Abstract
Visual masking provides several key advantages for exploring the earliest stages of visual processing in schizophrenia: it allows for control over timing at the millisecond level, there are several well-supported theories of the underlying neurobiology of visual masking, and it is amenable to examination by electroencephalogram (EEG) and functional magnetic resonance imaging (fMRI). In this paper, we provide an overview of the visual masking impairment schizophrenia, including the relevant theoretical mechanisms for masking impairment. We will discuss its relationship to clinical symptoms, antipsychotic medications, diagnostic specificity, and presence in at-risk populations. As part of this overview, we will cover the neural correlates of visual masking based on recent findings from EEG and fMRI. Finally, we will suggest a possible mechanism that could explain the patterns of masking findings and other visual processing findings in schizophrenia.
Keywords: visual masking, visual processing, neural tuning, schizophrenia
Overview of Visual Masking
Patients with schizophrenia experience a range of cognitive and perceptual deficits, including problems in processing visual stimuli. Many paradigms are available for exploring visual processing impairment in schizophrenia, and our laboratory, among others, has conducted systematic investigations with the visual masking paradigm.
In a visual masking paradigm, a visual target is followed shortly later (eg, 0–500 ms) by a “mask” that can either completely overlap the target or surround, but not touch, the target. This form of masking is known as visual backward masking. Masking can also occur when the mask precedes the target; this form of masking is known as visual forward masking. Masking occurs at stimulus onset asynchronies (SOAs) of approximately 0−100 ms in both forward and backward masking paradigms, but can extend over longer time ranges, depending on the specific paradigms and task parameters. While some of the studies reviewed below examined both forward and backward masking in schizophrenia, the emphasis in the field has been on backward masking, and we will primarily discuss that form of masking.
Visual masking possesses several key advantages as a tool for investigation: it allows for control over timing at the millisecond level, there are several well-supported theories of the underlying neurobiology of visual masking, and it is amenable to examination by electroencephalogram (EEG) and functional magnetic resonance imaging (fMRI). As part of this review of the visual masking impairment in schizophrenia, we will discuss its relationship to clinical symptoms, what is known about the influence of antipsychotic medications, diagnostic specificity (or lack thereof), and presence in at-risk populations. We will briefly discuss the implications of a masking deficit in schizophrenia for social cognition and functional outcome and provide an overview of the neural correlates of visual masking based on findings from EEG and fMRI. Finally, we will suggest an integrated mechanism that could explain a diverse pattern of masking deficits in schizophrenia. One goal of this review article is to track how our ideas have matured over time based on data from our laboratory. At the same time, we wish to acknowledge the substantial contributions from other laboratories that have guided much of our work. Given the limitations on words and number of references, we have added an online supplementary table that provides a list of notable articles on visual masking in schizophrenia.
Visual masking can affect visual processing at several different levels, and it does so through different types of mechanisms. When the visual stimulus reaches primary visual cortex, its basic features (eg, angles, lines, contrast, etc.) must be integrated into a percept that is then processed further at a later stage within and beyond the visual cortex. Traditionally, masking effects have been proposed to occur through 2 different mechanisms: masking by integration and masking by interruption.1 In masking by integration, the formation of the target percept essentially fuses with the mask percept, resulting in a jumbled object that is difficult to identify. In this type of masking, the shape of the response curve (ie, performance charted over SOAs) is generally monotonic, with performance at chance levels for an SOA of zero. In masking by interruption, it is thought that proper formation of the object percept has already occurred and the mask interrupts processing of the object percept at some later stage of visual processing, effectively precluding the object from reaching conscious awareness. In this type of masking, the response curve is generally a nonmonotonic U-shape, with performance at chance levels for SOAs of 20–100 ms, depending on the task parameters. These 2 types of masking are thought to rely on the interactions of 2 different visual pathways, namely the parvocellular and magnocellular pathways, and those will be discussed below.
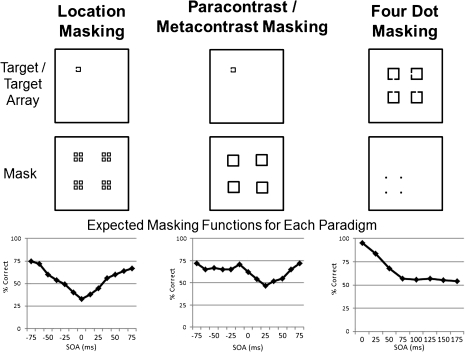
A more recent distinction in masking has focused on delineating an early fast-acting mechanism associated with object formation from a later mechanism that acts through object substitution.2 It is thought that perception is a consequence of recurrent communication between lower level and higher level neural areas that is needed to resolve initial ambiguity in a percept. During visual processing, information is initially processed by lower level units in a fast “feed forward” sweep. However, “reentrant” cortical feedback sweeps from higher to lower visual processing areas are necessary to refine the visual percept. Recent findings in cognitive and perceptual neuroscience have highlighted the importance of reentrant processes for conscious perception (eg, Dux et al3). Masking associated with object formation is similar to masking by integration as described above, whereby the mask percept fuses with the target percept. Masking by object substitution occurs when the mask replaces the target percept before reaching awareness and is maximal at delays greater than 100 ms; object substitution has some similarities to masking by interruption. One specialized masking paradigm, 4-dot masking, is limited to masking by object substitution and does not involve disruption of object formation.2 In this masking method, 4 relatively small dots arranged in a notional square serve as the mask. This type of mask provides no contour information and does not overlap with the target; thus, it cannot work through integration of the target and mask. A schematic of various masking functions is shown in figure 1.
Fig. 1.
Examples of targets, masks, and response functions from different masking paradigms. For these tasks, participants were asked to identify the location of gap in one side of a square. In the first two types of masking, the target could appear in one of four possible locations on the screen. In the case of 4-dot masking, the mask specified which square in an array of 4 squares was the target.
Visual Masking Impairment in Schizophrenia
Data from our research program and other laboratories consistently show impairment in schizophrenia during backward masking tasks compared to healthy controls (eg, Braff et al,4 Green et al,5 Schechter et al,6 Cadenhead et al,7 Butler et al,8 Rund et al,9 and online supplementary table of references). Specifically, patients with schizophrenia consistently exhibit a larger magnitude of the masking effect (ie, poorer performance), as well as a significant prolongation of the masking effect (ie, requiring longer SOAs to accurately identify the target) compared to healthy controls.
We have attempted to specify the masking deficit in schizophrenia by examining the effects of masking by integration and masking by interruption separately. In our experimental tasks, we biased masking toward either integration or interruption by manipulating the “energy” of the mask through changing its duration relative to target (energy is the product of contrast and duration). High-energy masking is thought to occur through a combination of integration and interruption, whereas low-energy masking is thought to occur mainly through interruption. We have found that patients with schizophrenia performed worse than controls on masking tasks of both types, with performance significantly lower across SOAs.5 These results indicate that masking impairment occurs even when there is little or no masking by integration (ie, no disruption of object formation).
Aside from manipulating energy, an alternative way to separate masking by interruption from integration is to use masks that surround, but do not touch, the target (see figure 1). This type of masking is called metacontrast for backward masking and paracontrast for forward masking. As the mask does not physically overlap with the target, integration does not occur and masking is limited to interruption.1 Masking performance in these conditions typically results in a nonmonotonic U-shaped function.10 Schizophrenia patients also exhibit deficits in both paracontrast and metacontrast masking, consistent with the conclusion that deficits exist even when masking is limited to interruption.10 Additionally, we recently reported that patients were more susceptible than controls to object substitution masking in a 4-dot task.11 Because 4-dot masking involves reentrant processes and does not work at the level of object formation, these results suggest that schizophrenia is associated with visual processing deficits that occur after the initial object formation stage.
It is logically possible that the masking deficits seen in the patients are due to a basic deficit in initial sensory input regardless of masking, but this does not seem to be the case. For example, we used a psychophysical staircase procedure to ensure that the initial visibility of the target (when presented without a mask) was equated between groups. For each participant, we systematically adjusted the contrast of the target such that identification of the unmasked target was kept at 84%.10,12 This individual contrast value for the target was then used in the masking procedures. Importantly, we did not find any between-group differences in the contrast values of the unmasked target, but the masking deficit remained. These results, as well as others, suggest that visual masking abnormalities in schizophrenia occur at stages of processing beyond basic sensory input.
It is increasingly clear that schizophrenia patients show deficits in both forward and backward masking, as well as when masking is limited to interruption (or more recently limited to object substitution masking), but it was not clear whether these deficits represent a single visual processing deficit or separable deficits involving different aspects of visual processing. One way to examine this question empirically is with structural equation modeling.10 Four different masking tasks were used, all examining both forward and backward masking: (1) a high-energy target identification task, (2) a high-energy target location task, (3) a low-energy target identification task, and (4) an equal-energy para-and metacontrast task. The first 2 tasks operate mainly through masking by both integration and interruption, whereas the latter 2 tasks operate mainly through masking by interruption. Separate models tested whether the different masking paradigms (ie, integration vs interruption) loaded onto a single latent variable (indicating a single masking factor with redundant information) or onto separate latent variables (indicating separable masking factors). The results showed that a model in which the separate masking paradigms loaded onto separate latent variables provided the best fit (though there were significant correlations among the latent variables). These results indicate that the different visual masking tasks tap into partially distinct visual processing mechanisms.
Although backward masking deficits have been highly replicable in schizophrenia, it should be noted that most of these studies were conducted on patients receiving antipsychotic medication, raising the question of medication effects on masking impairment. Results from several laboratories suggest that medication does not account for the deficits. For example, we found impairment in a sample of unmedicated patients in symptomatic remission.13 Results from other groups have shown that masking deficits are seen in unmedicated patients that are at least as severe as those displayed by medicated patients.14,15 These findings indicate that backward masking deficits are a core feature of schizophrenia and not likely due to antipsychotic medications.
Associations to Clinical Symptoms and Premorbid Functioning
One might think that masking deficits in schizophrenia patients are a result of active psychosis (ie, positive symptoms). However, early work from our laboratory demonstrated that psychotic symptom severity was not correlated with masking performance.16 In these studies, the SOA was systematically varied to determine the point at which masking no longer occurred (a point of unmasking, termed the critical stimulus interval). Multiple regression analyses showed that greater negative symptom severity was significantly associated with longer critical stimulus intervals. However, positive symptoms did not significantly predict the critical stimulus interval.16 Subsequent reports by other researchers have replicated the relationship between negative symptoms and visual masking deficits while also finding minimal or no evidence implicating psychotic symptoms in the observed deficits.17,18 Similar to the findings with negative symptoms, masking has also been examined in schizophrenia patients with good vs poor premorbid social functioning. These studies found that poor premorbid schizophrenia patients had significantly poorer performance compared to good premorbid patients.19
Backward Masking in At-Risk Populations
Visual backward masking may be a vulnerability indicator and a promising endophenotype for schizophrenia as it is seen in unaffected individuals who are considered to be at risk for schizophrenia. For example, we previously examined masking in a cohort of full siblings of schizophrenia patients who were asymptomatic and free from a psychiatric diagnosis within the schizophrenia spectrum (including schizophrenia, schizotypal or paranoid personality disorder, and bipolar disorder).20 The siblings had performance deficits compared to the control group on masking, consistent with what is expected from a marker of genetic liability.
Studies from a large number of laboratories have also found that that at-risk participants (including unaffected siblings, people who are considered to be prone to psychosis, and patients with remitted psychosis) all show performance deficits relative to healthy controls.13,20–22 Visual masking of nonclinical college-age students and community samples have indicated that high levels of schizotypal symptoms are also associated with diminished masking performance.23,24 Evidence of dysfunction on visual masking tasks across these various at-risk groups supports the conclusion that impairment in this type of visual processing represents a trait-like feature of the illness and is consistent with an endophenotype.
Diagnostic Specificity of Backward Masking Deficits
Visual masking impairment is not entirely diagnostically specific to schizophrenia as it is found in other conditions. Furthermore, the impairment does not appear to be specific to schizophrenia among chronic mental illnesses. There have been reports of visual processing impairments in bipolar disorder, but it is not clear whether, or how, these impairments differ from those in schizophrenia. Previously, we found visual masking deficits in bipolar disorder comparable to those in schizophrenia, but both clinical samples were chronic state hospital inpatients.5,25 A key question is whether such impairments exist in less severe forms of the illness. The results from 3 studies of visual masking in stable bipolar outpatients from different laboratories have been mixed.26,27 Hence, it appears at a general level that visual masking impairment is a feature of chronic mental illness, rather than schizophrenia in particular. However, this question has received little research attention and there still could be diagnostic differences at particular visual processing stages.
Functional and Social Cognitive Implications of Masking Impairment
As part of a shift toward a recovery focus in chronic mental illness, considerable efforts are underway to identify determinants of functioning in schizophrenia. Existing models of outcome have been informative but most have examined cognition-functioning relationships without including perceptual measures. However, studies from several laboratories have found that perceptual processing measures fit well into models of outcome, and they are consistent with bottom-up theoretical formulations.28–30 For example, we found that measures of masking correlate well with a measure of social perception.28,29,31 These relationships between visual masking and social cognition parallel other findings from other laboratories, eg, that measures of early visual processing (eg, contrast sensitivity) were related to community functioning.30
Outcome models that include perceptual variables are somewhat easier to interpret than those without because there is general agreement that perceptual variables assess earlier processes than neurocognitive and social cognitive factors. Although perceptual factors are good determinants in models of outcome, they are probably better at predicting relatively proximal constructs, such as social cognition and functional capacity compared with predictions of real world functioning.28,29 At any rate, the connections from early visual processing to daily functioning appear to require several intervening and mediating variables.
Neural Underpinnings of Visual Masking Impairment in Schizophrenia
Visual masking has been traditionally viewed in terms of the interaction between 2 key visual channels that form the basis for complex visual processing. The 2 channels are known by the anatomically based terms “parvocellular and magnocelluar” pathways or by the functionally based terms “sustained and transient” channels. The parvocelluar pathway has slower tonic responses related to stimulus identification, and the magnocelluar pathway is characterized by faster phasic responses relevant to stimulus onset, offset, and location. The 2 pathways convey visual information in parallel until they reach the level of primary visual cortex. The parvocelluar pathway is thought to provide input predominantly to the ventral “what” visual cortical areas and the magnocelluar pathway predominantly to the dorsal “where” visual cortical areas. According to this model, the parvocellular activity elicited by a stimulus conveys detailed information that is critical for identifying it, and the magnocelluar activity provides more rapid information that is needed to locate it. The visual masking effect can result from disruption of the parvocellular target information by either the magnocelluar or parvocelluar activity elicited by mask, depending on the particular paradigm.1 For example, backward masking through the interruption mechanism can occur when the parvocelluar activity of a target is interrupted by the magnocelluar activity of a mask.
Previous behavioral studies, including our own work, suggested a key role of the magnocelluar pathway in the visual masking deficits in schizophrenia.6,7,25 However, more recent studies of neural mechanisms of visual masking in schizophrenia with brain imaging methodologies present a more complex picture. One prominent theory about visual masking32 proposes that masking occurs when gamma range (30−70 Hz) activity in the parvocelluar pathway is disrupted by a mask. This theory raises the question of whether EEG-assessed gamma activity in schizophrenia is associated with visual masking performance. In 2 studies,12,33 schizophrenia patients showed reduced event-related gamma compared to controls during a backward masking task. They also failed to show a pattern seen in healthy controls of lateralized gamma activity in the right hemisphere. Patients with schizophrenia and controls did, however, show comparable event-related gamma activities when the target was presented without a mask. These findings suggest that aberrant gamma activity is related to visual masking performance in schizophrenia, but it remains unclear whether abnormal gamma activity is a primary cause of the masking impairment.
In a series of imaging studies, we examined the neural correlates of visual masking in schizophrenia with fMRI.34–36 Studies in healthy individuals have implicated the lateral occipital complex (LO) as a key brain area for detection of masked targets.37 For example, in healthy individuals, LO has shown sensitivity to the masking effect: ie, increased activation with increasing duration between target and mask.38 More generally, LO has been linked to object processing. The exact mechanism through which is it is linked to object processing is not known but may be related with the ability to segregate figure from background.39 Recently, we examined neural mechanisms associated with backward masking deficits in schizophrenia, primarily focusing on 3 key visual processing regions of interests (ROIs): LO, the human motion-sensitive area (hMT+) and the retinotopic area.34
We identified 3 key visual processing ROIs using independent functional localizer tasks.40 Among these 3 ROIs, we found sensitivity to the masking effect in both LO and hMT+, but not in the retinotopic areas, meaning that the activation in LO and hMT + increased as the target became more visible. Furthermore, the masking effect was more pronounced in LO than in hMT+, illustrating the expected role of LO in visual masking. Importantly, while both schizophrenia patients and controls showed increased LO activation as the masking effect became weaker, patients showed overall decreased LO activation compared to controls across all the SOAs. Outside the 3 ROIs, both schizophrenia patients and controls showed comparable sensitivity to the masking effect in several regions, including posterior cingulate cortex and inferior parietal lobule. These findings suggest that the blunted activation of LO during visual processing may be the neural basis for the visual masking deficit in schizophrenia. However, the blunting was seen across levels of visibility and so probably contributes to visual processing problems more generally, and not just limited to visual masking. In a separate study, we found that unaffected siblings of schizophrenia patients did not show blunted LO activation.35 Hence, blunted LO activation during a visual masking task might be a disease-specific factor, rather than a vulnerability marker.
In a subsequent study, we used the psychophysiological interaction (PPI) approach to further examine whether schizophrenia patients showed abnormal functional coupling between LO and other brain regions that are associated with visual perception.36 PPI examines how the functional connectivity with an a priori specified region changes in the presence of cognitive or perceptual task demands. We found that, compared to controls, schizophrenia patients showed altered dynamic coupling with LO in several high-level cortical areas including the left precuneus, left inferior frontal, and superior frontal gyri as a function of target visibility in the backward masking task. Note that we did not observe generally reduced coupling with LO in schizophrenia; patients only showed altered coupling with LO as target visibility was manipulated. Patients with schizophrenia, therefore, appeared to have altered (not overall reduced) dynamic coupling between LO and other cortical regions when processing visual information.
One limitation of these studies of fMRI and visual masking is that they all used the same type of target stimuli—a square with a gap on one side. The task was for the subject to identify which side had a gap (see figure 1). Hence, it is not known whether other types of masking paradigms would have yielded the same pattern of results. However, it is reassuring that our findings regarding LO activation during visual masking are consistent with findings from studies with healthy individuals that used more complex visual stimuli.
Visual Neural Tuning as an Explanation of Findings
One question from these findings is whether there is a common mechanism that can explain the range of phenomenon. Clues for such a mechanism have come from a relatively simple study. Our initial fMRI paper did not include visual masking at all but instead used functional localizers that were designed to activate the 3 visual processing regions mentioned above: retinotopic areas, motion sensitive hMT+, and LO. Although the groups did not differ in retinotopic areas or hMT+, they did differ in LO. Specifically, the patients had a broader extent of activation (ie, less focused) in LO in response to a localizer task (ie, the contrast between whole and scrambled objects). There were no differences in level of activation, only in the extent.40 This pattern of results suggests that LO, but not the other processing regions, is less specialized in its response to objects in schizophrenia. One possible explanation for this finding is that patients have broader visual neural tuning in LO.
Visual neural tuning refers to the graded pattern of selectivity for specific visual features that is shown by neurons in visual processing regions of cortex. For example, it has long been known that neurons in V1 are tuned for orientation; they respond maximally to a stimulus at one preferred orientation and their response rate decreases as stimulus orientation moves from the preferred one. Similarly, LO receives input from retinotopic areas and is tuned for object and object features. Neurons in LO give maximum response to a small set of objects, intermediate responses to similar objects, and low responses to objects that are visually quite different. Much of the effectiveness of tuning depends on the extent to which neurons have a connection to a “preferred” stimulus that elicits a greater response than other stimuli. It is possible that patients have broader tuning for objects: that is, less ability to generate specific responses to selected (preferred) visual objects. Therefore, they are less efficient at distinguishing between visual targets and visual noise.
This theory of an abnormality in visual tuning could account for other imaging findings from our group. For example, as mentioned above, we found that schizophrenia patients showed an overall blunted response on fMRI in LO compared to controls to visual targets in a masking task across all levels of visibility. This group difference was not seen in the other key visual processing regions (ie, retinotopic and hMT+).34 These findings suggest reduced discrimination between visual targets and visual noise (consistent with boarder neural tuning), particularly in LO compared with other regions, and particularly for objects. Furthermore, the results are consistent with EEG studies showing impaired closure negativity for object recognition in schizophrenia over LO.41
Neural tuning would also help to explain the fMRI connectivity analyses that showed patients had an abnormal pattern of coupling between LO and other brain regions involved with visual processing. Healthy controls showed increased coupling with key regions (eg, precuneus and inferior frontal lobe) with increased visibility, whereas the patients did not alter their LO coupling in response to visibility changes.36 However, patients and controls did not differ in their overall level of connectivity with LO, only with the modulation of it with visibility. In the context of a visual tuning problem, patients may be less efficient at distinguishing between visual targets and visual noise, resulting in a lack of modulation of coupling with changes in visibility.
Visual Tuning and Theories of Pathophysiology of Schizophrenia
This explanation of visual tuning connects well to current theories of the pathophysiology of schizophrenia. There is compelling support for gamma amino butyric acid (GABA) abnormalities in schizophrenia, particularly interneurons that express the calcium-binding protein parvalbumin.42 These findings include reduced expression of the GABA membrane transporter, GAT1, and a reduction in expression of GAD67, the enzyme that synthesizes GABA. Reduced GAD67 messenger RNA expression is considered one of the most consistent postmortem findings in schizophrenia.42 These GABA effects may not be the primary source of dysfunction; they could be downstream effects of abnormalities in the NMDA (N-methyl-D-aspartate) receptors that synapse on parvalbumin-expressing interneurons. However, the GABA system has direct implications for visual processing.
The GABA interneuron abnormalities in schizophrenia occur across the cortex, including the primary visual area,43 and they lead to rather specific predictions for visual processing. A key role for GABA in the visual system is to aid the tuning of individual neurons. Visual tuning involves lateral inhibition (reduced responding to nonselected stimuli) that is modulated by GABA interneurons. In the monkey, when a GABAA receptor antagonist (bicuculline methiodide) is applied to neurons in the object-sensitive region comparable to humans (area TE in the inferotemporal cortex), they lose tuning and respond to objects that do not elicit a response before or after the drug administration.44 Consistent with a GABA basis for perceptual abnormalities in schizophrenia, a recent MR spectroscopy study showed reduction in GABA concentration in visual cortex in schizophrenia.45
The GABA findings and tuning hypothesis help to explain an apparent paradox in our findings: that the abnormalities are more prominent in LO than in earlier visual processing regions. If a GABA problem occurs throughout cortex, why would its visual effects be more prominent in some regions than others? It appears that the importance of GABA for tuning likely increases as one moves up the processing hierarchy from V1 to LO.46 The reason for this pattern stems from classical vision theory that postulates tuning in the earliest part of visual cortex is based on feed-forward processes, as opposed to GABA modulated lateral inhibition. That is, the simple cells in V1 have orientation specificity due to the convergent input of thalamic cells that have receptive fields arranged in rows. In this situation, lateral inhibition is not needed. Although there is active debate in this area, findings from sensory physiology and computational modeling suggest tuning and selectivity in early visual areas can be accomplished without lateral inhibition. Not so for later regions, such as LO, which respond to more complex visual stimuli and require lateral inhibition for tuning.
Implications of a Visual Tuning Hypothesis
There are other possible explanations for this pattern of results in the imaging studies. One is that visual cortex shows an overall weaker response in schizophrenia, as opposed to a less tuned one. However, that explanation does not fit with the localizer data which showed a less focused response, not a smaller one. Nor does it fit with the connectivity data that show overall normal baseline connectivity with LO. Another alternative is that the findings reflect an abnormal magnocellular system. Previous behavioral data from our laboratory and others8,25,47,48 have suggested abnormalities in the magnocellular system in schizophrenia. However, we did not find evidence of group differences in area hMT+, which is part of the magnocellular system, either in the localizer or in the masking tasks. Of course, both factors may be present and we might have found group differences in this region with a larger sample.
If the proposed abnormality in visual tuning is supported, it would have treatment implications. Neural tuning in visual areas has shown excellent plasticity to both training and pharmacological manipulations. A recent behavioral study of perceptual learning in healthy controls demonstrated that training optimized neural tuning (using multivariate pattern analysis) by enhancing responses to preferred stimuli and reducing responses to nonpreferred stimuli.49 A pharmacological study in senescent monkeys showed that a GABAA agonist enhanced neural tuning.50 Another treatment implication is that neural tuning can be reliably assessed across species. A key challenge for cognitive treatment development initiatives is how to develop and adapt methods that assess key cognitive or perceptual subprocesses in patients and also have direct analogues in animal models because such models are needed to screen compounds in preclinical phases. Neural tuning can be assessed in a variety of animal models, including cats, nonhuman primates, and rodents.
Summary
Visual masking is a deceptively simple procedure: 2 briefly presented stimuli in which 1 interferes with the identification of the other. One might ask why a laboratory would devote so many years to study a procedure that has such simplicity. One reason is the strong parametric nature of the task. Visual masking can be manipulated at the millisecond level (now with off-the-shelf computer equipment) that allows the experimenter to have exquisite control over the visibility of stimuli. A second reason is that the procedure provided insights into the nature of the deficit. It behaves like a vulnerability indicator and impairments can be found in a range of at-risk samples. Third, it connects well to important clinical and functional features of schizophrenia. Our initial interest in visual masking involved its association with negative symptoms. More recent efforts are trying to determine how visual masking (and other visual perceptual tasks) fit into larger models of the determinants of functional outcome.
A fourth reason is that visual masking is tied to well-identified neural processes. There was early interest in the integrity and balance of parvocelluar and magnocellular visual pathways as an explanation for visual processing impairment. There is also considerable effort to use visual masking to parse the early stages of processing (object formation, object substitution, etc.). Our recent functional neuroimaging work has implicated a particular region, LO, as a pivotal site of dysfunction in schizophrenia. The findings from neuroimaging procedures lead to a fifth reason to study visual masking: it provides the basis for theorizing about visual processing problems in schizophrenia in general. The pattern of findings (both presence and absence of patient-control differences) has suggested that neural visual tuning in LO could be a parsimonious explanation. In essence, visual masking is a good visual workhorse for schizophrenia research: it is sufficiently precise to tie to specific neural systems but sufficiently broad so that any findings almost certainly apply to other paradigms and visual processing generally.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Institute of Mental Health Grants (043292 and 065707 to M.F.G.); VA Career Development Award (to J.K.W.).
Acknowledgments
Financial disclosure: None of the authors have any conflicts of interest to disclose.
References
- 1.Breitmeyer BG. Visual Masking: An Integrative Approach. New York, NY: Oxford University; 1984. [Google Scholar]
- 2.Enns JT. Object substitution and its relation to other forms of visual masking. Vision Res. 2004;44:1321–1331. doi: 10.1016/j.visres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Dux PE, Visser TA, Goodhew SC, Lipp OV. Delayed reentrant processing impairs visual awareness: an object-substitution-masking study. Psychol Sci. 2010;21:1242–1247. doi: 10.1177/0956797610379866. [DOI] [PubMed] [Google Scholar]
- 4.Braff DL, Saccuzzo DP, Geyer MA. Information processing dysfunctions in schizophrenia: studies of visual backward masking, sensorimotor gating, and habituation. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of Schizophrenia: Neuropsychology, Psychophysiology, and Information Processing. Vol 5. Amsterdam: Elsevier; 1991. pp. 303–334. [Google Scholar]
- 5.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania: I. Specifying a mechanism. Arch Gen Psychiatry. 1994;51:939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 6.Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- 7.Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiatry. 1998;43:132–138. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- 8.Butler PD, DeSanti LA, Maddox J, et al. Visual backward masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res. 2003;59:199–209. doi: 10.1016/s0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 9.Rund BR, Egeland J, Sundet K, et al. Early visual information processing in schizophrenia compared to recurrent depression. Schizophr Res. 2004;68:111–118. doi: 10.1016/S0920-9964(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 10.Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer BG, Mintz J. Visual processing in schizophrenia: structural equation modeling of visual masking performance. Schizophr Res. 2005;78:251–260. doi: 10.1016/j.schres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Green MF, Wynn JK, Breitmeyer B, Mathis KI, Nuechterlein KH. Visual masking by object substitution in schizophrenia[published online ahead of print Nov 16, 2010] Psychol Med. doi: 10.1017/S003329171000214X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green MF, Mintz J, Salveson D, et al. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry. 2003;53:1113–1119. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- 13.Green MF, Nuechterlein KH, Breitmeyer BG, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflection of aberrant cortical oscillation. Am J Psychiatry. 1999;156:1367–1373. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- 14.Braff DL, Saccuzzo DP. Effect of antipsychotic medication on speed of information processing in schizophrenic patients. Am J Psychiatry. 1982;139:1127–1130. doi: 10.1176/ajp.139.9.1127. [DOI] [PubMed] [Google Scholar]
- 15.Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol Psychiatry. 1996;40:295–298. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 16.Green MF, Walker E. Symptom correlates of vulnerability to backward masking in schizophrenia. Am J Psychiatry. 1986;143:181–186. doi: 10.1176/ajp.143.2.181. [DOI] [PubMed] [Google Scholar]
- 17.Braff DL. Sensory input deficits and negative symptoms in schizophrenic patients. Am J Psychiatry. 1989;146:1006–1011. doi: 10.1176/ajp.146.8.1006. [DOI] [PubMed] [Google Scholar]
- 18.Slaghuis WL, Bakker VJ. Forward and backward visual masking of contour by light in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1995;104:41–54. doi: 10.1037//0021-843x.104.1.41. [DOI] [PubMed] [Google Scholar]
- 19.Knight RA. Specifying cognitive deficiencies in premorbid schizophrenics. Prog Exp Pers Psychopathol Res. 1992;15:252–289. [PubMed] [Google Scholar]
- 20.Green MF, Nuechterlein KH, Breitmeyer BG. Backward masking performance in unaffected siblings of schizophrenic patients. Arch Gen Psychiatry. 1997;54:465–472. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- 21.Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychol Med. 2001;31:915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- 22.Green MF, Nuechterlein KH, Breitmeyer BG, Mintz J. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biol Psychiatry. 2006;59:446–451. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Bedwell JS, Orem DM. The effect of red light on backward masking in individuals with psychometrically defined schizotypy. Cogn Neuropsychiatry. 2008;13:491–504. doi: 10.1080/13546800802605755. [DOI] [PubMed] [Google Scholar]
- 24.Cadenhead KS, Perry W, Braff DL. The relationship of information-processing deficits and clinical symptoms in schizotypal personality disorder. Biol Psychiatry. 1996;40:853–858. doi: 10.1016/0006-3223(95)00547-1. [DOI] [PubMed] [Google Scholar]
- 25.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania: II. Specifying the visual channels. Arch Gen Psychiatry. 1994;51:939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 26.MacQueen GM, Young LT, Galway TM, Joffe RT. Backward masking task performance in stable, euthymic out-patients with bipolar disorder. Psychol Med. 2001;31:1269–1277. doi: 10.1017/s0033291701004597. [DOI] [PubMed] [Google Scholar]
- 27.Goghari VM, Sponheim SR. Divergent backward masking performance in schizophrenia and bipolar disorder: association with COMT. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:223–227. doi: 10.1002/ajmg.b.30583. [DOI] [PubMed] [Google Scholar]
- 28.Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- 29.Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med. 2011;41:487–497. doi: 10.1017/S0033291710001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res. 2003;59:233–241. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- 32.Purushothaman G, Ogmen H, Bedell HE. Gamma-range oscillations in backward masking functions and their putative neural correlates. Psychol Rev. 2000;107:556–577. doi: 10.1037/0033-295x.107.3.556. [DOI] [PubMed] [Google Scholar]
- 33.Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green MF, Lee J, Cohen MS, et al. Functional neuroanatomy of visual masking deficits in schizophrenia. Arch Gen Psychiatry. 2009;66:1295–1303. doi: 10.1001/archgenpsychiatry.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Cohen MS, Engel SA, et al. Regional brain activity during early visual perception in unaffected siblings of schizophrenia patients. Biol Psychiatry. 2010;68:78–85. doi: 10.1016/j.biopsych.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey P-O, Lee J, Cohen MS, et al. Altered dynamic coupling during visual perception in schizophrenia. Neuroimage. 2011;55:1219–1226. doi: 10.1016/j.neuroimage.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grill-Spector K, Kushnir T, Hendler T, Malach R. The dynamics of object-sensitive activation correlate with recognition performance in humans. Nat Neurosci. 2000;3:387–843. doi: 10.1038/77754. [DOI] [PubMed] [Google Scholar]
- 38.Green MF, Glahn D, Engel SA, et al. Regional brain activity associated with visual backward masking. J Cogn Neurosci. 2005;17:13–23. doi: 10.1162/0898929052880011. [DOI] [PubMed] [Google Scholar]
- 39.Appelbaum LG, Ales JM, Cottereau B, Norcia AM. Configural specificity of the lateral occipital cortex. Neuropsychologia. 2010;48:3323–3328. doi: 10.1016/j.neuropsychologia.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wynn JK, Green MF, Engel S, et al. Functional localization of visual processing regions in schizophrenia. Psychiat Res Neuroim. 2008;164:97–105. [Google Scholar]
- 41.Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 42.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Fujita I, Murayama Y. Neuronal mechanisms of selectivity for object features revealed by blocking inhibition in inferotemporal cortex. Nat Neurosci. 2000;3:807–813. doi: 10.1038/77712. [DOI] [PubMed] [Google Scholar]
- 45.Yoon JH, Maddock RJ, Rokem A, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kritzer MF, Cowey A, Somogyi P. Patterns of inter- and intralaminar GABAergic connections distinguish striate (V1) and extrastriate (V2, V4) visual cortices and their functionally specialized subdivisions in the rhesus monkey. J Neurosci. 1992;12:4545–4564. doi: 10.1523/JNEUROSCI.12-11-04545.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuck JR, Lee RG. Backward masking, information processing, and schizophrenia. Schizophr Bull. 1989;15:491–500. doi: 10.1093/schbul/15.3.491. [DOI] [PubMed] [Google Scholar]
- 48.Bedwell JS, Brown JM, Miller LS. The magnocellular visual system and schizophrenia: what can the color red tell us? Schizophr Res. 2003;63:273–284. doi: 10.1016/s0920-9964(02)00356-0. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Meeson A, Welchman AE, Kourtzi Z. Learning alters the tuning of functional magnetic resonance imaging patterns for visual forms. J Neurosci. 2010;30:14127–14133. doi: 10.1523/JNEUROSCI.2204-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]