Abstract
This study examined effects of cigarette smoking on mortality risk in 1213 persons aged 19–69 years with schizophrenia-related psychotic disorders admitted to State of Maryland Hospitals between 1994 and 2000. Inpatient medical records from 7 hospitals were reviewed to obtain demographic information, diagnosis, medication use, as well as smoking and other substance use. Social Security Death Index data were used to identify deaths in the study group between 1994 and 2004. Death records were reviewed to obtain manner of death and underlying disorders. Of the 1213, 55% were smokers and 71% abused substances. There was an age × smoking interaction (χ2 = 14.6, df = 1, P = .0001) for mortality, with estimated hazard ratios (HRs) for smokers vs nonsmokers of 2.1 among 35- to 54-year olds and HR of 0.7 among those aged 55–69 years. Five- and 10-year mortality rates for smokers aged 35–54 years were 7.0% and 14.2%, compared with 3.3% and 10.0% for nonsmokers, respectively (χ2 = 5.53, df = 1, P = .019). Cardiac causes were identified in 43% of deaths in smokers but only 19% of deaths in nonsmokers (P < .006). For those aged 35–54 years, the odds of cardiac related death was increased by 12 fold in smokers relative to nonsmokers (HR = 12.4, χ2 = 12.0, df = 1, P = .0005). Among people aged 35–54 years, those smoking greater than one pack daily have a significantly increased total mortality risk (HR = 2.7) vs nonsmokers. Cigarette smoking, particularly in people aged 35–54 years, contributes to an increased risk of death. Greater smoking severity significantly increases this risk. Smoking cessation in people with schizophrenia deserves significant attention.
Keywords: schizophrenia, mortality, cigarette smoking
Introduction
People with schizophrenia have a substantially increased risk for medical comorbidities and excess mortality.1–3 The mortality risk in this population is over twice that of the general population,4 resulting in about a 15-year reduction in average life span in people with schizophrenia.5 Early studies reported that increased mortality was due to a high rate of suicide.6–8 However, suicide is occurring at lower rates than once reported (5%), and even after accounting for the risk of suicide, life expectancy remains lower than the general population.9
The majority of excess mortality among persons with schizophrenia appears due to cardiovascular complications, notably coronary heart disease.10 Individuals diagnosed with schizophrenia have a significantly greater 10-year risk of developing cardiovascular disease as compared with the general population for both males (9.4% vs 7.0%) and females (6.3% vs 4.2%).11 A recent report notes that standardized mortality ratio for cardiovascular death is about 3 times greater in people with schizophrenia than the general population.12
Several modifiable risk factors contributing to excess mortality in people with schizophrenia have been suggested, including weight gain and metabolic complications as contributing factors to cardiovascular disease.3 Obesity contributes to a significantly elevated risk for a number of diseases. In recent years, antipsychotic-induced weight gain has been identified as significant risk factor for adverse health consequences such as diabetes.13 However, the relationship of weight changes on antipsychotic medications to increased mortality in schizophrenia has not yet been proven; and recent data suggest that differences in cardiovascular mortality risk among antipsychotics may not simply reflect differences in metabolic liabilities.12,14,15 Nevertheless, the high rates of metabolic syndrome and related consequences among people with schizophrenia suggest the importance of preventing and treating obesity, diabetes, lipid disorders, and hypertension in order to decrease morbidity and mortality.10,16,17
Cigarette smoking is one of the most important preventable causes of morbidity and premature mortality in the general population. Risk of heart disease is directly related to lifetime exposure to cigarette smoking.18 The impact of smoking on mortality in people with schizophrenia remains unknown. Despite much higher cigarette smoking rates (58%–90%) than the general population in people with schizophrenia,2,19–22 cancer, particularly lung cancer, has not emerged as a major factor contributing to high age-adjusted mortality rates in most studies in people with schizophrenia.2,23,24 This study examined the impact of cigarette smoking on mortality in people with schizophrenia and assessed the impact of greater smoking frequency (packs per day) on mortality risk. This population not only has a greater prevalence of smoking but may also have different reasons for use and patterns of use that may put them at a higher risk of smoking-related mortality than the general population. This study includes a large cohort (N = 1213) for which medical records including specific smoking data were available. Better understanding the risk of mortality in smokers with schizophrenia will enable the field to better target interventions.
Methods
Study Population
A total of 1213 unique patient records were identified and included from a State of Maryland database consisting of patients treated at 7 public inpatient mental health facilities. These patient records included patients initially treated at ages 19–69 years between the dates of January 1, 1994, and December 31, 2000, and who had a Diagnostic and Statistical Manual of Mental Disorders (Third Edition) or Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) diagnosis of schizophrenia, schizoaffective disorder, or psychosis not otherwise specified (NOS). The administrative database used to identify subjects was initiated to examine second-generation antipsychotic (SGA) use, and patients treated solely on first-generation agents or no antipsychotic were not included in this database. The year 1994 was selected as the start for follow-up of patients beginning SGA. This was the first year risperidone was marketed in the United States; prior to that year, clozapine was the only marketed SGA and follow-up from this earlier period would confound effects of antipsychotics (clozapine vs other SGA) with trends that may alter pre- and post- 1994 mortality. Additionally, only those records that contained information on smoking status were included in this analysis. There were 70 records not included due to missing smoking data. Thirty-six were excluded whose race was identified as “other” (not black or white) as this number was too small to allow reliable estimates of race effects on mortality in regression models. Thus, 1213 (92%) records were included from an original sample of 1319 inpatients aged 19–69 years with a schizophrenia-related disorder who were started on an SGA, and a chart was located. A larger sample of 2172 was originally identified; however, charts were not able to be retrieved for 853 patients due to missing or destruction of clinical records. This study was approved by the University of Maryland and State of Maryland Department of Health and Mental Hygiene institutional review boards and we received a waiver of the requirement to obtain written informed consent for use of administrative health records.
Identifying Deceased Persons
Deceased patients were identified through matching patient records with the Social Security Death Index (SSDI) either by their social security numbers or by their names with confirmed date of birth. The SSDI is a national computerized database that records death dates and locations that have been reported to the organization. Records were extracted from SSDI through December 31, 2004; patients were thus potentially followed for mortality for between 4 and 10 years after start of SGA treatment. In addition, the death certificates were collected from the Maryland Division of Vital Records to determine the immediate cause of death. Death certificates were available for all decedents in this study.
Clinical Data Collection
Between 2003 and 2007 (4 y), clinical data collection for all the subjects who were treated at some point in State of Maryland inpatient facilities took place. Medical records were reviewed to verify DSM-IV diagnoses, demographic information, comorbid diseases, smoking status, and severity of illness (Global Assessment of Functioning scores). Research staff collecting data included 2 research staff (doctoral or predoctoral interns) who were trained and educated to clinical charts and location of objective information. Standardized data collection forms were created and utilized for each subject. A detailed algorithm was created on where to locate clinical information. For smoking and substance use data, this included a DSM diagnosis and intake and substance abuse history forms. Occasional or infrequent use was not characterized as dependence or abuse of nicotine or substances. Only information from the first hospital admission during the period of study was collected if there were more than one. Medical record forms were standardized across all State of Maryland facilities.
The SGA prescribed at the time of their first hospitalization during the study period is recorded; however, antipsychotic treatment during follow-up was unknown and may have changed through the course of their treatment. Smokers were defined as subjects who were currently smokers or who had ever been dependent on cigarettes. Smokers were further categorized as subjects who smoked at the time of index admission either more than 1 pack daily, less than 1 pack daily, or unknown amount. While we attempted to collect pack per year histories, medical record reporting of previous years of exposure was sporadic. The majority of smokers were currently smoking; very few patients had a record of successfully quitting past cigarette smoking. No smoking restrictions were in place at these hospitals during the time of study. Substance use was collected and was defined as subjects who ever used any of the substances identified by name or corresponding street name: alcohol, cocaine, marijuana, heroin, lysergic acid diethylamide, phencyclidine, 3,4-methylenedioxy-N-methylamphetamine, inhalants, amphetamines, and or other hallucinogens (ie, mushrooms). Data collection also included height and weight to enable BMI calculations on patients as well as systolic and diastolic blood pressure and cardiac comorbidity data. This data are collected at the time of the index admission and do not represent measures at a subsequent point including prior to death.
Statistical Methods
Availability of smoking status at the time of patient's index SGA prescription in the medical charts examined varied from year to year and between decedents and patients surviving to the end of follow-up because some charts were not located. To account for this potential source of bias, the proportion of records for which baseline smoking status was available within each calendar year of first prescription was used as a sampling weight to adjust for secular variations in ascertainment of smoking. The weight for each patient was calculated from the inverse of the probability of having smoking information in the sample by calendar year of entry and survival status. Weighted Rao-Scott χ2 tests25 or Student t tests were employed to evaluate the differences between smokers (current or lifetime dependence) and nonsmokers in demographic and clinical characteristics, using SAS PROC SURVEYFREQ and PROC SURVEY MEANS.
The Kaplan-Meier method was used to assess the distribution of time to all-cause mortality according to patients’ smoking status; log-rank tests were used for unadjusted comparisons of mortality. These survival analyses were stratified by age in order to take account of the differences in the mortality among different age groups (19–34, 35–54, 55–69 y). Cox regression models were fitted, using sampling weights calculated as described above, to determine the association of mortality with patients’ smoking status after adjusting for the patient's characteristics (age group, gender, race, clozapine vs other antipsychotic). Interaction between age and smoking status was examined in the Cox models in order to evaluate whether survival trends differed by age. Variation in mortality by smoking severity (<1 packs per day, ≥1 packs per day, or unknown amount) was also examined in a Cox model. All tests were 2 sided with α = .05.
Results
Demographic Information
A total of 1213 patients, 651 with schizophrenia (53.7%), 408 with schizoaffective disorder (33.6%), and 154 with psychotic disorder NOS (12.7%) were included in the study. Fifty-five percent were cigarette smokers (664/1213) and of those 45% of the smoking group (298/664) smoked greater than 1 pack per day. The only demographic differences noted between smokers and nonsmokers were a higher percentage of males in the smoking group (Rao-Scott χ2 = 7.1, df = 1, P = .0077), and a significantly greater percentage of smokers than nonsmokers were prescribed clozapine vs other antipsychotics (Rao-Scott χ2 = 16.8, df = 1, P < .0001). There were no differences in age, race, and global functioning between smokers and nonsmokers. Patients who were smokers were more likely to use substances compared with those who were nonsmokers (Rao-Scott χ2 = 25.18, df = 1, P < .0001). No differences in body mass index, blood pressure, or the presence of preexisting cardiac disease were evident between smokers and nonsmokers (see table 1).
Table 1.
Characteristics of Subjects by Baseline Smoking Status
| Characteristics | Smoker (N = 664), N (%) | Nonsmoker (N = 549), N (%) | Statistics |
||
| Rao-Scott χ2 | df | P | |||
| Age (y), mean (SE) | 40.8 (0.4) | 40.1 (0.5) | t = −1.3a | 1114 | .193 |
| Age category | |||||
| <35 y | 197 (30.3) | 198 (36.5) | 8.1 | 2 | .018 |
| 35–54 y | 387 (58.2) | 275 (50.0) | |||
| 55–69 y | 80 (11.5) | 76 (13.5) | |||
| Sex (male) | 434/663 (65.5) | 319 (58.0) | 7.10 | 1 | .008 |
| Race (white) | 348/585 (60.5) | 281/495 (57.4) | 1.04 | 1 | .309 |
| Greater than 1 pack per day | 298 (44.9) | ||||
| Global Assessment of Functioning score, mean (SE) | 37.4 (0.5) | 38.3 (0.5) | t = 1.22a | 1021 | .223 |
| BMI (kg/m2, mean, SE) | 27.0 (0.3) | 26.7 (0.3) | t = −0.6a | 724 | .558 |
| Systolic blood pressure (mm Hg) | 118.4 (0.7) | 119.3 (0.9) | t = 1.02a | 734 | .310 |
| Diastolic blood pressure (mm Hg) | 75.7 (0.5) | 75.4 (0.7) | t = −0.22a | 557 | .825 |
| Cardiac disease | 139/615 (22.5) | 105/515 (20.7) | 0.530 | 1 | .467 |
| Antipsychotic treatment (clozapine) | 241 (38.7) | 140 (27.2) | 16.8 | 1 | <.0001 |
| Substance abuser | 439/568 (76.9) | 233/374 (61.5) | 25.2 | 1 | <.0001 |
| Use of substances | |||||
| Alcohol | 365/568 (63.8) | 184/374 (48.7) | 20.7 | 1 | <.0001 |
| Cannabis | 209/568 (36.8) | 117/374 (30.6) | 3.7 | 1 | .053 |
| Cocaine | 175/568 (30.5) | 83/374 (21.8) | 8.4 | 1 | .004 |
| Heroin | 77/568 (13.1) | 32/374 (8.4) | 4.9 | 1 | .027 |
| Other | 158/568 (27.9) | 68/374 (18.5) | 10.5 | 1 | .001 |
Student t test.
Mortality Risk
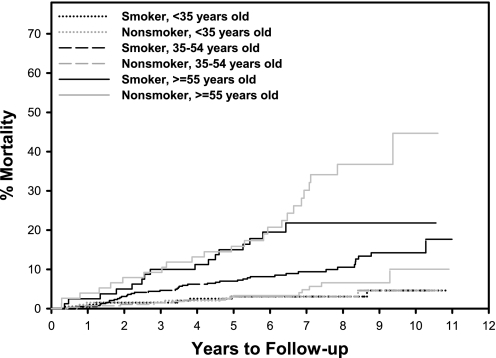
Crude mortality rates were 65 of 664 (9.8%) in smokers and 43 of 549 (7.8%) in nonsmokers. Among patients 19–34 years old (N = 395), 5- and 10-year mortality rates were 3.1% and 4.6% in smokers, respectively, compared with 2.0% and 6.1% in nonsmokers, respectively (χ2 = 0.229, df = 1, P = .632); among patients 35–54 years old (N = 662), 5- and 10-year mortality rates were 7.0% and 14.2%, respectively, in smokers, compared with 3.3% and 10.0% in nonsmokers, respectively (χ2 = 5.53, df = 1, P = .019 for difference in mortality); and among patients 55–69 years old (N = 156), 5- and 10-year mortality rates were 15.0% and 21.8% in smokers, respectively, compared with 15.9% and 44.7% in nonsmokers, respectively (χ2 = 1.402, df = 1, P = .237). The difference in mortality across smoking by age groups was statistically significant (χ2 = 87.12, df = 5, P < .0001) (see figure 1).
Fig. 1.
Time to Death in Smokers Vs Nonsmokers by Age Group. Kaplan-Meier Estimates of Cumulative Probability of All-Cause Death by Years of Follow-up, Age, and Smoking Status. Tests of mortality difference from the Cox proportional hazards model—smoking (age < 35 y): hazard ratio (95% confidence interval [CI]) = 1.24 (0.57–2.70), P = .590; smoking (age 35–54 y): hazard ratio (95% CI) = 2.06 (1.37–3.10), P = .0006; smoking (age ≥55 y): hazard ratio (95% CI) = 0.65 (0.42–0.99), P = .047; difference in hazard ratio age <35 y vs age 35–54 y: P =.258; difference in hazard ratio age 35–54 y vs age≥55 y: P = .0001.
Among all deaths, cardiac related causes of death accounted for 43.1% (28/65) and 18.6% (8/43) of deaths among smokers and nonsmokers, respectively. Cardiac related mortality was higher for smokers than nonsmokers in the 35- to 54-year-old age group (χ2 = 51.78, df = 5, P < .0001). Other causes of death were cerebrovascular disease (N = 2, 3.1%, vs N = 8, 7.0%), cancer (N = 7, 10.8%, vs N = 4, 9.3%), human immunodeficiency virus (HIV)/AIDS (N = 2, 3.1%, vs N = 1, 2.3%), non-HIV infection (N = 9, 13.8%, vs N = 8, 18.6%), diabetes (N = 1, 1.5%, vs N = 1, 2.3%), respiratory disease (N = 2, 3.1%, vs N = 4, 9.3%), suicide (N = 1, 1.5% vs N = 2, 4.7%), and others (N = 13, 20.0% vs N = 12, 27.9%) in the smoking and nonsmoking groups, respectively.
Risk Factors for Mortality (Hazard Ratio of Cox Regression)
The association between smoking status and mortality was examined after adjusting for age, gender, race, and antipsychotic treatment using the Cox proportional hazards model (table 2). Males had a 1.4 times higher risk of mortality than females (hazard ratio [HR] = 1.4, χ2 = 6.3, df = 1, P = .01); African Americans had about 40% lower mortality risk than whites (HR = 0.57, χ2 = 14.2, df = 1, P = .0002). There was a significant age × smoking interaction (χ2 = 14.6, df = 1, P < .0001), with estimated HRs of 2.1 for smokers vs nonsmokers among 35- to 54-year olds (P < .007) and 0.7 among those 55–69 years old (P = .047). Smoking status in the Cox Model for cardiac related mortality (age 35–54 y) increased the HR to 12.4 (95% confidence interval [CI] = 3.0–51.4; χ2 = 12.0, df = 1, P = .0005). The HR for 55–69 years vs 35–55 years for cardiac mortality was 1.38 (95% CI = 0.71–2.68, χ2 = 0.908, df = 1, P = .341). Cardiac death was too infrequent in 19- to 34-year olds to estimate risk compared with older ages. Other causes of death were too infrequent to model predictors of cause-specific mortality.
Table 2.
All-Cause Mortality: Hazard Ratios by Smoking Status Adjusted for Age, Race, Sex, and Antipsychotic treatment
| Variable | Hazard Ratio (95% Confidence Interval) | Statistics |
|
| χ2 (df = 1) | P | ||
| Male | 1.42 (1.08–1.88) | 6.30 | .012 |
| Age: 19–34 y | 0.47 (0.24–0.94) | 4.58 | .032 |
| Age: ≥55 y | 6.81 (4.34–10.66) | 70.12 | <.001 |
| Black | 0.57 (0.43–0.76) | 14.19 | .0002 |
| Clozapine treatment | 0.77 (0.58–1.01) | 3.49 | .062 |
| Smoking (age: 19–34 y) | 1.24 (0.57–2.70) | 0.291 | .590 |
| Smoking (age: 35–54 y) | 2.06 (1.37–3.10) | 11.87 | .0006 |
| Smoking (age: 55–69 y) | 0.654 (0.42–0.99) | 3.95 | .047 |
Note: Tests for differences by age in excess risk due to smoking: age of 19–34 y vs age of 35–54 y (χ2 = 1.278, df = 1, P = .258); age of 35–54 y vs age of 55–69 y (χ2 = 14.57, df = 1, P = .0001).
Cox model results for association between smoking severity (packs per day) and all-cause mortality by age are presented in table 3. Smoking severity was not associated with excessive risk of mortality among patients 19–34 years old. Among patients 35–54 years of age, heavy smokers (≥1 pack daily, N = 177) had approximately 170% increased risk of mortality than nonsmokers (N = 275) (HR = 2.7, χ2 = 18.6, df = 1, P < .0001), although no increased risk was seen in light smokers (<1 pack per day). Among patients 55–69 years old, compared with nonsmokers, the mortality risk was lower in heavy smokers (N = 34, HR = 0.47, χ2 = 5.2, df = 1, P = .022), and there was a trend for reduced mortality in light smokers (N = 26, HR = 0.5, χ2 = 3.7, df = 1, P = .056).
Table 3.
All-Cause Mortality: Age-Specific HRs Relative to Nonsmokers by Smoking Severity, Adjusted for Race, Sex, and Antipsychotic Treatment
| Age, y | Smoking Severity | HR | 95% CI | χ2 | P Value |
| <35 | <1 ppd | 1.82 | 0.71–4.69 | 1.54 | .215 |
| ≥1 ppd | 0.45a | 0.11–1.87 | 1.20 | .272 | |
| Unknown | 1.83 | 0.65–5.11 | 1.32 | .250 | |
| 35–54 | <1 ppd | 1.05 | 0.55–1.99 | 0.02 | .891 |
| ≥1 ppd | 2.66 | 1.71–4.16 | 18.62 | <.001 | |
| Unknown | 2.08 | 1.23–3.53 | 7.49 | .006 | |
| 55–69 | <1 ppd | 0.51b | 0.26–1.02 | 3.67 | .056 |
| ≥1 ppd | 0.47c | 0.25–0.90 | 5.24 | .022 | |
| Unknown | 1.28 | 0.71–2.31 | 0.66 | .418 |
Note: ppd, pack per day.
Hazard ratio (HR) different from age 35–54 y (P = .020).
HR different from age <35 y (P = .033).
HR different from age 35–54 y (P < .001).
Discussion
People with schizophrenia are known to be at higher risk of premature death; however, the contributions of modifiable risk factors to mortality risk in this population remain largely unknown. In our study, we report the lifetime prevalence of smoking to be 55% in people with schizophrenia. Our findings show that the risk of increased mortality from smoking is significant (HR = 2.1) and is first evident in the middle ages (35–54 y). We did not find an increased risk of mortality in people aged 19–34 years, likely because total mortality in this group was very low and cumulative and detrimental effects of smoking have not had sufficient time to accrue in younger people. Our risk estimates are similar to recent general population estimates as White26 reported that in the general population risk of cardiovascular death in smokers was about twice that of nonsmokers. Of further interest is the 12-fold risk of cardiac related mortality in the middle ages compared with nonsmokers. Overall, these data underscore the importance of planning for the assessment and treatment of cigarette smoking in people with this illness as they are at a significantly increased risk of mortality, particularly, cardiac mortality compared with people with schizophrenia who do not smoke cigarettes.
Our most puzzling finding was that in the older ages (55–69 y) mortality risk was lower for smokers unlike the pattern in the general population. A few reasons may explain this finding. First, our sample included a limited number of people in this age bracket (N = 156), possibly due in part to the low average life span (61 y) in patients with schizophrenia. Second, older patients have many comorbid health problems that may confound any impact of smoking on total mortality.27 Lastly, in keeping with the sharply decreased average in people with schizophrenia compared with the general population, patients who smoke may have died prior to the age of 55 years from cardiovascular related mortality so that older surviving smokers in our sample may have been selected from those least likely to develop smoking-related complications.
Our results also suggest that the greater the number of cigarettes smoked daily, the greater the risk for mortality in persons with schizophrenia. We found that those aged 35–54 years who smoke greater than a pack per day had a 170% increased absolute mortality risk (HR = 2.7) as compared with nonsmokers. These findings are similar to general population data that suggest that disease risk is highest in those who smoke more than 20 cigarettes per day. Additionally, it is known that few systems of the body are unaffected by smoking.28 Smoking is undoubtedly a risk factor for cardiovascular disease and associated mortality (ischemic heart disease, cerebrovascular disease, atherosclerosis, aneurysm); however it is also associated in the general population with an increased risk for mortality from malignant neoplasm (lung, laryngeal, pharyngeal, digestive tract, colorectal, and oral)29,30 and respiratory diseases (pneumonia, chronic obstructive pulmonary disease).18 Thus, all-cause mortality was examined in our current study to include morbidities related to all body systems. Of particular note, cardiovascular disease was the most frequently occurring cause of death, and cardiac related mortality risk was greatly elevated compared with nonsmokers in this our sample. Cancer mortality in this sample (7 cases of cancer death among 664 smokers vs 4 cases among 594 nonsmokers) was too infrequent to ascertain whether smoking increased cancer death risk in this population.
The strengths of this study include the large sample size and specific clinical chart information regarding smoking and substance use. Other studies have relied upon Medical claim data,31 small samples,32 and unclear or combined diagnoses.31,33 The additional chart data we have gathered in this study are significantly richer than administrative data that have many limitations.34 First, diagnoses in administrative data are often inaccurate. Second, listed causes of death such as “cardiac arrest,” eg, may not always reflect the root cause of a problem because many conditions in addition to cardiovascular disease can lead to cardiac arrest. We were careful to include all underlying medical disorders and contributing factors listed on the death records to help determine if deaths listed as cardiac were due to primary or secondary cardiac cause. Third, administrative data may not accurately reflect true medical conditions. Previous studies have found that over 25% of medical record diagnoses do not match administrative data.35 This type of clinical data has many strengths over using administrative or Medicaid data by not being tied to claims. Another strength of this study is the follow-up period of 4–10 years.
Limitations of this study include the retrospective nature of the data gathering and the lack of pack years on all patients who are smokers. Also, the number of deaths for causes other than cardiac disease was too small to analyze for differences in cause-specific mortality. Additionally, patients were identified by having an inpatient hospitalization in state facilities, thus representing a group who likely received medical assistance, had more severe mental illness at the start of mortality follow-up, and who may have been more or less likely to receive adequate medical treatment. The initial sample was identified as being treated with SGAs between 1994 and 2000; however, antipsychotic treatment changed during their course of treatment and use of other concomitant medications or first-generation antipsychotics, as well as information on subsequent antipsychotic changes, were not recorded. All analyses were controlled for clozapine vs other antipsychotic treatment to account for antipsychotic treatment with a high weight gain liability; clozapine treatment was not associated with an increased risk of mortality. Other antipsychotics and concomitant medications may contribute to a differential mortality risk due to different side effect profiles, however, this analysis was unable to control for other treatments. Given the inclusion criteria, these subjects identified through inpatient hospitalization records may not be representative of patients treated entirely in the outpatient sector.
There has been increasing attention focused on the need to improve care for individuals with co-occurring mental illness and cigarette smoking or substance use disorders.36 This underserved population requires the development of evidence-based approaches to improve their lives. This study demonstrates that smoking should be a top priority in the treatment of people with schizophrenia as lives are considerably shortened with tobacco use.
Funding
National Institutes of Mental Health (R03 MH076985-01 to D.L.K.); Advanced Centers for Intervention and Services Research (P50 MH40279).
Acknowledgments
Kelly is a member of advisory board of Solvay, Bristol Myers Squibb, and Janssen. Dr McMahon, Dr Wehring, Ms Liu, Ms Mackowick, Dr Boggs, Dr Warren, Ms Feldman, Dr Shim, and Dixon report no competing interests. Love is a stock shareholder of Glaxo Smith Kline. Preliminary data from this manuscript were presented at the American College of Neuropsychopharmacology Meeting, December 2008, Scottsdale, AZ, and the Society for Research in Nicotine and Tobacco, April 2008, Dublin, Ireland.
References
- 1.Hannerz H, Borga P, Borritz M. Life expectancies for individuals with psychiatric diagnoses. Public Health. 2001;115:328–337. doi: 10.1038/sj.ph.1900785. [DOI] [PubMed] [Google Scholar]
- 2.Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66:183–194. doi: 10.4088/jcp.v66n0205. quiz 147, 273–184. [DOI] [PubMed] [Google Scholar]
- 3.Kelly DL, Boggs DL, Conley RR. Reaching for wellness in schizophrenia. Psychiatr Clin North Am. 2007;30:453–479. doi: 10.1016/j.psc.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 5.Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36:239–245. doi: 10.1177/070674379103600401. [DOI] [PubMed] [Google Scholar]
- 6.Allebeck P. Schizophrenia: a life-shortening disease. Schizophr Bull. 1989;15:81–89. doi: 10.1093/schbul/15.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Allebeck P, Wistedt B. Mortality in schizophrenia. A ten-year follow-up based on the Stockholm County inpatient register. Arch Gen Psychiatry. 1986;43:650–653. doi: 10.1001/archpsyc.1986.01800070036005. [DOI] [PubMed] [Google Scholar]
- 8.Black DW, Warrack G, Winokur G. Excess mortality among psychiatric patients. The Iowa Record-Linkage Study. JAMA. 1985;253:58–61. [PubMed] [Google Scholar]
- 9.Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia: a reexamination. Arch Gen Psychiatry. 2005;62:247–253. doi: 10.1001/archpsyc.62.3.247. [DOI] [PubMed] [Google Scholar]
- 10.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. [Google Scholar]
- 12.Kelly DL, McMahon RP, Liu F, et al. Cardiovascular Disease Mortality in Patients with Chronic Schizophrenia Treated with Clozapine: A Retrospective Cohort Study. J Clin Psychiatry. 2010;71:304–311. doi: 10.4088/JCP.08m04718yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66:1116–1121. doi: 10.4088/jcp.v66n0905. [DOI] [PubMed] [Google Scholar]
- 14.Kelly DL, Wehring HJ, Linthicum J, et al. Cardiac-related findings at autopsy in people with severe mental illness treated with clozapine or risperidone. Schizophr Res. 2009;107:134–138. doi: 10.1016/j.schres.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiihonen J, Lonnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) Lancet. 2009;374:620–627. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- 16.Seeman MV. An outcome measure in schizophrenia: mortality. Can J Psychiatry. 2007;52:55–60. doi: 10.1177/070674370705200109. [DOI] [PubMed] [Google Scholar]
- 17.Auquier P, Lancon C, Rouillon F, Lader M. Mortality in schizophrenia. Pharmacoepidemiol Drug Saf. 2007;16:1308–1312. doi: 10.1002/pds.1496. [DOI] [PubMed] [Google Scholar]
- 18.CDC. The health consequences of smoking: a report of the Surgeon General. www.cdc.gov/tobacco/data_statistics/sgr/2004/index.htm. Accessed November 24, 2009. [PubMed] [Google Scholar]
- 19.de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM. Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry. 1995;152:453–455. doi: 10.1176/ajp.152.3.453. [DOI] [PubMed] [Google Scholar]
- 20.National Household Survey on Drug Use and Health (NSDUH) 2009. www.oas.samhsa.gov/nsduhlatest.htm. Accessed November 24, 2009. [Google Scholar]
- 21.Chaves L, Shirakawa I. Nicotine use in patients with schizophrenia evaluated by the Fagerstrom Tolerance Questionnaire: a descriptive analysis from a Brazilian sample. Rev Bras Psiquiatr. 2008;30:350–352. doi: 10.1590/s1516-44462008005000014. [DOI] [PubMed] [Google Scholar]
- 22.Moeller-Saxone K. Cigarette smoking and interest in quitting among consumers at a Psychiatric Disability Rehabilitation and Support Service in Victoria. Aust N Z J Public Health. 2008;32:479–481. doi: 10.1111/j.1753-6405.2008.00283.x. [DOI] [PubMed] [Google Scholar]
- 23.Catts VS, Catts SV, O'Toole BI, Frost AD. Cancer incidence in patients with schizophrenia and their first-degree relatives—a meta-analysis. Acta Psychiatr Scand. 2008;117:323–336. doi: 10.1111/j.1600-0447.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 24.Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- 25.Rao JNK, Scott AJ. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat. 1984;12:46–60. [Google Scholar]
- 26.White WB. Smoking-related morbidity and mortality in the cardiovascular setting. Prev Cardiol. 2007;10(suppl 1):1–4. doi: 10.1111/j.1520-037x.2007.06050.x. [DOI] [PubMed] [Google Scholar]
- 27.Folsom DP, Lebowitz BD, Lindamer LA, Palmer BW, Patterson TL, Jeste DV. Schizophrenia in late life: emerging issues. Dialogues Clin Neurosci. 2006;8:45–52. doi: 10.31887/DCNS.2006.8.1/dfolsom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mucha L, Stephenson J, Morandi N, Dirani R. Meta-analysis of disease risk associated with smoking, by gender and intensity of smoking. Gend Med. 2006;3:279–291. doi: 10.1016/s1550-8579(06)80216-0. [DOI] [PubMed] [Google Scholar]
- 29.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 30.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 31.Maynard C, Cox GB, Hall J, Krupski A, Stark KD. Substance use and five-year survival in Washington State mental hospitals. Adm Policy Ment Health. 2004;31:339–345. doi: 10.1023/b:apih.0000028896.44429.ca. [DOI] [PubMed] [Google Scholar]
- 32.Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212–217. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- 33.Dickey B, Dembling B, Azeni H, Normand SL. Externally caused deaths for adults with substance use and mental disorders. J Behav Health Serv Res. 2004;31:75–85. doi: 10.1007/BF02287340. [DOI] [PubMed] [Google Scholar]
- 34.Hennessy S, Bilker WB, Knauss JS, et al. Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ. 2002;325:1070. doi: 10.1136/bmj.325.7372.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staffa JA, Jones JK, Gable CB, Verspeelt JP, Amery WK. Risk of selected serious cardiac events among new users of antihistamines. Clin Ther. 1995;17:1062–1077. doi: 10.1016/0149-2918(95)80085-9. [DOI] [PubMed] [Google Scholar]
- 36.Ziedonis DM, Smelson D, Rosenthal RN, et al. Improving the care of individuals with schizophrenia and substance use disorders: consensus recommendations. J Psychiatr Pract. 2005;11:315–339. doi: 10.1097/00131746-200509000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]