Abstract
Comparison of current and estimated premorbid IQ in schizophrenia suggests that there are subgroups with low IQ, deteriorated IQ (DIQ), or preserved IQ and that this is established by psychosis onset. There are no controlled studies examining the trajectory of these IQ subgroups longitudinally or their relationship with clinical and social outcomes. Of 129 individuals with first-episode schizophrenia or schizoaffective disorder, 25% showed stable low IQ, 31% showed stable IQ in the average/high range, and 44% demonstrated intellectual deterioration by 10 points or more. Patients in the low and deteriorated groups were equally impaired on tests of memory and executive function compared with the preserved average/high-IQ group and controls and showed more negative and disorganization symptoms than the preserved average/high-IQ group. Sixty patients and 27 controls were assessed again 1 and 3 years later. There was no evidence that those with IQ deterioration at baseline continued on a declining cognitive trajectory or that those with preserved average/high IQ experienced subsequent IQ decline. The low IQ group showed no change in IQ, whereas both the DIQ and the preserved IQ groups improved. However, the rate of improvement of these 2 subgroups was no greater than that of the healthy controls, suggesting that this reflected practice effects. Both the low and the deteriorated groups had longer index admissions, more core negative symptoms, and worse occupational outcomes at 3 years. These data suggest that following psychosis onset, IQ is stable and that it is IQ at psychosis onset rather than premorbid IQ predicts a more severe illness.
Keywords: schizophrenia, cognition, trajectory, premorbid IQ, WAIS, WTAR
Introduction
Studies have found that individuals who later develop schizophrenia have lower IQ scores than their peers prior to the development of psychosis and as far back as infancy.1–7 This has been estimated as an average of 0.5 SDs below the population mean.8 Some studies have found that there is a decline in IQ during adolescence,4,9 and others found that intellectual underperformance is greatest in those nearest to the onset of psychosis10,11 or that IQ deteriorates over the transition to psychosis.12–14
When the extent of the IQ decrement is assessed in patients with established schizophrenia using standard estimates of premorbid IQ, approximately 40% of patients show a decline of 10 points or more, whereas the remaining patients have either preserved average/high IQ or low IQ that has not changed.15–18 This pattern of heterogeneity in premorbid and current IQ differences is present at the time of the first episode16 and, together with the studies finding direct evidence of IQ decline, suggests that a large subgroup of patients are on a deteriorating cognitive trajectory at the time of psychosis onset.
It is not known whether patients characterized in this way continue to deteriorate once psychosis has developed or whether those whose IQ appears to be preserved show deterioration at a later stage. We also do not know if these profiles are predictive of later clinical and functional outcomes. There are several reasons why these are important questions. One is that recent neuroimaging studies have found progressive reduction in cortical gray matter volume around the time of psychosis onset19 and over subsequent years.19,20 A behavioral correlate of this may be progressive intellectual impairment, and, if so, the ability to identify patients deteriorating at the behavioral level would aid strategies aimed at cognitive enhancement. Longitudinal neuropsychological studies following the first psychotic episode tend to find no evidence of a decline in cognitive functions,21 but it remains possible that deterioration pertains to a subgroup. Relatively few longitudinal first-episode studies have examined IQ alongside more specific cognitive domains22–26 or have assessed healthy controls in parallel to patients.24,27–31 None to our knowledge has examined IQ trajectory in patients and healthy controls.
A second reason to examine IQ trajectory concerns the concept of cognitive reserve32 as applied to schizophrenia.33 The cognitive reserve hypothesis proposes that those with higher premorbid intellectual function are more able to cope with the impact of neural insult either because of higher brain structural reserve or because of better functional capacity to use compensatory forms of neural processing. Barnett et al33 have proposed that in schizophrenia, better cognitive reserve may result in fewer psychotic symptoms either because of superior reasoning skills or because of the ability to inhibit the abnormal neural processing that mediates psychotic symptoms. They also suggested that higher cognitive reserve would result in better functional outcome because greater insight would lead to improved treatment adherence. This predicts that patients with higher premorbid IQ will have better outcomes with respect to both symptom remission and social function. We previously found that both premorbid IQ and IQ at illness onset were significant predictors of functional outcome 4 years following a first episode of psychosis, but the relationship with IQ at onset was stronger.34 van Winkel et al,35 on the other hand, found that the reverse was true; ie, premorbid IQ but not IQ at the first psychotic episode predicted 10-year functional outcome. Thus, the latter study supports the cognitive reserve hypothesis, but our findings suggest that it is the IQ measured after the development of psychosis is more important for prognosis.
The current study investigated the stability of IQ following the first psychotic episode to establish whether there are further changes and whether different IQ trajectories have an impact on symptoms and social outcomes. Initially, we assessed patients to establish whether our previous finding of different premorbid current IQ subgroups at psychosis onset16 could be demonstrated in a different first-episode group. We then examined patients in 3 IQ categories, preserved average/high IQ, low IQ, and deteriorated IQ (DIQ), at 2 further time points over the following 3 years. We compared IQ trajectory with that of verbal memory and executive function because these are thought to be specifically impaired in schizophrenia and may be more relevant to outcome. We also included potential moderating factors in our analysis, including age of onset of psychosis, duration of untreated psychosis, premorbid social adjustment (PSA), insight, and adherence to medication. Finally, we compared our neuropsychological findings in patients with healthy controls tested at the same time points.
Method
Participants
One hundred twenty-nine patients with a first-episode psychosis (108 schizophrenia and 21 schizoaffective) were recruited into the West London First Episode Psychosis Study. Patients eligible for the study were screened with the World Health Organization Psychosis Screen36 and were recruited if they were between 16 and 60 years old, presenting for the first time with a psychotic illness, and had received no more than 12 weeks of antipsychotic medication. The diagnosis was ascertained by means of a structured interview, the diagnostic module of the Diagnosis Interview for Psychosis,37 which includes items from the Operational Criteria Checklist for Psychosis (OPCRIT)38 and the World Health Organization Schedules for Clinical Assessment in Neuropsychiatry.39 A computerized algorithm generates diagnoses under several classification systems, including Diagnostic and Statistical Manual of Mental Disorders (DSM)-IIIR and International Classification of Diseases-10 (DSM-IIIR diagnoses were then checked against DSM-IV criteria using OPCRIT for Windows [http://sgdp.iop.kcl.ac.uk/opcrit/]).
Five patients were medication free, 5 prescribed first-generation antipsychotics, 118 prescribed second-generation antipsychotics, and 1 prescribed a combination of first- and second-generation antipsychotics. One hundred twenty healthy controls were recruited from the same catchment area that the patients derived from, with the exclusion criterion of a history of psychiatric illness in themselves or first-degree relatives. Demographic information on the control group and patient subgroups is shown in table 1.
Table 1.
Comparison of First-Episode Measures of Demographics and Neuropsychological Functioning in the Controls and WTAR-WAIS III Patient Subgroups. Group Means are Presented With SDs in Parenthesis
| Low IQ IQ Subgroup | DIQ Subgroup | Preserved IQ Subgroup | Control | Statistic | Post Hoc (Tukey’s HSD) | |
| N | 32 (24.8%) | 57 (44.2%) | 40 (31.0%) | 120 | ||
| Age baseline | 24.209 (6.67) | 25.14 (7.79) | 27.13 (9.24) | 26.94 (7.00) | F3,248 = 1.74, P = .149 | |
| Sex | 22M/10F | 36M/21F | 27M/13F | 65M/55F | χ2 = 3.90, P = .273 | |
| Years of education | 11.97 (1.91) | 11.46 (2.40) | 13.33 (2.09) | 14.18 (2.05) | F3,248 = 24.88, P < .001 | LIQ, DIQ < PIQ < NC |
| WTAR estimated premorbid IQ | 79.31 (6.70) | 91.26 (11.31) | 102.83 (7.61) | 100.59 (9.49) | F3,248 = 55.30, P < .001 | LIQ < DIQ < PIQ, NC |
| WAIS current IQ | 78.63 (7.38) | 73.53 (11.37) | 104.85 (11.89) | 105.62 (14.09) | F3,248 = 112.11, P < .001 | LIQ, DIQ < PIQ, NC |
| Immediate verbal memory | 4.58 (1.65) | 4.32 (1.48) | 5.80 (1.57) | 6.17 (1.85) | F3,248 = 18.75, P < .001 | LIQ, DIQ < PIQ, NC |
| Verbal learning | 32.16 (11.21) | 32.47 (9.88) | 44.88 (8.43) | 50.08 (9.59) | F3,248 = 52.42, P < .001 | LIQ, DIQ < PIQ < NC |
| Spatial working memory span | 4.88 (1.36) | 4.73 (1.33) | 6.00 (1.30) | 6.45 (1.28) | F3,246 = 28.05, P < .001 | LIQ, DIQ < PIQ, NC |
| Spatial working memory manipulation | 38.67 (17.42) | 42.85 (14.30) | 27.44 (16.61) | 17.83 (15.57) | F3,231 = 34.12, P < .001 | LIQ, DIQ > PIQ > NC |
| Planning | 6.00 (2.32) | 6.02 (2.49) | 8.00 (2.40) | 8.59 (2.04) | F3,241 = 22.20, P < .001 | LIQ, DIQ < PIC, NC |
Note: WTAR, Weschler Test of Adult Reading; WAIS, Wechsler Adult Intelligence Scale; DIQ, deteriorated IQ; NC, Normal Controls; HSD, honestly significant difference.
Seventy-eight patients (61 schizophrenia and 17 schizoaffective) were followed up approximately 1 year after the initial assessment and 60 (48 schizophrenia and 12 schizoaffective) 1 and 3 years after the initial assessment; 27 controls were assessed on all 3 occasions. The mean number of weeks between baseline and second assessment was 59.22 (17.23) for patients and 59.40 (32.37) for controls and between baseline and third assessment was 143.42 (44.97) for patients and 130.39 (41.54) for controls. Diagnostic assessments were conducted at each time point; the diagnosis reached was that from the most recent assessment, which was consistent in those assessed at both the 1- and the 3-year follow-up time points. Permission to conduct the study was obtained from appropriate research ethics committees. Participants gave written informed consent and were paid an honorarium for their time.
Clinical Assessments
The range and severity of psychotic symptoms were assessed using the Scales for the Assessment of Positive and Negative Symptoms,40 and scores for the 3 symptom-derived syndromes of schizophrenia (negative, positive, and disorganization) were calculated.41 Social function was assessed using the Social Function Scale (SFS)42 where individuals rate their abilities in 7 areas including employment or occupational activity, and an overall score is also calculated. Affective symptoms were measured by the Hamilton Rating Scale for Depression43 and the Young Mania Rating Scale.44 To establish the timing of onset of the psychotic illness, the Nottingham Onset Scale45 was used. The length of index admission was also obtained from clinical notes, scored as 0 where treatment was as an outpatient. Premorbid function was assessed with the PSA scale.46 Insight was assessed using the Schedule for the Assessment of Insight.47 Adherence with medication was assessed using the Compliance Rating Scale.48
Cognitive Assessments
Current IQ was measured using a short form of the Wechsler Adult Intelligence Scale (WAIS) III49 composed of 4 subtests: information, arithmetic, block design, and digit symbol and developed for use in schizophrenia.50 Prorated full-scale IQ (FSIQ) was calculated. Premorbid IQ was estimated using the Weschler Test of Adult Reading (WTAR).51
Immediate verbal memory and learning were measured using the Rey Auditory Verbal Learning Task52 in which subjects are asked to read a list of 15 nouns. Immediate recall of the words was recorded as immediate memory and learning as the sum of words recalled over 5 trials, with the list being reread between each trial.
Other tests of memory and executive function were taken from the Cambridge Automated Neuropsychological Test Battery53 as follows: working memory span, taken from the spatial span task. This test of forward spatial span is akin to the Corsi block test. The maximum number of consecutively presented spatial locations that were successfully recalled was measured. Working memory manipulation, taken from the spatial working memory task. This is a self-ordered search task whereby participants need to recall where previous “tokens” were found from a random array of “boxes” in order to maximize success at finding subsequent tokens. The number of search errors was measured. Planning, this is analogous to the Tower of London task. In a series of problems varying in difficulty, subjects plan and execute a sequence of moves of stimuli in a visual array to match a goal array. The number of moves required range from 2 to 5 with 12 trials in total. The total number of perfect solutions was measured.
IQ Subgroups
The patient group was divided according to current and premorbid IQ. low IQ: WAIS and WTAR <90 and WTAR within 10 points of WAIS, preserved IQ: WTAR and WAIS ≥90 and a WTAR within 10 points of WAIS, and DIQ: WTAR greater than WAIS by more than 10 points.
Analysis
Data were analyzed using SPSS v15.0. Duration of untreated psychosis (DUP) was non-normally distributed and log transformed before analysis. Categorical data were analyzed using chi-square and continuous data using one-way analysis of variance (ANOVA). Linear mixed models were used to assess change over time and between groups separately for each cognitive measure and IQ. This approach allows inclusion of all cases, including those with missing data points, and thus makes use of incomplete longitudinal data. Because many patients were asymptomatic at follow-up, the symptoms scores were not normally distributed nor could they be transformed to return them to normal distribution. Therefore, Friedman’s nonparametric ANOVA by ranks was used to assess change over time in symptoms. To compare psychotic syndrome scores and affective symptom scores between groups at 3-year follow-up, Kruskal-Wallis nonparametric ANOVA was used. Length of index admission, compliance, and insight scores were also non-normally distributed and could not be returned to normal by transformation, so were also analyzed using Kruskal-Wallis nonparametric ANOVA.
Results
Table 1 shows the demographic and neuropsychological measures at the initial assessment for controls and IQ subgroups, which were matched for age and sex. There were no differences between preserved IQ and control groups on premorbid and current IQ. These 2 groups were also similar on verbal immediate memory, spatial span, and planning, but the controls were better on spatial working memory manipulation and verbal learning.
The mean premorbid IQ of the DIQ group was in the normal range, although this was significantly less than the preserved IQ group. The current IQ of the DIQ group indicated that IQ in this group had declined to the level of the low IQ group at baseline. The DIQ and low IQ groups were equally worse on all neuropsychological tests compared with controls.
Table 2 shows that, within the schizophrenia subgroups, there were no differences in age at onset, DUP, PSA, and affective or positive psychotic symptoms at presentation. The preserved IQ group had less severe negative and disorganization symptoms compared with the other 2 groups, which were not different.
Table 2.
Comparison of First-Episode Measures of Clinical Functioning in the Patient IQ Subgroups. Group Means are Presented With SDs in Parenthesis
| Low IQ Subgroup | DIQ Subgroup | Preserved IQ Subgroup | Statistic | Post Hoc | |
| Days between start of treatment and neuropsychological assessment | 59.06 (51.56) | 56.65 (72.87) | 62.73 (40.37) | F2,126 = 0.12, P = .885 | |
| DUP weeksa | 18.22 (23.56) | 56.54 (107.52) | 49.95 (92.46) | F2,128 = 2.16, P = .120 | |
| Premorbid social adjustment | 21.80 (8.24) | 19.89 (6.86) | 20.67 (8.20) | F2,104 = 0.50, P = .610 | |
| Age of onset | 23.88 (6.40) | 24.16 (7.21) | 26.38 (9.24) | F2,128 = 1.26, P = .288 | |
| Negative syndrome score | 0.41 (0.28) | 0.40 (0.25) | 0.23 (0.22) | F2,128 = 6.79, P = .002 | PIQ < LIQ, DIQ |
| Core negative symptoms | 4.69 (3.61) | 4.07 (3.27) | 2.33 (2.99) | F2,128 = 5.34, P = .006 | PIQ < LIQ, DIQ |
| Positive syndrome score | 0.80 (0.20) | 0.73 (0.23) | 0.69 (0.21) | F2,128 = 2.32, P = .102 | |
| Disorganization syndrome score | 0.48 (0.31) | 0.50 (0.32) | 0.32 (0.26) | F2,128 = 4.69, P = .011 | PIQ < LIQ, DIQ |
| Hamilton Depression Rating Scale | 12.06 (7.31) | 14.56 (8.97) | 12.75 (8.96) | F2,127 = 1.01, P = .368 | |
| Young Mania Rating Scale | 7.73 (7.24) | 10.16 (11.73) | 7.58 (10.01) | F2,126 = 0.94, P = .393 |
Note: DIQ, deteriorated IQ; DUP, duration of untreated psychosis.
Analysis performed on log-transformed score.
Follow-Up
To determine whether individuals who were successfully followed up were representative of the whole baseline group, we compared those who completed all 3 assessments with those who were assessed at baseline only on age, sex, years of education, and premorbid and current IQ; controls and patient IQ subgroups were examined separately. Completers vs noncompleters from the control, low IQ, and DIQ groups did not significantly differ on these measures. The preserved IQ group did not differ on premorbid IQ, current IQ, or age but did differ on sex, with female patients being overrepresented in the follow-up group, and years of education, with those who were successfully followed up having more years of education than those who were not. The group characteristics of those who were included in the follow-up analyses and comparisons of completers vs noncompleters are provided in the Supplementary table.
Cognitive Change
Both unstructured and autoregressive linear mixed models were used to assess change over time across all groups (table 3). The autoregressive model was a better fit in all cases according to Schwarz’s Bayesian Criterion (BIC) and thus are reported here.
Table 3.
Findings From First-Order Autoregressive Linear Mixed Models Comparing Cognitive Measures in the Controls and low IQ (LIQ), deteriorated IQ, and preserved IQ (PIQ) Groups. Effects at P < .01 Were Considered Significant. Where Interactions Were Significant, Those Time and Group Points That Significantly Differed From the Whole Model Are Reported (n = 129)
| Measure | Group Difference | Change Over Time (All Groups) | Coefficients of Significant Differences | Time By Group Interaction | Coefficient of Significant Differences |
| Current IQ | F3,272.8 = 109.66, P < .001 | F2,262.9 = 13.00, P < .001 | Baseline: −8.89; 1 year: −4.73; 3 years: reference | F6,262.4 = 3.22, P = .005 | LIQ baseline: 7.23; P = .029; all others NS |
| Immediate verbal memory | F3,247.0 = 27.09, P < .001 | F2,332.4 = 4.99, P = .007 | Baseline: −0.85; 1 year: −0.87; 3 years: reference | F6,227.2 = 0.75, P = .614 | n/a |
| Verbal learning | F3,263.5 = 62.44, P < .001 | F2,298.5 = 13.20, P = .007 | Baseline: −5.90; 1 year: −7.18; 3 years: reference | F6,296.1 = 1.22, P = .300 | n/a |
| Spatial working memory span | F3,246.7 = 26.52, P < .001 | F2,283.8 = 6.73, P = .001 | Baseline: −0.41; 1 year: −0.31; 3 years: reference | F6,281.9 = 1.11, P = .350 | n/a |
| Spatial working memory manipulation | F3,277.7 = 21.77, P < .001 | F2,188.3 = 2.57, P = .079 | n/a | F6,188.3 = 1.34, P = .243 | n/a |
| Planning | F3,235.2 = 20.18, P < .001 | F2,314.17 = 3.59, P = .029 | n/a | F6,312.97 = 1.77, P = .104 | n/a |
Note: NS, non-significant; n/a, not applicable.
Intelligence Quotient.
Overall, there was a significant improvement over time in IQ, and there was a significant interaction between group and time. This interaction reflected less change between baseline and 3-year follow-up in the low IQ group than the other groups.
Memory and Executive Function.
Immediate verbal memory, verbal learning, and spatial working memory span all improved significantly over time, but none showed a significant interaction between time and group. Performance on spatial working memory manipulation and planning did not change over time, and there was no significant interaction between time and group for either.
Clinical and Functional Outcome
The IQ subgroups that completed all 3 assessments were compared on the trajectory of their symptoms. All 3 groups improved on positive, negative, and disorganization symptoms; depression; and mania ratings with the exception of the preserved IQ group, which did not show an improvement in the negative syndrome score (table 4).
Table 4.
Results of Analyses of Change in Psychosis and Affective Symptoms Over Time in the low IQ (LIQ), deteriorated IQ (DIQ), and preserved IQ (PIQ) Groups (n = 60)
| Measure | LIQ (n = 12) | DIQ (n = 27) | PIQ (n = 21) |
| Negative syndrome score | χ2 = 10.31, P = .006 | χ2 = 8.77, P = .001 | χ2 = 2.98, P = .225 |
| Positive syndrome score | χ2 = 11.70, P = .003 | χ2 = 22.18, P < .001 | χ2 = 26.43, P < .001 |
| Disorganization syndrome score | χ2 = 16.29, P < .001 | χ2 = 34.28, P < .001 | χ2 = 19.77, P < .001 |
| Hamilton Depression Rating Scale | χ2 = 14.00, P = .001 | χ2 = 7.70, P = .021 | χ2 = 26.00, P < .001 |
| Young Mania Rating Scale | χ2 = 5.82, P = .05 | χ2 = 21.88, P < .001 | χ2 = 19.00, P < .001 |
At 3-year follow-up, there were no significant differences between the groups for the positive (χ2 = 0.10, P = .755) or disorganization (χ2 = 0.17, P = .677) syndromes. There was a nonsignificant trend for the negative syndrome to be different (χ2 = 2.99, P = .084), and when the analysis was restricted to the core negative symptoms of affective flattening and alogia, there was a significant difference between the groups (χ2 = 6.63, P = .036) reflecting more symptoms in the DIQ than in the preserved IQ group (χ2 = 6.44, P = .011). There was no difference between the groups in terms of insight (χ2 = 0.52, P = .772) or medication adherence (χ2 = 0.53, P = .771) at 3-year follow-up.
There was no difference between the 3 patient groups at 3 years in total SFS score (F2,59 = 0.42, P = .657), but there was a significant difference in the employment/occupation SFS subscale (F2,59 = 4.22, P = .019), and post hoc analyses showed a significant difference between the preserved IQ and DIQ groups (P = .029), trend level difference between the preserved IQ and low IQ groups (P = .054), and no difference between the low IQ and DIQ groups (P = .978). At follow-up, 62% of the preserved IQ group were engaged in work or study compared with 30% in the DIQ group and 33% in the low IQ group. The groups differed significantly in length of index admission (χ2 = 6.05, P = .049), and pairwise comparisons showed that the low IQ and DIQ groups did not differ (χ2 = 0.803, P = .370), but the patients in the preserved IQ group had shorter admissions compared with the low IQ group (χ2 = 5.43, P = .020) and DIQ group (χ2 = 3.27, P = .071) at a trend level of significance.
Further Post Hoc Analyses
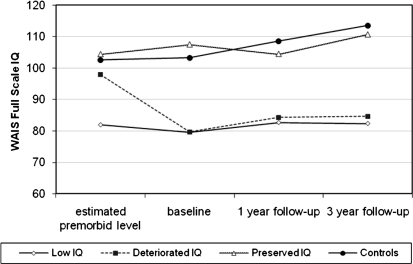
To investigate whether premorbid IQ or IQ decline in the DIQ group determined outcome, we reanalyzed the data, including only those patients in the DIQ group with WTAR ≥90 (n = 35 in study and n = 19 completed follow-up). Examining the trajectory of WAIS IQ over the 3 time points, there remained a significant interaction between time and group (F6,239.6 = 3.71, P = .002), with the low IQ group showing less improvement over time. Figure 1 shows the IQ trajectory of those controls and IQ subgroups that completed all 3 assessments, with only those in the DIQ group with average/high premorbid IQ included. In terms of outcome, the groups remained significantly different on SFS employment/occupation score (F2,51 = 4.93, P = .011); 32% of this subset of the DIQ group were engaged in work or study at 3 years. There was a nonsignificant trend for the length of index admission to differ among the groups (χ2 = 5.24, P = .073), with the preserved IQ group being shorter than low IQ group and the DIQ group being intermediate between the two. There were no differences in core negative symptoms at 3 years (χ2 = 2.75, P = .253).
Fig. 1.
Estimated Premorbid IQ Using Weschler Test of Adult Reading (WTAR) and 3-Year Trajectory of Current IQ in the Controls and Low IQ, Deteriorated IQ (WTAR ≥90 Only), and preserved IQ Subgroups. WAIS, Wechsler Adult Intelligence Scale.
To further investigate the differential impact of current IQ and premorbid IQ on cognitive and clinical outcomes, we also examined the correlation between both IQ scores and measures of outcome across all patients, regardless of their IQ subgroup. Collinearity between the premorbid and current IQ scores means that if one is a strong predictor of a particular outcome measure, the other is likely to also predict that same outcome measure. Nevertheless, current IQ correlated more highly with outcome than premorbid IQ. All 3-year cognitive measures, SFS employment/occupation, core negative symptoms, and length of index admission, were correlated with current IQ at P < .01. Only immediate verbal memory, verbal learning, and planning were correlated with premorbid IQ at P < .01. Table 5 shows the correlations.
Table 5.
Pearson’s r or Spearman’s rho
| Estimated Premorbid IQ | Baseline Current IQ | |
| Immediate verbal memory | 0.50* | 0.55* |
| Verbal learning | 0.45* | 0.54* |
| Spatial working memory span | 0.30** | 0.45* |
| Spatial working memory manipulation (error score) | −0.20 | −0.38*** |
| Planning | 0.37** | 0.46* |
| Negative syndrome scorea | −0.27** | −0.25 |
| (Core negative symptoms only)a | −0.26** | −0.33*** |
| Positive syndrome scorea | 0.06 | −0.05 |
| Disorganization syndrome scorea | −0.10 | −0.07 |
| Hamilton Depression Rating Scalea | 0.15 | 0.09 |
| Young Mania Rating Scalea | −0.05 | −0.20 |
| Medication adherencea | 0.15 | 0.14 |
| Insighta | 0.24 | 0.19 |
| Length of index admissiona | −0.33** | −0.36*** |
| SFS overall score | 0.14 | 0.16 |
| SFS employment/occupation subscale score | 0.32** | 0.40*** |
Note: SFS, Social Function Scale.
Correlations between premorbidor current IQ at baseline and outcome measures in all patients who completed 3 assessments (n = 60).
**P < .05, ***P < .01, and *P < .001.
Discussion
In this study, we replicated our previous finding of heterogeneity in the relationship between premorbid and current IQ16 in a different group of patients with first-episode schizophrenia or schizoaffective disorder. Thus, 44% of patients showed a 10-point or greater decline in IQ, whereas the IQ of the remaining patients was stable up to the first psychotic episode, with 25% having low IQ values and the remaining patients having preserved IQ in the normal range or higher. This finding is also in agreement with other studies examining patients with established schizophrenia.15,17,18 At first episode, the preserved IQ group had less severe negative and disorganization symptoms than the other 2 groups, which were not different, but no other clinical feature distinguished the groups, including the level of premorbid social function, age at onset, duration of untreated psychosis, and severity of affective or positive psychotic symptoms.
During the 3 years following psychosis onset, there was no evidence that those with IQ deterioration continued on a declining cognitive trajectory or that those with preserved average/high IQ experienced IQ decline following onset. The low IQ group showed no statistically significant change in IQ, whereas both the DIQ and the preserved IQ groups improved. However, the rate of improvement of these 2 subgroups was no greater than that of the healthy controls, suggesting that this reflected practice effects. These findings support previous controlled studies that showed improvement in IQ following psychosis onset that was equal to or less than that of healthy controls23,24 and suggests that the improvement in IQ after the first episode seen in uncontrolled studies is nonspecific.22,25,26
To our knowledge, only 1 other study has compared estimates of premorbid IQ with IQ trajectory following psychosis onset, and this did not include healthy controls.54 In this study, patients were dichotomized according to estimated premorbid IQ; the lower premorbid IQ group showed no change in IQ when assessed both at first episode and at 6–14 years later, whereas the higher premorbid IQ group showed a decline in IQ at first episode, which subsequently improved to premorbid levels. In our study, by separating patients with and without evidence of IQ decline at first episode,18 we showed that, although patients with IQ decline improved between first psychotic episode and later follow-up, this was no greater than that seen in healthy controls. We could find no evidence that even those patients with relatively high premorbid IQ (>90) who had declined by psychosis onset subsequently improved to premorbid levels.
The same group35 also reported that IQ at psychosis onset did not predict functional outcome, whereas premorbid IQ was a significant predictor but only when adjusted for the confounding effects of DUP, gender, and age of onset. We have previously shown in a different group of first-episode patients that both premorbid IQ and IQ at first episode predicted 4-year functional outcome but that IQ at onset was the stronger predictor, and neither findings were confounded.34 In the current study, we replicated this finding in a new group of patients by examining the level of correlation between outcome measures and measures of premorbid and current IQ at baseline for the group of patients who had 3 assessments. Because premorbid and current IQ are intercorrelated, it is impossible to completely tease apart the contribution of these 2 measures; however, the results suggest that current baseline IQ was a better predictor of outcome, being more highly correlated with several cognitive measures, core negative symptoms, and occupational functioning at 3-year follow-up, as well as the length of index admission. The current study extends these findings by showing that those with preserved IQ in the average/high range had better outcomes than the other 2 groups in that they had less disorganization and negative symptoms at onset, shorter index admissions, less core negative symptoms, and better occupational outcome at 3 years. The group that showed a decline in IQ closely resembled the low IQ group in their current IQ at first episode, length of index admission, and occupational function at 3 years in addition to showing more core negative symptoms than the average/high-IQ group. Within the DIQ group, even those who declined from average/high levels had as poor an occupational outcome as those with lower premorbid IQ. These results suggest that it was not premorbid IQ but the IQ that the DIQ group arrived at by the time of the first psychotic episode that predicted a more severe illness.
These findings also have implications for the cognitive reserve hypothesis, which proposes that the higher the premorbid cognitive ability, the more resilient individuals are to the impact of cerebral dysfunction.32,33 Our findings suggest that in schizophrenia, there are some patients who benefit from cognitive reserve because they have premorbid ability in the average/high range, which remains stable following the onset of psychosis. However, there are others with similar levels of premorbid ability who undergo a decline in cognitive ability sufficient to cause diminished cognitive reserve and worse outcome. We were unable to identify any predictive measures that could distinguish these 2 groups, such as age at onset, duration of untreated psychosis, and PSA. Thus, the elucidation of factors that confer vulnerability to cognitive change during the development of psychosis requires further study because this group may be particularly amenable to intervention if detected early enough.
The current findings and those of others21 have failed to elucidate possible cognitive correlates of the continuing reductions in gray matter volume following psychosis onset seen in neuroimaging studies.19,20 Gray matter volume loss early in the illness is clearly of clinical relevance because Cahn et al55 found that those with the greatest volume loss over the first year had the highest negative symptoms and poorest functional outcome. One explanation is that the structural changes giving rise to cognitive impairment have occurred by the time of presentation with psychosis, and therefore, the neural context for poor outcome is already established for some patients at this stage. Although the continuing volumetric reductions detected on MRI could represent the manifestation of these changes, their functional consequences may already have been declared. Our data support the view that the ability to detect patients with a deteriorating cognitive course very early in the development of psychosis, at the ultra high-risk stage, will be important for neuroprotective strategies in schizophrenia.56–58
At baseline, the low IQ and DIQ groups performed equivalently on tests of verbal learning and memory, working memory, and planning and significantly worse than the controls and preserved IQ groups. These findings are compatible with those of Kremen et al17 who found that subgroups of schizophrenia patients with preserved IQ and DIQ, matched on current IQ, demonstrated similar neuropsychological performance. Our DIQ group had comparable current IQ to our low IQ group, and because performance on all cognitive measure was the same in these 2 patient groups, our findings support their conclusion that in schizophrenia; current neuropsychological performance is a function of current IQ rather than prior intellectual trajectory.
At follow-up, there was evidence of improvement over time on verbal immediate memory and learning and spatial working memory span, but this was equivalent across IQ subgroups and controls. Improvement in the controls indicated that practice enhanced performance, and this finding emphasizes the importance of a comparison control group when assessing change in performance of specific cognitive functions over time in keeping with a study of improvement in cognition following treatment with antipsychotic medication.29 Despite performing better than the low IQ and DIQ groups, those with preserved IQ were significantly impaired compared with controls on verbal learning and spatial working memory manipulation. We and others have previously found that preserved IQ subgroups have impaired executive function and verbal memory.16–18,59 However, when IQ and specific measures of memory and executive function were compared as predictors of outcome, we previously found that only IQ consistently predicted functional outcome whether measured as a premorbid estimate or at 3 time points over 4 years following onset.34
A limitation of this study is the use of an indirect measure of premorbid IQ. There are many studies substantiating the use of tests of irregular word pronunciation in normal volunteers and in a variety of neuropsychiatric disorders.60–63 The majority of studies comparing patients with chronic stable or acutely symptomatic schizophrenia with matched controls and other patient groups have found these tests to be a valid measure of premorbid IQ,64,65 and other studies have found estimated IQ to be stable over time.66,67 Nevertheless, the use of tests of irregular word pronunciation in schizophrenia has been criticized on the grounds that the disorder itself may be related to impairment in verbal ability, thus causing IQ to be underestimated.68 Against this are studies that have found that current vocabulary approximates direct measures of premorbid IQ in schizophrenia.69,70 Conversely, another criticism is that irregular word-reading tasks overestimate IQ at the lower FSIQ range, giving a spurious impression of IQ decline.70 We do not think that this explains our findings because 25% of patients were classified as having low current IQ, characterized as equivalent estimated premorbid and current IQ. Another limitation is that, although we completed all assessments for 60 patients when these were divided into IQ groups, the numbers were inevitably rather small. Our findings therefore require further study in larger high-risk groups where direct measures of premorbid IQ can be ascertained.
Supplementary Material
Supplementary table is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Wellcome Trust (064607).
Supplementary Material
Acknowledgments
We are grateful to Isobel Harrison and Stan Mutsatsa for clinical assessments and the consultants and nurses of West London and South West London and St George’s Mental Health National Health Service Trusts for greatly facilitating the study. E.M.J. and V.C.L. acknowledge support from the Raymond Way Fund. T.R.E.B. has acted as a consultant for Servier, Johnson & Johnson, and Bristol-Myers Squibb. The other authors have no biomedical financial interests or potential conflicts of interest.
References
- 1.David AS, Malmberg S, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychol Med. 1997;27:1311–1323. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- 2.Davidson M, Reichenberg A, Rabinowitz J, et al. Behavioral and intellectual markers for Schizophrenia in apparently healthy male adolescents. Am J Psychiatr. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- 3.Jones P, Murray R, Jones P, Rodgers B, Marmot M. Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 4.Kremen WS, Buka SL, Seidman LJ, et al. IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study. Am J Psychiatr. 1998;155:672–677. doi: 10.1176/ajp.155.5.672. [DOI] [PubMed] [Google Scholar]
- 5.Cannon TD, Bearden CE, Hollister JM, et al. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- 6.Zammit S, Allebeck P, David AS, et al. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatr. 2004;61:354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
- 7.Bilder RM, Reiter G, Bates JA, et al. Cognitive development in schizophrenia: follow back from first episode. J Clin Exp Neuropsychol. 2006;28:270–282. doi: 10.1080/13803390500360554. [DOI] [PubMed] [Google Scholar]
- 8.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatr. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 9.Fuller R, Nopoulos P, Arndt S, et al. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatr. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowitz J, Reichenberg A, Weiser M, et al. Cognitive and behavioural functioning in men with schizophrenia both before and shortly after first admission to hospital. Cross-sectional analysis. Br J Psychiatr. 2000;177:26–32. doi: 10.1192/bjp.177.1.26. [DOI] [PubMed] [Google Scholar]
- 11.Gunnell D, Harrison G, Rasmussen F, Fouskakis D, Tynelius P. Associations between premorbid intellectual performance, early-life exposures and early-onset schizophrenia: cohort study. Br J Psychiatr. 2002;181:298–305. doi: 10.1192/bjp.181.4.298. [DOI] [PubMed] [Google Scholar]
- 12.Cosway R, Byrne M, Clafferty R, et al. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychol Med. 2000;30:1111–1121. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- 13.Caspi A, Reichenberg A, Weiser M, et al. Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophr Res. 2003;65:87–94. doi: 10.1016/s0920-9964(03)00056-2. [DOI] [PubMed] [Google Scholar]
- 14.Lencz T, Smith CW, McLaughlin D, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatr. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Badcock JC, Dragovic M, Waters FAV, Jablensky A. Dimensions of intelligence in schizophrenia: evidence from patients with preserved, deteriorated and compromised intellect. J Psychiatr Res. 2005;39:11–19. doi: 10.1016/j.jpsychires.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Joyce EM, Hutton SB, Mutsatsa SH, Barnes TRE. Cognitive heterogeneity in first-episode schizophrenia. Br J Psychiatr. 2005;187:516–522. doi: 10.1192/bjp.187.6.516. [DOI] [PubMed] [Google Scholar]
- 17.Kremen WS, Seidman LJ, Faraone SV, Tsuang MT. IQ decline in cross-sectional studies of schizophrenia: methodology and interpretation. Psychiatry Res. 2008;158:181–194. doi: 10.1016/j.psychres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Weickert TW, Goldberg TE, Gold JM, et al. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatr. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 19.Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cahn W, Rais M, Stigter FP, et al. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2009;19:147–151. doi: 10.1016/j.euroneuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Szoke A, Trandafir A, Dupont ME, et al. Longitudinal studies of cognition in schizophrenia: meta-analysis. Br J Psychiatr. 2008;192:248–257. doi: 10.1192/bjp.bp.106.029009. [DOI] [PubMed] [Google Scholar]
- 22.Gold S, Arndt S, Nopoulos P, O'Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatr. 1999;156:1342–1348. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- 23.Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;78:27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Frangou S, Hadjulis M, Vourdas A. The Maudsley early onset schizophrenia study: cognitive function over a 4-year follow-up period. Schizophr Bull. 2008;34:52–59. doi: 10.1093/schbul/sbm124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Scottish Schizophrenia Research Group. The Scottish first episode schizophrenia study V. One-year follow-up. Br J Psychiatr. 1988;152:470–476. doi: 10.1192/bjp.152.4.470. [DOI] [PubMed] [Google Scholar]
- 26.Townsend LA, Norman RMG, Malla AK, Rychlo AD, Ahmed RR. Changes in cognitive functioning following comprehensive treatment for first episode patients with schizophrenia spectrum disorders. Psychiatry Res. 2002;113:69–81. doi: 10.1016/s0165-1781(02)00236-6. [DOI] [PubMed] [Google Scholar]
- 27.Albus M, Hubmann W, Mohr F, et al. Neurocognitive functioning in patients with first-episode schizophrenia: results of a prospective 5-year follow-up study. Eur Arch Psychiatry Clin Neurosci. 2006;256:442–451. doi: 10.1007/s00406-006-0667-1. [DOI] [PubMed] [Google Scholar]
- 28.Hill SK, Keshavan MS, Thase ME, Sweeney JA. Neuropsychological dysfunction in antipsychotic-naive first-episode unipolar psychotic depression. Am J Psychiatr. 2004;161:996–1003. doi: 10.1176/appi.ajp.161.6.996. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg TE, Goldman RS, Burdick KE, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatr. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 30.Addington J, Saeedi H, Addington D. The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophr Res. 2005;78:35–43. doi: 10.1016/j.schres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Crespo-Facorro B, Rodriguez-Sanchez JM, Perez-Iglesias R, et al. Neurocognitive effectiveness of haloperidol, risperidone, and olanzapine in first-episode psychosis: a randomized, controlled 1-year follow-up comparison. J Clin Psychiatr. 2009;70:717–729. doi: 10.4088/JCP.08m04634. [DOI] [PubMed] [Google Scholar]
- 32.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 33.Barnett JH, Salmond CH, Jones PB, Sahakian BJ. Cognitive reserve in neuropsychiatry. Psychol Med. 2006;36:1053–1064. doi: 10.1017/S0033291706007501. [DOI] [PubMed] [Google Scholar]
- 34.Leeson VC, Barnes TRE, Hutton SB, Ron MA, Joyce EM. IQ as a predictor of functional outcome in schizophrenia: a longitudinal, four-year study of first-episode psychosis. Schizophr Res. 2009;107:55–60. doi: 10.1016/j.schres.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Winkel R, Myin-Germeys I, De Hert M, et al. The association between cognition and functional outcome in first-episode patients with schizophrenia: mystery resolved? Acta Psychiatr Scand. 2007;116:119–124. doi: 10.1111/j.1600-0447.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- 36.Jablensky A, Sartorius N, Ernberg G, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- 37.Jablensky A, McGrath J, Herrman H, et al. Psychotic disorders in urban areas: an overview of the Study on Low Prevalence Disorders. Aust N Z J Psychiatr. 2000;34:221–236. doi: 10.1080/j.1440-1614.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- 38.McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatr. 1991;48:764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- 39.Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatr. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 40.Andreasen N. Basel, Switzerland: Karger; 1990. Methods for assessing positive and negative symptoms. [DOI] [PubMed] [Google Scholar]
- 41.Liddle PF, Barnes TRE. Syndromes of chronic schizophrenia. Br J Psychiatr. 1990;157:558–561. doi: 10.1192/bjp.157.4.558. [DOI] [PubMed] [Google Scholar]
- 42.Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatr. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatr. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 45.Singh SP, Cooper JE, Fisher HL, et al. Determining the chronology and components of psychosis onset: the Nottingham Onset Schedule (NOS) Schizophr Res. 2005;80:117–130. doi: 10.1016/j.schres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Foerster A, Lewis S, Owen M, Murray RM. Pre-morbid adjustment and personality in psychosis: effects of sex and diagnosis. Br J Psychiatr. 1991;158:171–176. doi: 10.1192/bjp.158.2.171. [DOI] [PubMed] [Google Scholar]
- 47.David AS. Insight and psychosis. Br J Psychiatr. 1990;156:798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- 48.Hayward P, Chan N, Kemp R, Youle S, David AS. Medication self management: a preliminary report on an intervention to improve medication compliance. J Ment Health. 1995;4:511–517. [Google Scholar]
- 49.Wechsler D. San Antonio, TX: Harcourt Assessment; 1997. Wechsler Adult Intelligence Scale-3rd Edition (WAIS-3) [Google Scholar]
- 50.Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46:209–215. doi: 10.1016/s0920-9964(00)00017-7. [DOI] [PubMed] [Google Scholar]
- 51.Wechsler D. San Antonio, TX: The Psychological Corporation; 2001. The Wechsler Test of Adult Reading (WTAR) [Google Scholar]
- 52.Lezak MD. New York: Oxford University Press; 2004. Neuropsychological assessment. [Google Scholar]
- 53.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 54.van Winkel R, Myin-Germeys I, Delespaul P, et al. Premorbid IQ as a predictor for the course of IQ in first onset patients with schizophrenia: a 10-year follow-up study. Schizophr Res. 2006;88:47–54. doi: 10.1016/j.schres.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 55.Cahn W, van Haren NEM, Pol HEH, et al. Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. Br J Psychiatr. 2006;189:381–382. doi: 10.1192/bjp.bp.105.015701. [DOI] [PubMed] [Google Scholar]
- 56.Yung AR, Killackey E, Hetrick SE, et al. The prevention of schizophrenia. Int Rev Psychiatr. 2007;19:633–646. doi: 10.1080/09540260701797803. [DOI] [PubMed] [Google Scholar]
- 57.Lieberman JA, Buckley PF, Perkins DO. Neuroprotection: a new strategy in the treatment of schizophrenia. CNS Spectrums. 2007;12(10 suppl 18):1–16. [Google Scholar]
- 58.Cohen JD, Insel TR. Cognitive neuroscience and schizophrenia: translational research in need of a translator. Biol Psychiatr. 2008;64:2–3. doi: 10.1016/j.biopsych.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 59.Leeson VC, Robbins TW, Franklin C, et al. Dissociation of long term verbal memory and fronto-executive impairment in first-episode psychosis. Psychol Med. 2009;39:1799–1808. doi: 10.1017/S0033291709005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crawford JR, Parker DM, Stewart L. Prediction of WAIS IQ with the National Adult Reading Test; cross validation and extension. Br J Clin Psychol. 1989;28:267–273. [Google Scholar]
- 61.Crawford JR, Deary IJ, Starr J, Whalley JL. The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66-year interval. Psychol Med. 2001;31:451–458. doi: 10.1017/s0033291701003634. [DOI] [PubMed] [Google Scholar]
- 62.Nelson HE, O'Connell A. Dementia: the estimation of pre-morbid intelligence levels using the new adult reading test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- 63.O'Carroll R. The assessment of pre-morbid ability. A critical review. Neurocase. 1995;1:83–89. [Google Scholar]
- 64.O'Carroll R, Walker M, Duncan J. Selecting controls for schizophrenia research studies: the use of the National Adult Reading Test (NART) as a measure of pre-morbid ability. Schizophr Res. 1992;8:137–141. doi: 10.1016/0920-9964(92)90030-9. [DOI] [PubMed] [Google Scholar]
- 65.Crawford JR, Besson JA, Bremner M, et al. Estimation of premorbid intelligence in schizophrenia. Br J Psychiatr. 1992;161:69–74. doi: 10.1192/bjp.161.1.69. [DOI] [PubMed] [Google Scholar]
- 66.Morrison G, Sharkey V, Allardyce J. Nithsdale schizophrenia surveys: a longitudinal study of National Adult Reading Test stability. Psychol Med. 2000;30:717–720. doi: 10.1017/s0033291799001920. [DOI] [PubMed] [Google Scholar]
- 67.Smith D, Roberts S, Brewer W, Pantelis C. Test-retest reliability of the National Adult Reading Test (NART) as an estimate of premorbid IQ in patients with schizophrenia. Cognit Neuropsychiatry. 1998;3:71–80. [Google Scholar]
- 68.Barber F, Pantelis C, Bodger S, Nelson HE. Chichester, UK: John Wiley; 1996. Intellectual functioning in schizophrenia: natural History. [Google Scholar]
- 69.Eberhard J, Riley F, Levander S. Premorbid IQ and schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2003;253:84–88. doi: 10.1007/s00406-003-0412-y. [DOI] [PubMed] [Google Scholar]
- 70.Russell AJ, Munro J, Jones P, et al. The National Adult Reading Test as a measure of premorbid IQ in schizophrenia. Br J Clin Psychol. 2000;39:297–305. doi: 10.1348/014466500163301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.