Abstract
There is growing recognition that neural oscillations are important in a wide range of perceptual and cognitive functions. One of the key issues in electrophysiological studies of schizophrenia is whether high or low frequency oscillations, or both, are related to schizophrenia because many brain functions are modulated with frequency specificities. Many recent electrophysiological studies of schizophrenia have focused on high frequency oscillations at gamma band and in general support gamma band dysfunction in schizophrenia. We discuss the concept that gamma oscillation abnormalities in schizophrenia often occur in the background of oscillation abnormalities of lower frequencies. The review discusses the basic neurobiology for the emergence of oscillations of all frequency bands in association with networks of inhibitory interneurons and the convergence and divergence of such mechanisms in generating high vs low frequency oscillations. We then review the literature of oscillatory frequency abnormalities identified in each frequency band in schizophrenia. By describing some of the key functional roles exerted by gamma, low frequencies, and their cross-frequency coupling, we conceptualize that even isolated alterations in gamma or low frequency oscillations may impact the interactions of high and low frequency bands that are involved in key cognitive functions. The review concludes that studying the full spectrum and the interaction of gamma and low frequency oscillations may be critical for deciphering the complex electrophysiological abnormalities observed in schizophrenia patients.
Keywords: oscillation, schizophrenia, gamma, theta
Overview
Neural oscillations are electrical activities of the brain measurable at different frequencies. They are typically described as low frequency bands at delta (<4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12-30 Hz) to high frequencies at gamma band that spans from roughly gamma (30–80 Hz) to high gamma (>80 Hz). These oscillatory activities can be obtained at many levels, ranging from single cell to local field potentials in animals, to large-scale synchronized activities in human scalp. Patients with schizophrenia exhibit impaired neural oscillatory activities during sensory and cognitive tasks. One of the oldest but also reemerging debates in electrophysiology is what is the key frequency relevant to the pathophysiology of schizophrenia, given that abnormalities of neural oscillations are found essentially in all frequency bands in schizophrenia patients. Research into the specificity of frequency abnormality, if found, could provide key biological markers linking disease mechanism to the clinical dysfunctions in schizophrenia.
Many known brain functions are associated with electrical activities at specific frequencies. Recent event-related potential (ERP) studies of schizophrenia have focused on gamma band because of its critical role in cognitive functions1 and in general support gamma band reduction in schizophrenia.2,3 In comparison, earlier electroencephalographic (EEG)/ERP studies focused more on lower frequencies and also found substantial evidence of abnormalities.4 In this review, we first briefly describe the underlying neurobiology of oscillations and then provide a balanced review of the current evidence of gamma and low frequency oscillatory abnormalities in schizophrenia. The review focuses on gamma oscillation abnormalities in schizophrenia that often occur in the background of oscillation abnormalities of lower frequencies. We suggest that studying the full spectrum and the interaction of gamma and low frequency oscillations may be critical for deciphering the complex electrophysiological abnormalities observed in schizophrenia patients.
Neurobiology of Neural Oscillations
In laboratories, intracellular single unit recordings measure the oscillatory activity of a neuron, while extracellular local field potentials measure the summation of oscillatory activity originating from a group of neurons. In humans, oscillations are typically recorded by scalp EEG and magnetoencephalography (MEG) and occasionally by cortical and depth electrodes during surgery, which all yield complicated oscillatory waveforms comprised of energy in different frequency bands. Unlike animal recordings, scalp-recorded oscillations do not measure direct neuronal bursting, but instead measure synchronization of activities directly or indirectly reflecting neural communication processes. They are considered an assembly of local field potentials representing synchronous activity of a large set of neurons or neural circuits.
The cerebral cortex is composed of 2 major types of cells: excitatory pyramidal cells, which project to other parts of the cortex or brain and several types of nonpyramidal cells, the most important being interneurons. Interneurons are small neural cells that communicate via inhibitory synapses involving gamma aminobutyric acid (GABAA) receptors and are thought to be critical for the formation of oscillations. They generally make multiple contacts on to a single pyramidal cell, and also contact multiple pyramidal cells, allowing them to exert a powerful influence over pyramidal cell excitation. The emergence of oscillations in all frequency bands is associated with networks of inhibitory interneurons. The coordinated modulation of membrane potentials of networks of pyramidal cells allows groups of neurons to fire synchronously in the form of oscillatory electrical activities. Computer simulation models as well as the application of pharmacological agents that can alter the length of inhibition both demonstrate that the frequency of network oscillation depends on the duration of the inhibitory postsynaptic potentials created by the action of interneurons: the longer the inhibition, the slower the oscillatory frequencies.
Optogenetic studies have demonstrated that selective excitation of a specific subtype of GABAergic interneurons that are positive for parvalbumin induces gamma oscillations.5 Altered GABA function in schizophrenia is strongly supported by postmortem studies revealing loss of GABA markers in several cortical regions,6 leading to current theories that oscillatory dysfunctions may be associated with GABAergic abnormalities in schizophrenia. In the hippocampus and the cortex, parvalbumin-positive interneurons can fire in synch with gamma or theta oscillations. Animal models with reduced synaptic inhibition on to parvalbumin-positive interneurons are characterized by significantly reduced theta oscillations and theta-gamma coupling (see below) in the hippocampus,7 suggesting the possibility that GABA alteration in schizophrenia could be related to both gamma and low frequency band dysfunctions.
Neurotransmitters other than GABA also influence oscillations. For example, the cholinergic agent carbachol induces beta and theta oscillatory activities. In response to excitatory activation of metabotropic glutamate receptors, network inhibitory postsynaptic oscillations emerge and entrain pyramidal cell discharge at gamma-beta frequencies.6 Both cholinergic and glutamatergic dysfunctions are also closely tied to schizophrenia and could potentially play significant roles in the formation of various abnormal oscillatory activities found in schizophrenia.
While the neurochemical rhythmatogenesis of neural oscillations is under intense investigation, the genetic origin of these oscillations is an exciting but emerging field. It is known that spontaneous neural oscillations in the form of background EEG represent highly heritable traits in humans, up to 90% in heritability in some frequency bands.8 Gating of the theta-alpha band oscillations in a paired-click paradigm also showed higher heritability compared with averaged ERP supporting the view that oscillations may be closer to gene effects than standard ERP measures.9 Studies have successfully used neural oscillations to identify candidate genes, eg, the link between GABRA2 (GABAA alpha2 receptor gene) and beta1 (12.5–16 Hz) resting EEG and between CHRM2 (muscarinic 2 cholinergic receptor gene) and oddball derived theta/delta activities.10 These efforts provide supportive evidence that neural oscillatory phenotypes can facilitate disease genetic studies. The genetics of abnormal oscillatory activities in schizophrenia has not been systematically studied but may represent a particularly high-yield research approach for gene identification and more importantly for linking the genetics of schizophrenia pathophysiology to clinical problems experienced by patients.
Is there a Critical Frequency Linked to Schizophrenia?
Gamma frequency: Gamma oscillations (>30 Hz) have recently received great attention given their role in cognition.1 Gamma band findings in schizophrenia have been frequently reviewed.11 Reduced gamma band power and synchronization are reported in schizophrenia in steady state, sensory gating, arithmetic task, speech, oddball, Gestalt perception, and working memory conditions.12,13 However, inconsistent and opposite findings exist. Increased gamma has been described under working memory, somatosensory stimulation, visual recognition tasks, and unmedicated conditions in schizophrenia.2,11,14 Higher gamma synchrony is correlated with more severe psychotic symptoms2 or the opposite.11 The question of whether there is a “gamma band reduction in schizophrenia” becomes a prominent question by itself. Available evidence appears to suggest that there are gamma band reductions during impaired cognitive functions but sometimes no abnormalities or even increased gamma band activities at rest or during less cognitively demanding conditions.
Beta frequency: Schizophrenia patients demonstrated reduced beta and gamma intertrial phase-locking prior to sensorimotor action.15 In others reports, schizophrenia patients had a higher level of EEG noise at beta but reduced beta power in response to beta frequency stimulation (see review13). Unmedicated patients had higher beta and gamma power at all stages of sleep.16 Finally, patients treated with clozapine had increased beta power compared with healthy controls or patients treated with haloperidol.17
Alpha frequency: Reduced power and coherence of alpha activity in schizophrenia was reported during resting EEG4 and sustained attention. Unmedicated patients showed reduced alpha EEG power (along with increased neighboring frequencies at theta and beta) or coherence. Replication of some of these findings was reported in larger samples of medicated patients who showed diminished alpha (along with augmented delta and theta) compared with normal subjects (see review18). EEG alpha has been found to correlate with negative symptoms. Improvement of negative symptoms by repetitive transcranial magnetic stimulation was reported to be associated with increased alpha amplitude.19
Theta and delta frequency: Increased EEG theta/delta is one of the more consistent observations in schizophrenia EEG/ERP studies, which occurs locally and globally, in unmedicated, in first episode, and in chronic patients.4 Interestingly, the increases become more widespread after clozapine treatment (see review18). Less suppression of the alpha-theta activities is the most significant oscillatory component marking the genetic liability for schizophrenia during sensory gating.9 A recent review of MEG studies of spontaneous activity revealed converging evidence of increased theta and delta oscillations in subjects with schizophrenia, most prominent in the temporal lobe.20 Like other frequencies, contradictory and negative findings exist. For example, in sleep, unmedicated schizophrenia patients showed reduced delta/theta power.21
The above review by no means covers the rich EEG/ERP data on frequency-specific abnormalities in schizophrenia. It intends to illustrate that oscillatory abnormalities in schizophrenia is not simply about gamma reduction. Both gamma band reductions and increases have been associated with schizophrenia, and more importantly, they likely occur in the context of multiple oscillatory abnormalities in other frequencies. A better understanding of the relationships of high vs low frequency oscillations during cognitive functions is necessary to guide future electrophysiology studies in schizophrenia.
Functional Role of High vs Low Frequency Oscillations
A general principle of the functional role of oscillations in various frequency bands is that because of conduction delays in the brain, slow oscillations (eg, theta) are able to travel further distances and link remote areas of the brain. Fast oscillations such as gamma are in general less capable to traverse large distances and are therefore more likely to be restricted to local circuits. This was elegantly demonstrated by a series of EEG studies in which localized gamma activity was found in a visual task, beta synchronization between neighboring temporal and parietal cortices was found in a multimodal semantic processing task, and synchronization in the theta and alpha bands was involved in a working memory task that recruited frontal and parietal areas.22
Gamma is often the first component in response to a sensory stimulus, interestingly not only in auditory but also in visual, somatosensory, and olfactory or even cellular levels. In the auditory domain, gamma first occurs within ∼20–100 ms window after stimulus onset. Early gamma may not vary much with the degree of task complexity and may be primarily a sensory phenomenon.14 In comparison, later gamma represents perceptual and cognitive processing and is subject to selective attention and memory modulations.
On the lower frequencies, alpha rhythm is often observed in the resting state with closed eyes and was previously thought to reflect idling or internal mental processes, as it is reduced during movement or preparation for movement.23 However, recent studies suggest that alpha activity may be due to top-down inhibitory processes. For example, greater prestimulus alpha was observed in a more difficult task when a salient feature had to be ignored.24 Theta oscillations are linked to spatial exploration, rapid eye movement sleep, and memory. In rats, stimulation at the peak of a theta wave promotes long-term potentiation, whereas stimulation at the trough leads to long-term depression. In humans, theta oscillations have also been linked to memory retrieval. Prestimulus theta oscillations in the medial temporal lobe before presentation of a word was associated with successful remembering,25 and intracranial recordings in epilepsy patients showed that prestimulus alpha and theta activity in the rhinal cortex and hippocampus predicted correct remembering of a previously presented word.26 Low frequency oscillations may provide an essential role in engaging gamma rhythms and determining their behavioral consequence in attention.27
These experiments illustrate that individual high and low frequency oscillations are associated with both overlapped and distinct sensory and cognitive functions. Another important aspect is their cross-frequency communications. Cross-frequency coupling refers to interactions between oscillations of different frequency bands. The most well studied example is the observation of gamma frequency engagement in certain phases of theta cycles (theta-gamma coupling).
When a rat explores a familiar environment, hippocampal neurons called “place cells” are activated when the rat moves into its place field (the spatial location encoded by the place cell). Theta frequency oscillatory cycles can be recorded during such travel. Gamma oscillations are shown to be coupled to the phase of the theta cycle. Each successive gamma cycle represents the spatial location of the rat. As the rat enters the place field, gamma oscillations occur starting at the peak of the theta cycle. As it continues to move through the place field, successive gamma oscillations occur earlier and earlier in the theta phase, a process know as “phase precession.” Theta-gamma coupling is enhanced when rats correctly remember spatial information.23
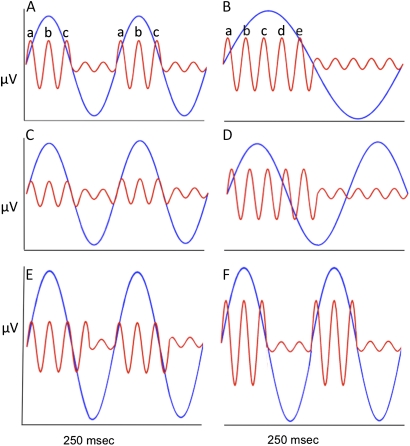
Emerging evidence of theta-gamma coupling has also been demonstrated in humans but has not yet been related to schizophrenia, although it has been proposed.28 Indeed, in humans, Canolty et al29 found that across a wide range of tasks, gamma and theta oscillations interacted such that the amplitude of high gamma oscillations (80–150 Hz) was increased at the trough of theta oscillations. It has been hypothesized that the coupling of theta and gamma may represent how numerous items are stored in working memory (figure 1A). Working memory capacity has been shown to be 7 ± 2 items, which approximates the number of gamma oscillations that occur within one theta cycle. Thus, working memory capacity may be constrained by the number of gamma cycles that occur in a theta cycle,28 and theta-gamma coupling may serve to link multiple items together as a unitary memory. One intriguing study in support of this hypothesis used intracranial recordings in the hippocampus of humans during a working memory task. Theta oscillation slowing was observed as the number of items to be remembered increased, allowing more gamma cycles to occur in each theta phase (figure 1B).30 Because working memory impairment is one of the core deficits in schizophrenia, abnormal theta-gamma coupling, which has been linked to successful memory in humans, could be a contributing factor and warrants further investigation. Hypothetical models of potential modes of abnormal theta-gamma coupling are depicted in figure 1C–F.
Fig. 1.
Hypothetical models of normal and abnormal high vs low frequency oscillations on cross-frequency coupling functions. A-B represent possible dynamics of normal gamma-theta coupling function: (A) Cross-frequency coupling during memory task in which gamma amplitude (red, 40 Hz) increases at peak of theta phase (blue, 8 Hz). (B) Increased working memory load associated with theta phase slowing (from 8 to 4 Hz) while gamma frequency stays constant, allowing more items to be incorporated within the theta cycle and remembered. C-F represent several hypothetical disease models of abnormal coupling of high and low frequency oscillations: (C) Gamma amplitude abnormalities: blunted gamma amplitude (while theta is held constant) could alter the salience of theta-gamma coupling. (D) Phase shift abnormalities: Theta phase slowing in order to allow more gamma cycles per theta cycle is dysfunctional such that, in comparison to B where theta frequency decreased from 8 Hz to 4 Hz, in this disease model, theta frequency only decreased from 8 Hz to 6 Hz. Thus, the coupling of increased gamma amplitude to the positive phase of theta only occurred for the first 4 gamma cycles instead of the first 5 gamma cycles. (E) Abnormally increased theta amplitude decreases the relative salience of gamma amplitude (which remains relatively constant) at the peak of theta phase. (F) Alternatively, increased theta amplitude leads to a greater increase in gamma amplitude and abnormally enhances theta-gamma coupling. Such abnormally enhanced theta-gamma coupling could make switching between coupling and decoupling more difficult and decrease the dynamic flexibility of network modulation.
Is Schizophrenia Related to a High or a Low Frequency Oscillation Problem?
The ever-deepening understanding of the neurobiology and cognitive functions of neural oscillations should help us rethink the implications of the oscillatory abnormalities associated with schizophrenia. Are we asking an overly simplified question when we focus only on gamma band, only on alpha band, theta band, and so on? Should we instead focus on the full spectrum of neural oscillations as well as their potential dysfunctional interactions?
A neural oscillation abnormality may be due to different disease mechanisms: (1) a frequency-specific dysfunction could be due to an inherent difficulty in the ability of neural circuits to oscillate at that frequency. In that case any process invoking primarily that oscillation would likely obligate an abnormality; (2) the capacity to oscillate at the frequency may be intact; an abnormal oscillatory response or its interaction with one or more frequencies may be the carrier or serve as a marker for a particular sensory/cognitive process that is affected by schizophrenia. In either case, defining the fundamental oscillatory problems associated with the core pathophysiology of schizophrenia is an important first step. Our review intends to draw attention to the low frequency abnormalities and their interaction with gamma, an area that may potentially be a high-yield research area. Because the relative energy from low frequency oscillations is proportionally larger than the energy from gamma in many EEG/ERP/MEG recordings, low frequency oscillations could exert a powerful influence on gamma activities.
Funding
This work was supported by the National Institutes of Health (MH085646, DA027680, T32MH067533).
Acknowledgments
All authors report no conflict of interests.
References
- 1.Tiitinen H, Sinkkonen J, Reinikainen K, et al. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- 2.Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31:37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 5.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wulff P, Ponomarenko AA, Bartos M, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106:3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Beijsterveldt CE, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Hum Genet. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- 9.Hong LE, Summerfelt A, Mitchell BD, et al. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Arch Gen Psychiatry. 2008;65:1008–1016. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porjesz B, Almasy L, Edenberg HJ, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 12.Uhlhaas PJ, Linden DE, Singer W, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 14.Basar-Eroglu C, Brand A, Hildebrandt H, et al. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Ford JM, Roach BJ, Faustman WO, Mathalon DH. Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biol Psychiatry. 2008;63:736–743. doi: 10.1016/j.biopsych.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tekell JL, Hoffmann R, Hendrickse W, et al. High frequency EEG activity during sleep: characteristics in schizophrenia and depression. Clin EEG Neurosci. 2005;36:25–35. doi: 10.1177/155005940503600107. [DOI] [PubMed] [Google Scholar]
- 17.Sperling W, Vieth J, Martus M, Demling J, Barocka A. Spontaneous slow and fast MEG activity in male schizophrenics treated with clozapine. Psychopharmacology (Berl) 1999;142:375–382. doi: 10.1007/s002130050902. [DOI] [PubMed] [Google Scholar]
- 18.Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99:225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y, Potkin SG, Kemp AS, et al. Therapeutic effects of individualized alpha frequency transcranial magnetic stimulation (alphaTMS) on the negative symptoms of schizophrenia. Schizophr Bull. 2006;32:556–561. doi: 10.1093/schbul/sbj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siekmeier PJ, Stufflebeam SM. Patterns of spontaneous magnetoencephalographic activity in patients with schizophrenia. J Clin Neurophysiol. 2010;27:179–190. doi: 10.1097/WNP.0b013e3181e0b20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keshavan MS, Reynolds CF, III, Miewald MJ, et al. Delta sleep deficits in schizophrenia: evidence from automated analyses of sleep data. Arch Gen Psychiatry. 1998;55:443–448. doi: 10.1001/archpsyc.55.5.443. [DOI] [PubMed] [Google Scholar]
- 22.von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 23.Buzsaki G. Rhythms of the Brain. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 24.Min BK, Herrmann CS. Prestimulus EEG alpha activity reflects prestimulus top-down processing. Neurosci Lett. 2007;422:131–135. doi: 10.1016/j.neulet.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Guderian S, Schott BH, Richardson-Klavehn A, Duzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci U S A. 2009;106:5365–5370. doi: 10.1073/pnas.0900289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fell J, Ludowig E, Staresina BP, et al. Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial EEG. J Neurosci. 2011;31:5392–5397. doi: 10.1523/JNEUROSCI.3668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canolty RT, Edwards E, Dalal SS, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axmacher N, Henseler MM, Jensen O, et al. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci U S A. 2010;107:3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]