Abstract
Impairments in neuropsychological functioning have been described in subjects clinically at high risk for psychosis, but the specific cognitive deficits in different clinical high-risk groups remain to be elucidated. The German Research Network on Schizophrenia employs a heuristic 2-stage model: a putatively late prodromal state (LPS), characterized by the onset of attenuated positive or brief psychotic symptoms, and an early prodromal state (EPS), mainly characterized by the presence of basic symptoms, which are predictive for psychosis within the next 10 years.
A total of 205 subjects met the criteria for either an EPS or an LPS of psychosis and were assessed with a comprehensive neuropsychological test battery. Neurocognitive profiles of high-risk groups were compared with data of 87 healthy controls comparable with regard to gender, age, and premorbid verbal IQ.
Patients in the LPS were impaired in all neurocognitive domains (memory/learning, executive control/processing speed, and working memory) examined, with memory being the worst. Deficits were less pronounced in patients in the EPS, with a specific deficit in the executive control/processing speed domain. Consistent with a progressive neurodevelopmental disorder, some cognitive abilities were already impaired in patients in the EPS, followed by further deterioration in the LPS. Specifically, deficits in executive control functioning were related to the presence of basic symptoms, indicating a vulnerability for psychosis. Memory deficits were associated with the onset of psychotic symptoms indicating further disease progression in the trajectory to psychosis and, thus, may be useful in predicting psychosis and targeting early intervention.
Keywords: clinical high risk, cognition, neuropsychology, memory, executive function
Introduction
Prospective longitudinal studies of birth cohorts and genetic high-risk samples have elucidated the following risk factors and predisposing signs of schizophrenia: family history, obstetric complications (OCs), urban residence, season of birth, low IQ, and delayed developmental milestones.1 Retrospective studies suggest that subjects with a first episode of schizophrenia typically have passed through 3 successive prodromal stages2,3: (1) a nonspecific phase with affective and anxiety symptoms, (2) an early prodromal phase with prominent negative symptoms (mean duration of 5 years), and (3) a late prodromal phase with subthreshold psychotic symptoms (1.1 years on average).
The ultra-high-risk (UHR) criteria outlined by Yung and McGorry4 correspond to the late prodromal phase, which is characterized by attenuated positive symptoms (APS) or brief limited intermittent psychotic symptoms (BLIPS). Patients meeting the UHR criteria have a transition rate to psychosis of approximately 30%–35% within a follow-up period of 1–3 years.5–7
The work of Huber, Gross, and Klosterkötter suggests that some cognitive basic symptoms allow the identification of subjects in an even earlier prodromal state.8,9 Basic symptoms describe subtle, self-experienced, and self-reported deficits in thought and perception. In a clinical sample, Huber’s basic symptoms had high positive predictive values (0.71–0.91) and high specificity. Seventy percent of individuals who showed basic symptoms at baseline developed schizophrenic psychosis in a mean time of 5.6 years.10 Thus, Huber’s basic symptoms are manifested during the early prodromal phase of psychosis.
Neurocognitive investigations have provided further evidence about the developmental course of the disease. Neurocognitive deficits, considered an integral part of the illness,11 are present at the beginning of the first psychotic episode in patients with schizophrenia.12 They are stable over time,13–16 largely independent of positive symptomatology,17,18 and partly independent of medication treatment.19–21 Furthermore, neurocognitive deficits are present in patients before the presence of psychotic symptoms. Prospective birth cohort studies22–25 and genetic high-risk studies26–32 have found specific deficits in measures of verbal memory, working memory, executive function, and attentional functioning in individuals who developed schizophrenia.
Therefore, neurodevelopmental models have become the dominant hypotheses used to explain the pathogenesis of schizophrenia,33,34 which indicate that neuromotor deficits, cognitive abnormalities, and physiological alterations are already present early in life and vary qualitatively and quantitatively with brain development and disease progression.
The aim of the German Research Network on Schizophrenia (GRNS) is to study in depth the appearance of deficits and symptoms in the early course of the illness. It has aimed to replace the established pragmatic (treatment-oriented) definition of subjects at UHR with definitions of an early prodromal state (EPS) and a late prodromal state (LPS), respectively, thereby integrating the basic symptom and the UHR approaches.
The present study focuses on the neurocognitive profiles of these 2 groups. Previous studies of clinical high-risk subjects indicated that they perform at an intermediate level between healthy controls and first-episode psychosis patients in multiple cognitive functional domains.35–39 In particular, patients who fulfilled UHR criteria have shown impairments in tasks involving executive control, verbal learning/memory, motor control, and general intellectual functioning.36,40–43
However, the generalizability of the results and the ability to draw conclusions about specific deficits were limited because of several methodological issues. Most studies used healthy control groups that were not comparable with regard to premorbid intellectual functioning, educational level, or demographical characteristics,37,40,42,43 whereas others used population norms of individual tests obtained from different normative samples.36,41 Furthermore, some used small and selective test batteries38,42,43 or compared data of single tests35,36,40,42 or composite scores with unconfirmed factor structures.38,41 In addition, sample sizes were small and consisted mainly of patients with APS. Possible differences between patients with APS and those with BLIPS and the effects of psychotropic medication on neuropsychological functioning were not generally addressed in previous studies. Finally, the issue of specific and generalized impairments has received little attention so far in UHR studies. Lencz et al44 addressed many of the above-mentioned criticisms in their study. They found lower current estimated IQ scores than predicted from premorbid IQ scores in UHR individuals. In addition, they reported generalized deficits across cognitive domains and specific deficits in verbal learning and executive functions, whereas visual–spatial functions were relatively spared in UHR patients compared with individuals in a healthy control group, who were comparable with regard to years of education and demographical characteristics. Verbal memory deficits in UHR patients predicted psychosis within a mean follow-up time of 2 years.
To summarize, previous studies with subjects fulfilling UHR criteria provide clear evidence for cognitive dysfunction; however, the time point at which the generalized or specific deficit emerges is unclear. Only 2 recent studies considered neuropsychological functioning of subjects in a putatively earlier prodromal state and compared their performances with UHR subjects in an LPS.37,45 Both failed to find substantial differences between high-risk groups. Pukrop et al37 reported significant impairments in the EPS compared with controls in measures of verbal learning and verbal fluency; however, Simon et al45 did not find neurocognitive impairments compared with help-seeking patient controls. Small sample sizes and small effect sizes might account for these conflicting results.
Thus, it is not clear whether high-risk individuals in an EPS have any cognitive deficits at all and, if so, whether these deficits are qualitatively similar to those found in UHR individuals in an LPS. This study expands the scope of previous investigations by addressing the neurocognitive functions of 2 different high-risk groups in comparison to a healthy control group (comparable with respect to demographical data and general intellectual ability) and by investigating whether generalized or specific cognitive deficits exist and whether these deficits are related to current medication treatment, current (negative, positive, or depressive) symptoms, and general functioning in everyday life.
Our hypotheses are as follows:
High-risk subjects in an EPS and those in an LPS have generalized neurocognitive deficits compared with subjects in the healthy control group.
Measures of executive function and verbal memory are more impaired than those of other domains in the LPS.
Individuals in an EPS of psychosis perform intermediate between the subjects in the healthy control group and individuals in the LPS of psychosis.
Methods
Data presented here were collected at the Early Recognition and Intervention Centers of the Departments of Psychiatry at the Universities of Bonn, Cologne, Düsseldorf, and Munich as part of the GRNS. The basic design of this early detection and intervention study has been outlined elsewhere.46
Briefly, a 2-step approach was employed to identify individuals with a high risk to develop psychosis. A screening instrument was used to help seek people who had approached general practitioners or mental health professionals, followed by a detailed assessment at the Early Recognition and Intervention Centers using the Early Recognition Inventory/Interview for the Retrospective Assessment of the Onset of Schizophrenia (ERIraos).46 Patients were included in the study trial if they met the criteria for the EPS or LPS (Appendix A) and did not fulfill exclusion criteria (Appendix B). All participants spoke German fluently and had normal or corrected-to-normal vision, normal hearing, and normal motor limb function.
Subjects
A total of 205 subjects fulfilled the inclusion criteria for either an EPS (n = 116) or an LPS (n = 89). Eighty-seven healthy controls who were comparable with subjects in the clinical high-risk groups with regard to age, sex, and verbal intelligence were recruited from the same geographical region by advertising in local newspapers or through word of mouth. Controls were screened to exclude subjects with any past or present psychiatric, neurological, or somatic disorder possibly affecting cognition, and all negated current use of psychotropic medication or illicit drugs. Moreover, none of the control subjects fulfilled inclusion criteria for an EPS or LPS (Appendix A).
After a complete description of the recognition and intervention study was provided, written informed consent was obtained from all participants. This study was approved by the institutional review boards of all participating universities.
Psychopathological Assessment
Following inclusion, subjects underwent a detailed assessment of psychopathological symptoms with the ERIraos.46A Global Assessment of Functioning (GAF47) score was obtained on a 100-point numeric scale that provides an index of overall psychological, social, and occupational functioning. The Positive and Negative Syndrome Scale (PANSS) was employed to evaluate the presence and absence of positive, negative, and general psychopathology of schizophrenia.48 The Montgomery-Åsberg Depression Rating Scale (MADRS), scored on the basis of semi-structured interviews, was used to assess the affective, cognitive, and vegetative dimensions of depression.49 To ensure eligibility for the study, participants were interviewed by trained psychiatrist or psychologist using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).50
Neuropsychological Assessment
The assessment procedure consisted of the administration of 8 tests in a fixed order. The tests measured neuropsychological functions that are known to be impaired in patients with schizophrenia.51,52 In addition, a short motor examination was conducted. Verbal IQ was examined with a word recognition test.53 The tests and cognitive functions under examination are outlined in table 1. Most of the tests are well described elsewhere,55,61 and brief descriptions of the tests and measures are given in the Supplementary materials.
Table 1.
Neuropsychological Assessment Battery
| Functional Domain (A Priori) | Test | Variable Employed |
| Premorbid verbal IQ | Vocabulary test (Mehrfachwahl-Wortschatz-Intelligenztest53) | Raw score correct |
| Executive control/processing speed | ||
| Visual motor speed | Trail-Making Test, parts A and B54 | Time to completion |
| Verbal fluency | Verbal Fluency55 | Sum of correct responses |
| Perceptual motor speed | Digit Symbol Coding Test56 | Raw score correct |
| Working memory | ||
| Sustained attention | Continuous Performance Test—Identical Pairs57 | Signal detection indices D′ |
| Working memory | Letter-Number Sequencing56 | Raw score correct |
| Memory/learning | ||
| Visual memory | Self-ordered Pointing Task, 12-item version58 | Number of errors |
| Verbal learning and memory | Rey Auditory Verbal Learning Test T1, ∑T1–T5, Delayed Recall, and Recognition59 | Number of correct words |
| Motor control | Brief Motor Scale60 | Grades |
Data Analysis
Clinical and neuropsychological data at baseline assessment were analyzed in this study. Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS 14.0; SPSS Inc, Chicago, Illinois). The SPSS Missing Value Analysis was performed to estimate missing data. Subjects with more than 3 missing values of 30 neuropsychological measures were excluded from the analyses. Each variable in the analysis had no more than 3% missing values. Missing values were estimated with the expectation maximization algorithm.62
The distribution of variables was examined within and between groups. Scaling procedures were applied to improve psychometric properties before subjecting variables to variance analytic procedures or to the computation of cognitive domain scales. Each variable was examined for outliers. Outliers were replaced by a score of 3.5 SDs below or above the mean. Neuropsychological test scores were standardized by computing z scores based on the performance of the control group separately for each center. Negative z scores indicate worse performance than controls.
For the purposes of data reduction and the examination of generalized and specific deficits across cognitive domains, 12 neuropsychological test variables were selected and grouped according to neuropsychological conventions and previous literature findings (eg, Lencz et al44). Three cognitive domains were constructed: memory/learning, executive control/processing speed, and working memory. Scores for each cognitive domain were computed by averaging the z scores on contributing variables. Domain scores were then subjected to z transformation so that deficits in high-risk patients could be displayed relative to control group performance. Factor analysis was used to assess the validity of the a priori assignments of variables to scales. Using the correlation matrix (Supplementary table 1), we evaluated neuropsychological test results to assess relationships among neuropsychological measures. Principal component analysis (PCA), followed by varimax rotation and an eigenvalue cutoff of 1, was performed to extract components. The following measures were selected for analysis:
Trail-Making Test (TMT): time taken to complete each part of this 2-part neuropsychological test (TMT-A and TMT-B),
Verbal fluency: sum of correctly produced words beginning with the letters S, A, B, and N, respectively,
Continuous Performance Test—Identical Pairs: the signal detection indices D′ for the number and shapes subtest,
Self-Ordered Pointing Task (SOPT): errors in the 12-item version of the SOPT,
Letter-Number Sequencing (Wechsler Adult Intelligence Scale III [WAIS-III]): number of correctly ordered sequences,
Digit Symbol Coding Test (WAIS-III): number of correct symbols drawn within 90 s, and
Auditory Verbal Learning Test: 4 measures were selected: the delayed recall score, the recognition score, the verbal learning score (∑T1–T5), and the number of words recalled after the first learning trial (T1).
Following the PCA, 3 components emerged from these 12 variables, which explained 60% of the variance. The loadings of the test scores on the varimax-rotated factors confirmed the a priori assignment of the test to the 3 cognitive domains (Supplementary table 2).
In addition, a motor control score was computed by summing up the scores (each ranging from 0 to 2) of 2 selected motor tasks of the Brief Motor Scale (BMS).
Statistical Analyses
Demographic information and psychopathological scores were compared among the 2 high-risk groups and the control group using chi-square tests for categorical variables and 1-way analysis of variance (ANOVA) for continuous variables. Post hoc comparisons between study groups were conducted by performing least significant difference tests.
Two-way multivariate analysis of variance (MANOVA)—with the cognitive domain scale as the repeated measures factor and the group as the between-subject factor—was conducted to assess overall differences between groups and profile shapes. If a significant interaction between the cognitive domain and the group factor emerged, deviations from flatness in the high-risk group profiles could be assumed. The deviations were assessed to examine relative impairment within a high-risk group by contrasting the mean for each cognitive domain with the mean of the remaining domains using paired t tests. The purpose was to show impairment relative to the other scales in the high-risk groups. In cases of such a relative impairment, we further examined whether the impairment (compared with controls) was independent of performance in other cognitive domains by using analysis of covariance, with the domain scale as the dependent variable and the other cognitive domain scales as covariates.
Following the repeated measures analysis, a univariate ANOVA was performed for each individual domain score. Multiple pairwise contrasts were carried out and the Bonferroni correction for multiple comparisons was applied (familywise α of 5%) if significant main effects for a group were found. Group differences in our motor coordination scale were analyzed using contingency tables and the Fisher exact test.
The relationships between cognitive domain scales and psychopathological scales were examined within patient groups using Pearson’s correlation coefficients. To limit multiple testing, correlation analysis was restricted to the 3 cognitive domain scores and to scales measuring depression (MADRS) and positive and negative symptoms (PANSS), respectively. To avoid false-positive results without being overly conservative, we employed an α error of 1% (2-tailed) for these correlations.
The possible confounds of current psychotropic medication were analyzed by adding psychotropic medication (yes/no) to the MANOVA model. In addition, group sizes permitted us to examine differences between subgroups of patients in the EPS who were included because they either fulfilled the combined trait and state criteria outlined by Yung and McGorry4 or possessed basic symptoms only. In the LPS group, we compared individuals with BLIPS and those with APS only.
Results
Demographic and Clinical Characteristics
Demographic and clinical data of the study groups are displayed in table 2. The 3 groups did not significantly differ in terms of mean age, gender distribution, and mean premorbid intellectual functioning. Most patients (∼95%) in both EPS and LPS groups reported at least 1 basic symptom. In the LPS group, most patients (∼95%) had APS. However, only about half of the LPS group (∼55%) had ever experienced a BLIPS episode.
Table 2.
Demographic and Clinical Characteristics of the Study Sample (N = 292)
| Control Group | Early Prodromal State | Late Prodromal State | Test Statistic | P | |
| N | 87 | 116 | 89 | ||
| Age, mean (±SD; range) | 25.5 (±4.4; 18–39) | 25.6 (±6.1; 17–40) | 25.3 (±6.4; 17–41) | F(2, 289) = 0.09 | .918 |
| Gender (male/female), N (%) | 49/38 (56.3/43.7) | 76/40 (65.5/34.5) | 53/36 (59.6/40.4) | χ2(2) = 1.87 | .392 |
| Premorbid verbal intelligence, mean (±SD) | 108.8 (±13.1) | 108.5 (±12.6) | 106.2 (±13.8) | F(2, 289) = 1.11 | .331 |
| ERIraos, N (%) | |||||
| Brief limited intermittent psychotic symptoms | — | 0 (0) | 49 (55.1) | ||
| Attenuated positive symptoms | — | 0 (0) | 85 (95.5) | ||
| Basic symptoms | — | 112 (96.6) | 84 (94.4) | ||
| Genetic risk | — | 25 (21.6) | 17 (19.1) | ||
| Obstetric risk | — | 26 (22.4) | 10 (11.2) | ||
| GAF reduction | — | 78 (67.2) | 58 (65.2) | ||
| PANSS, mean (±SD) | |||||
| PANSS positive | — | 9.1 (±2.3) | 12.9 (±3.9) | F(1, 188) = 70.5 | <.001 |
| PANSS negative | — | 10.8 (±3.6) | 15.0 (±4.8) | F(1, 188) = 45.8 | <.001 |
| PANSS global | — | 29.1 (±6.5) | 32.4 (±7.4) | F(1, 188) = 10.7 | .001 |
| Montgomery-Åsberg Depression Rating Scale, mean (±SD) | — | 19.5 (±7.9) | 17.6 (±7.2) | F(1, 188) = 2.98 | .086 |
| GAF, mean (±SD) | — | 58.9 (±10.7) | 59.1 (±12.2) | F(1, 179) = 0.01 | .914 |
Note: GAF, Global Assessment of Functioning; PANSS, Positive and Negative Syndrome Scale; ERIraos, Early Recognition Inventory/Interview checklist.
A total of 25 patients (21.6%) in the EPS group and 17 patients (19.1%) in the LPS group had a first-degree relative with schizophrenia spectrum disorder. Pregnancy or delivery complications, termed collectively as OCs, were reported in 26 patients (22.4%) of the EPS sample and in 10 patients (11.2%) of the LPS sample. A GAF score reduction of about 30 points was common in both high-risk groups. A combination of genetic risk plus GAF reduction was present in 19 participants (16.4%) in the EPS sample. The distribution and overlap of risk factors in the study sample are illustrated in Supplementary figure 1.
Patients in the LPS group had higher positive, negative, and global PANSS scores than those in the EPS group. These differences are expected because there is some overlap between symptoms on these scales and group assignment criteria; however, the focus of group assignment criteria is on lifetime appearance rather than the acute expression of symptoms.
The level of depressive symptoms was not significantly different between high-risk groups. In the EPS and LPS groups, the majority of subjects did not receive any psychotropic medication at the time of testing (75.9% and 76.4%, respectively) or over the lifetime (67.2% and 70%, respectively). In the EPS group, 22 patients were currently treated with antidepressants, 3 patients with tranquilizer, and 6 patients with other psychotropic medications. In the LPS group, 9 patients were treated with neuroleptics, 11 patients with antidepressants, and 6 patients with tranquilizer.
Cognitive Domain Scales
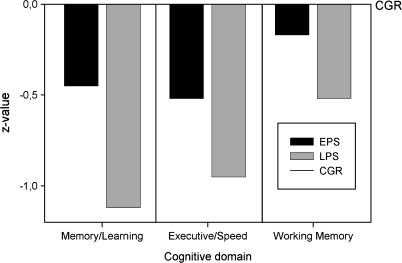
Neuropsychological profiles for the high-risk groups are illustrated in figure 1. The LPS group was impaired relative to the healthy control group for every cognitive domain score, with effect sizes (z scores) ranging from −1.12 to −0.52. The EPS group performed intermediate to the LPS group and the control group, with effect sizes ranging from −0.52 to −0.17.
Fig. 1.
The Mean Scale Scores for the 3 Cognitive Domains of Subjects in the Early Prodromal State (EPS) and of Those in the Late Prodromal State (LPS), Presented as z Score Deficits Relative to Healthy Control Group (CGR) Subjects (Mean Set to 0 and SD Set to 1).
The MANOVA of all 3 domain scores revealed a significant main effect for group factor, which indicated that groups differed with regard to cognitive functioning (F(2, 289) = 25.15, P < .001, η2 = .15). Both the LPS (Cohen’s D = −1.0) and the EPS (Cohen’s D = −0.48) groups showed a significant general cognitive deficit relative to healthy controls. Additionally, the LPS group performed worse than the EPS group when averaged over cognitive domains (all simple contrasts between groups with P < .001).
The profile of the high-risk groups deviated significantly from flatness as indicated by the significant interaction between group and cognitive domain factors (F(4, 578) =2.675, P = .031, η2 = .018). This finding suggests a selective pattern of impairment. In the EPS group, the executive control domain was significantly more impaired (paired t(115) = −1.98, P =.025) and the working memory scale was significantly less impaired (paired t(115) = 2.76, P < .001) in comparison to the mean of the remaining domains. In the LPS group, the memory domain was more impaired (paired t(88) = −2.74, P = .007) and the working memory domain was less impaired (paired t(88) = 3.87, P < .001) in comparison to the mean of the remaining domains.
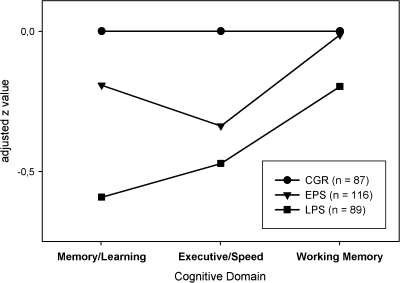
Results from univariate ANOVA for each cognitive domain demonstrated that effect sizes were medium on the memory (η2 = .12) and executive control (η2 = .10) scales and small on the working memory (η2 = .038) scale (table 3). Simple comparisons revealed that on the memory and executive control scales, the EPS group had lower scores than the healthy control group. However, the working memory performance values did not differ between the EPS and control subjects. The impairment on the executive control scale in the EPS group relative to controls remained significant after the domain score was residualized against performance on the other cognitive domains, but the significant difference on the verbal memory scale disappeared (figure 2).
Table 3.
Comparison of Cognitive Domain Scales Among EPS and LPS Patients and CGR
| Cognitive Domain | High-Risk Subjects |
One-way ANOVA |
||||||||
| Scale |
EPS (n = 116) |
LPS (n = 89) |
||||||||
| Alpha | SEM | Mean | SD | Mean | SD | F | df | P | Significant Post Hoc Test | |
| Memory/learning | .83 | 0.26 | −0.45 | 1.21 | −1.12 | 1.37 | 19.296 | 2.289 | <.001 | LPS < EPS < CGR |
| Executive control/processing speed | .76 | 0.22 | −0.52 | 1.19 | −0.95 | 1.02 | 16.758 | 2.289 | <.001 | LPS < EPS < CGR |
| Working memory | .60 | 0.13 | −0.17 | 1.02 | −0.52 | 1.11 | 5.663 | 2.289 | .004 | LPS < CGR |
Note: Healthy comparison group (CGR) has by definition of scales means and SD of 0 ± 1 and were omitted from table; 1-way ANOVA was computed for each measure with group (CGR, EPS, and LPS) as between-subject factor. For a familywise significance level of α = .05, testwise significance level was Bonferroni corrected to α = .0042. EPS, early prodromal state; LPS, late prodromal state; CGR, control group; ANOVA, analysis of variance; SEM, standard error of the mean.
Fig. 2.
Profiles of the Mean Scale Scores for Each Cognitive Domain of Subjects in the Early Prodromal State (EPS) and of Those in the Late Prodromal State (LPS), Calculated as z Scores Relative to Healthy Control Group (CGR) Subjects, Taking Into Account the Influence of the 2 Remaining Scores.
The LPS group had significantly lower scores than the EPS and healthy control groups on the verbal memory and executive control scales. Working memory performance scores of the LPS group significantly differed from those of the healthy control group but not from those of the EPS group. The relative impairment of verbal memory function in the LPS group remained significantly relative to the healthy control group and the EPS group even when the verbal memory score was controlled for performance on other cognitive scales (figure 2).
Neuropsychological Test Scores
The performances of both high-risk groups are compared with those of healthy controls for each individual neuropsychological test in table 4. This table also summarizes results of univariate ANOVA, together with paired contrasts for multiple comparisons. The nonparametric analysis of the motor control scale showed that more subjects in the EPS (27%, V = 8.0, P = .017) and LPS (24.6%, V = 6.6, P = .033) had motor control impairments in comparison to healthy controls (13.3%).
Table 4.
Scores, z Values, and Univariate ANOVAs on Neuropsychological Tests Among EPS and LPS Group and Healthy CGR
| Measure | CGR (n = 87) |
High-Risk Groups |
One-way ANOVA |
|||||||||
| EPS (n = 116) |
LPS (n = 89) |
|||||||||||
| Mean | SD | Mean | SD | z Score | Mean | SD | z Score | F | df | P | Significant Post Hoc Test (Uncorrected) | |
| Memory/learning | ||||||||||||
| AVLT_RE | 14.27 | 1.15 | 14.29 | 1.26 | −0.02 | 13.67 | 1.59 | −0.59 | 5.485 | 2.289 | .005 | LPS < EPS, CGR |
| AVLT_DR | 13.05 | 2.13 | 12.71 | 2.56 | −0.27 | 11.60 | 3.00 | −0.84 | 9.802 | 2.289 | <.001 | LPS < EPS, CGR |
| AVLT_∑T1–T5 | 61.47 | 7.90 | 58.59 | 8.34 | −0.63 | 54.49 | 8.84 | −1.20 | 24.697 | 2.289 | <.001 | LPS < EPS < CGR |
| AVLT_T1 | 8.84 | 2.61 | 8.12 | 2.04 | −0.65 | 7.47 | 1.99 | −1.00 | 21.78 | 2.289 | <.001 | LPS < EPS < CGR |
| SOPT | 2.64 | 1.96 | 2.45 | 1.94 | −0.06 | 3.08 | 2.15 | −0.54 | 5.261 | 2.289 | .006 | LPS < EPS, CGR |
| Executive control/processing speed | ||||||||||||
| TMT-B | 56.61 | 15.74 | 65.25 | 21.30 | −0.68 | 74.89 | 23.95 | −1.44 | 21.868 | 2.289 | <.001 | LPS < EPS < CGR |
| TMT-A | 23.76 | 6.12 | 26.23 | 8.55 | −0.56 | 28.81 | 9.97 | −1.13 | 13.984 | 2.289 | <.001 | LPS < EPS < CGR |
| DSC | 62.69 | 10.67 | 60.97 | 11.09 | −0.36 | 57.31 | 11.71 | −0.77 | 11.282 | 2.289 | <.001 | LPS < EPS, CGR |
| Verbal fluency | 60.60 | 13.83 | 53.85 | 12.64 | −0.51 | 50.13 | 11.71 | −0.81 | 18.333 | 2.289 | <.001 | LPS, EPS < CGR |
| Working memory | ||||||||||||
| CPT-Shapes_D′ | 2.00 | 0.72 | 2.02 | 0.70 | 0.01 | 1.78 | 0.71 | −0.29 | 2.692 | 2.289 | .069 | — |
| CPT-Numbers_D′ | 1.77 | 0.73 | 1.62 | 0.71 | −0.20 | 1.40 | 0.75 | −0.47 | 5.062 | 2.289 | .007 | LPS < CGR |
| LNS | 17.62 | 2.85 | 17.34 | 3.08 | −0.18 | 16.73 | 3.15 | −0.36 | 2.492 | 2.289 | .085 | — |
Note: One-way ANOVA was computed for each measure with group (CGR, EPS, and LPS) as between-subject factor. Simple contrasts were uncorrected for multiple testing. EPS, early prodromal state; LPS, late prodromal state; CGR, control group; AVLT, Auditory Verbal Learning Test; RE, Recognition; DR, Delayed Recall; TMT, Trail-Making Test; DSC, Digit Symbol Coding Test; CPT, Continuous Performance Test; LNS, Letter-Number Sequencing.
Moderator Variable Analysis
Depressive symptoms were not correlated with memory, executive control, and working memory scores in either patient group (all |r| < .10). In the EPS group, memory was related to the PANSS positive symptom scale (r = −.26, P = .006). In the LPS group, executive control was correlated with the PANSS negative symptom scale (r = −.30, P = .007). No other association reached significance.
When we repeated the MANOVA and included medication as a factor, we did not find a significant main effect of medication (P > .5). We also did not detect significant interactions with the neuropsychological domain factor (P > .5) or the group factor (P > .5) Thus, no differences in neurocognitive functioning were detected between subjects who were currently treated with a psychotropic substance and those who were not treated.
Subgroups of High-Risk Individuals
No significant differences were revealed between individuals with basic symptoms only in the EPS group and those with genetic risk plus GAF reduction over all cognitive domains in the EPS group (F(1, 112) = 0.336, P = .563, η2 = .003). We also did not detect any interactions between the group and cognitive domain factors (F(2, 224) = 1.054, P = .316, η2 = .010). In the LPS group, ANOVA revealed no significant differences between individuals with BLIPS and those with APS only, neither to the overall effect (F(1, 87) = 0.006, P = .939, η2 < .001) nor to the interaction between factors (F(2, 174) = 0.918, P = .401, η2 = .010).
Discussion
This study, which employs one of the largest systematically obtained samples of putatively prodromal subjects thus far, sheds new light on the neurocognitive profiles of at-risk mental states in presumably subsequent stages of schizophrenia. There are 3 main findings of the study. First, both EPS and LPS subjects had some general cognitive dysfunction with respect to healthy controls, which was more pronounced in the LPS compared with the EPS. Second, specific executive control deficits were present in the EPS, which were over and above deficits in other cognitive domains. Third, the verbal memory deficit was specifically increased in the LPS, independent of impairments in other cognitive domains.
Neuropsychological Impairments
Averaged over all cognitive domains, performance was impaired in both high-risk groups in comparison to the control group and in the LPS group in comparison to the EPS group. The effect size of impairment relative to controls was large (Cohen’s D ∼ 1.0) for the LPS and medium (Cohen’s D ∼ 0.5) for the EPS, despite group matching for verbal (premorbid) IQ.
Our findings are in general agreement with those of several previous studies, which examined samples of individuals comparable to our LPS group.36,38,40,43,44 The moderate deficits in the EPS are significant in the current large sample and are similar in size to the deficits described in 2 previous smaller studies, which examined subjects with basic symptoms.37,45
Beyond this deficit, we found that executive control, verbal memory, and working memory performance deficits are present in late high-risk stages (LPS).35,36,38,40,44 Patients in the EPS of psychosis performed intermediate between patients in the LPS and control subjects on tasks measuring executive control and verbal memory function. The working memory performance of subjects in the EPS did not differ from those in the control group. In addition, verbal memory and executive control performances were more impaired in the LPS than in the EPS. Within the limits imposed by the cross-sectional design, this pattern is consistent with the hypothesis that the trajectory through the prodromal stages of psychosis is associated with increasing levels of cognitive impairment. Furthermore, in partly overlapping samples, additional differences among patients in an EPS and patients in an LPS related to neurophysiological measures of information processing63,64 and structural brain abnormalities were found.65
The hypothesis of increased impairments during prodromal states is also supported by prospective longitudinal studies, which have revealed small to medium deficits during childhood and adolescence in subjects who later developed schizophrenia.30,66 For example, 1 study found that conscripts at 17 years who had lower than expected IQ scores compared with estimated IQ scores during childhood (based on reading and spelling abilities) had an increased risk for hospitalization for schizophrenia.67 Other studies revealed that intellectual deficits are greater in those closer to psychosis onset.25,68,69
Taken together, these findings suggest mild cognitive impairments early in childhood as manifestation of neurodevelopmental abnormalities70,71 and an increase of neurocognitive impairment in successive stages with the appearance of prodromal symptomatology.34
These neuropsychological impairments could be the consequences of the presence of depressive symptoms in high-risk individuals. However, we did not detect a correlation between depressive symptoms (assessed with the MADRS) and cognitive performance.
The verbal IQ appears to be slightly above average in all groups with means ranging from 106 to 108. Most likely, this is a consequence of outdated test norms. We employed the Mehrfachwahl-Wortschatztest-Intelligenztest (MWT-B), which had been standardized in a representative German population in 1975. Satzger et al72 showed that the MWT-B overestimates verbal IQ, relative to the verbal IQ obtained with the German Wechsler Adult Intelligence Scale-Revised. This underlines the importance of studying control groups rather than relying on published test norms to identify cognitive deficits and deficit profiles in clinical groups.
Executive Control Impairment as a Putative Vulnerability Marker
The executive control domain score was already impaired in the EPS of psychosis relative to healthy controls. This deficit in executive control was greater than—and independent of—impairments in other cognitive domains. Following the adjustment of the executive control domain scores for deficits in both the other cognitive domains, the differences between healthy controls and both high-risk groups remained significant. However, the differences between the high-risk groups disappeared, indicating that executive control is affected early in the prodromal state (before the appearance of first positive symptoms). We also found that executive performance did not differ between individuals with only basic symptoms and patients with a genetically high risk and GAF reduction, suggesting that both sets of criteria for EPS identify risk groups with similar slight cognitive deficits.
The executive control/processing speed domain scale in this study comprised a variety of tasks that require the rapid coordination of internal and external stimulus processing, response selection, and execution. Deficits in similar tasks have been reported in subjects as early antecedents of psychosis. In the Dunedin study, Cannon et al73 found impairments on the TMT, a verbal fluency task, and a motor coordination task (Pegboard) in subjects aged 13 years who later developed a schizophreniform disorder. Such deficits were not found in adolescents who developed affective disorder. In another birth cohort study, Niendam et al22 analyzed 7 subtests of the Wechsler Intelligence Scale for Children, which had been administered to 32 individuals aged 7 years who developed schizophrenia in adulthood and to 25 of their nonschizophrenic siblings. Compared with their nonschizophrenic siblings, patients exhibited more severe deficits only on the digit symbol coding task. As found with individuals in the EPS, these studies indicate that executive control impairments occur many years before the onset of overt psychosis.
Correlations between cognitive performance and PANSS symptoms were generally low and nonsignificant. One exception to this was that executive control performance was associated with the severity of negative symptoms in the LPS group but not in the EPS group. Because the most prominent deficits in the LPS group were in the memory domain, this may seem counterintuitive. However, executive dysfunctions tend to correlate most closely with negative symptoms also in patients with schizophrenia, despite patients tend to have marked memory impairments.74–77 As a group, LPS can be considered to be closer to the onset of schizophrenia, or to be more likely to convert, than EPS. The specific correlation between poor executive control and more negative symptoms found both in patients with schizophrenia and in patients in LPS further underlines the similarity between these groups.
Antecedents of deficits in the control of execution seen in clinical high-risk individuals, as well as in birth cohort studies indicating abnormalities in brain development, might include early motor developmental deviances.78,79 In our study, both high-risk groups showed abnormal motor control performance measured with the BMS, which is consistent with findings in subjects with an at-risk mental state.42
Memory Impairment as a Putative Marker of Disease Progression
Verbal memory deficit has been regarded as one of the major deficits in schizophrenia. Meta-analyses of verbal memory deficits in patients with schizophrenia report effect sizes of 1.21–1.41 in measures of learning and effect sizes of 0.90–1.20 in measures of delayed free recall.51,80,81 Clinical high-risk studies have consistently found verbal memory impairments in the LPS of psychosis38,40,41 and that the presence of these deficits predicts who later develops psychosis.44
We found that verbal memory performance was impaired in the LPS group compared with the EPS and healthy control groups even if the verbal memory deficit was controlled for the performance in the other cognitive domains. Thus, our study suggests that a verbal memory deficit may be a marker of disease progression in clinical high-risk individuals. This agrees with the finding that verbal memory performance was inversely correlated with the severity of positive symptoms in individuals in an EPS of psychosis. The lack of such a correlation in the LPS group, despite prominent memory deficits, is again reminiscent to findings in schizophrenia, where verbal memory deficits are largely independent of psychotic symptoms and stable over time.82 However, in individuals in a high-risk state of psychosis, the presence of positive symptoms may indicate disease progression, which might be related to the severity of the verbal memory dysfunction. Cosway et al28 found that an increase in psychotic symptoms between repeated assessments of neuropsychological function in genetic high-risk individuals was related to a decline in verbal memory performance and general IQ. Furthermore, volumetric reductions in prefrontal-hippocampal networks subserving verbal memory functions have been shown to mark the transition to psychosis in UHR individuals.83–85
Verbal memory performance is influenced by numerous neuropsychological functions.82 As shown in table 4, individuals in the EPS also had deficits in some memory parameters, indicating an encoding deficit rather than a recall deficit. By contrast, recognition and recollection were also impaired in subjects in the LPS, suggesting a deficit in storage or retention. In a recent structural magnetic resonance imaging study of a subgroup of the EPS and LPS subjects included in the present study, we found evidence of reduced hippocampal volume in both the EPS and the LPS.86 However, a low hippocampal volume was related to poor recall performance only in the LPS. This finding suggests that subjects in the EPS are still able to compensate functionally for a mediotemporal structural vulnerability, whereas those in the LPS no longer possess this ability.
In our present study, the number of prodromal subjects with BLIPS was relatively high (55%). The proportion of subjects with BLIPS varies considerably between studies, and the sources of this variation are largely unclear. For example, Yung et al6 reported 24.5% UHR subjects with BLIPS, while in another larger sample of the Melbourne group, no subject met BLIPS criteria.87 The North American Prodrome Longitudinal Study7 reported 3.7% with BLIPS, while Mason et al88 in their UHR sample in Canberra, Australia, reported 31% with BLIPS. In our study, the high proportion of subjects with BLIPS in the LPS group may have been due to a higher age of prodromal subjects, different recruitment pathways, and the grouping of subjects with a genetic risk factor plus functional deterioration (but without APS, n = 25) into EPS. Importantly, however, we found no neuropsychological differences between LPS subjects with or without BLIPS, suggesting that the prior occurrence of BLIPS is not related to cognitive functioning.
Working Memory Deficits
Among the 3 domains examined, working memory appears to be least affected in high-risk individuals. Compared with controls, individuals in the EPS were not impaired, and the impairment was only moderate in those in the LPS. The negative finding in the EPS is in-line with some other data.37,45 Implications of impairments in working memory and sustained attention in individuals in the LPS and their putative roles as neurocognitive markers and predictors of later outcomes are comprehensively discussed elsewhere.26,27,43,89,90
Conclusions
We found the cognitive abilities of subjects in 2 putatively sequential prodromal stages of psychosis to be differentially impaired. Executive control seems to be compromised in the EPS (prior to the onset of positive symptoms), whereas verbal memory dysfunctions appear to evolve during a later prodromal stage. The qualitative differences between deficits in the early and late high-risk subjects cannot be explained by assuming that a larger proportion of “true” prodromal subjects exist in the LPS group, as a result of more valid clinical risk criteria. Rather, the overall pattern of findings suggests that progressive cognitive deficits exist in parallel with supposed disease progression. However, the cross-sectional nature of this study limits conclusions regarding the temporal sequence of impairment. Together with small-scale longitudinal data,85 the present findings contribute to the emerging view of the prodrome as a period of time when marked dynamic brain changes occur.91 Additional longitudinal studies, including those involving neuroimaging,92–94 are needed to understand, and possibly combat, the dynamic biological changes that occur during this critical time window.
Some data indicate that cognitive deficits are more pronounced in “true prodromals,” which develop frank psychosis.39,44,95 The longitudinal clinical data of our study will reveal whether different patterns of cognitive impairment predict psychosis in early or late prodromal stages.
In patients with schizophrenia, neurocognitive deficits, more than negative or positive symptoms, determine social and vocational outcome.96 Therefore, cognition has become a treatment target in itself. The present results, together with prior evidence, imply that many subjects at risk for psychosis already have substantial cognitive deficits, and these deficits most likely limit the ability of patients to achieve their personal goals. A cognitive assessment should therefore be offered to help-seeking subjects with “prodromal symptoms” in order to detect problems that might compromise educational or occupational success. In addition, treatment trials in this population should consider cognition as an outcome in order to improve deficits or even to prevent cognitive decline, which apparently occurs very early in the prodrome.97
Supplementary Material
Supplementary materials are available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Bundesministerium für Bildung und Forschung (BMBF 01 GI 9934, 01 GI 0234).
Supplementary Material
Acknowledgments
We thank Mrs Gabi Herrmann, who assisted with the acquisition of data. The funding source had no further role in the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the paper for publication.
Contributors
Frommann, Brinkmeyer, Ruhrmann (MD), Pukrop (MD), Berning, Decker, Bechdolf (MD), Wölwer (PhD), Klosterkötter (MD), Maier (MD), and Wagner (PhD). W.W., W.M., and J.K. conceived the study and obtained funding. M.W. and I.F. supervised the study. Frommann was responsible for data management and analysis and wrote the first draft of the manuscript. All authors have contributed to data acquisition and to revising the manuscript and have approved the final manuscript.
Appendix A. Inclusion Criteria for the Intervention Studies
Inclusion Criteria for EPS
Self-Experienced Cognitive Thought and Perception Deficits (Basic Symptoms)
One or more of the following basic symptoms appeared in the last 3 months, several times a week:
Thought interferences
Thought perseverations
Thought pressure
Thought blocking
Disturbances of receptive language, either heard or read
Decreased ability to discriminate between ideas and perception, fantasy, and true memory contents
Tendency to delusion of reference (“subject centrism”)
Derealization
Visual perception disturbances
Acoustic perception disturbances
and/or
One of the following risk factors plus a reduction in the GAF score (DSM-IV) of at least 30 points (within the past year)
First-degree relative with a lifetime diagnosis of schizophrenia or a schizophrenia spectrum disorder and
Pre- or perinatal complications.
Inclusion Criteria for LPS
Subsyndromal Psychotic Symptoms Like BLIPS and/or APS
BLIPS, defined as appearance of one of the following psychotic symptoms more than twice for less than 1 week (interval between episodes at least 1 week), spontaneous remission:
Ideas of reference
Unusual thoughts and magical ideation
Unusual perceptual experiences
Odd thinking or speech
Suspicion or paranoid ideation
and/or
Presence of at least one of the following APS within the last 3 months, appearing several times per week for a period of at least 1 week:
Hallucinations
Delusions
Formal thought disorder
Gross disorganized or catatonic behavior
Appendix B. Exclusion Criteria
DSM-IV diagnosis of schizophrenia, schizophreniform, schizoaffective, delusional or bipolar disorder at any time of life,
DSM-IV diagnosis of brief psychotic episode with a duration of more than 1 week at any time of life or overt psychotic symptoms within 1 week before inclusion (inclusion was possible if a spontaneous remission within the BLIPS time frame occurred),
DSM-IV diagnosis of delirium, dementia, amnestic and other cognitive disorders, mental retardation, mental disorders due to a general medical condition or mental disturbances due to psychotropic substances,
Abuse of alcohol or drugs within the last 3 months prior to the study; in case of drug abuse, it had to be determined whether present prodromal symptoms appeared before any drug abuse; if not, symptoms had to persist after a drug-free period of at least 3 months (hallucinogens, amphetamines) or at least 4 weeks (cannabis),
Continuous treatment with high potency neuroleptics for more than 1 week at any time of life or any use of neuroleptics during 6 months prior to the study,
Any contraindication as described for amisulpride,
Women of childbearing risk not practicing contraception, and
Additional exclusion criteria were related to somatic disturbances like pathological electrocardiogram aberrations.
References
- 1.Maier W, Cornblatt BA, Merikangas KR. Transition to schizophrenia and related disorders: toward a taxonomy of risk. Schizophr Bull. 2003;29:693–701. doi: 10.1093/oxfordjournals.schbul.a007039. [DOI] [PubMed] [Google Scholar]
- 2.Häfner H, an der Heiden W. Course and outcome of schizophrenia. In: Hirsch SR, Weinberger DR, editors. Schizophrenia. Oxford, UK: Blackwell Publishing; 2003. pp. 101–141. [Google Scholar]
- 3.Häfner H, an der Heiden W. Epidemiology of schizophrenia. Can J Psychiatry. 1997;42:139–151. doi: 10.1177/070674379704200204. [DOI] [PubMed] [Google Scholar]
- 4.Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 5.Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull. 2003;29:633–651. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- 6.Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60:21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 7.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber G. Reine Defektsyndrome und Basis-Stadien endogener Psychosen. Fortschr Neurol Psychiatr. 1966;34:409–426. [Google Scholar]
- 9.Huber G, Gross G, Schüttler R. Schizophrenie. Eine Verlaufs- und sozialpsychiatrische Langzeitstudie. Berlin, Germany: Springer; 1979. [Google Scholar]
- 10.Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 11.Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60:229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- 12.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 13.Hoff AL, Sakuma M, Wieneke M, et al. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am J Psychiatry. 1999;156:1336–1341. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- 14.Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;78:27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Gold S, Arndt S, Nopoulos P, O’Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry. 1999;156:1342–1348. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- 16.Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- 17.Lucas S, Fitzgerald D, Redoblado-Hodge MA, et al. Neuropsychological correlates of symptom profiles in first episode schizophrenia. Schizophr Res. 2004;71:323–330. doi: 10.1016/j.schres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Rhinewine JP, Lencz T, Thaden EP, et al. Neurocognitive profile in adolescents with early-onset schizophrenia: clinical correlates. Biol Psychiatry. 2005;58:705–712. doi: 10.1016/j.biopsych.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Weickert TW, Goldberg TE. First- and second-generation antipsychotic medication and cognitive processing in schizophrenia. Curr Psychiatry Rep. 2005;7:304–310. doi: 10.1007/s11920-005-0085-5. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg TE, Goldman RS, Burdick KE, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 21.Sergi MJ, Green MF, Widmark C, et al. Social cognition [corrected] and neurocognition: effects of risperidone, olanzapine, and haloperidol. Am J Psychiatry. 2007;164:1585–1592. doi: 10.1176/appi.ajp.2007.06091515. [DOI] [PubMed] [Google Scholar]
- 22.Niendam TA, Bearden CE, Rosso IM, et al. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- 23.Cannon M, Jones P, Huttunen MO, et al. School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry. 1999;56:457–463. doi: 10.1001/archpsyc.56.5.457. [DOI] [PubMed] [Google Scholar]
- 24.Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- 25.Gunnell D, Harrison G, Rasmussen F, Fouskakis D, Tynelius P. Associations between premorbid intellectual performance, early-life exposures and early-onset schizophrenia. Cohort study. Br J Psychiatry. 2002;181:298–305. doi: 10.1192/bjp.181.4.298. [DOI] [PubMed] [Google Scholar]
- 26.Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Dev Psychopathol. 1999;11:487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- 27.Erlenmeyer-Kimling L, Rock D, Roberts SA, et al. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- 28.Cosway R, Byrne M, Clafferty R, et al. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychol Med. 2000;30:1111–1121. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- 29.Schubert EW, McNeil TF. Neurobehavioral deficits in young adult offspring with heightened risk for psychosis who developed schizophrenia-spectrum disorder. Schizophr Res. 2007;94:107–113. doi: 10.1016/j.schres.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Niemi LT, Suvisaari JM, Tuulio-Henriksson A, Lonnqvist JK. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res. 2003;60:239–258. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- 31.Byrne M, Clafferty BA, Cosway R, et al. Neuropsychology, genetic liability, and psychotic symptoms in those at high risk of schizophrenia. J Abnorm Psychol. 2003;112:38–48. [PubMed] [Google Scholar]
- 32.Whyte MC, Brett C, Harrison LK, et al. Neuropsychological performance over time in people at high risk of developing schizophrenia and controls. Biol Psychiatry. 2006;59:730–739. doi: 10.1016/j.biopsych.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 34.Lencz T, Cornblatt B, Bilder RM. Neurodevelopmental models of schizophrenia: pathophysiologic synthesis and directions for intervention research. Psychopharmacol Bull. 2001;35:95–125. [PubMed] [Google Scholar]
- 35.Hambrecht M, Lammertink M, Klosterkotter J, Matuschek E, Pukrop R. Subjective and objective neuropsychological abnormalities in a psychosis prodrome clinic. Br J Psychiatry Suppl. 2002;43:s30–s37. doi: 10.1192/bjp.181.43.s30. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins KA, Addington J, Keefe RS, et al. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr Res. 2004;67:115–122. doi: 10.1016/j.schres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Pukrop R, Schultze-Lutter F, Ruhrmann S, et al. Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J Clin Exp Neuropsychol. 2006;28:1388–1407. doi: 10.1080/13803390500434425. [DOI] [PubMed] [Google Scholar]
- 38.Eastvold AD, Heaton RK, Cadenhead KS. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophr Res. 2007;93:266–277. doi: 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brewer WJ, Wood SJ, Phillips LJ, et al. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006;32:538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brewer WJ, Francey SM, Wood SJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162:71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- 41.Niendam TA, Bearden CE, Johnson JK, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res. 2006;84:100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Gschwandtner U, Pfluger M, Aston J, et al. Fine motor function and neuropsychological deficits in individuals at risk for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256:201–206. doi: 10.1007/s00406-005-0626-2. [DOI] [PubMed] [Google Scholar]
- 43.Pflueger MO, Gschwandtner U, Stieglitz RD, Riecher-Rossler A. Neuropsychological deficits in individuals with an at risk mental state for psychosis—working memory as a potential trait marker. Schizophr Res. 2007;97:14–24. doi: 10.1016/j.schres.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Lencz T, Smith CW, McLaughlin D, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Simon AE, Cattapan-Ludewig K, Zmilacher S, et al. Cognitive functioning in the schizophrenia prodrome. Schizophr Bull. 2007;33:761–771. doi: 10.1093/schbul/sbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Häfner H, Maurer K, Ruhrmann S, et al. Early detection and secondary prevention of psychosis: facts and visions. Eur Arch Psychiatry Clin Neurosci. 2004;254:117–128. doi: 10.1007/s00406-004-0508-z. [DOI] [PubMed] [Google Scholar]
- 47.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: APA; 1994. [Google Scholar]
- 48.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 49.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 50.Wittchen HU, Fydrich T. Strukturiertes Klinisches Iterview für DSM-IV. Manual zum SKID-I und SKID-II. [Structured Clinical Interview for DSM.IV] Göttingen, Germany: Hogrefe; 1997. [Google Scholar]
- 51.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 52.Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 53.Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest (MWT-B) Auflage 2 Erlangen, Germany: Perimed; 1989. [Google Scholar]
- 54.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 55.Spreen O, Strauss E. A Compendium of Neuropsychological Tests. 2nd ed. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 56.Wechsler D. Wechsler Adult Intelligence Scale. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 57.Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- 58.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 59.Rey A. L’examen clinique en psychologie. Paris, France: Presses Universitaires de France; 1958. [Google Scholar]
- 60.Jahn T, Cohen R, Hubmann W, et al. The Brief Motor Scale (BMS) for the assessment of motor soft signs in schizophrenic psychoses and other psychiatric disorders. Psychiatry Res. 2006;142:177–189. doi: 10.1016/j.psychres.2002.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Lezak MD. Neuropsychological Assessment. 3rd ed. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 62.SPSS Inc. Missing Value Analysis (MVA). SPSS Statistics 17.0 Command Syntax Reference. Chicago, IL: SPSS Inc.; 2008. pp. 1249–1251. [Google Scholar]
- 63.Quednow BB, Frommann I, Berning J, Kuhn KU, Maier W, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol Psychiatry. 2008;64:766–773. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 64.Frommann I, Brinkmeyer J, Ruhrmann S, et al. Auditory P300 in individuals clinically at risk for psychosis. Int J Psychophysiol. 2008;70:192–205. doi: 10.1016/j.ijpsycho.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Koutsouleris N, Schmitt GJ, Gaser C, et al. Neuroanatomical correlates of different vulnerability states for psychosis and their clinical outcomes. Br J Psychiatry. 2009;195:218–226. doi: 10.1192/bjp.bp.108.052068. [DOI] [PubMed] [Google Scholar]
- 66.Jones PB, Tarrant CJ. Specificity of developmental precursors to schizophrenia and affective disorders. Schizophr Res. 1999;39:121–125. doi: 10.1016/s0920-9964(99)00110-3. discussion 161. [DOI] [PubMed] [Google Scholar]
- 67.Reichenberg A, Weiser M, Rapp MA, et al. Elaboration on premorbid intellectual performance in schizophrenia: premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62:1297–1304. doi: 10.1001/archpsyc.62.12.1297. [DOI] [PubMed] [Google Scholar]
- 68.Rabinowitz J, Reichenberg A, Weiser M, Mark M, Kaplan Z, Davidson M. Cognitive and behavioural functioning in men with schizophrenia both before and shortly after first admission to hospital. Cross-sectional analysis. Br J Psychiatry. 2000;177:26–32. doi: 10.1192/bjp.177.1.26. [DOI] [PubMed] [Google Scholar]
- 69.Caspi A, Reichenberg A, Weiser M, et al. Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophr Res. 2003;65:87–94. doi: 10.1016/s0920-9964(03)00056-2. [DOI] [PubMed] [Google Scholar]
- 70.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 71.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 72.Satzger W, Fessmann H, Engel RR. The equivalence of three German vocabulary tests and the German Version of the Wechsler Adult Intelligence Scale-Revised (HAWIE-R) Zeitschrift für Differentielle und Diagnostische Psychologie. 2002;23:159–170. [Google Scholar]
- 73.Cannon M, Moffitt TE, Caspi A, Murray RM, Harrington H, Poulton R. Neuropsychological performance at the age of 13 years and adult schizophreniform disorder: prospective birth cohort study. Br J Psychiatry. 2006;189:463–464. doi: 10.1192/bjp.bp.105.020552. [DOI] [PubMed] [Google Scholar]
- 74.Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT. Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry. 1994;51:804–811. doi: 10.1001/archpsyc.1994.03950100052005. [DOI] [PubMed] [Google Scholar]
- 75.Heydebrand G, Weiser M, Rabinowitz J, Hoff AL, DeLisi LE, Csernansky JG. Correlates of cognitive deficits in first episode schizophrenia. Schizophr Res. 2004;68:1–9. doi: 10.1016/S0920-9964(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 76.Brazo P, Delamillieure P, Morello R, Halbecq I, Marie RM, Dollfus S. Impairments of executive/attentional functions in schizophrenia with primary and secondary negative symptoms. Psychiatry Res. 2005;133:45–55. doi: 10.1016/j.psychres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Dibben CR, Rice C, Laws K, McKenna PJ. Is executive impairment associated with schizophrenic syndromes? A meta-analysis. Psychol Med. 2009;39:381–392. doi: 10.1017/S0033291708003887. [DOI] [PubMed] [Google Scholar]
- 78.Isohanni M, Murray GK, Jokelainen J, Croudace T, Jones PB. The persistence of developmental markers in childhood and adolescence and risk for schizophrenic psychoses in adult life. A 34-year follow-up of the Northern Finland 1966 birth cohort. Schizophr Res. 2004;71:213–225. doi: 10.1016/j.schres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Murray GK, Jones PB, Moilanen K, et al. Infant motor development and adult cognitive functions in schizophrenia. Schizophr Res. 2006;81:65–74. doi: 10.1016/j.schres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 80.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 81.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 82.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- 83.Pantelis C, Velakoulis D, McGorry P, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudonal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 84.Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 85.Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hurlemann R, Jessen F, Wagner M, et al. Interrelated neuropsychological and anatomical evidence of hippocampal pathology in the at-risk mental state. Psychol Med. 2008;38:843–851. doi: 10.1017/S0033291708003279. [DOI] [PubMed] [Google Scholar]
- 87.Yung AR, Stanford C, Cosgrave E, et al. Testing the Ultra High Risk (prodromal) criteria for the prediction of psychosis in a clinical sample of young people. Schizophr Res. 2006;84:57–66. doi: 10.1016/j.schres.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 88.Mason O, Startup M, Halpin S, et al. Risk factors for transition to first episode psychosis among individuals with ‘at-risk mental states’. Schizophr Res. 2004;71:227–237. doi: 10.1016/j.schres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Wood SJ, Pantelis C, Proffitt T, et al. Spatial working memory ability is a marker of risk-for-psychosis. Psychol Med. 2003;33:1239–1247. doi: 10.1017/s0033291703008067. [DOI] [PubMed] [Google Scholar]
- 90.Francey SM, Jackson HJ, Phillips LJ, et al. Sustained attention in young people at high risk of psychosis does not predict transition to psychosis. Schizophr Res. 2005;79:127–136. doi: 10.1016/j.schres.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 91.Cannon TD. Neurodevelopment and the transition from schizophrenia prodrome to schizophrenia: research imperatives. Biol Psychiatry. 2008;64:737–738. doi: 10.1016/j.biopsych.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 92.Borgwardt SJ, Riecher-Rossler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 93.Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Koutsouleris N, Meisenzahl EM, Davatzikos C, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66:700–712. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkotter J. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr Res. 2007;92:116–125. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 96.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 97.Harvey PD. When does cognitive decline occur in the period prior to the first episode of schizophrenia? Psychiatry (Edgmont) 2009;6:12–14. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.