Abstract
Background: Similar to patients with other chronic disorders, patients with serious mental illness (SMI) are often poorly adherent with prescribed medications. Objective: We conducted a randomized controlled trial examining the effectiveness of a pharmacy-based intervention (Meds-Help) in increasing antipsychotic medication adherence among Department of Veterans Affairs (VA) patients with SMI. We also examined the impact of Meds-Help on psychiatric symptoms, quality of life, and satisfaction with care. Methods: We enrolled 118 patients from 4 VA facilities with schizophrenia, schizoaffective, or bipolar disorder who were on long-term antipsychotics but had antipsychotic medication possession ratios (MPRs) <0.8 in the prior year. Patients were randomized to usual care (UC; n = 60) or the pharmacy-based intervention (Meds-Help; n = 58). We reassessed adherence at 6 and 12 months, at which time patients completed Positive and Negative Symptom Scales (PANSS), Quality of Well-being Scales (QWB), and Client Satisfaction Questionnaires (CSQ-8). Results: Prior to enrollment, Meds-Help and UC patients had mean antipsychotic MPRs of 0.54 and 0.55, respectively. At 6 months, mean MPRs were 0.91 for Meds-Help and 0.64 for UC patients; at 12 months, they were 0.86 for Meds-Help and 0.62 for UC patients. In multivariate analyses adjusting for patient factors, Meds-Help patients had significantly higher MPRs at 6 and 12 months (P < .0001). There were no significant differences between groups in PANSS, QWB, or CSQ-8 scores, but power to detect small effects was limited. Conclusions: Congruent with prior studies of patients with other disorders, a practical pharmacy-based intervention increased antipsychotic adherence among patients with SMI. However, SMI patients may require additional care management components to improve outcomes.
Keywords: adherence, antipsychotic medications, health services
Introduction
The long-term use of antipsychotic medications is an essential treatment component for many patients with serious mental illness (SMI).1 Unfortunately, similar to patients with other chronic disorders, patients with SMI are often only partially adherent with prescribed medications. In a systematic review of 39 studies that assessed adherence using a variety of methods, approximately 40% of patients with schizophrenia were partially or nonadherent with antipsychotic medications.2 Fully 40% of Department of Veterans Affairs (VA) patients with schizophrenia obtain less than 80% of the medication supplies needed to take their antipsychotic doses continuously.3 Similarly, almost half of VA patients with bipolar disorder obtain less than 80% of supplies needed for continuous use of either antipsychotics or mood stabilizers.4,5 Systematic approaches to improving adherence are needed.
Because partial adherence is a multidetermined phenomenon, there are many potential targets for adherence-enhancing interventions.2 Depot antipsychotic medications eliminate the need for daily ingestion of oral antipsychotics and may increase adherence,6 but depot medications have not been widely adopted in the United States, potentially because of concerns about pain and coerciveness.7 Psychoeducation programs have been studied, with researchers finding that simple didactic approaches are not as successful in improving adherence as are individualized, multicomponent, behavioral, and family interventions.8–11 However, more complex interventions are often difficult to deliver, requiring specialized therapist training or frequent patient or family visits, and these approaches have not been widely disseminated.
Based on studies suggesting that concrete problem-solving approaches accompanied by “technical” aids may be helpful in improving adherence among patients with chronic medical conditions,9–14 we developed a low-complexity pharmacy-based intervention for patients with SMI. This intervention was informed by the Health Belief Model and designed to reduce medication access barriers and to provide “cues to action” to help patients remember to refill prescriptions and take scheduled doses.15
The primary aim of this study was to assess the effectiveness of this pharmacy-based intervention, Meds-Help, in improving antipsychotic adherence among patients with SMI. Our secondary aims were to examine the effects of the intervention on patients’ psychiatric symptoms, quality of life, and satisfaction with health services.
Methods
The study was registered at ClinicalTrials.gov (Identifier: NCT00057135), approved by Human Subjects Committees at each of the 4 participating study sites, and involved human participants between November 18, 2002, and September 30, 2005. Study data and findings are reported in accordance with the Consolidated Standards of Reporting Trials.16–18
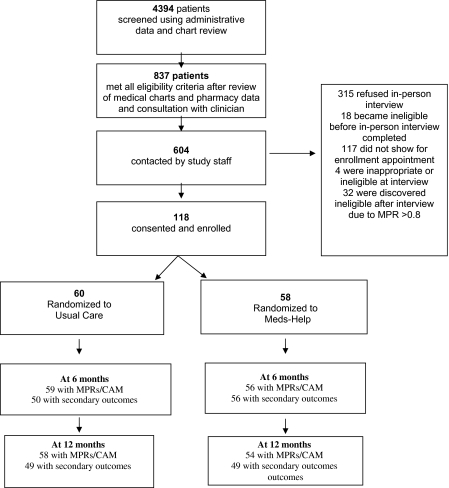
Figure 1 outlines the recruitment of study participants. Using VA administrative data, we identified 4394 patients prescribed antipsychotic medications with ≥2 outpatient mental health visits in the prior 12 months. Research staff reviewed patients’ chart notes, pharmacy data, and consulted with their psychiatrists to confirm that patients met other eligibility criteria, including having clinical diagnoses of schizophrenia, schizoaffective, or bipolar disorder; a treatment plan that included long-term antipsychotic treatment, antipsychotic medication possession ratios (MPRs) of <0.8 in the prior 12 months; and no clinical contraindications to study participation. Clinical rather than semi-structured research diagnoses were used as entry criteria, as clinical diagnoses are more likely to be used in any clinical implementation. For patients on more than one antipsychotic, the weighted average antipsychotic MPR during the prior 12 months had to be <0.8.
Fig. 1.
Patient Enrollment.
Patients were excluded if they: no longer appeared to be in active VA care (eg, no visits in the prior 6 months and no visits scheduled or chart notes indicating a transfer of care), were receiving antipsychotic medications outside the VA system, or had a life expectancy of less than a year. Patients were also excluded if they were receiving “stronger interventions” to promote adherence (eg, the VA adaptation of Assertive Community Treatment, staff administration of medications, depot antipsychotics, or clozapine with its attendant close supervision). Living situation was not a specific inclusion/exclusion criterion, as long as paid staff did not assist patients in managing their medications.
Because of Institution Review Board requirements, study staff stopped reviewing charts as soon as any exclusion criterion was noted; 837 patients were identified as potentially eligible for participation. These patients were sent letters informing them about the study and were subsequently contacted by telephone. Patients who were interested in participation after a scripted telephone discussion were invited to an in-person meeting to complete an informed consent process; 154 patients attended this in-person meeting.
During the informed consent process, visual aids were used to explain the key elements of the study, and standardized queries were made to ensure that patients understood these elements. If patients had guardians, their guardians were informed about the study and consent forms mailed. Four patients were excluded at this time because they failed to understand key study elements or did not have a mailing address or telephone access. An additional 32 patients were excluded because their most recent MPR exceeded 0.8.
In all, 118 patients were randomized to UC alone or UC plus Meds-Help. Randomization was completed centrally at the coordinating site, using a blocked randomization scheme by site based on the patient's level of adherence in the prior 12 months (MPR of <0.4, 0.4–0.59, or 0.6–0.79) and the presence of concurrent substance use. Substance use was considered “present” if any patient chart note in the prior 12 months indicated difficulties with drugs or alcohol or if the patient reported that in the prior 6 months he/she had used any illicit drugs, had an episode of binge drinking, or consumed ≥7 drinks per week (for women) or ≥14 drinks per week (for men). Site research associates (RAs) called into the central research coordinator to receive newly enrolling patients’ treatment assignment.
UC consisted primarily of treatment in VA outpatient mental health clinics and included psychiatrist visits, non-MD mental health visits, and group visits. During the study period, patients completed an average of 8 visits with psychiatrists; 49% had visits with non-MD mental health clinicians and 23% had group visits.
The Meds-Help intervention consisted of (1) unit-of-use packaging that included all patients’ medications for psychiatric and general medical conditions (figure 2), (2) a medication and packaging education session, (3) refill reminders mailed 2 weeks before scheduled refill dates, and (4) notification of clinicians when patients failed to fill antipsychotic prescriptions within 7–10 days of a fill date. Meds-Help staff served as contacts for patient questions regarding pharmacy services or doctors’ prescriptions. Pharmacy technicians, with oversight by pharmacists, completed many of these intervention components.
Fig. 2.
Unit-of-Use Packaging.
The medication education session was conducted by a pharmacist, usually in-person, but occasionally by telephone. During this session, the pharmacist reviewed patients’ prescribed medications, including treatment indications. The pharmacist also explained unit-of-use medication packaging (eg, package usage and warning labels, lack of child proofing) and plans for interim use of pill boxes when medication changes were made by clinicians before the next shipment of medication packages.
Measures
The primary outcome measure was medication adherence as measured by the MPR. A more stringent “composite adherence measure” (CAM) was also assessed.
The MPR is the ratio of the number of outpatient days’ supply of medication that a patient has received during the designated time period divided by the number of days’ supply they needed to receive to take their prescribed dose of antipsychotic continuously during noninstutionalized days. The MPR was the primary outcome measure because it is based on data (pharmacy fills) that can be collected unobtrusively, making it less likely to affect adherence behaviors; it can be calculated for patients even if they stop actively participating in the study; and it is associated with important outcomes in observational studies.3,19
MPRs were calculated for 3 periods: the 12 months prior to enrollment (baseline), 0–6 months following enrollment, and 6–12 months following enrollment, unless participants had fewer than 90 noninstitutionalized days in a specified time period because of death, transfer of care outside the VA, or long-term inpatient stays. If depot antipsychotic medications were instituted after enrollment, we did not calculate MPRs after the switch.
Because patients may fill prescriptions but not ingest their antipsychotic medications, the more stringent CAM was based on multiple data sources. Patients were considered adherent on the CAM only if: (1) their MPR during the study time periods was ≥0.8, (2) they reported they “always” took their antipsychotics or only missed antipsychotics “a couple of times” in response to questions from Schizophrenia Outcomes Module, and (3) their blood test indicated the presence of some antipsychotic medication. The rationale for requiring patients to meet all 3 criteria is that an indication of poor adherence on any of these measures is likely associated with less-than-optimal adherence. Our a priori research plan was to consider blood levels to be negative when patients refused blood draws or did not show for follow-up assessments. If a physician discontinued antipsychotic medication because a patient refused it, blood levels were not drawn and assumed to be negative; if a physician discontinued antipsychotics because the medications were no longer considered necessary, the CAM was assessed only to the point of discontinuation.
Secondary outcomes were psychiatric symptoms as measured by the Positive and Negative Symptom Scale (PANSS), quality of life as measured by the Quality of Well-Being Scale (QWB), and patient satisfaction as measured by Client Satisfaction Questionnaire (CSQ-8) scale. The PANSS and QWB were completed by the research associates at each of the study sites. All these scales are widely used and have demonstrated reliability and validity.20–24 Exploratory outcomes included VA psychiatric hospitalizations, ascertained through chart reviews.
As with many health services interventions, patients could not be blinded to study assignment. Research associates were also not blinded due to the costs and logistics of hiring blinded assessors for each site and the likelihood that assessors would be “unblinded” by patient comments. However, the primary outcomes of adherence were based on longer term pharmacy fill patterns and less likely to be affected by subtle biases on the part of patients or interviewers.
Prior to enrolling patients, RAs received training in PANSS administration and scoring using standardized videotapes. RAs also received periodic training during the study, using tapes of study patients. For the total PANSS score, the inter-rater reliability, as assessed by an intraclass correlation coefficient (ICC), was 0.94 for the first 2 RAs enrolling patients; at a second time point, the ICC was 0.77 for 4 study RAs, and additional training was provided. At a third time point, the ICC for the 4 RAs was 0.93. In-person follow-up assessments were conducted in a private room at the patient's local VA facility.
Data Analyses
Analyses were conducted on an intent-to-treat basis. We describe means (±SD) for the continuous MPR measure and frequencies for the CAM categories (fully adherent/poorly adherent) at baseline, 6, and 12 months. The primary outcome of MPR was examined at 6 and 12 months using multiple linear regression; the CAM was evaluated as these time points using logistic regression analyses. Multivariable analyses included the baseline MPR as a covariate to adjust for levels of adherence prior to enrollment. We also adjusted for potential confounding variables of race, age, psychiatric diagnosis, substance use, and study site. Analyses for secondary outcomes of psychiatric symptoms, quality of well-being, and satisfaction were conducted similarly, with multiple linear regression analyses that adjusted for race, age, psychiatric diagnosis, and study site. The study was powered to detect a medium-sized effect in the primary outcome of MPR.
Results
Patient Characteristics
As presented in table 1, mean patient age was 49.9 (SD ± 11.3) years; 97% were men. Sixty patients (51%) were nonwhite (53 were black, 4 Hispanic, and 3 were Asian); 67% had a diagnosis of schizophrenia or schizoaffective disorder and 33% had a diagnosis of bipolar disorder. Approximately 31% had problematic substance use. Study groups were balanced on all factors, although the difference between groups in the percentages of patients with a bipolar diagnosis bordered on statistical significance.
Table 1.
Patient Characteristics (N = 118)
| All |
Control |
Intervention |
P Value | ||||
| N or Mean | % or SD | N or Mean | % or SD | N or Mean | % or SD | ||
| Mean age (mean, SD) | 49.9 | 11.3 | 50.2 | 11.7 | 49.6 | 11.0 | .78 |
| Women (N, %) | 4 | 3.4 | 3 | 5.0 | 1 | 1.7 | .33 |
| Nonwhite (N, %) | 60 | 50.8 | 34 | 56.7 | 26 | 44.8 | .20 |
| Diagnosis | .06 | ||||||
| Schizophrenia (N, %) | 79 | 66.9 | 45 | 75.0 | 34 | 58.6 | |
| Bipolar (N, %) | 39 | (33.1) | 15 | 25.0 | 24 | 41.4 | |
| Substance use (N, %) | 36 | (30.5) | 19 | 31.7 | 17 | 29.3 | 0.78 |
| Site | 0.60 | ||||||
| A (N, %) | 34 | (28.8) | 20 | 33.3 | 14 | 24.1 | |
| B (N, %) | 26 | (22.0) | 14 | 23.3 | 12 | 20.7 | |
| C (N, %) | 21 | (17.8) | 9 | 15.0 | 12 | 20.7 | |
| D (N, %) | 37 | (31.4) | 17 | 28.3 | 20 | 34.5 | |
Patients were receiving a variety of antipsychotic medications at the time of enrollment with no significant differences between groups regarding the agents prescribed. Olanzapine and risperidone were the most frequently prescribed; 35% of intervention and 33% of UC patients were receiving olanzapine and 42% of intervention and 44% of UC patients were receiving risperidone. At the time of enrollment, most patients (64%) lived with a partner or family member, 6% lived with friends, 2% lived in group homes, and 28% lived alone. There were no significant differences between groups in reported living situation.
Enrolled patient attitudes’ toward medications as measured by items on the Ratings of Medication Influence Scale were similar to those previously reported for a population of 307 patients with schizophrenia25; 84% reported that reasons for a “willingness to take their medications” included mild or strong beliefs that the medication made them feel better, whereas 45% reported that reasons for being reluctant to take their medication included distressing side effects.
Study Retention
Because the primary outcome, MPRs, could be calculated using administrative pharmacy data, we were able to ascertain this outcome for 56 (97%) of the Meds-Help and 59 (98%) of UC patients at 6 months and for 54 (93%) of Meds-Help and 58 (97%) of UC patients at 12 months. Because MPRs and the CAM were not calculated for patients who did not have ≥90 noninstitutionalized days in a follow-up period, MPRs could not calculated at 6 months for 2 intervention patients and one control patient and could not be calculated at 12 months for 4 intervention and 2 control patients due to deaths, long-term hospitalizations, withdrawal of consent, or switch to depot medications.
Unlike adherence measures, the secondary outcomes of the PANSS, QWB, and CSQ-8 required that patients complete in-person interviews within the appropriate time frame. These outcomes were calculated for 56 (97%) of intervention patients and 50 (83%) of UC patients at 6 months and for 49 (84%) of intervention patients and 49 (82%) of UC patients at 12 months. Patients missed in-person interviews for a variety of reasons including the deaths noted above, no shows or interview refusals, loss to follow-up, geographic moves, or jail time.
Acceptability and Completion of the Intervention
The intervention was acceptable, with only 7% of intervention patients requesting discontinuation of Meds-Help. However, an additional 19% did not continue the intervention for the full 12 months for other reasons, including death, geographic moves, long-term hospitalizations, transferring care outside the VA, switch to depot antipsychotics, or physician discontinuation of regularly dosed antipsychotics. Thus, 74% of the intervention patients could be considered “completers” of the full 12-month intervention.
Effect of the Intervention on Adherence
Tables 2–4 present the outcomes of our intent-to-treat analyses. Table 2 presents the unadjusted estimates of patient outcomes while tables 3 and 4 presents estimates that are adjusted for patient characteristics and study site.
Table 2.
Adherence and Other Study Measures at Baseline, 6, and 12 Months
| Baseline |
P Value | 6 Months |
Bivariate P Value | 12 Months |
P Value | |||||||
| N | # or Mean | % or SD | N | # or mean | % or SD | N | # or mean | % or SD | ||||
| MPR (mean, SD) | ||||||||||||
| Intervention | 58 | 0.54 | 0.20 | .7676 | 56 | 0.91 | 0.23 | <.0001 | 54 | 0.86 | 0.30 | <.0001 |
| Control | 60 | 0.55 | 0.19 | 59 | 0.64 | 0.33 | 58 | 0.62 | 0.33 | |||
| CAM criteria (number, %) | ||||||||||||
| Intervention | 58 | 0 | 0 | NA | 52 | 26 | 50.0 | .0003 | 50 | 17 | 34.0 | .06 |
| Control | 60 | 0 | 0 | 53 | 9 | 17.0 | 51 | 9 | 17.7 | |||
| PANSS positive (mean, SD) | ||||||||||||
| Intervention | 58 | 15.0 | 5.4 | .3101 | 56 | 13.5 | 4.9 | .84 | 49 | 13.0 | 4.7 | .16 |
| Control | 60 | 16.4 | 6.0 | 50 | 15.6 | 6.1 | 49 | 14.6 | 5.3 | |||
| PANSS total (mean, SD) | ||||||||||||
| Intervention | 58 | 62.3 | 16.3 | .492 | 56 | 58.8 | 15.0 | .29 | 49 | 55.3 | 14.3 | .46 |
| Control | 60 | 64.6 | 15.0 | 50 | 61.7 | 14.5 | 49 | 57.1 | 12.4 | |||
| QWB (mean, SD) | ||||||||||||
| Intervention | 58 | 0.59 | 0.09 | .0737 | 56 | 0.58 | 0.09 | .27 | 47 | 0.60 | 0.11 | .24 |
| Control | 60 | 0.62 | 0.10 | 50 | 0.61 | 0.12 | 49 | 0.61 | 0.10 | |||
| CSQ-8 (mean, SD) | ||||||||||||
| Intervention | 58 | 25.9 | 4.5 | .0788 | 55 | 27.3 | 4.3 | .96 | 49 | 26.4 | 5.7 | .51 |
| Control | 59 | 27.4 | 3.8 | 50 | 27.1 | 5.0 | 49 | 27.0 | 5.2 | |||
Note: MPR, medication possession ratio; CAM, composite adherence measure; PANSS, Positive and Negative Symptom Scales; QWB, Quality of Well-being Scales; CSQ-8, Client Satisfaction Questionnaires.
Table 3.
Effects of Intervention on MPR (Linear Regression Model)
| 6 Months |
12 Months |
|||
| β | P Value | β | P Value | |
| Baseline MPR | 0.67 | <.0001 | 0.53 | .0009 |
| Intervention | 0.28 | <.0001 | 0.26 | <.0001 |
| Bipolar | 0.08 | .1906 | −0.002 | .98 |
| Nonwhite | 0.06 | .2542 | 0.06 | .41 |
| Age | 0.004 | .0735 | −0.00003 | .99 |
| No substance use | 0.09 | .0961 | 0.10 | .14 |
| Site B | −0.15 | .0568 | −0.05 | .59 |
| Site C | −0.11 | .1319 | −0.12 | .18 |
| Site D | −0.18 | .0046 | −0.04 | .59 |
Note: MPR, medication possession ratio.
Table 4.
Effects of Intervention on Composite Adherence Measure
| 6 Months |
12 Months |
|||
| OR | 95% CI | OR | 95% CI | |
| ▵ 0.1 Baseline MPR | 1.90 | (1.31, 2.75) | 2.51 | (1.49, 4.21) |
| Intervention | 7.40 | (2.59, 21.16) | 5.41 | (1.61, 18.12) |
| Bipolar | 0.58 | (0.17, 2.01) | 0.44 | (0.11, 1.79) |
| Nonwhite | 1.52 | (0.47, 4.89) | 4.28 | (1.04, 17.61) |
| Age | 1.01 | (0.96, 1.05) | 1.03 | (0.97, 1.08) |
| No substance use | 1.58 | (0.51, 4.94) | 2.73 | (0.76, 9.80) |
| Site B | 0.34 | (0.07, 1.59) | 0.44 | (0.08, 2.30) |
| Site C | 0.50 | (0.10, 2.47) | 0.06 | (0.01, 0.63) |
| Site D | 1.05 | (0.28, 3.86) | 1.14 | (0.26, 4.99) |
Note: OR, odds ratio; CI, confidence interval; MPR, medication possession ratio.
At baseline, the mean antipsychotic MPR was 0.54 for the Meds-Help group and 0.55 for the UC group. At 6 months follow-up, the mean MPR was 0.91 for Meds-Help patients and 0.64 for UC patients (▵ 0.26 in MPR by group). On the more stringent CAM, 50% of intervention patients and 17% of UC patients met criteria for adherence at 6 months.
At 12-month follow-up, the mean MPR was 0.86 for Meds-Help patients and 0.62 for UC patients (▵ 0.25 increase in MPR by group). At this juncture, 34% of intervention and 18% of UC patients met the criteria for adherence on the CAM. Patients were characterized as having a negative CAM for a variety of reasons; most negative CAMs were the result of failing to meet 2 or more of the 3 adherence criteria.
As presented in table 3, in multiple linear regression analyses adjusting for baseline MPR, patient diagnosis, race, age, problematic substance use, and study site, intervention patients had significantly higher MPRs than UC patients, both at 6 and 12 months (P < .0001). As presented in table 4, in logistic regression analyses, adjusting for the same factors, intervention patients had odds ratios of 7.4 and 5.4 for meeting the CAM compared with UC patients at 6 and 12 months, respectively.
Few other factors were significantly associated with adherence (MPR or CAM) after adjustment for patient factors and baseline adherence aside from a study site and nonwhite race which were associated with the CAM at 12 months.
Effect of the Intervention on Secondary Outcomes
At baseline, Meds-Help patients had a mean total PANSS score of 62 and UC patients had a mean total score of 65. These scores are in the range for what is generally considered “mildly ill” for patients with schizophrenia.26 In multiple linear regression analyses (table 5), differences in PANSS scores between groups at 6 or 12 months postenrollment were not statistically significant. This was true for the overall PANSS scores and for the positive PANSS subscale, considered separately. However, the study was powered to assess the significance of medium sized effects, rather than possible small effects of the intervention on the positive PANSS score at 6 (0.38) and 12 months (0.32).
Table 5.
Effects of Intervention on Secondary Outcomes
| 6 Months |
12 Months |
|||
| β | P Value | β | P Value | |
| PANSS | ||||
| Baseline PANSS | 0.68 | <.0001 | 0.58 | <.0001 |
| Intervention | −1.71 | .42 | 0.95 | .67 |
| Bipolar | 4.17 | .10 | −0.30 | .91 |
| Nonwhite | −0.41 | .87 | 0.64 | .79 |
| Age | 0.06 | .57 | 0.04 | .74 |
| No substance use | −1.95 | .41 | 1.49 | .53 |
| Site B | 3.07 | .35 | −1.73 | .61 |
| Site C | 2.05 | .55 | −8.76 | .02 |
| Site D | 1.44 | .60 | 2.74 | .34 |
| Positive PANSS | ||||
| Baseline PANSS | 0.65 | <.0001 | 0.57 | <.0001 |
| Intervention | −1.16 | .18 | −0.28 | .75 |
| Bipolar | 0.39 | .70 | 0.75 | .48 |
| Nonwhite | −0.88 | .37 | 0.56 | .57 |
| Age | −0.02 | .67 | 0.02 | .65 |
| No substance use | −0.57 | .56 | 0.30 | .76 |
| Site B | 0.24 | .85 | −0.67 | .63 |
| Site C | 0.03 | .98 | −2.61 | .07 |
| Site D | 1.12 | .31 | 0.56 | .62 |
| QWB | ||||
| Baseline QWB | 0.51 | <.0001 | 0.63 | <.0001 |
| Intervention | −0.01 | .76 | −0.01 | .65 |
| Bipolar | −0.03 | .10 | −0.03 | .21 |
| Nonwhite | 0.02 | .28 | 0.005 | .79 |
| Age | −0.002 | .08 | −0.001 | .25 |
| No substance use | 0.02 | .23 | −0.002 | .91 |
| Site B | 0.03 | .27 | 0.04 | .10 |
| Site C | 0.03 | .31 | 0.06 | .04 |
| Site D | 0.05 | .04 | 0.05 | .03 |
| CSQ-8 | ||||
| Baseline CSQ8 | 0.60 | <.0001 | 0.69 | <.0001 |
| Intervention | 1.36 | 0.09 | 0.77 | .39 |
| Bipolar | −1.28 | 0.18 | −1.15 | .28 |
| Non-White | −0.17 | 0.85 | 1.76 | .08 |
| Age | 0.04 | 0.34 | 0.11 | .02 |
| No Substance Use | 1.58 | 0.07 | 2.90 | .003 |
| Site B | −1.62 | 0.20 | −2.11 | .14 |
| Site C | 0.16 | 0.89 | 1.04 | .45 |
| Site D | −0.42 | 0.68 | −1.13 | .33 |
Note: PANSS, Positive and Negative Symptom Scales; QWB, Quality of Well-being Scales.
At baseline, Meds-Help patients had a mean QWB score of 0.59 and UC patients had a mean score of 0.62. These scores are in the range that has been reported for stable outpatients with schizophrenia.22,27 In multiple linear regression analyses, there were no significant differences in QWB scores between groups, and effect sizes were small (0.29 at 6 months and 0.09 at 12 months). At baseline, intervention patients had a mean total CSQ-8 score of 26 and UC patients had a mean total score of 27, indicating high levels of satisfaction with health services. Again, there were no significant differences in mean CSQ-8 scores at either 6 or 12 months postenrollment, and effect sizes were small (0.04 at 6 months and 0.11 at 12 months).
Few other patient factors were significantly associated with secondary outcomes in analyses adjusting for baseline scores and other patient factors, although there were study site effects at 12 months for PANSS and QWB and an age and substance use were associated with CSQ-8 at 12 months.
At enrollment, 21% of intervention patients and 10% of UC patients had had a psychiatric hospitalization in the prior 12 months. In multivariate analyses, there were no significant differences in the likelihood of a psychiatric hospitalization at 12 months postenrollment. In an exploratory “completer” analysis (n = 87), results were similar to those reported for the intent-to-treat analyses, except the difference in positive PANSS scores was significant in unadjusted (but not adjusted) analyses at 6 months.
Adverse Events
One of the 2 deaths in the 12-month study period was in the intervention group and was considered “possibly” related to the intervention. This death was attributed to intoxication with cocaine, citalopram, and nortriptyline. The patient had had no changes in amitriptyline refilling patterns in the 11 months prior to death, and the medication was prescribed in usual doses. The high medication levels may have occurred for a number of reasons (intentional overdose, counterbalancing of a cocaine high with amitriptyline, etc.). However, if the patient ingested his amitriptyline more regularly with the intervention and was a slow metabolizer, this may have contributed to high nortriptyline levels. The data safety monitoring board and local IRBs reviewed this event and allowed the study to continue without change. No hospitalizations during the study were deemed related to the intervention.
Discussion
We found that a low-complexity pharmacy-based intervention improved antipsychotic adherence among patients with SMI, a patient population in which poor adherence is common. The differential increase in MPR among Meds-Help patients compared with UC (Δ 0.25 MPR) was consistent with that previously reported for patients with diabetes and hypertension who received unit-of-use packaging and refill reminders for oral hypoglycemic and antihypertensive medications.12,13 Thus, a pharmacy-based intervention with these components appears to be as effective in increasing adherence for patients with SMI as for patients with other chronic medical conditions.
Although there was a site difference in the impact of the Meds-Help measure on one measure of adherence (CAM) at 12 months, multivariate analyses that controlled for site suggest that the Meds-Help program had a positive impact on adherence (MPR and CAM), even when site differences in pharmacy staffing and procedures were taken into consideration.
The increase in adherence observed with Meds-Help is also congruent with a recent study examining a pharmacy-based intervention for elderly patients with coronary risk factors who were taking ≥4 medications. This intervention included unit-of-use packaging along with bimonthly visits with a clinical pharmacist. During the study's observational phase when all study participants received the intervention, the percentage of medications taken increased from 61% to 97%; during a subsequent phase when the intervention was withdrawn for randomly selected patients, those who continued to receive the intervention sustained high levels of adherence while those who were withdrawn from the intervention returned to taking 69% of prescribed medications.28
The current study and these prior studies suggest that health systems should consider pharmacy-based interventions for patients with chronic conditions and poor adherence. Although the “active” ingredients in these studies’ multicomponent interventions cannot be positively identified, the Meds-Help intervention was based on components of Health Belief Model, and both reduced access barriers and provided strong visual and written cues for action (through unit-of-use packaging and mailed refill reminders). Thus, study results are consistent with cues to action and the reduction in access barriers improving adherence. A recent systematic review reported evidence for the effectiveness of unit-of-dose packaging in improving adherence among patients with a variety of medical conditions.29
In this study, pharmacy technicians completed most of the prescription tracking and packaging. Although pharmacy technicians have relevant training and are lower cost than many health system employees, they have seldom been incorporated into mental health and other chronic care teams. Further utilizing these personnel to provide pharmacy-based services may be an affordable avenue for health systems attempting to address the chronic problem of adherence.
Secondary Symptomatic Outcomes
Given numerous observational studies reporting a strong association between higher levels of antipsychotic adherence and decreased psychiatric hospitalization,3,30 we had expected that symptomatic improvements would accompany increases in antipsychotic adherence. Unfortunately, we were not able to demonstrate significant improvements in symptoms, although we were underpowered to assess small effects.
A recent comprehensive review of adherence-enhancing RCTs in a variety of medical disorders reported that only a minority of studies showed both an increase in adherence and accompanying changes in symptomatic outcomes.31 Our study findings are congruent with recent study of a more intensive adherence-enhancing intervention for patients with schizophrenia, medication-focused cognitive adaptation training (Pharm-CAT).32 Patients who received Pharm-CAT also had significant improvements in antipsychotic adherence along with some functional improvements; however, they did not experience greater reductions in positive psychotic symptoms compared with UC patients.32
There are several potential explanations for findings of increased adherence without an accompanying finding of significant symptom improvement. Potentially, patients may have refilled their medications more regularly with Meds-Help but failed to increase their medication ingestion. However, our a priori CAM included components related to ingestion and still showed robust increases with the intervention. In addition to patients with schizophrenia, we included patients with bipolar disorder on long-term antipsychotics. These patients had lower levels of psychotic symptoms at baseline, which could potentially limit our ability to detect symptomatic changes. However, decreases in PANSS scores from baseline to 12 months were similar for patients with schizophrenia and bipolar disorder. We note that the increase in overall dose from improved adherence may simply have been too small to impact symptoms (25% more outpatient days with medications “on hand”), the follow-up period may have been too short to fully capture the benefits of increased adherence, or the relatively stable outpatients enrolling in this study may have been receiving the maximum benefit from their antipsychotic medications despite incomplete adherence. Patients with SMI may need broader-based interventions than those focused on medication adherence if their outcomes are to be improved.
Other Limitations
Measures of adherence in this study are approximations of actual adherence behaviors. A direct measure of patients’ medication taking behavior would be the ultimate gold standard but is seldom achievable in adherence research.33 Although our participation rate is similar to that of most RCTs, patients recruited into a randomized trial may not be typical of all patients with SMI. More paranoid patients or those who were completely unwilling to take medications may have been underrepresented in the study sample—and this should be considered when generalizing study results to clinical populations. However, study participants’ reported attitudes toward antipsychotic medications were similar to those reported for another population of patients with schizophrenia.25
Our study was not double blind, and a blinded assessment of outcomes would have been desirable. However, primary study outcomes were based on longer term patterns of medication filling which may be less susceptible to subtle biases on the part of interviewers or patients.
Summary
We found that a low-complexity pharmacy-based intervention increased antipsychotic adherence among patients with SMI. This finding is congruent with several studies that have reported improved adherence with prescription tracking and unit-of-dose packaging by pharmacy personnel for patients with other chronic illnesses. The intervention is feasible in clinical practice and is now implemented in 5 facilities in Veterans Integrated Services Network 11. Health systems may find this an affordable and logistically feasible method for addressing poor adherence among their patient populations. However, to improve the outcomes of patients with SMI, additional chronic care management components may be needed.
Funding
Department of Veterans Affairs Health Services Research and Development (IIR 01-174-01).
Acknowledgments
An earlier version of this article was presented in presentations at the Department of Veterans Affairs Health Services Research and Development National Meeting, Arlington, VA, February 2007, and the Academy Health Annual Research Meeting, Orlando, FL, June 2007.
References
- 1.Viguera AC, Baldessarini RJ, Hegarty JD, van Kammen DP, Tohen M. Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry. 1997;54:49–55. doi: 10.1001/archpsyc.1997.01830130055011. [DOI] [PubMed] [Google Scholar]
- 2.Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892–909. doi: 10.4088/jcp.v63n1007. [DOI] [PubMed] [Google Scholar]
- 3.Valenstein M, Copeland LA, Blow FC, et al. Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med Care. 2002;40:630–639. doi: 10.1097/00005650-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58:855–863. doi: 10.1176/ps.2007.58.6.855. [DOI] [PubMed] [Google Scholar]
- 5.Sajatovic M, Valenstein M, Blow FC, Ganoczy D, Ignacio R. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8:232–241. doi: 10.1111/j.1399-5618.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- 6.Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. (2nd ed.) 2004;161:1–56. [PubMed] [Google Scholar]
- 7.Glazer WM, Kane JM. Depot neuroleptic therapy: an underutilized treatment option. J Clin Psychiatry. 1992;53:426–433. [PubMed] [Google Scholar]
- 8.Dolder CR, Lacro JP, Leckband S, Jeste DV. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol. 2003;23:389–399. doi: 10.1097/01.jcp.0000085413.08426.41. [DOI] [PubMed] [Google Scholar]
- 9.Zygmunt A, Olfson M, Boyer CA, Mechanic D. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159:1653–1664. doi: 10.1176/appi.ajp.159.10.1653. [DOI] [PubMed] [Google Scholar]
- 10.Kelly GR, Scott JE, Mamon J. Medication compliance and health education among outpatients with chronic mental disorders. Med Care. 1990;28:1181–1197. doi: 10.1097/00005650-199012000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lincoln TM, Wilhelm K, Nestoriuc Y. Effectiveness of psychoeducation for relapse, symptoms, knowledge, adherence and functioning in psychotic disorders: a meta-analysis. Schizophr Res. 2007;96:232–245. doi: 10.1016/j.schres.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Skaer TL, Sclar DA, Markowski DJ, Won JK. Effect of value-added utilities on prescription refill compliance and health care expenditures for hypertension. J Hum Hypertens. 1993;7:515–518. [PubMed] [Google Scholar]
- 13.Skaer TL, Sclar DA, Markowski DJ, Won JK. Effect of value-added utilities on prescription refill compliance and Medicaid health care expenditures—a study of patients with non-insulin-dependent diabetes mellitus. J Clin Pharm Ther. 1993;18:295–299. doi: 10.1111/j.1365-2710.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 14.van Dulmen S, Sluijs E, van Dijk L, de Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC Health Serv Res. 2007;7:55. doi: 10.1186/1472-6963-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker M. Theoretical models of adherence and strategies for improving adherence. In: Schumaker SA, Schron EB, Ockene JK, editors. The Handbook of Health Behavior Change. New York: Springer Publishing; 1990. [Google Scholar]
- 16.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 19.Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161:692–699. doi: 10.1176/appi.ajp.161.4.692. [DOI] [PubMed] [Google Scholar]
- 20.Kay SR, Opler LA, Lindenmayer JP. The. Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl. 1989;(7):59–67. [PubMed] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Patterson TL, Kaplan RM, Grant I, et al. Quality of well-being in late-life psychosis. Psychiatry Res. 1996;63:169–181. doi: 10.1016/0165-1781(96)02797-7. [DOI] [PubMed] [Google Scholar]
- 23.Depp CA, Davis CE, Mittal D, Patterson TL, Jeste DV. Health-related quality of life and functioning of middle-aged and elderly adults with bipolar disorder. J Clin Psychiatry. 2006;67:215–221. doi: 10.4088/jcp.v67n0207. [DOI] [PubMed] [Google Scholar]
- 24.Attkisson CC, Zwick R. The Client Satisfaction Questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann. 1982;5:233–237. doi: 10.1016/0149-7189(82)90074-x. [DOI] [PubMed] [Google Scholar]
- 25.Loffler W, Kilian R, Toumi M, Angermeyer MC. Schizophrenic patients’ subjective reasons for compliance and noncompliance with neuroleptic treatment. Pharmacopsychiatry. 2003;36:105–112. doi: 10.1055/s-2003-39985. [DOI] [PubMed] [Google Scholar]
- 26.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR, et al. What does the PANSS mean? Schizophr Res. 2005;79:231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Kasckow JW, Twamley E, Mulchahey JJ, et al. Health-related quality of well-being in chronically hospitalized patients with schizophrenia: comparison with matched outpatients. Psychiatry Res. 2001;103:69–78. doi: 10.1016/s0165-1781(01)00260-8. [DOI] [PubMed] [Google Scholar]
- 28.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 29.Connor J, Rafter N, Rodgers A. Do fixed-dose combination pills or unit-of-use packaging improve adherence? A systematic review. Bull World Health Organ. 2004;82:935–939. [PMC free article] [PubMed] [Google Scholar]
- 30.Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55:886–891. doi: 10.1176/appi.ps.55.8.886. [DOI] [PubMed] [Google Scholar]
- 31.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X, et al. Interventions for enhancing medication adherence. Cochrane Database of Syst Rev. 2008;(3):16. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 32.Velligan DI, Diamond PM, Mintz J, et al. The use of individually tailored environmental supports to improve medication adherence and outcomes in schizophrenia. Schizophr Bull. 2008;34:483–493. doi: 10.1093/schbul/sbm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velligan DI, Lam YW, Glahn DC, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32:724–742. doi: 10.1093/schbul/sbj075. [DOI] [PMC free article] [PubMed] [Google Scholar]