Abstract
Background: It remains unknown as to whether the antipsychotic dose needed for the acute-phase treatment of schizophrenia is also necessary for relapse prevention. Aim: To compare the efficacy between standard dose [(World Health Organization daily defined dose (DDD)] vs low dose (≥50% to <1 DDD) or very low dose (<50% DDD) for relapse prevention in schizophrenia. Data source: Double-blind, randomized, controlled trials with a follow-up duration of ≥24 weeks, including ≥2 dosage groups of the same antipsychotic drug for relapse prevention in schizophrenia, were searched using MEDLINE, the Cochrane Central Register of Controlled Trials, and EMBASE (last search: August 2009). Data extraction: Data on overall treatment failure, hospitalization, relapse, and dropouts due to side effects were extracted and combined in a meta-analysis. Data synthesis: Thirteen studies with 1395 subjects were included in this meta-analysis. Compared with the standard-dose treatment, the low-dose therapy did not show any statistically significant difference in overall treatment failure or hospitalization, while the standard dose showed a trend-level (P = .05) superiority in risk of relapse. The very low–dose group was inferior to the standard-dose group in all efficacy parameters. No significant difference was found in the rate of dropouts due to side effects between either standard dose vs low dose or very low dose. Conclusions: Although antipsychotic treatment with ≥50% to <1 DDD may be as effective as standard-dose therapy, there are insufficient clinical trial data to draw firm conclusions on standard- vs low-dose maintenance antipsychotic therapy for schizophrenia.
Keywords: antipsychotic, dose, neuroleptic, relapse prevention, schizophrenia
Introduction
Antipsychotic drugs have played a central role in the treatment of schizophrenia for more than 50 years.1 Antipsychotic treatment significantly reduces the risk of relapse; however, this also causes various side effects, including motor, metabolic, and cardiovascular side effects,2–5 which contributes to poor adherence and undesirable outcome.6 Given that the risk for these adverse effects from antipsychotic drugs is often dose-related,2,4,5 the use of the lowest possible effective antipsychotic dose for relapse prevention is critically important to enhance the overall treatment outcome. While dosing issues of antipsychotic treatment to relieve acute psychotic symptoms of schizophrenia have been widely investigated,7,8 data are still limited on the therapeutic dose for relapse prevention. One clinically important question remains unanswered: “Is the dose needed for the acute phase also necessary for relapse prevention?” In fact, major treatment guidelines suggest opposite treatment strategies.9–11 For example, the practice guidelines by the American Psychiatric Association (APA) recommend the use of the lowest possible effective dose for the maintenance treatment,9 while the Expert Consensus Guidelines generally advocate the continuous use of antipsychotic dose that was effective in the acute phase also for relapse prevention.10 To address this gap in knowledge, we conducted a meta-analysis to compare the efficacy and safety of standard dose (ie, equal to or more than the defined daily dose [DDD] by the World Health Organization) vs low dose (ie, less than the defined dose, but equal to or greater than half the dose) or very low dose (ie, less than half the defined dose) for relapse prevention in the treatment of schizophrenia or schizoaffective disorder.
Methods
Study Selection
Double-blind randomized controlled trials (RCTs) including at least 2 dosage groups of the same antipsychotic drug for relapse prevention in the maintenance treatment of schizophrenia or schizoaffective disorder were identified. MEDLINE (1966–August 2009), the Cochrane Central Register of Controlled Trials (last search: August 2009), EMBASE (1980–August 2009), Current Contents (2001–August 2009), Science Citation Index Expanded (2005–August 2009), E-Journals (1832–August 2009), and Science Citation Index Expanded (1900–August 2009) were searched by using the following search terms: neuroleptic, antipsychotic, maintenance, relapse, schizophrenia, and schizoaffective disorder. The reference lists of the relevant study reports, review articles, and Cochrane reviews were also examined. No language restriction was applied. All the titles or abstracts were independently read by 2 of the authors (H.U. and H.T.), and relevant articles were selected and reviewed. In turn, the reference sections of articles identified in the search were reviewed for relevant articles. Pharmaceutical companies that produced second-generation antipsychotics (SGAs; Astellas, AstraZeneca, Dainippon-Sumitomo Pharma, Eli Lilly, Janssen Pharmaceutical, Novartis, Otsuka, and Pfizer) were asked whether they were aware of further trials and also invited to provide results of unpublished trials when available.
Inclusion Criteria
Double-blind RCTs were included if they included patients with schizophrenia or schizoaffective disorder (according to the Research Diagnostic Criteria or the Diagnostic and Statistical Manual of Mental Disorders, the third edition or later) and had a minimum follow-up duration of 24 weeks. In addition, to specifically examine the effect of antipsychotics in relapse prevention, we only included trials that included patients with stable psychopathology at baseline systematically defined in each individual study. We also restricted our analysis to trials that involved a standard-dose group and at least one of very low–dose and low-dose groups: (a) standard-dose group, using a mean dose of ≥1 DDD unit and less than the upper limit of locally approved dose range in the origin of the trials; (b) very low–dose group, using a mean dose of <0.5 DDD unit; and (c) low-dose group, using a mean dose of ≥0.5 and <1 DDD unit. This unit of measurement used for standardizing antipsychotic doses was developed by the World Health Organization Collaborating Centre for Drug Statistics Methodology System of Defined Daily Doses.12 The DDD unit is the assumed average dose (mg) per day for a drug used for its main indication in adults (eg, schizophrenia for antipsychotics) (table 1). In light of the inherent limitations of the DDD unit system, we also analyzed the data using dosage ranges recommended by the Schizophrenia Patient Outcomes Research Team (PORT) Treatment Recommendations (table 1).11 In this second analysis, all treatment arms in the trials that were identified through the literature search as described above were sorted to one of the following dose groups: (a) standard-dose group, using a mean dose within the PORT-recommended dose range for the respective medications; (b) very low–dose group, using a mean dose of less than half the lower limit of the PORT-recommended dose range; and (c) low-dose group, using a mean dose of ≥0.5 and <1 of the lower limit.
Table 1.
DDDs and the PORT-Recommended Dose Ranges of Included Antipsychotic Drugs for the Maintenance Treatment of Schizophrenia
| Drug Name | DDD12 | PORT-Recommended Dose Range11 |
| Quetiapine | 400 | 300–750 mg/d |
| Ziprasidone | 80 | 120–160 mg/d |
| Olanzapine | 10 | 10–20 mg/d |
| Long-acting risperidone | 1.8a | n.a. |
| Haloperidol decanoate | 3.3b | 50–200 mg/4 wks |
| Fluphenazine decanoate | 1c | 6.25–25 mg/2 wks |
| Pimozide | 4 | n.a. |
| Propericyazine | 50 | n.a. |
Note: DDD, defined daily dose; PORT: Schizophrenia Patient Outcomes Research Team; n.a., not available.
Equivalent to 25.2 mg/2 wks.
Equivalent to 92.4 mg/4 wks.
Equivalent to 14 mg/2 wks.
In the event of several publications generated by the same group of investigators that were clearly based on overlapping samples of subjects, we included data from the publication with the longest follow-up duration and/or provided the most detailed information.
Outcome Parameters
The primary outcome of interest was overall treatment failure, which was defined as the rate of subjects who prematurely discontinued their assigned treatment for any reason. The secondary outcome parameter was the rate of subjects who required hospitalization. The rate of subjects who met relapse criteria as defined by the individual studies was also evaluated. Dropouts due to side effects were analyzed as a measure of tolerability. Corresponding authors were contacted for missing data. All these parameters were obtained based on an intention-to-treat basis. All data were extracted independently by 2 of us (H.U. and H.T.).
Statistical Method
The outcome data were combined in a meta-analysis and were compared between the 3 dose groups as defined above. The risk difference and its 2-sided 95% confidence interval (CI) were calculated, with risk difference defined as (nE = 1, e/ne) – (nE = 1, c/nc) where nE = 1, e is the number of patients who experienced an event in the experimental group (ie, low- or very low–dose group), ne is the total number of patients in the experimental group, nE = 1, c is the number of patients with the event in the control group (ie, standard-dose group), and nc is the total number of patients in the control group. In the case of significant differences between groups, the number of subjects needed to treat (NNT) or the number of subjects needed to harm (NNH) was calculated. NNT or NNH was derived from the risk difference by the formula NNT or NNH = 1/risk difference, with the 95% CIs of NNT or NNH being the inverse of the upper and lower limits of the 95% CI of the risk difference. We also calculated odds ratios and relative risks. The random-effects model of DerSimonian and Laird13 was used in all cases because they take heterogeneity among studies into account. Study heterogeneity was sought for visual inspection of the forest plots and by using tau2, chi-square, and the Higgins I2 tests. Significance levels of P < .1 were set a priori to assume the presence of heterogeneity. The overall test statistic is given by z = risk difference/SE (risk difference), odds ratio/SE (odds ratio), or relative risk/SE (relative risk), where SE is a standard error. Funnel plots were drawn for all outcome parameters to examine publication bias. Specifically, the effect sizes of the individual studies were plotted against the standard errors of those effect sizes.14 These analyses were also performed in a subgroup of studies that used depot antipsychotics. All the calculations were performed with Review Manager 5,15 a meta-analytic standard software used by The Cochrane Collaboration. We used P values of <.05 and 2-sided 95% CIs (according to whether the CI included the null value) to assess significance.
Results
Included Studies
A total of 13 studies, involving 1395 subjects (739 in standard-dose groups, 457 in low-dose groups, and 199 in very low–dose groups), were included in this meta-analysis (tables 2 and 3).16–28 The reasons for excluding the remaining studies are detailed in figure 1. No additional data were provided by any of the pharmaceutical companies approached by the investigators. We could not locate any relevant unpublished study with another search in the ClinicalTrials.gov or the Food and Drug Administration Web site. Eight and seven studies included low- and very low–dose groups, respectively. Long-acting injectable antipsychotic drugs were included in 1 out of the 4 studies using SGAs, and 7 out of the 9 studies using first-generation antipsychotics (FGAs). The study duration of included studies ranged between 24 and 104 weeks, and a follow-up at 1 year or beyond was performed in all but 2 studies. The mean age of the subjects ranged from 28.3 to 50.1 years. Twelve studies included clinically stable ambulatory patients, and one study included hospitalized patients who presented with stable psychopathology. Funnel plots did not suggest any obvious publication bias for efficacy outcome parameters, whereas the plots on dropouts due to side effects in comparisons between low dose vs standard dose and between very low dose vs standard dose were asymmetrical.
Table 2.
Characteristics of the 13 Included Studies
| Study | Antipsychotic | Standard-Dose Group: Dose (N) | Low-Dose Group: Dose (N) | Very Low–Dose Group: Dose (N) | Study Duration (wks) | Mean Age of Subjects (y) |
| Second generation antipsychotics | ||||||
| Dellva et al16 | Olanzapine (oral) | 5–15 (mean, 11.5) mg/d (N = 48) | — | 1 mg/d (N = 14) | 46 | 37.0 |
| Velligan et al17 | Quetiapine | 600 mg/d (N = 52) | 300 mg/d (N = 41) | — | 24 | 40.7a |
| Arato et al18 | Ziprasidone (oral) | 80 or 160 mg/d (N = 135) | 40 mg/d (N = 72) | — | 52 | 50.1 |
| Simpson et al19 | Risperidone (long-acting) | 50 mg/2 wks (N = 161) | 25 mg/2 wks (N = 162) | — | 52 | 40.9 |
| First generation antipsychotics | ||||||
| Kane et al20 | Fluphenazine decanoate | 12.5–50 mg/2 wks (N = 64) | — | 1.25–5 mg/2 wks (N = 62) | 52 | 28.9 |
| Nishikawa et al21 | Propericyazine | 60 mg/d (N = 12) | 30 mg/d (N = 12) | 10 mg/d (N = 13) | 52 | 39.3 |
| Nishikawa et al22 | Pimozide | 6 mg/d (N = 11) | 2 mg/d (N = 13) | — | 52 | 38.6 |
| Marder et al23 | Fluphenazine decanoate | 25 mg/2 wks (N = 31) | — | 5 mg/2 wks (N = 35) | 104 | 36.5 |
| Hogarty et al24 | Fluphenazine decanoate | Mean, 25 mg/2 wks (N = 33) | — | Mean 3.8 mg/2 wks (N = 37) | 104 | 28.3 |
| Inderbitzin et al25 | Fluphenazine decanoate | Mean, 47.2 mg/4 wks (N = 20)b | Mean, 22.7 mg/4 wks (N = 23)b | — | 52 | 40.9b |
| Huttunen et al26 | Haloperidol decanoate | 150 mg/4 wks (N = 13) | — | 25 mg/4 wks (N = 13) | 104 | <45 |
| Schooler et al27 | Fluphenazine decanoate | 12.5–50 mg/2 wkse (N = 107)c | 2.5–10 mg/2 wksd (N = 106) | — | 104 | 29.6e |
| Kane et al28 | Haloperidol decanoate | 100 or 200 mg/4 wks (N = 52) | 50 mg/4 wks (N = 28) | 25 mg/4 wks (N = 25) | 52 | 38.5 |
Note: BPRS, Brief Psychiatric Rating Scale.
The mean age in 43 completers.
Data from participants who completed all study procedures.
When participants showed prodromal sign of relapse, rescue medication (either oral fluphenazine or fluphenazine decanoate) was added until patients were restabilized. The mean value (ie, the dosage for the first 6-mo period, including rescue medication) was 30.9 mg/2 wks.
When participants showed prodromal sign of relapse, rescue medication (either oral fluphenazine or fluphenazine decanoate) was added until patients were restabilized. The mean value (ie, the dosage for the first 6-mo period, including rescue medication) was 13.3 mg/2 wks.
Data from the entire sample, including targeted dose group.
Table 3.
Summary of Definitions of Remission and Relapse in the 13 Included Studies
| Study | Main Definition of Remission or Partial Remission at Baseline | Definition of Relapse |
| Second generation antipsychotics | ||
| Dellva et al16 | Outpatients who had responded to acute therapy, using the same dose (ie, >40% reduction in the BPRS total score) | Hospitalization for psychopathology |
| Velligan et al17 | Outpatients; BPRS: ≤3 on conceptual disorganization unusual thought content, hallucinatory behavior, and suspiciousness | Not indicated |
| Arato et al18 | Inpatients; CGI-S: ≤5; PANSS: ≤4 on hostility and uncooperativeness | ≥6 on CGI-I or ≥6 on PANSS hostility and/or uncooperativeness |
| Simpson et al19 | Outpatients; no hospitalization for psychopathology within the past 4 mo | Hospitalization for psychopathology, ≥6 on CGI-I, or ≥25% increase in total PANSS score |
| First generation antipsychotics | ||
| Kane et al20 | Outpatients; GAS: ≥35; BPRS: ≤4 on conceptual disorganization and hallucinatory behavior, ≤5 on suspiciousness, and ≤3 on unusual thought content | For patients entering with scores ≥2 below the maximum allowable, an increase in any of the 4 BPRS items in the inclusion criteria to the maximum allowable. For patients entering with scores <2 below the maximum allowable, a ≥2 increase in any of the 4 BPRS items |
| Nishikawa et al21 | Outpatients; “the recovery stage of remission or residual phase” | Clinical worsening on physicians’ judgment |
| Marder et al23 | Outpatients; being stabilized with ≤25 mg of fluphenazine decanoate/2 wks for ≥2 mo | Continuation of a ≥3 increase on either the thought disturbance cluster (conceptual disorganization, hallucination, and unusual thought content) or paranoid cluster (hostility, uncooperativeness, and suspiciousness) in the BPRS, following the dose incrementa,b |
| Hogarty et al24 | Outpatients; the lack of marked influence of hallucinations or delusions on behavior; no evidence of moderate or severe deterioration | Changes from 1 or 2 to 4 or 5 on conceptual disorganization and/or unusual thought content, hallucinations, and delusions in the BPRS |
| Inderbitzin et al25 | Outpatients; receiving ≥20 mg of fluphenazine decanoate/4 wks for ≥2 mo | Any exacerbation requiring hospitalization and/or addition of neuroleptic or anxiolytic |
| Huttunen et al26 | Outpatients or inpatients who were just about to be discharged | Not indicated (the number of patients requiring hospitalization was available) |
| Schooler et al27 | Outpatients; BPRS: ≤4 on conceptual disorganization, grandiosity, hallucinatory behavior, and unusual thought content | ≥4 on conceptual disorganization, grandiosity, hallucinatory behavior, or unusual thought subscale in the BPRS exceeded (the number of relapsed patients was not available) |
| Kane et al28 | Outpatients; BPRS: ≤3 on conceptual disorganization unusual thought content and ≤4 on hallucinatory behavior and suspiciousness | A ≥3-point increase on any of the 4 BPRS items listed in the inclusion criteria |
Note: BPRS, Brief Psychiatric Rating Scale; CGI-S, Clinical Global Impression, severity of illness; CGI-I, Clinical Global Impression, global improvement; PANSS, Positive and Negative Syndrome Scale; GAS, Global Assessment Scale.
When a ≥3 increase on either the thought disturbance cluster (conceptual disorganization, hallucination, and unusual thought content) or paranoid cluster (hostility, uncooperativeness, and suspiciousness) in the BPRS occurred, the dose was increased to 50 mg/2 wks.
When a ≥3 increase on either the thought disturbance cluster (conceptual disorganization, hallucination, and unusual thought content) or paranoid cluster (hostility, uncooperativeness, and suspiciousness) in the BPRS occurred, the dose was increased to 10 mg/2 wks.
Fig. 1.
Flowchart of Literature Search.
Overall Treatment Failure
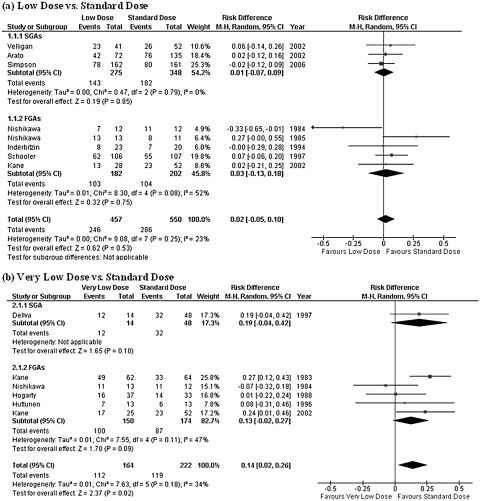
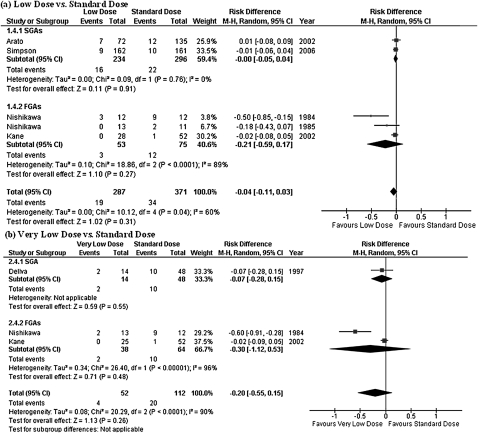
Low-dose group did not show any statistically significant difference in comparison with standard-dose group in terms of the rate of subjects who discontinued their assigned treatment for any reason (figure 2). The upper limit of the 95% CI of risk difference was as low as 0.10. On the other hand, the very low–dose treatment was found to be inferior to the standard-dose therapy in overall treatment failure (NNH = 8, 95% CI = 4–50; figure 2).
Fig. 2.
Risk of Overall Treatment Failure. SGA: second-generation antipsychotic; FGA: first-generation antipsychotic; CI: confidence interval.
Hospitalization
The rate of subjects who needed hospitalization was reported in a total of 9 studies (5 studies each comparing low vs standard doses and very low vs standard doses). While no significant difference was found in subsequent admission to hospital between low-dose and standard-dose therapies, the very low–dose treatment was inferior to the standard-dose therapy (NNH = 9, 95% CI = 6–25; figure 3).
Fig. 3.
Risk of Hospitalization. SGA: second-generation antipsychotic; FGA: first-generation antipsychotic; CI: confidence interval.
Relapse
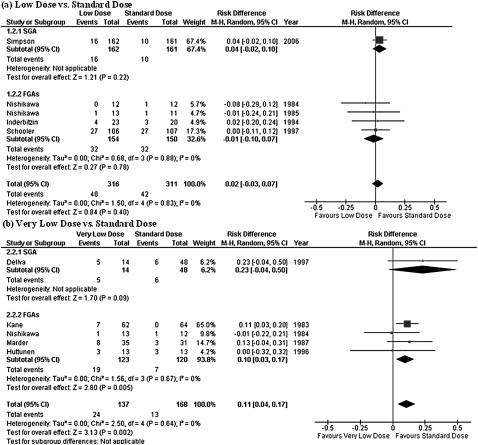
Definitions of relapse differed widely among studies, and 3 studies did not report any data on relapse. Since the rate of subjects who needed hospitalization was available in 2 of those 3 studies, the need for hospitalization was taken as a proxy for relapse in our analysis for these 2 studies. Hence, the rate of subjects who relapsed was available in a total of 12 studies. Similar to the findings in the hospitalization rate, the very low–dose treatment was inferior to the standard-dose therapy (NNH = 4, 95% CI = 3–8). No statistically significant difference was found in risk of relapse between low-dose and standard-dose therapies; however, it should be noted that the P value barely exceeded .05 (figure 4). The results were heterogeneous in the analysis comparing between very low dose vs standard dose (tau2 = 0.02; chi square = 12.1, degrees of freedom [df] = 5, P = .03; I2 = 59%).
Fig. 4.
Risk of Relapse. SGA: second-generation antipsychotic; FGA: first-generation antipsychotic; CI: confidence interval.
Side Effects
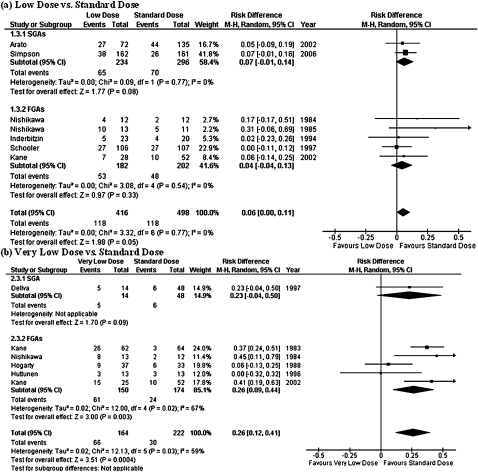
No significant difference was found in the rate of subjects who withdrew from their assigned medication due to side effects between either low dose vs standard dose or very low dose vs standard dose (figure 5). This finding was replicated also when SGAs and FGAs were separately analyzed. The results were heterogeneous in the analyses comparing both between very low dose vs standard dose (tau2 = 0.00; chi square = 10.1, df = 4, P = .04; I2 = 60%) and between low dose vs standard dose (tau2 = 0.08; chi square = 20.3, df = 2, P < .0001; I2 = 90%).
Fig. 5.
Risk of Dropout due to Side Effects. SGA: second-generation antipsychotic; FGA: first-generation antipsychotic; CI: confidence interval.
Relative Risks and Odds Ratios
The presence and lack of statistically significant differences when risk differences were calculated were also observed when relative risks or odds ratios were obtained, respectively, other than the relative risk for dropouts due to side effects between very low–dose and standard-dose therapies (table 4).
Table 4.
Relative Risks and Odds Ratios
| Outcome Parameters | Relative Risk |
Odds Ratio |
||||||
| Ratio | 95% CI | z | P | Ratio | 95% CI | z | P | |
| Low dose vs standard dose | ||||||||
| Overall treatment failure | 1.05 | 0.93–1.17 | 0.78 | .43 | 1.09 | 0.84–1.40 | 0.62 | .53 |
| Hospitalization | 1.12 | 0.77–1.62 | 0.58 | .56 | 1.16 | 0.73–1.85 | 0.65 | .52 |
| Relapse | 1.25 | 1.00–1.55 | 1.98 | .05 | 1.35 | 0.99–1.84 | 1.91 | .06 |
| Dropouts due to side effects | 0.70 | 0.41–1.19 | 1.32 | .19 | 0.60 | 0.26–1.39 | 1.20 | .23 |
| Very low dose vs standard dose | ||||||||
| Overall treatment failure | 1.24 | 1.02–1.52 | 2.12 | .03 | 2.07 | 1.24–3.45 | 2.77 | .006 |
| Hospitalization | 2.21 | 1.16–4.23 | 2.40 | .02 | 2.70 | 1.21–6.02 | 2.42 | .02 |
| Relapse | 2.75 | 1.56–4.84 | 3.50 | .0005 | 4.16 | 1.85–9.38 | 3.44 | .0006 |
| Dropouts due to side effects | 0.38 | 0.15–0.95 | 2.06 | .004 | 0.27 | 0.05–1.42 | 1.54 | .12 |
CI: confidence interval.
Low Dose vs Very Low Dose
A total of 2 studies, involving 78 subjects (40 in low-dose groups and 38 in very low–dose groups), included low-dose and very low–dose treatment arms (tables 1 and 2).21,28 Despite a small number of included studies, we found that the very low–dose treatment was inferior to the low-dose therapy in overall treatment failure and relapse (NNH = 5, 95% CI = 3–33; NNH = 5, 95% CI = 3–50, respectively; figure 6).
Fig. 6.
Comparison of Low Dose and Very Low Dose. CI: confidence of interval.
Depot Antipsychotics
A total of 8 studies, involving 1395 subjects (481 in standard-dose groups, 319 in low-dose groups, and 172 in very low–dose groups), used depot antipsychotics (tables 2 and 3).19,20,23–28 Results of this subgroup analysis were similar to those that were demonstrated in the whole data set. Low-dose group did not show any statistically significant difference in comparison with standard-dose group in terms of any of the outcome measures, including the rate of subjects who discontinued their assigned treatment for any reason (Supplementary Figures 1–4). On the other hand, the very low–dose treatment was found to be inferior to the standard-dose therapy in overall treatment failure, hospitalization, and relapse (NNH = 6, 95% CI = 4–20; NNH = 10, 95% CI = 6–25; NNH = 5, 95% CI = 3–25, respectively; Supplementary Figures 1–4).
Dose Group Classification According to the PORT Treatment Recommendations
Out of the 13 studies identified through the literature search, 7 studies were excluded for the following reasons: (1) recommended dose range was not available for studied antipsychotics—3 studies: Simpson et al,19 Nishikawa et al,21 and Nishikawa22; (2) all tested doses were within the PORT-recommended dose range—3 studies: Velligan et al,17 Inderbitzin et al,25 and Schooler et al27; and (3) since a mean dose was not specified, it was not possible to group the treatment arm (eg, fluphenazine decanoate 1.25–5 mg/2 wks) to either low- or very low–dose group—1 study: Kane et al.20 Thus, the remaining 6 studies16,18,23,24,26,28 were included (Supplementary Table 1). Low-dose group did not show any statistically significant difference in comparison with standard-dose group in any of the outcome measures, including the rate of subjects who discontinued their assigned treatment for any reason (Supplementary Figures 5–8). Similarly, no significant difference was found between very low– or low- and standard-dose treatments (Supplementary Figure 9).
Discussion
To our knowledge, this is the first meta-analysis of the long-term efficacy of very low, low, and standard antipsychotic dose therapies for schizophrenia. The systematic review revealed a lack of sufficient clinical trial data to draw firm conclusions on the maintenance of antipsychotic dose in schizophrenia and schizoaffective disorder. This notwithstanding, the analyses yield 2 important findings: (a) low-dose therapy may be as effective as standard-dose therapy in terms of efficacy, and (b) less than half the standard dose is likely to be associated with the increased risk of treatment failure. This is in contrast to the contention that the dose needed for relapse prevention should be the same as that for the acute-phase treatment.10 The study has several limitations, which we discuss below, but, notwithstanding these limitations, our findings provide an opportunity to reconsider an effective psychopharmacological treatment strategy for relapse prevention in clinically stable patients with schizophrenia or schizoaffective disorder.
Overall treatment failure due to any reason has recently been suggested to be a clinically pragmatic treatment outcome.29 A decision to continue or stop medication reflects the combined evaluation of the efficacy and safety of the treatment by the patient and clinician.29 For this reason, this outcome measure has extensively been used in many pivotal clinical trials, including Clinical Antipsychotic Trials of Intervention Effectiveness.30 Another relevant and explicit index may be hospitalization that patients needed. Although demonstrating a noninferiority of the treatment of interest to a standard treatment is often associated with difficulties,31 the finding that the upper limit of 95% CI of risk difference for overall treatment failure and hospitalization were as low as 0.10 and 0.07, respectively, may be suggestive of a comparable efficacy between low- and standard-dose treatments. On the other hand, our analysis showed that very low dose was associated with worse outcomes in terms of those 2 outcome measures as compared with standard dose and clearly do not support the use of less than half the standard dose for relapse prevention. However, clinical conditions that result in treatment failure or require hospitalization are likely to widely differ among treatment or research settings. It would have been ideal to compare changes in psychopathology assessed with a rating scale; however, the detailed data were not available in a majority of studies that were included in this meta-analysis. These facts highlight an urgent need of well-designed prospective studies in schizophrenia to further address the need to balance drug exposure and relapse prevention.
The treatment strategy for the maintenance phase of schizophrenia is still under debate; indeed, polar recommendations are issued by some currently published treatment guidelines. While the practice guidelines by the APA9 and the Schizophrenia PORT Treatment Recommendations11 support the low-dose treatment to minimize antipsychotic side effects, the Expert Consensus Guidelines generally recommend the use of the antipsychotic dose that was needed for acute-phase treatment to lower the risk of relapse.10 These inconsistent recommendations may be in part due to a difference in timing of publications; however, interestingly, even in the Expert Consensus Guidelines, the recommendations by experts were split, indicating an absence of consensus on this issue in the field. Although both treatment strategies seem to have rationales, maintaining acute doses is a strategy that seems to be heavily based on the results of previous trials that compared very low dose with standard dose.28,32 In fact, the trials conducted in the 1980s, including a very low dose (eg, fluphenazine decanoate ≤5 mg/2 wks),20,23,24 have been often cited to support this contention. The only and first meta-analysis on the maintenance dose of antipsychotics32 before the present article was also substantially influenced by those trials, which in turn led to a conclusion that standard-dose treatment was more efficacious than “low”-dose therapy. Thus, it is this lack of distinction between low and very low doses in the earlier studies that led to the discrepant conclusions.
In the present study, we did not find any statistically significant difference in the rate of subjects who discontinued their assigned treatment due to side effects among dosage groups. However, a variety of side effects, including motor and cardiac side effects, have been reported to occur in a dose-dependent fashion.2,4,5 An association between a greater degree of exposure to antipsychotic drugs and a higher risk for sudden cardiac death has also been confirmed for both typical and atypical antipsychotic drugs.4 Furthermore, a higher dose of several antipsychotics results in a greater prolactin level elevation,33 which is suggested to be associated with problematic adverse effects, including menstrual disturbances. In addition, somnolence has been reported to occur more frequently with standard-dose therapy (20 and 24 mg/d of sertindole; 8 and 16 mg/d of haloperidol) than low-dose treatment (12 mg/d of sertindole; 4 mg/d of haloperidol), respectively.34 Given these dose-dependent side effects of antipsychotics, a moderately low dose may be the optimal target in clinical practice in order to balance the desired maintenance of therapeutic benefits and the dose-dependent adverse effects. All the studies included in this meta-analysis were not specifically designed to evaluate dose effects of antipsychotic drugs on side effects, and the lack of detailed information on side effects in those trials limits the interpretation of our results. Given the lack of significant finding in this meta-analysis, this potential dose effect on side effects that has been reported in the literature has to be confirmed in future investigations.
Several limitations qualify our conclusions. First, the number of studies included in the present meta-analysis was relatively small, especially for SGAs. Although a subgroup of 4 trials, using SGAs, demonstrated similar results to those that were found in FGA studies, a relative underestimation of studies on SGAs should be acknowledged and warrants further investigations. Second, study designs, including selection criteria, definition of stable psychopathology at baseline, outcome measures, and studied antipsychotic drugs, differ among individual trials, although this is not a unique limitation to our meta-analysis. However, the use of DDD and common outcome measures, such as overall treatment failure and hospitalization instead of “relapse,” are expected to counteract this limitation to some extent. In fact, the DDD system has been developed as an international standard for drug utilization studies, and 1 DDD of each drug has a robust rationale. The fact that we found similar findings when using PORT-recommended dosage ranges support our findings with DDD. However, given a very small number of included studies, the lack of statistically significant difference in efficacy between dose groups may be due to a type-II error, which warrants further well-designed prospective clinical trials on the maintenance antipsychotic treatment for schizophrenia. Third, the low dose and very low dose were conveniently defined, using a cut-off point of 0.5 DDD. However, this categorical distinction between “very low” and “low” suggests a discrete dose threshold that divides them, while it is more likely that the continuous nature of dose-response may necessitate a more gradual dose adjustment. For example, 0.5 DDD may not be the same as 0.9 DDD in terms of therapeutic or side effects, although they would be sorted into the same low-dose group. In fact, a treatment arm with 25 mg/2 wks of long-acting risperidone was included in the low-dose group though its DDD was very close to 1.0. This fact has to be acknowledged, especially when applying the findings of this study to daily clinical practice.
In conclusion, we report on the systematic review and meta-analysis of dose effects of antipsychotics for relapse prevention in schizophrenia. The currently available evidence suggests that the efficacy of low- and standard-dose antipsychotic medication may be comparable in preventing relapse in schizophrenia and schizoaffective disorders, and that less than half the standard dose may increase the risk of treatment failure. However, these results have to be interpreted in light of a small number of relevant studies in the literature. We acknowledge individual differences in patient's response to antipsychotics on various pharmacokinetic and pharmacodynamic levels,35–37 precluding our ability to propose a uniformed treatment approach for relapse prevention.38 Still, the intriguing observations from this meta-analysis have important implications for the dosing of antipsychotics in patients with schizophrenia and urge the field to perform further well-designed prospective clinical trials to address this controversial dosing issue.
Supplementary Material
Supplementary Figures 1–9 and Table 1 are available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We would like to thank Dr Nishikawa for kindly providing us with unpublished data. No funding support was obtained for the present work. Previous presentation: Some of these data were presented at the 22nd European College of Neuropsychopharmacology, Istanbul, September 14, 2009. Conflict of Interest: Dr H.U. has received grants, speaker's honoraria, or manuscript fees from Pfizer Health Research Foundation, GlaxoSmithKline, Otsuka, Dainippon Sumitomo Pharma, Janssen Pharmaceutical, and Pfizer. Dr T.S.’s fellowship has been supported by the Japanese Society of Clinical Psychopharmacology, Government of Canada Post-Doctoral Research Fellowships, and Kanae Foundation. Dr D.C.M. has received grants or consultant fees from the Bristol-Myers Squibb and Pfizer and has received speaker's honoraria from AstraZeneca. Other authors have nothing to disclose.
References
- 1.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Lemmens P, Brecher M, Van Baelen B. A combined analysis of double-blind studies with risperidone vs. placebo and other antipsychotic agents: factors associated with extrapyramidal symptoms. Acta Psychiatr Scand. 1999;99:160–170. doi: 10.1111/j.1600-0447.1999.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 3.Nasrallah H. A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology. 2003;28(suppl 1):83–96. doi: 10.1016/s0306-4530(02)00114-2. [DOI] [PubMed] [Google Scholar]
- 4.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. New Engl J Med. 2009;360:225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeste DV, Caligiuri MP, Paulsen JS, et al. Risk of tardive dyskinesia in older patients. A prospective longitudinal study of 266 outpatients. Arch Gen Psychiatry. 1995;52:756–765. doi: 10.1001/archpsyc.1995.03950210050010. [DOI] [PubMed] [Google Scholar]
- 6.Rettenbacher MA, Hofer A, Eder U, et al. Compliance in schizophrenia: psychopathology, side effects, and patients’ attitudes toward the illness and medication. J Clin Psychiatry. 2004;65:1211–1218. doi: 10.4088/jcp.v65n0908. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC, Posever T, Herz L, et al. An exploratory haloperidol-controlled dose-finding study of ziprasidone in hospitalized patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 1998;18:296–304. doi: 10.1097/00004714-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Beasley CM, Dellva MA, Tamura RN, et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry. 1999;174:23–30. doi: 10.1192/bjp.174.1.23. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Practice Guideline for the Treatment of Patients with Schizophrenia. 2nd ed. American Psychiatric Association. Am J Psychiatry. 2004; 161(Suppl. 2): 1–56. [Google Scholar]
- 10.Kane JM, Leucht S, Carpenter D, Docherty JP. Expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64(suppl 12):5–19. [PubMed] [Google Scholar]
- 11.Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull. 1998;24:1–10. doi: 10.1093/oxfordjournals.schbul.a033302. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Collaborating Centre for Drug Statistics Methodology. 2009. Available at: http://www.whocc.no/atcddd/. Accessed Febraury 25, 2009. [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 15.Version 5.0. Copenhagen: The Cochrane Collaboration; 2008. Review Manager (RevMan) [computer program] [Google Scholar]
- 16.Dellva MA, Tran P, Tollefson GD, Wentley AL, Beasley CM., Jr Standard olanzapine versus placebo and ineffective-dose olanzapine in the maintenance treatment of schizophrenia. Psychiatr Serv. 1997;48:1571–1577. doi: 10.1176/ps.48.12.1571. [DOI] [PubMed] [Google Scholar]
- 17.Velligan DI, Newcomer J, Pultz J, et al. Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res. 2002;53:239–248. doi: 10.1016/s0920-9964(01)00268-7. [DOI] [PubMed] [Google Scholar]
- 18.Arato M, O'Connor R, Meltzer HY. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone extended use in schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17:207–215. doi: 10.1097/00004850-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Simpson GM, Mahmoud RA, Lasser RA, et al. A 1-year double-blind study of 2 doses of long-acting risperidone in stable patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2006;67:1194–1203. doi: 10.4088/jcp.v67n0804. [DOI] [PubMed] [Google Scholar]
- 20.Kane JM, Rifkin A, Woerner M, et al. Low-dose neuroleptic treatment of outpatient schizophrenics. I. Preliminary results for relapse rates. Arch Gen Psychiatry. 1983;40:893–896. doi: 10.1001/archpsyc.1983.01790070083010. [DOI] [PubMed] [Google Scholar]
- 21.Nishikawa T, Tsuda A, Tanaka M, Hoaki Y, Koga I, Uchida Y. Prophylactic effect of neuroleptics in symptom-free schizophrenics: a comparative dose-response study of haloperidol and propericiazine. Psychopharmacology (Berl) 1984;82:153–156. doi: 10.1007/BF00427763. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa T, Tsuda A, Tanaka M, Koga I, Uchida Y. Prophylactic effects of neuroleptics in symptom-free schizophrenics: roles of dopaminergic and noradrenergic blockers. Biol Psychiatry. 1985;20:1161–1166. doi: 10.1016/0006-3223(85)90174-x. [DOI] [PubMed] [Google Scholar]
- 23.Marder SR, Van Putten T, Mintz J, Lebell M, McKenzie J, May PR. Low- and conventional-dose maintenance therapy with fluphenazine decanoate. Two-year outcome. Arch Gen Psychiatry. 1987;44:518–521. doi: 10.1001/archpsyc.1987.01800180028005. [DOI] [PubMed] [Google Scholar]
- 24.Hogarty GE, McEvoy JP, Munetz M, et al. Dose of fluphenazine, familial expressed emotion, and outcome in schizophrenia. Results of a two-year controlled study. Arch Gen Psychiatry. 1988;45:797–805. doi: 10.1001/archpsyc.1988.01800330021002. [DOI] [PubMed] [Google Scholar]
- 25.Inderbitzin LB, Lewine RR, Scheller-Gilkey G, et al. A double-blind dose-reduction trial of fluphenazine decanoate for chronic, unstable schizophrenic patients. Am J Psychiatry. 1994;151:1753–1759. doi: 10.1176/ajp.151.12.1753. [DOI] [PubMed] [Google Scholar]
- 26.Huttunen MO, Tuhkanen H, Haavisto E, et al. Low- and standard-dose depot haloperidol combined with targeted oral neuroleptics. Psychiatr Serv. 1996;47:83–85. doi: 10.1176/ps.47.1.83. [DOI] [PubMed] [Google Scholar]
- 27.Schooler NR, Keith SJ, Severe JB, et al. Relapse and rehospitalization during maintenance treatment of schizophrenia. The effects of dose reduction and family treatment. Arch Gen Psychiatry. 1997;54:453–463. doi: 10.1001/archpsyc.1997.01830170079011. [DOI] [PubMed] [Google Scholar]
- 28.Kane JM, Davis JM, Schooler N, et al. A multidose study of haloperidol decanoate in the maintenance treatment of schizophrenia. Am J Psychiatry. 2002;159:554–560. doi: 10.1176/appi.ajp.159.4.554. [DOI] [PubMed] [Google Scholar]
- 29.Swartz MS, Perkins DO, Stroup TS, McEvoy JP, Nieri JM, Haak DC. Assessing clinical and functional outcomes in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial. Schizophr Bull. 2003;29:33–43. doi: 10.1093/oxfordjournals.schbul.a006989. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 31.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295:1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 32.Barbui C, Saraceno B, Liberati A, Garattini S. Low-dose neuroleptic therapy and relapse in schizophrenia: meta-analysis of randomized controlled trials. Eur Psychiatry. 1996;11:306–313. doi: 10.1016/S0924-9338(96)89899-3. [DOI] [PubMed] [Google Scholar]
- 33.Volavka J, Czobor P, Cooper TB, et al. Prolactin levels in schizophrenia and schizoaffective disorder patients treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychiatry. 2004;65:57–61. doi: 10.4088/jcp.v65n0109. [DOI] [PubMed] [Google Scholar]
- 34.Zimbroff DL, Kane JM, Tamminga CA, et al. Controlled, dose-response study of sertindole and haloperidol in the treatment of schizophrenia. Sertindole Study Group. Am J Psychiatry. 1997;154:782–791. doi: 10.1176/ajp.154.6.782. [DOI] [PubMed] [Google Scholar]
- 35.Uchida H, Mamo DC, Mulsant BH, Pollock BG, Kapur S. Increased antipsychotic sensitivity in elderly patients: evidence and mechanisms. J Clin Psychiatry. 2009;70:397–405. doi: 10.4088/jcp.08r04171. [DOI] [PubMed] [Google Scholar]
- 36.Uchida H, Kapur S, Mulsant BH, Graff-Guerrero A, Pollock BG, Mamo DC. Sensitivity of older patients to antipsychotic motor side effects: a PET study examining potential mechanisms. Am J Geriatr Psychiatry. 2009;17:255–263. doi: 10.1097/JGP.0b013e318198776d. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen HB, Timm S, Wang AG, et al. Association between the CCR5 32-bp deletion allele and late onset of schizophrenia. Am J Psychiatry. 2006;163:507–511. doi: 10.1176/appi.ajp.163.3.507. [DOI] [PubMed] [Google Scholar]
- 38.Uchida H, Pollock BG, Bies RR, Mamo DC. Predicting age-specific dosing of antipsychotics. Clin Pharmacol Ther. 2009;86:360–362. doi: 10.1038/clpt.2009.133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.