2009;15:2535–2549. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- 78.Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.