Abstract
Despite robust evidence of hippocampal abnormalities in schizophrenia, it is unclear whether hippocampal dysfunction predates the onset of psychosis. We used functional magnetic resonance imaging to investigate hippocampal function in subjects with an at-risk mental state (ARMS). Eighteen subjects meeting criteria for an ARMS and 22 healthy controls, matched for age, gender, and premorbid IQ, were scanned while performing a version of the Deese-Roediger-McDermott false memory task. During an encoding phase, subjects read lists of words aloud. Following a delay, they were presented with 24 target words, 24 semantically related lure words, and 24 novel words and required to indicate if each had been presented before. Behaviorally, the ARMS group made more false alarm responses for novel words than controls (P = .04) and had a lower discrimination accuracy for target words (P = .02). During encoding, ARMS subjects showed less activation than healthy controls in the left middle frontal gyrus, the bilateral medial frontal gyri, and the left parahippocampal gyrus. Correct recognition relative to false alarms was associated with differential engagement of the hippocampus bilaterally in healthy controls, but this difference was absent in the ARMS group. The ARMS was associated with altered function in the medial temporal cortex, as well as in the prefrontal regions, during both verbal encoding and recognition. These neurofunctional differences were associated with diminished recognition performance and may reflect the greatly increased risk of psychosis associated with the ARMS.
Keywords: episodic memory, schizophrenia, fMRI, prefrontal cortex, parahippocampal gyrus
Introduction
Prodromal symptoms and signs of psychosis, such as attenuated psychotic symptoms and a decline in social and occupational function,1–3 are associated with a high risk of psychosis and can be described as an at-risk mental state (ARMS). Neuropsychological studies indicate that the ARMS is associated with a range of cognitive impairments, particularly on tasks that engage executive functions, working, and episodic memory.4–6 More importantly, research into the ARMS has attempted to identify definitive markers that distinguish those who go on to develop psychosis from those who do not. One promising risk marker for the development of schizophrenia-spectrum psychotic disorders is verbal memory impairment.4,5 The successful encoding, storage, and retrieval of verbal episodic memories relies on a distributed network of cortical and subcortical regions, particularly the medial temporal and prefrontal cortex.7 Neuropsychological impairment on verbal memory tasks seen in ARMS subjects suggests the presence of a prefrontal-hippocampal neurodevelopment abnormality.8 However, measures of verbal memory are neuropsychologically complex, and it is perhaps only those measures that require prefrontal rather than hippocampal engagement are predictive.9 Indeed, despite overwhelming evidence of functional10–16 and structural17–20 hippocampal abnormalities in established schizophrenia, the small number of imaging studies in the ARMS have provided mixed evidence for hippocampal pathology prior to transition to psychosis.21 Although 1 early cross-sectional study reported smaller hippocampal volumes in ARMS groups as a whole,22 a much larger study in the same cohort did not support this finding.23 However, 1 volumetric magnetic resonance imaging study of subjects with an ARMS did report hippocampal volume reductions compared with healthy controls,24 albeit at a liberal statistical threshold. Furthermore, in a cross-sectional volumetric comparison, ARMS individuals who developed psychosis had less gray matter in the right medial temporal cortex as well as lateral temporal regions, inferior frontal cortex, and cingulate cortex bilaterally compared with those subjects who did not develop the illness.25 Pantelis et al25 also report longitudinal data showing that ARMS individuals who had developed psychosis had a reduction in gray matter in the LPHG, fusiform, orbitofrontal and cerebellar cortices, and the cingulate gyri. In those who had not become psychotic, longitudinal changes were restricted to the cerebellum. However, 2 further longitudinal studies failed to find gray matter volume reductions in the medial temporal cortex in ARMS subjects who developed psychosis.26,27
Inconsistencies in the structural neuroimaging data may be because early impairments are relatively subtle in ARMS individuals. If changes in the medial temporal cortex predate the onset of psychosis, these may be easier to detect using functional rather than structural neuroimaging. To date, there are only 2 functional magnetic resonance imaging (fMRI) studies in ARMS groups28,29 and neither have had a specific focus on fronto-medial temporal activation, both instead using tasks largely associated with prefrontal executive function. Thus, the first aim of the present study was to use an fMRI activation task engaging verbal episodic memory to examine hippocampal and prefrontal cortex function in ARMS subjects and compare this with that seen in a healthy control group. A further objective was to investigate whether functional alterations were evident during the encoding of stimuli and/or their subsequent recognition.
To examine hippocampal and prefrontal functions in the ARMS during verbal episodic memory, we used a modified version of the Deese-Roediger-McDermott memory paradigm.30,31 The task involves the repeated presentation of words during encoding that are semantically linked to a nonpresented lure word presented during a subsequent recognition phase. It is therefore sensitive to the effects of organizational encoding strategies reflecting semantic processes subserved by prefrontal cortex.32 In healthy subjects, semantic associations formed during encoding influence the likelihood of the subsequent misrecognition of words semantically related to presented items. The task also allowed us to examine the extent to which subjects made “false alarms” when a previously unpresented word is misidentified as having been seen before. A propensity to make false alarms has been widely reported in patients with schizophrenia during memory tasks33–35 and has also been found in the first-degree relatives of patients.36 Moreover, the increased rate of false alarms to novel stimuli during recognition in schizophrenia has been associated with decreased hippocampal activation.37
Using an fMRI task in which both encoding and recognition phases were scanned separately, we tested 2 hypotheses. First, we predicted that during verbal encoding, the ARMS group would have reduced activation in the prefrontal and medial temporal cortex relative to matched control subjects. We then tested the hypothesis that during a subsequent recognition task, the ARMS group would show impairment in recognition accuracy of both target and semantically related lure words and demonstrate a tendency to misidentify previously unpresented words (ie make false alarms). We predicted that relative to controls, the ARMS group would show impaired hippocampal activation during correct recognition and false alarm trials.
Methods
Participants
Forty subjects (22 healthy controls and 18 with an ARMS) participated in the study. All were right handed, spoke English as their first language, and had no history of neurological illness and drug or alcohol dependence. The study had National Health Service UK Research Ethics Committee approval, and all participants gave informed consent. All participants had an estimated premorbid IQ in the normal range as assessed using the Wide Range Achievement Test-Revised.38 Working memory maintenance and manipulation were assessed using the digit span subtest of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III39). Handedness was assessed using the Lateral Preference Inventory.40 Mean age, estimated premorbid IQ, years of education, and WAIS-III digit span (raw scores) are reported in table 1.
Table 1.
Participant Demographics, Neuropsychological and Clinical Measures
| Healthy controls (n = 22) | ARMS (n = 18) | Analysis | |
| Age (y) | 27.55 (1.6) | 27.10 (4.95) | t = 0.25, P = .81 |
| Years of education | 15.15 (3.11) | 13.45 (2.06) | t = 1.72, P = .12 |
| WRAT-estimated premorbid IQ | 105 (9.1) | 99 (17.18) | t = 1.54, P = .13 |
| Gender | 14M:8F | 10M:8F | χ2 = 0.57, P = .45 |
| WAIS-III digit span | 19.00 (4.76) | 17.61 (4.06) | t = 1.07, P = .29 |
| Symptoms | |||
| PANSS total | 45 (13) | ||
| PANSS positive | 11 (3) | ||
| PANSS negative | 10 (4) | ||
| PANSS general | 25 (7) |
Note: ARMS, at-risk mental state; WRAT, Wide Range Achievement Test-Revised; WAIS-III, Wechsler Adult Intelligence Scale-Third Edition; PANSS, positive and negative symptom scales.
Subjects with an ARMS were recruited via Outreach and Support in South London, a clinical service for people at high risk of developing psychosis.41 Diagnosis of an ARMS was made via a detailed clinical assessment using the Comprehensive Assessment of At-Risk Mental States.2,42 Subjects met one or more of the following criteria: (a) attenuated psychotic symptoms, (b) brief limited intermittent psychosis (BLIP; psychotic symptom that last for <1 week), or (c) a recent decline in function, together with either schizotypal personality disorder or a first-degree relative with a psychotic disorder. All ARMS subjects were experiencing attenuated psychotic symptoms, 4 had also experienced a BLIP, and 3 had a family history together with a decline in function. The mean Global Assessment of Function score of the group at initial assessment was 61. Psychopathology on the day of scanning was assessed using the positive and negative symptom scales43 and are presented in table 1. Sixteen of the subjects were naïve to antipsychotic medication. Two had received low doses of risperidone and quetiapine. None of the ARMS subjects were receiving antidepressant medication. Their self-reported ethnicity was White British (n = 12), Black (n = 2), and mixed (n = 4).
Healthy controls were recruited from the local community through advertisements. Participants with a history of medical or psychiatric disorders or who were receiving prescription medications were excluded. Their self-reported ethnicity was White British (n = 16), Black (n = 4), and Asian (n = 2).
fMRI Task and Procedure
The encoding paradigm consisted of alternating epochs of experimental ENCODE and baseline CONTROL conditions. During ENCODE blocks, subjects were required to read aloud and memorize commonly occurring English nouns presented one at a time on a computer screen. They were instructed to read the words out loud and try to memorize as many as possible because recognition would be tested later. Sixteen lists were presented with 1 list per block. Each list contained 10 words semantically related to a critical nonpresented word taken from an established list of semantically related words.44 For example, the items “hiking,” “tent,” “trail,” “scout,” “cabin,” “summer,” “lake,” “canvas,” and “holiday” comprised the list related to the critical nonpresented word “camp.” Each word was presented for 2.5 s followed by a fixation cross for 1.5 s such that a new word appeared every 4 s. Encoding blocks alternated with control blocks, which comprised 4 consecutive presentations of the word “wait” at 4-s intervals. Participants were told to read the word aloud each time it was presented.
Recognition was tested after a delay of 12 min during which an unrelated attention task was performed to curb possible rehearsal of stimuli. During recognition testing, the probe words were presented individually (using an event-related design) on a screen and subjects used a button press to indicate whether the word had been presented before. Participants were instructed to make 1 of 3 possible responses: “remember” if they were confident they had seen the word during the encoding phase, “know” if the word seemed familiar but they were less certain, or “new” if they thought it had not been presented. These responses correspond to different recognition memory states of recollection and familiarity.45 The recognition list consisted of 28 targets (words presented and articulated during the encoding phase), 28 lures (nonpresented words semantically associated with the target items), and 28 novel words (nonpresented words not semantically associated with targets). The first and last encoding lists as well as the first and last words in each of the lists in between were excluded from the recognition task to control for recency and primacy effects. Targets were the second and seventh words in each list. Novel words were taken from separate lists that were not presented. All participants were trained on the encoding and recognition task (using a practice word list not used in the fMRI experiment) prior to entering the scanner. Visual stimuli were back projected with a LCD projector on to a screen 2.5 m from the subject’s head and were visible via a prism mounted on the head coil.
fMRI Data Acquisition
Images were acquired in a 1.5 T Magnet (Signa LX;GE, Milwaukee, Wisconsin) at the Institute of Psychiatry, London. During the encoding phase, echo-planar images were acquired using a compressed acquisition sequence46 to allow for overt verbal articulation of the word stimuli in the absence of acoustic scanner noise and to reduce motion artefacts related to movement caused by articulation (overall TR = 4000 ms, silent period = 2500 ms). A total of 228 image volumes were acquired in a single functional run. The recognition phase involved 2 separate runs each containing 197 volumes (TR = 2000 ms). Whole brain coverage was achieved using 16 noncontiguous axial planes parallel to the intercommissural plane with the following characteristics: TE = 40 ms, slice thickness = 7 mm, slice skip = 0.7 mm, and in-plane resolution = 3 × 3 mm2.
Analysis
fMRI Data
Preprocessing was performed using SPM2 software (http://www.fil.ion.ucl.ac.uk/spm/), running in Matlab 6.5 (Mathworks Inc, Sherborn, Massachusetts). All volumes from each subject were realigned and unwarped using the first as reference and resliced with sinc interpolation. The functional images were spatially normalized47 to a standard MNI-305 template using nonlinear-basis functions. Functional data were spatially smoothed with a 6 mm full width at half-maximum isotropic Gaussian kernel to compensate for residual variability in functional anatomy after spatial normalization and to permit application of Gaussian random field theory for adjusted statistical inference.
Statistical Parametric Mapping.
A standard random effects statistical analysis of regional responses was performed to identify regional activations in each subject independently. To remove low-frequency drifts, the data were high pass filtered using a set of discrete cosine basis functions with a cutoff period of 128 s. For the encoding data, blocks containing word lists were modeled independently by convolving the onset times. Word list blocks were then contrasted against the control blocks to create first-level SPMs in each subject. Parameter estimates were calculated for all brain voxels using the general linear model, and encoding group maps were constructed using 1-sample t tests. The group comparison for encoding was conducted using a 2-sample test. In the recognition phase, events were modeled according to subjects’ behavioral responses. In the present study, remember and know responses were collapsed into an “OLD” (recognition) response to ensure that there were sufficient events to provide optimal statistical power in the image analysis. All potential recognition memory states were modeled independently by convolving onset times with a canonical hemodynamic response function: correct recognition (target say OLD), forgetting (target say NEW), false recognition (lure say OLD), correct rejection of lures (lure say NEW), false alarms (novel say OLD), and correct rejection of novel words (novel say NEW). Trials were modeled against a low-level baseline consisting of a visual fixation cross.
To test our a priori hypothesis, we examined activation associated with correct recognition and false alarms and the interaction with group using a between-group 2 × 2 factorial analysis of variance (ANOVA) based on subjects’ recognition response. We also conducted exploratory analyses to test (a) correct recognition trials vs false recognition trials, (b) correct rejection trials vs false alarm trials, (c) false recognition trials vs false alarm trials, and (d) false recognition trials vs correct rejection of lure trials. Forgetting trials (target say NEW) were excluded from the analyses as the vast majority of subjects had insufficient events (<10) in this response condition. Subjects with <10 false alarm trials were excluded from the recognition analyses (these were healthy controls, n = 8, and ARMS, n = 5, leaving 14 healthy controls and 13 ARMS subjects in our analyses). No subjects had <10 trials in any of the other recognition response conditions. In the context of the general linear model, for subjects with sufficient trials per response, imbalances in the number of trials across conditions do not bias the statistical comparison (contrasts) of these conditions because the estimated contrast variance properly reflects differences in trial numbers per condition. As we were testing a priori hypotheses regarding group effects in the prefrontal cortex and the medial temporal lobe, we constrained second-level group contrasts using an anatomical mask generated by Wake Forest University Pickatlas (http://www.fmri.wfubmc.edu/).48 For the prefrontal cortex, the mask consisted of bilateral Brodmann areas: 8, 9, 46, 10, 11, 47, 45, and 44. For the medial temporal lobe, the mask consisted of bilateral hippocampus and parahippocampal gyrus. All encoding and recognition results are reported at a voxel-wise level corrected for multiple comparisons (family wise error [FWE], P < .05). For correlational analyses, data were extracted from significant clusters identified by group contrasts (mean cluster parameter estimates) using the MarsBar toolbox (http://marsbar.sourceforge.net/).
Behavioral Data
Accuracy data from the recognition condition were normally distributed in both groups. Response accuracy was assessed using factorial ANOVA in SPSS version 15. Responses (remember and know) for word types (target, lure, and novels) were compared across groups (healthy controls vs ARMS). In order to more accurately measure recognition memory performance, discrimination indices developed by Snodgrass and Corwin49 were calculated. Recognition accuracy is estimated by the discrimination index (Pr: hit rate − false alarm rate, where hit rate = hits + 0.5/number of OLD items + 1 and false alarm rate = false alarms + 0.5/number of OLD items + 1), which represents the probability that an item will be correctly recognized as OLD above and beyond the tendency to make false alarms. Response bias (Br = false alarm rate/[1 − Pr]) was also calculated. Br represents the probability of an individual saying “OLD” to an item when uncertain. A Br value <0.5 indicates a conservative response bias and a value >0.5 indicates a liberal response bias. Discrimination indices were compared between groups using independent sample t tests. Correlational analysis between behavioral measures and SPM parameter estimates was conducted using Spearman’s test (2 tailed).
Results
Behavioral Data
Examination of audio recordings and response files during word encoding revealed that all participants were able to successfully complete the task.
Word Type.
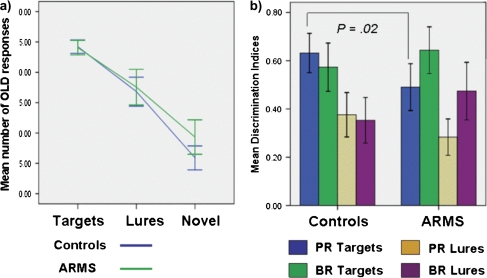
The mean number of OLD responses for each word type is shown in figure 1a. ANOVA revealed a significant main effect for word type (F2,76 = 195.45, P < .001), with all subjects making more OLD responses for targets and lures than for novel words (t39 = 11.00, P < .01). There was a significant interaction between word type and group (F2,76 = 3.11, P = .04), with ARMS subjects making significantly more OLD responses for novel words than healthy controls (ie false alarms; t38 = −2.14, P = .04). Analysis of discrimination indices (figure 1b) revealed that, compared with healthy controls, ARMS subjects had a significantly lower accuracy (Pr) for target items (t38 = 2.35, P = .02) but not for lure words (t38 = 1.5, P = .13). Although ARMS subjects showed a more liberal response bias for target and lure words than healthy controls, these differences were nonsignificant (t38 = −1.05, P = .28) and (t38 = −1.6, P = .11), respectively.
Fig. 1.
a) Plot of mean number of OLD responses by word type and group and b) mean discrimination indices by group; recognition accuracy (Pr) = (number of target say OLD + 0.5/number of targets + 1)/(number of novel say OLD + 0.5/number of targets + 1), mean response bias (Br) = false alarm rate/(1 − Pr) by group. Error bars represent 95% confidence intervals. Recognition accuracy is estimated by the discrimination index (Pr: hit rate − false alarm rate, where hit rate = hits + 0.5/number of OLD items + 1 and false alarm rate = false alarms + 0.5/number of OLD items + 1). Response bias (Br = false alarm rate/[1 − Pr]) was also calculated. ARMS, at-risk mental state.
Response Type.
To examine whether recognition states differed between groups, we compared the number of remember and know responses made by ARMS and healthy controls subjects. There was a significant main effect for response type (F1,38 = 8.3, P < .01), with all subjects making more remember than know responses. The interaction between response type and group was nonsignificant (F1,39 = 2.8, P = .13).
fMRI Results
Activation Related to Encoding.
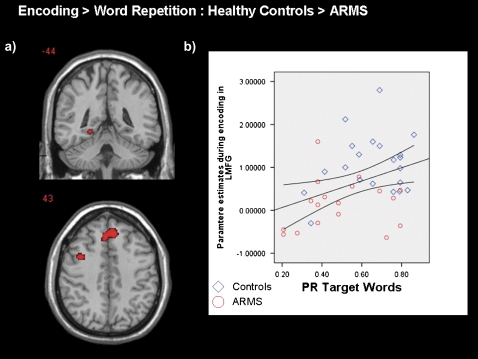
In healthy controls, activation related to encoding was restricted to the left hemisphere, with engagement of the inferior frontal, middle frontal, and precentral gyri; the inferior parietal lobule; and the fusiform gyrus. In ARMS subjects, activation was also left sided but restricted to the inferior and middle frontal gyri (see table 2). A between-group contrast (table 2; figure 2a) revealed that during encoding, ARMS subjects showed less activation than healthy controls in the medial frontal gyrus bilaterally, in a left middle frontal gyrus, and the posterior part of the left middle parahippocampal gyrus. We examined activation during encoding (in the 3 regions where a significant group effect was seen) and its association with recognition performance (to correct for multiple correlations, we report at a P value <.05/3 = .016). Only parameter estimates in the medial frontal gyrus (r = .39, P = .01) were positively correlated with Pr for target words (figure 2b).
Table 2.
Region Activations and Group Contrasts During Encodinga
| Region | x | y | z | z score | BA |
| Healthy controls | |||||
| Left fusiform gyrus | −44 | −66 | −14 | 5.42 | 19 |
| Left inferior frontal sulcus | −42 | 10 | 32 | 5.35 | 8 |
| Left precentral gyrus | −40 | 6 | 24 | 5.02 | 6 |
| Left precuneus | −30 | −68 | 40 | 5.01 | 7 |
| Left middle frontal gyrus | −40 | 4 | 46 | 4.85 | 6 |
| Left inferior parietal lobule | −30 | −64 | 44 | 4.15 | 7 |
| ARMS | |||||
| Left inferior frontal gyrus | −46 | 10 | 24 | 4.95 | 9 |
| −54 | 20 | 24 | 3.94 | 9 | |
| Left inferior/middle frontal gyrus | −48 | −12 | 36 | 4.91 | 9 |
| Healthy controls > ARMS | |||||
| Right medial frontal gyrus | 6 | 36 | 42 | 4.17 | 8 |
| Left medial frontal gyrus | −8 | 30 | 42 | 3.98 | 8 |
| Left middle frontal | −40 | 8 | 40 | 3.80 | 8 |
| Left parahippocampal gyrus | −24 | −44 | −8 | 3.67 | — |
| ARMS > Healthy controls | |||||
| No regions of significant difference |
Note: ARMS, at-risk mental state; BA, Brodmann areas.
All results are reported at corrected voxel level (FWE, P < .05) The x, y, z coordinates of local maxima are listed according to the MNI coordinate system.
Fig. 2.
a) SPM maps displaying coronal and axial sections of regions activated in healthy controls greater than those in the ARMS group during word encoding (FWE, P < .05) and b) Scatter plots showing association between LMFG activation during encoding and Pr for target words in controls subjects (blue) and ARMS subjects (red). The left side of the brain is shown on the left of the image. ARMS, at-risk mental state; LMFG, left middle frontal gyrus.
Activation Related to Recognition.
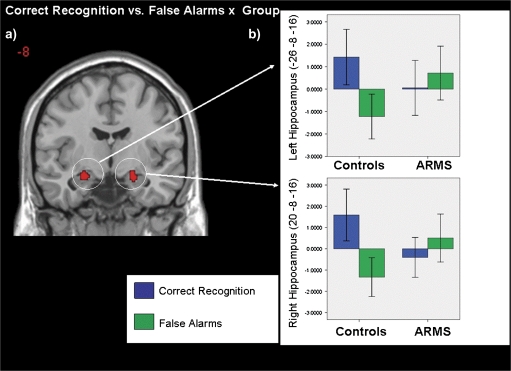
To test our hypothesis regarding correct recognition and false alarm trials, we compared these trials and their interaction with group. At a corrected threshold, the main effect for correct recognition vs false alarms was nonsignificant, although an effect for correct recognition > false alarms was observed in the right middle frontal gyrus at an uncorrected level (P < .001). No regions showed relatively greater activation for false alarms. There was a significant group by response interaction in the hippocampus bilaterally (table 3; figure 3a). Examination of the parameter estimates from the hippocampal regions revealed that controls displayed greater hippocampal activation during correct recognition than during false alarm trials. In contrast, hippocampal activation in ARMS subjects was greater during false alarms relative to correct recognition trials (figure 3b). There was also a significant group interaction in the left hippocampus for correct rejection vs false alarm trials (table 3). As in the previous contrast, examination of parameter estimates from the left hippocampal region revealed that controls displayed greater hippocampal activation during correct rejection of novel words relative to false alarm trials. In contrast, ARMS subjects showed greater hippocampal activation during false alarm trials than for correct rejection of novel word. Main effects and group interaction associated with correct recognition vs false recognition, false recognition vs false alarms, and false recognition vs correct rejection of lures were nonsignificant at corrected thresholds.
Table 3.
Regions Activated by Main Effects and Group Interactions for Recognition Trialsa
| Region | x | y | z | z score | BA |
| Correct recognition vs false alarms | |||||
| No significant main effect | |||||
| Group interaction | |||||
| Left hippocampus | −26 | −8 | −16 | 3.90 | — |
| Right hippocampus | 20 | −8 | −16 | 3.85 | — |
| Correct recognition vs false recognition | |||||
| No significant main or interaction effect | |||||
| Correct rejection vs false alarms | |||||
| No significant main effect | |||||
| Group interaction | |||||
| Left hippocampus | −26 | −12 | −16 | 3.68 | — |
| False recognition vs false alarms | |||||
| No significant main effect or interaction | |||||
| False recognition vs correct rejection of lures | |||||
| No significant main effect or interaction |
Note: Abbreviations are explained in the first footnote to table 2.
All results reported at corrected voxel level (FWE, P < .05). The x, y, z coordinates of local maxima are listed according to the MNI coordinate system.
Fig. 3.
a) SPM map displaying coronal section of bilateral hippocampus activated for correct recognition vs false alarms × group interaction. The left side of the brain is shown on the left of the image and b) mean parameter estimates for correct recognition and false alarms in controls and ARMS subjects in the bilateral hippocampus. ARMS, at-risk mental state.
Discussion
The aim of the present study was to examine hippocampal and prefrontal function during a verbal episodic memory task in the ARMS. As only 2 ARMS participants had been exposed to antipsychotic medication, the group differences in activation were not attributable to effects of treatment. Furthermore, it is unlikely that behavioral and functional differences can be accounted for by variations in age, premorbid IQ, and attention span. Contrary to our hypothesis, the ARMS and healthy controls groups did not differ in the number of target or lure words they recognized. However, ARMS subjects did differ from controls in showing a significantly lower recognition accuracy (Pr), which has been shown to provide a better index of hippocampal function than proportional hit rate.49 Subsequently, it became apparent that ARMS subjects’ performance during recognition was artificially inflated by a tendency to mistakenly claim that they had said novel items before. Higher rates of false alarms during tests of verbal memory are commonly reported in patients with schizophrenia50–53 and may reflect an increased tendency to believe that an event has occurred in the case of uncertainty34 (ie a recognition response bias). However, although ARMS subjects demonstrated a more liberal response bias than controls, this effect was nonsignificant. Moreover, it is unclear if the putative response bias is independent of memory impairments or is a downstream consequence of mnemonic inefficiency.
During verbal encoding, both healthy controls and ARMS subjects engaged left prefrontal regions. Controls also engaged parietal and temporal regions, which is consistent with previous neuroimaging studies of verbal episodic encoding.54,55 As the encoding tasked used a low-level baseline, it is possible that some of the regions activated were associated with processes other than verbal encoding such as the reading of words. However, relative to controls, ARMS subjects showed less activation in the bilateral medial prefrontal cortex, left middle frontal gyrus, and parahippocampal gyrus. Reduced activation in prefrontal and medial temporal regions has been reported in patient samples and is thought to underlie the marked mnemonic dysfunction in patients with schizophrenia.15 Furthermore, activation in the left middle frontal gyrus, part of the dorsolateral prefrontal cortex, and a region involved in the organization of material during encoding7,56 was associated with subsequent recognition performance. Reduced activation in ARMS subjects was also seen in medial prefrontal regions. During encoding, medial prefrontal cortex is associated with the processing of self-relevant or self-generated information,7,57 although its role in the current encoding task is not entirely clear.
Altered hippocampal and parahippocampal activation during encoding has been widely reported in patients with schizophrenia14,58,59 and may be related to an impairment in the encoding of contextual information60 and the formation of representations that support recollection.16 However, reduced engagement of ventrolateral prefrontal cortex often reported in schizophrenia,15 thought to reflect deficits in semantic processing,7,61 was not evident in ARMS subjects. This is consistent with intact recognition performance of the ARMS group for lure words and evidence of normal prefrontal activation during successful associative encoding in patients with first-episode schizophrenia.62
As predicted, there were also group differences in cortical activation during the recognition phase of the task. In control subjects, both correct recognition and correct rejection of target words were associated with greater hippocampal activation than were false alarm trials. Thus, in control subjects, hippocampal activation was associated with correctly recognizing a previously presented target word and the correct rejection of a word that had been not presented. Significantly, the ARMS group did not show the same dissociation in hippocampal activation across these recognition memory states. The ARMS group differed from controls in that false alarm trials were associated with a greater degree of hippocampal activation compared with correct recognition/rejection trials (particularly in the left hippocampus).
Altered hippocampal activation during recognition and poorer recognition performance evident in the ARMS group may have been a downstream consequence of reduced parahippocampal activation during encoding. Danion and colleagues63 argue that, in patients with schizophrenia, episodic memory dysfunction results from a predominant failure of binding processes during encoding, although the impairment of retrieval processes cannot be ruled out. The findings of this study suggest that impaired encoding precedes impoverished recognition memory.
In summary, our functional findings in the ARMS are in line with studies of patients with schizophrenia that report medial temporal cortex dysfunction during episodic encoding and retrieval16 that is frequently accompanied by prefrontal cortex dysfunction.14,15 To our knowledge, the present investigation is the first functional neuroimaging study to have found evidence of hippocampal dysfunction in the ARMS in the context of a cognitive task. Our results are consistent with neuropsychological data showing impaired episodic memory performance in the ARMS4,5 and a recent report that resting state blood volume in the hippocampus is increased in the ARMS relative to controls.64 Our findings of reduced frontal activations in the ARMS are consistent with data from previous fMRI studies of tasks that engage executive functions and working memory in this group28,65 and with evidence of prefrontal cortical volume reductions in the ARMS.66
The study has some limitations. We were unable to compare ARMS subjects based on their clinical outcome. We are currently in the process of identifying the ARMS subjects who have subsequently made the transition to first-episode psychosis. This will allow us to establish if the hippocampal functional deficits are robust markers of transition to psychosis. A further limitation was that, due to the response profile of the groups, we were unable to analyze recognition data according to response type (ie remember/know). We acknowledge that recollection and familiarity are distinct recognition states associated with different networks60. However, in the present study we were interested in getting robust estimates of the recognition state as a whole. Given findings of impoverished recollection in patients with schizophrenia,63 we decided to include a measure of remember/know to ensure that our results were not driven by ARMS subjects’ tendency to make less remember (recollective) responses than controls. The behavioral data suggest that this is not the case. Lastly, it was necessary to remove a number of subjects (8 controls and 5 ARMS subjects from our recognition analyses) due to insufficient numbers of false alarm trials. While this reduced our sample size for recognition analyses, we feel that this was a methodologically rigorous step to ensure that there was sufficient power.
In conclusion, our data suggest that impaired episodic memory performance is evident in ARMS is associated with dysfunction in hippocampal and prefrontal networks. We postulate that the functional and corresponding behavioral abnormalities represent an increased vulnerability to psychosis. The extent to which they predict the subsequent onset of psychosis needs to be clarified by establishing which individuals have made the transition to psychosis.67
Funding
National Alliance for Research on Schizophrenia and Depression Young Investigator Awards to P.A. and M.L.S.
Acknowledgments
P.A. takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have agreed to its submission in this form, and we do not have any interests that might be interpreted as influencing its content.
References
- 1.Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. J Abnorm Psychol. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- 2.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 3.Hafner H. Prevention and early intervention in schizophrenia: facts and visions. Seishin Shinkeigaku Zasshi. 2002;104:1033–1054. [PubMed] [Google Scholar]
- 4.Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkotter J. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr Res. 2007;92:116–125. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Brewer WJ, Francey SM, Wood SJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162:71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- 6.Eastvold AD, Heaton RK, Cadenhead KS. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophr Res. 2007;93:266–277. doi: 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- 8.Lencz T, Smith CW, McLaughlin D, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Brewer WJ, Wood SJ, Phillips LJ, et al. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006;32:538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 11.Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scand J Psychol. 2001;42:239–250. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- 12.Crespo-Facorro B, Wiser AK, Andreasen NC, et al. Neural basis of novel and well-learned recognition memory in schizophrenia: a positron emission tomography study. Hum Brain Mapp. 2001;12:219–231. doi: 10.1002/1097-0193(200104)12:4<219::AID-HBM1017>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofer A, Weiss EM, Golaszewski SM, et al. Neural correlates of episodic encoding and recognition of words in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. Am J Psychiatry. 2003;160:1802–1808. doi: 10.1176/appi.ajp.160.10.1802. [DOI] [PubMed] [Google Scholar]
- 14.Ragland JD, Gur RC, Valdez J, et al. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- 16.Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenton ME, Gerig G, McCarley RW, Szekely G, Kikinis R. Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry Res. 2002;115:15–35. doi: 10.1016/s0925-4927(02)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 19.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 20.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 21.Wood SJ, Pantelis C, Velakoulis D, Yucel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull. 2008;34:322–329. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips LJ, Velakoulis D, Pantelis C, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr Res. 2002;58:145–158. doi: 10.1016/s0920-9964(01)00392-9. [DOI] [PubMed] [Google Scholar]
- 23.Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 24.Borgwardt SJ, Riecher-Rossler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 26.Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broome MR, Matthiasson P, Fusar-Poli P, et al. Neural correlates of executive function and working memory in the ‘at-risk mental state’. Br J Psychiatry. 2009;194:25–33. doi: 10.1192/bjp.bp.107.046789. [DOI] [PubMed] [Google Scholar]
- 30.Deese J. On the prediction of occurences of particular verbal intrusion in immediate recall. J Exp Psychol. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- 31.Roediger HL, McDermott KB. Creating false memories. Remembering words that were not on a list. J Exp Psychol. 1995;21:803–814. [Google Scholar]
- 32.Dannhauser TM, Shergill SS, Stevens T, et al. An fMRI study of verbal episodic memory encoding in amnestic mild cognitive impairment. Cortex. 2008;44:869–880. doi: 10.1016/j.cortex.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Brebion G, Amador X, Smith MJ, Gorman JM. Mechanisms underlying memory impairment in schizophrenia. Psychol Med. 1997;27:383–393. doi: 10.1017/s0033291796004448. [DOI] [PubMed] [Google Scholar]
- 34.Brebion G, Amador X, Smith MJ, Malaspina D, Sharif Z, Gorman JM. Opposite links of positive and negative symptomatology with memory errors in schizophrenia. Psychiatry Res. 1999;88:15–24. doi: 10.1016/s0165-1781(99)00076-1. [DOI] [PubMed] [Google Scholar]
- 35.Brebion G, Smith MJ, Gorman JM, Amador X. Discrimination accuracy and decision biases in different types of reality monitoring in schizophrenia. J Nerv Ment Dis. 1997;185:247–253. doi: 10.1097/00005053-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Vollema MG, Postma B. Neurocognitive correlates of schizotypy in first degree relatives of schizophrenia patients. Schizophr Bull. 2002;28:367–377. doi: 10.1093/oxfordjournals.schbul.a006946. [DOI] [PubMed] [Google Scholar]
- 37.Weiss AP, Schacter DL, Goff DC, et al. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry. 2003;53:48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- 38.Jastak S, Wilkinson SG. The Wide Range Achievement Test: Revised Administration Manual. Wilmington, DE: Jastak Associates; 1984. [Google Scholar]
- 39.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. (WAIS-III) San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 40.Coren S. Measurement of handedness via self-report: the relationship between brief and extended inventories. Percept Mot Skills. 1993;76(pt 1):1035–1042. doi: 10.2466/pms.1993.76.3.1035. [DOI] [PubMed] [Google Scholar]
- 41.Broome MR, Woolley JB, Johns LC, et al. Outreach and support in south London (OASIS): implementation of a clinical service for prodromal psychosis and the at risk mental state. Eur Psychiatry. 2005;20:372–378. doi: 10.1016/j.eurpsy.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Phillips LJ, Yung AR, McGorry PD. Identification of young people at risk of psychosis: validation of Personal Assessment and Crisis Evaluation Clinic intake criteria. Aust N Z J Psychiatry. 2000;34(suppl):S164–S169. doi: 10.1080/000486700239. [DOI] [PubMed] [Google Scholar]
- 43.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 44.Stadler MA, Roediger HL, III, McDermott KB. Norms for word lists that create false memories. Mem Cognit. 1999;27:494–500. doi: 10.3758/bf03211543. [DOI] [PubMed] [Google Scholar]
- 45.Tulving E. Multiple memory systems and consciousness. Hum Neurobiol. 1987;6:67–80. [PubMed] [Google Scholar]
- 46.Amaro E, Jr., Williams SC, Shergill SS, et al. Acoustic noise and functional magnetic resonance imaging: current strategies and future prospects. J Magn Reson Imaging. 2002;16:497–510. doi: 10.1002/jmri.10186. [DOI] [PubMed] [Google Scholar]
- 47.Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- 48.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 49.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 50.Brebion G, David AS, Jones H, Pilowsky LS. Hallucinations, negative symptoms, and response bias in a verbal recognition task in schizophrenia. Neuropsychology. 2005;19:612–617. doi: 10.1037/0894-4105.19.5.612. [DOI] [PubMed] [Google Scholar]
- 51.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 52.Weiss AP, Dodson CS, Goff DC, Schacter DL, Heckers S. Intact suppression of increased false recognition in schizophrenia. Am J Psychiatry. 2002;159:1506–1513. doi: 10.1176/appi.ajp.159.9.1506. [DOI] [PubMed] [Google Scholar]
- 53.Weiss AP, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Biol Psychiatry. 2004;55:668–675. doi: 10.1016/j.biopsych.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Tulving E, Kapur S, Markowitsch HJ, Craik FI, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc Natl Acad Sci U S A. 1994;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Otten LJ, Rugg MD. Electrophysiological correlates of memory encoding are task-dependent. Brain Res Cogn Brain Res. 2001;12:11–18. doi: 10.1016/s0926-6410(01)00015-5. [DOI] [PubMed] [Google Scholar]
- 56.Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. I. Encoding. Brain. 1998;121(pt 7):1239–1248. doi: 10.1093/brain/121.7.1239. [DOI] [PubMed] [Google Scholar]
- 57.Fossati P, Hevenor SJ, Lepage M, et al. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22:1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 58.Leube DT, Rapp A, Buchkremer G, et al. Hippocampal dysfunction during episodic memory encoding in patients with schizophrenia-an fMRI study. Schizophr Res. 2003;64:83–85. doi: 10.1016/s0920-9964(02)00503-0. [DOI] [PubMed] [Google Scholar]
- 59.Jessen F, Scheef L, Germeshausen L, et al. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am J Psychiatry. 2003;160:1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- 60.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Dempster FN, Brainerd CJ. Interference and Inihibition in Cognition. San Diego, CA: Acacdemic Press; 1995. [Google Scholar]
- 62.Achim AM, Bertrand MC, Sutton H, et al. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry. 2007;64:999–1014. doi: 10.1001/archpsyc.64.9.999. [DOI] [PubMed] [Google Scholar]
- 63.Danion JM, Huron C, Vidailhet P, Berna F. Functional mechanisms of episodic memory impairment in schizophrenia. Can J Psychiatry. 2007;52:693–701. doi: 10.1177/070674370705201103. [DOI] [PubMed] [Google Scholar]
- 64.Schobel SA, Lewandowski NM, Corcoran CM, et al. Differential targeting of the CA1 subfield of the hippocampal formation byschizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broome MR, Matthiasson P, Fusar-Poli P, et al. Neural correlates of executive function and working memory in the ‘at-risk mental state’. Br J Psychiatry. 2009;194:25–33. doi: 10.1192/bjp.bp.107.046789. [DOI] [PubMed] [Google Scholar]
- 66.Meisenzahl EM, Koutsouleris N, Gaser C, et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res. 2008;102:150–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 67.Cannon TD, Cornblatt B, McGorry P. The empirical status of the ultra high-risk (prodromal) research paradigm. Schizophr Bull. 2007;33:661–664. doi: 10.1093/schbul/sbm031. [DOI] [PMC free article] [PubMed] [Google Scholar]