Abstract
[URE3] is an amyloid-based prion of Ure2p, a regulator of nitrogen catabolism in Saccharomyces cerevisiae. The Ure2p of the human pathogen Candida albicans can also be a prion in S. cerevisiae. We find that overproduction of the disaggregating chaperone, Hsp104, increases the frequency of de novo [URE3] prion formation by the Ure2p of S. cerevisiae and that of C. albicans. This stimulation is strongly dependent on the presence of the [PIN+] prion, known from previous work to enhance [URE3] prion generation. Our data suggest that transient Hsp104 overproduction enhances prion generation through persistent effects on Rnq1 amyloid, as well as during overproduction by disassembly of amorphous Ure2 aggregates (generated during Ure2p overproduction), driving the aggregation toward the amyloid pathway. Overproduction of other major cytosolic chaperones of the Hsp70 and Hsp40 families (Ssa1p, Sse1p, and Ydj1p) inhibit prion formation, whereas another yeast Hsp40, Sis1p, modulates the effects of Hsp104p on both prion induction and prion curing in a prion-specific manner. The same factor may both enhance de novo prion generation and destabilize existing prion variants, suggesting that prion variants may be selected by changes in the chaperone network.
THE [URE3] prion is a self-propagating amyloid (a β sheet-rich filament) of the Saccharomyces cerevisiae Ure2 protein that largely prevents Ure2p’s repression of catabolism of poor nitrogen sources, leaving it stuck in the “on” position (Lacroute 1971; Wickner 1994; Taylor et al. 1999; Brachmann et al. 2005). [PSI+] is an amyloid prion of Sup35p, abrogating Sup35p's essential translation termination function enough to allow some read-through of termination codons (Cox 1965; Wickner 1994; Glover et al. 1997; King et al. 1997; Paushkin et al. 1997; King and Diaz-Avalos 2004; Tanaka et al. 2004). The [PIN+] prion was discovered on the basis of its requirement for efficient generation of the [PSI+] prion by overproduction of Sup35p (Derkatch et al. 1997). It was later shown to be an amyloid of Rnq1p (Sondheimer and Lindquist 2000;Derkatch et al. 2001) and to promote de novo formation of [URE3] as well (Bradley et al. 2002). Full-length recombinant Sup35p, Ure2p, and Rnq1p form amyloid fibers spontaneously at neutral pH, and these fibers can infect yeast cells with the corresponding prion (King and Diaz-Avalos 2004; Tanaka et al. 2004; Brachmann et al. 2005; Patel and Liebman 2007). These and other yeast and fungal prions (Saupe 2007; Wickner et al. 2010) have been useful models of the mammalian prion diseases, the transmissible spongiform encephalopathies (TSEs) (Collinge and Clarke 2007; Aguzzi et al. 2008; Caughey et al. 2009).
A single prion protein sequence can form any of several prion variants, with distinct biological properties, due to differences in amyloid structure, a phenomenon first demonstrated in mammal TSEs (Bessen and Marsh 1992; Caughey et al. 1998; Bruce 2003) and now known in several yeast prion systems as well (Derkatch et al. 1996; King 2001; Schlumpberger et al. 2001; Bradley et al. 2002; King and Diaz-Avalos 2004; Tanaka et al. 2004; Brachmann et al. 2005; Toyama et al. 2007). Yeast prion variants may be distinguished biologically by the intensity of the prion phenotype, the stability of prion propagation, the sensitivity to deficiency or overproduction of various chaperones, or the ease of transmission to other species (Derkatch et al. 1996; Newnam et al. 1999; Kushnirov et al. 2000; Borchsenius et al. 2006; Edskes et al. 2009).
[PSI+], [URE3], and [PIN+] all require the disaggregating chaperone Hsp104 for their propagation (Chernoff et al. 1995; Derkatch et al. 1997; Moriyama et al. 2000). Hsp104, along with other cooperating chaperones, breaks long amyloid filaments into shorter ones, thereby creating new prion seeds (reviewed by Romanova and Chernoff 2009; Haslberger et al. 2010). In addition, overproduction of Hsp104 efficiently cures [PSI+], but not [URE3] or [PIN+] (Chernoff et al. 1995; Derkatch et al. 1997; Moriyama et al. 2000).
Hsp70 and Hsp40 chaperones also play a prominent role in prion propagation, with considerable specificity in which chaperone helps or hinders which prion (reviewed by Sharma and Masison 2009). Mutants of the cytoplasmic Hsp70 ssa1 lose [PSI+], but not [URE3], while mutants of ssa2 lose [URE3] but not [PSI+] (Jung et al. 2000; Roberts et al. 2004). Overproduction of the Hsp40 family chaperone Ydj1p cures [URE3] via an interaction with Hsp70s (Moriyama et al. 2000; Sharma et al. 2009), but does not cure [PSI+] (Kushnirov et al. 2000). Sis1p, another Hsp40, is necessary for the propagation of [URE3], [PSI+], and [PIN+] (Sondheimer et al. 2001; Higurashi et al. 2008). The 34-residue repeat (“tetratricopeptide”)—containing cochaperones, Sti1p and Cpr7p, that mediate Hsp70—Hsp90 interactions have also been found to be involved in prion propagation (Jones et al. 2004; Reidy and Masison 2010), as have nucleotide exchange factors for Hsp70s, namely Sse1p and Fes1p (Fan et al. 2007; Kryndushkin and Wickner 2007). In addition to molecular chaperones, cytoskeletal proteins, elements of the ubiquitin system, and other factors modulate prion propagation in S. cerevisiae (Bailleul et al. 1999; Bailleul-Winslett et al. 2000; Allen et al. 2007; Kryndushkin et al. 2008).
Compared to prion propagation, much less is known about cellular proteins that can influence yeast prion generation. It was shown that either [PIN+] or overproduction of one of the several polyglutamine-rich proteins, including Rnq1p, is necessary for efficient [PSI+] de novo induction (Derkatch et al. 2001; Osherovich and Weissman 2001). Chaperone Ssa1p, a member of the Hsp70 family, was reported to enhance [PSI+] generation (Allen et al. 2005). In addition, deficiency of the ribosome-associated Hsp70s, called Ssb1/2, results in increased spontaneous or induced [PSI+] formation (Chernoff et al. 1999). Since cellular chaperones actively interact with misfolded protein aggregates, it is reasonable to suggest that other chaperones may influence prion generation in vivo. Consistent with this hypothesis, several chaperones influence the rate of spontaneous assembly of both Sup35p and Ure2p into amyloid-like fibers in vitro. Members of Hsp70 and Hsp40 families were reported to reduce the polymerization rate for both proteins (Krzewska and Melki 2006; Savistchenko et al. 2008; Shorter and Lindquist 2008). In contrast, Hsp104p was the only factor reported to promote amyloid fibrillization in vitro of both Sup35p and Ure2p (Krzewska and Melki 2006; Shorter and Lindquist 2006). Hsp104’s fiber-severing action may be speeding the gross appearance of amyloids, not by forming the very first seeds, a largely undetectable event, but by accelerating the amplification process, and so appear to be stimulating initiation.
In this study, we show that overproduction of chaperone Hsp104p can enhance [URE3] prion generation induced by excess of Ure2p, whereas other chaperones act in an opposite direction, inhibiting prion generation. A similar, but much stronger effect was obtained with a prion, [URE3alb], formed by Ure2p from Candida albicans (Ure2alb) in S. cerevisiae. Analysis of prion-specific chaperone action profiles reveals that the same factor may both increase de novo prion generation and destabilize existing prions.
MATERIALS AND METHODS
Strains, media, and plasmids:
Strains BY241 (MATa ura3leu2trp1 PDAL5-ADE2 PDAL5-CAN1kar1 [URE3] or [ure-o] [PIN+]) and BY251 (MATα leu2trp1his3 PDAL5-ADE2 PDAL5-CAN1kar1 [URE3] or [ure-o] [PIN+] were described previously (Brachmann et al. 2005). DK174 (MATa ura3leu2trp1his3 PDAL5-ADE2 PDAL5-CAN1kar1 [ure-o] [PIN+]) was made by mating between BY241 and BY251. Strain 1075 (MATα ura3leu2trp1his3 PDAL5-ADE2kar1 [URE3] or [ure-o] [PIN+]) was a gift of Dr. Daniel Masison (Sharma and Masison 2008). BY302 has the URE2 cerevisiae open reading frame (ORF) replaced with the C. albicans URE2 ORF, but driven by the constitutive cerevisiae URE2 promoter (Edskes et al. 2011). All of the above strains contain the ADE2 gene under control of the DAL5 promoter, allowing detection of the prion state of Ure2p (or Ure2palb) and even different [URE3] prion variants by colony color (Brachmann et al. 2005). The [ure-o][pin−] derivatives were obtained by growth on media with 5 mm GuHCl. For [PSI+] experiments, strain 74-D694 (MATa ade1-14 ura3leu2trp1his3 [PIN+] [psi−]) (Chernoff et al. 1995) was used.
Standard yeast media and cultivation procedures were used (Sherman 1991). To obtain better red color development, 1/2 YPD medium, containing half the normal amount of yeast extract, was used instead of YPD. For prion selection, −Ade medium consists of standard synthetic defined (SD) medium supplemented only with standard amounts of substances required for growth (0.002% of tryptophan, histidine, uracil, and 0.01% leucine). During prion induction, raffinose medium contains 2% raffinose as a carbon source, whereas galactose–raffinose medium contains 2% galactose and 1% raffinose instead of dextrose as a carbon source; no additional amino acids except those required for growth were added.
Plasmids used are listed in Table 1. Plasmid construction is described in supporting information.
TABLE 1.
Plasmids used in this study
| Plasmid name | Plasmid description | Promoter | Marker | Copy number | Source |
| DK30 | pRS425–Ssa1 | ADH1 | Leu2 | High copy | Kryndushkin and Wickner (2007) |
| DK26 | pRS425–Sse1 | SSE1 | Leu2 | High copy | Kryndushkin and Wickner (2007) |
| DK5 | pRS425–Ydj1 | TEF1 | Leu2 | High copy | Kryndushkin and Wickner (2007) |
| DK8 | Yep181–Hsp104 | HSP104 | Leu2 | High copy | This study |
| DK117 | Yep181–Sis1 | SIS1 | Leu2 | High copy | This study |
| DK119 | Yep181–Hsp104–Sis1 | native | Leu2 | High copy | This study |
| DK68 | PGAL–URE2 | GAL1 | Ura3 | High copy | This study |
| DK69 | PGAL–SUP35 | GAL1 | Ura3 | High copy | This study |
| pH 729 | PGAL–URE2alb | GAL1 | Trp1 | High copy | This study |
| pVTG12 | Ure2N–GFP | URE2 | Leu2 | Centromeric | Edskes et al. (1999) |
| RNQ1–GFP | RNQ1–GFP | ADH1 | Leu2 | Centromeric | Nakayashiki et al. (2005) |
Prion curing and induction experiments:
To measure [URE3] or [URE3alb] loss under chaperone overproduction, ∼20 random yeast colonies from a transformation plate were inoculated in liquid YPD media and grown overnight to allow plasmid loss (which was ∼70–90% complete according to further analysis); then 5000 cells were spread on a 1/2 YPD plate. The ratio of red to white colonies was scored.
To perform prion induction, the nonprion strain containing either the empty vector or a high-copy plasmid with a chaperone gene of interest was transformed with the corresponding overexpression plasmid (a prion gene under control of a galactose-inducible promoter). Transformants were grown overnight in liquid raffinose medium and then were shifted to galactose–raffinose medium for 2 days. Finally, for selection of prion-containing cells, aliquots were plated on −Ade medium, where the PGAL promoter is repressed. After 5 days of growth at 30°, medium and large Ade+ colonies were counted. The high-copy plasmid with a chaperone gene was maintained during the induction in galactose–raffinose medium, but was not selected during growth on −Ade plates; since overproduction of some chaperones can cure [URE3] or [URE3alb], we did not want to unnecessarily destabilize the prions that did arise.
Induction of [URE3] is particularly sensitive to the composition of selective media (−Ade). Absolute numbers for induction are greatly dependent on the presence of particular amino acids and their amounts in the medium (see also Brachmann et al. 2006). To get consistent results, it is very important to follow the same recipe for all experiments. We chose to use minimal SD media with only four supplements (Leu, Trp, His, and Ura). This recipe ensured the most reproducible results for all strains tested, including BY302.
Plasmid loss in Figure 3 was performed by growing yeast in YPD and streaking to single colonies on synthetic complete (SC) −Trp plates (to keep the PGAL–URE2alb plasmid). Colonies were tested for the presence of the HSP104 plasmid by replica plating on the SC −Leu. A colony that lost the HSP104 plasmid was grown further and used in induction experiments. The result was confirmed for three independent colonies that lost the HSP104 plasmid.
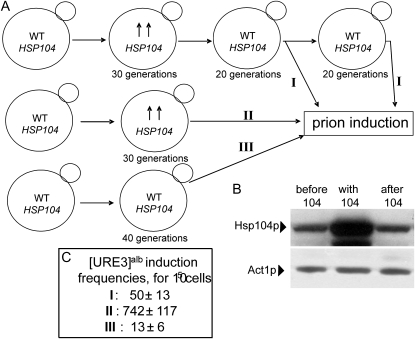
Figure 3.—
The presence of overproduced Hsp104p for a limited time creates long-lasting conditions for increased prion generation. (A) Scheme of the experiment. The upper line (I): Strain BY302 bearing the PGAL–URE2alb plasmid was transformed with the HSP104 overexpression plasmid. After growth for 30 generations the plasmid with HSP104 was lost and cells were grown further for 20 generations. An aliquot of cells was taken and the standard prion induction was performed; the rest of the culture was grown for 20 more generations and then also subjected to the prion induction procedure. The middle line (II): The same strain was subjected to prion induction after transformation with the HSP104 plasmid and growth for 30 generations. The bottom line (III): Prion induction was performed on the same strain without the HSP104 plasmid. (B) Western blotting with antiHsp104 antibodies showing that the level of Hsp104p returned to normal after the HSP104 plasmid was lost and cells were grown further for 20 generations. (C) Prion induction frequencies measured for I, II, and III. The frequency in I was indistinguishable when measured at 20 or 40 generations after the HSP104 plasmid was lost.
RESULTS
Hsp104p dramatically promotes the prion conversion of Ure2p from C. albicans:
We used the cerevisiae strain BY302 having the C. albicans URE2 open reading frame in place of that of cerevisiae, but driven by the cerevisiae URE2 promoter (Edskes et al. 2011). Among the genes derepressed on inactivation of Ure2p by prion formation is DAL5, encoding the allantoate permease (Turoscy and Cooper 1987). BY302 also carries ADE2 under control of the DAL5 promoter so that an active Ure2alb gives red Ade− colonies and an inactive Ure2alb results in white Ade+ clones (Brachmann et al. 2005). The C. albicans Ure2 protein (Ure2palb) can form the [URE3alb] prion in S. cerevisiae (Edskes et al. 2011) and, as with other prions (Wickner 1994), the frequency of prion formation is dramatically increased when this protein is overexpressed (Edskes et al. 2011). BY302 also carries the [PIN+] prion that enhances [URE3] generation in cells initially lacking the prion ([ure-o]) (Bradley et al. 2002). Using two stable [URE3alb] isolates, we examined the effects of overexpression of cellular chaperones that were shown to influence the stability of other yeast prions. These include Ssa1p (a member of the yeast Hsp70 family), Sse1p (a nucleotide-exchange factor for yeast Hsp70s), and Ydj1p and Sis1p (different members of the yeast Hsp40 family). We found that the curing profile of [URE3alb] was similar to that observed for conventional [URE3] (Table 2) (Moriyama et al. 2000; Schwimmer and Masison 2002; Kryndushkin and Wickner 2007). Ydj1p efficiently cured [URE3alb], while Sse1p and Ssa1p showed moderate curing. No curing was observed for Hsp104p or Sis1p. Overexpression of chaperones was confirmed by Western blotting (Figure S1).
TABLE 2.
[URE3alb] and [URE3] prion curing after chaperone overproduction
| Overproduced proteins; percentage of prion loss |
||||||
| Yeast strains | Control | Ssa1 | Ydj1 | Sse1 | Sis1 | Hsp104 |
| BY302 [URE3alb] | 1 ± 0.2 | 4 ± 0.8 | 60 ± 10 | 10 ± 1 | 1 ± 0.2 | 1 ± 0.2 |
| BY241 [URE3] | 0 ± 0 | 20 ± 2 | 55 ± 10 | 85 ± 10 | 0 ± 0 | 8 ± 1 |
[URE3alb] and [URE3] cells were transformed with chaperone overexpression plasmids. After overnight growth in rich medium, the percentage of cells that lost prion was scored. Overproduction of the indicated chaperone proteins is documented in Figure S1.
Data shown are the average of three experiments.
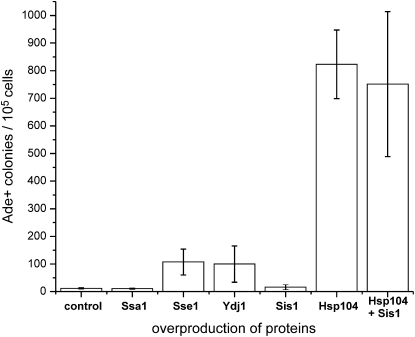
Further, we asked how the chaperones affect de novo induction of [URE3alb] prions in S. cerevisiae. The same set of chaperones was tested. Since the frequency of spontaneous prion induction is low (about 1 per million cells) and is difficult to estimate due to chromosomal mutations arising, we used transient overproduction of Ure2palb to induce [URE3alb] in strain BY302. BY302, containing either the empty vector or a high copy plasmid with a chaperone gene, was transformed with the PGAL–Ure2alb plasmid. For Ure2palb overproduction, transformants were grown overnight in liquid raffinose medium and then were shifted to galactose–raffinose medium for 2 days. Finally, for [URE3alb] detection, cells were plated on dextrose −Ade medium, where the PGAL promoter is repressed. Induction of [URE3alb] inactivates Ure2palb, allowing ADE2 transcription. Thus, the number of colonies on −Ade medium reflects [URE3alb] induction frequency. Remarkably, we found that overproduction of Hsp104p dramatically increases (∼70-fold) the induction of [URE3alb] (Figure 1). In addition, overproduction of Sse1p or Ydj1p also had a moderate stimulatory effect on [URE3alb] formation (∼8-fold), whereas excess of either Ssa1p or Sis1p had no effect (Figure 1). We confirmed that the vast majority of Ade+ colonies were indeed [URE3alb] by growth on medium containing GuHCl, an agent eliminating all known [URE3]s (Edskes et al. 2009). The result with Sse1p and Ydj1p is particularly surprising since these chaperones can cure an established [URE3alb]. It suggests that cellular chaperones can remodel amyloid aggregates, resulting either in generation of a new prion variant or in prion loss.
Figure 1.—
Effects of different chaperones on de novo [URE3alb] prion induction. BY302 was transformed with both a chaperone overexpression plasmid and the PGAL–URE2alb plasmid. A total of 105 cells were spread on −Ade plates to detect and compare [URE3alb] induction frequencies. At least three independent experiments were performed and average numbers were plotted. Error bars represent standard deviation.
Effects of Hsp104p and other chaperones on [URE3] prion generation:
To determine the specificity of the observed effect for Hsp104p, we tested whether the same set of chaperones can also influence the formation of the cerevisiae [URE3]. The basic setup of the experiments was the same as was used for [URE3alb]: strain BY241 [ure-o] [PIN+], containing either empty vector or a high-copy plasmid with a chaperone gene, was transformed with the PGAL–URE2 plasmid, and prion induction experiments were performed. We could still detect a stimulatory effect of overproduced Hsp104p on [URE3] de novo prion formation, although much smaller (∼3-fold; Figure 2A; Table 3) compared to the effect on [URE3alb] (∼70-fold; Figure 1). In contrast, overproduction of Ssa1p, Sse1p, and Ydj1p reproducibly showed a mild inhibitory effect on [URE3] formation (Table 3), consistent with their [URE3] curing effects (Table 2). Since the absolute effects of chaperones on [URE3] induction were relatively small, we reproduced our findings in two other [ure-o] [PIN+] yeast strains: DK174 (with related background) and 1075 (Sharma and Masison 2008) (with unrelated background). In each case we found an ∼3-fold increase of [URE3] prion formation when an elevated level of Hsp104p was present in cells, whereas an excess of Ssa1p, Sse1p, or Ydj1p repressed [URE3] induction (Table 3). Again, the majority of colonies on −Ade plates were indeed [URE3], as confirmed by GuHCl curing. Different numbers of colonies on −Ade plates were not related to any kind of toxicity, because simultaneous plating of cells on rich medium resulted in equal colony number with and without overproduced Hsp104p (data not shown). However, the effect of overproduced Sis1p was dependent on genetic background. Elevated levels of Sis1p enhanced [URE3] de novo prion induction in BY241 and the related strain DK174, while reducing [URE3] induction in 1075.
Figure 2.—
Hsp104 can influence both [URE3] and [PSI+] prion induction. (A) Strain DK174 having the PGAL–URE2 plasmid was transformed with either control vector or the HSP104 overexpression plasmid. After the standard induction procedure, 106 cells were spread on −Ade plates. (B) A Similar induction assay was performed for the strain 74D-694 [psi−] [PIN+] having the PGAL–SUP35 plasmid and either control vector or the HSP104 overexpression plasmid.
TABLE 3.
Effects of different chaperones on de novo [URE3] induction by excess of Ure2p
| Overproduced proteins (Ade+ colonies per 106 cells) |
|||||||
| Yeast strains | Control | Ssa1 | Sse1 | Ydj1 | Sis1 | Hsp104 | Sis1 +Hsp104 |
| BY241 | 56 ± 4 | 21 ± 4 | 36 ± 6 | 32 ± 5 | 91 ± 10 | 146 ± 19 | 165 ± 22 |
| DK174 | 21 ± 3 | 10 ± 2 | 12 ± 1 | 12 ± 3 | 55 ± 18 | 66 ± 12 | 153 ± 13 |
| 1075 | 58 ± 3 | 45 ± 4 | 42 ± 4 | 40 ± 5 | 25 ± 6 | 182 ± 10 | 38 ± 7 |
Three different yeast strains were transformed with both a chaperone overexpression plasmid and the PGAL–URE2 plasmid. A total of 106 cells were spread on –Ade plates to detect and compare [URE3] induction frequencies. Numbers are averages of three independent induction experiments.
To investigate the effect of Sis1p further, we constructed a high-copy plasmid that contained both SIS1 and HSP104 genes. Simultaneous overproduction of Sis1p and Hsp104p resulted in an additive increase of [URE3] induction in strains BY241 and DK174 (Table 3). The observed effect is consistent with the proposed cooperation between Sis1p and Hsp104p during prion propagation (Tipton et al. 2008). While Hsp104 is believed to fragment prion fibers, Sis1p is involved in the delivery of prion substrates to Hsp104p (Tipton et al. 2008).
Hsp104p was shown in vitro to be able to eliminate the lag phase of recombinant Sup35NM amyloid assembly, a result interpreted to mean that Hsp104 catalyzes the formation of prion nuclei (Shorter and Lindquist 2004). If this were the mechanism of Hsp104 stimulation of [URE3] and [URE3alb] formation, then the stimulatory effect on prion induction should be observed even in the absence of the seeding factor, [PIN+]. To test this hypothesis, we repeated the induction experiments described above, using the isogenic strains BY241 [ure-o] [pin−] or BY302 [pin−], obtained by eliminating the [PIN+] determinant by growth on rich YPD medium supplemented with 5 mm GuHCl. We confirmed the absence of [PIN+] by introducing the centromeric plasmid pH 126 RNQ1–GFP and subsequent fluorescent microscopy analysis that showed diffuse cytoplasmic staining of RNQ1–GFP. Unlike the stimulation seen with a [PIN+] strain, we saw no stimulation of [URE3] or [URE3alb] generation in the [pin−] cells by overproduction of Hsp104p (Table 4). This result argues that in vivo Hsp104p can not efficiently produce initial amyloid seeds; rather, it makes the Rnq1p amyloid a better primer for Ure2p and/or accelerates the amyloid formation after seeds are formed.
TABLE 4.
Effects of Hsp104 on de novo [URE3] and [URE3alb] induction by excess of corresponding prion proteins in the absence of [PIN+]
| Yeast strains | Ade+ colonies/107 cells |
| URE2cerevisiae [pin−] + control | 2 ± 1 |
| URE2cerevisiae [pin−] + hcHSP104 | 2 ± 2 |
| URE2albicans [pin−] + control | 1 ± 1 |
| URE2albicans [pin−] + hcHSP104 | 2 ± 2 |
| URE2cerevisiae [PIN+] + control | 56 ± 4 |
Standard induction procedure was performed for [pin−] strains. A total of 107 cells were spread on –Ade plates. Three independent experiments were performed. hc, high copy. The URE2cerevisiae strain is BY241 and the URE2albicans strain is BY302. The [PIN+] control data are from Table 3.
Sis1p modifies the Hsp104p curing of yeast prions:
Having shown a potential cooperation between Sis1p and Hsp104p during induction of [URE3], we turned to the question of whether Sis1p can modify Hsp104p-mediated curing of yeast prions. This effect is highly prion specific, with [PSI+] much more sensitive to Hsp104p overproduction compared to [URE3] and [PIN+]. We tested two different strains of both [PSI+] and [URE3] and compared the efficiency of prion curing by high-copy plasmids expressing either Hsp104p alone or Hsp104p together with Sis1p. Surprisingly, we found that Sis1p modifies the Hsp104p curing effect dependent on the prion tested: Sis1p enhanced curing of [PSI+] by Hsp104p, but protected [URE3] from curing by Hsp104p (Table 5). This effect was specific for Sis1 since overproduction of another Hsp40 member, Ydj1p, did not show an additive effect in [PSI+] curing (Table 5). Currently it is not clear why Sis1p acts differently on these two yeast prions. It might reflect the difference in the substrate specificity for Sis1p or may be a consequence of different actions of Hsp104p on yeast prions. Certainly the two amyloids are quite different, with Ure2p amyloid being extremely stable, while that of Sup35p is more easily dissociated into monomers (Baxa et al. 2004; Kryndushkin and Wickner 2007; Toyama et al. 2007).
TABLE 5.
Sis1p modifies the Hsp104p curing of yeast prions
| Overproduced proteins (% prion loss) |
|||||
| Yeast strains | Control | Sis1 | Hsp104 | Sis1 + Hsp104 | Ydj1 + Hsp104 |
| BY241[URE3] | 0 | 0 | 8 ± 1 | 2.0 ± 0.6 | NDa |
| 1075 [URE3] | 0 | 0 | 1.0 ± 0.2 | 0.2 ± 0.1 | NDa |
| 74D-694 [PSI+] | 0 | 0 | 15 ± 4 | 44 ± 5 | 15 ± 4 |
| 779-6A [PSI+] | 0 | 0 | 84 ± 6 | 94 ± 3 | 85 ± 7 |
The curing assay was performed as described in Table 2. Numbers are averages of three independent curing experiments.
Experiments were not performed, because Ydj1p cures [URE3] efficiently, making a comparison with Sis1p senseless.
Effects of Hsp104p on [PSI+] prion generation:
Severing of prion fibers by Hsp104p is believed to underlie the stability of prion propagation for all amyloid-based yeast prions. We tested the induction of [PSI+] in the presence of elevated amounts of Hsp104p. Strain 74-D694 [psi−] [PIN+], bearing a control vector or the Hsp104p overexpression plasmid, was transformed with the PGAL–SUP35 plasmid. Prion induction experiments were performed as described above for [URE3]. The ade1-14 allele in 74-D694 allows selection of [PSI+]-containing cells on −Ade plates due to functional inactivation of Sup35p. Although overproduction of Hsp104p efficiently cures [PSI+] (Chernoff et al. 1995), we detected de novo generated [PSI+] clones (Figure 2B, Table 6), as did others (Borchsenius et al. 2006). As in [URE3] induction, we did not select for plasmid retention during selection on −Ade medium, so cells can lose the HSP104 expression plasmid during growth on −Ade plates. In fact most Ade+ cells had lost this plasmid during colony formation (data not shown). We assume that some [PSI+] prion variants are more resistant than others to the curing action of Hsp104p. For example, “stronger” prion variants recognized by greater Sup35p functional inactivation are less sensitive in general to Hsp104p overproduction compared to “weaker” variants (Cox et al. 2007). Indeed, despite an overall decrease in numbers of generated [PSI+] clones, the fraction of strong [PSI+] variants was substantially increased (Table 6).
TABLE 6.
Induction of [PSI+] by excess Sup35p
| 74D-694 [PIN+] | Strong Ade+ | Weak Ade+ | % of strong variants |
| PGAL–Sup35 + control | 21 ± 8 | 294 ± 31 | 7 |
| PGAL–Sup35 + hcHSP104 | 40 ± 14 | 142 ± 41 | 22 |
The [psi−][PIN+] strain was transformed with both the HSP104 overexpression plasmid and the PGAL–SUP35 plasmid. A total of 106 cells were spread on –Ade plates to detect and compare [PSI+] induction frequencies. Averages of three independent induction experiments are shown.
Hsp104p has little effect on spontaneous prion formation:
Spontaneous prion formation is a rare and poorly understood event. Little is known about modulators of this process. We tested whether Hsp104p can promote spontaneous prion formation in the same way as it does for prion formation induced by excess of prion protein. BY241, DK174, and BY302 containing either empty vector or high-copy plasmid with HSP104 were grown overnight in liquid minimal medium selective for the presence of plasmids. About 5 × 107 cells from each culture were spread on −Ade plates to select for spontaneously induced [URE3] or [URE3alb]. Surprisingly, almost no difference in the numbers of Ade+ colonies was observed (Table 7), indicating that Hsp104 has no stimulatory role on spontaneous [URE3] or [URE3alb] induction. The result with [URE3alb] is especially striking, considering the very high stimulatory effect of Hsp104p on induced [URE3alb] prion formation (Figure 1). Since the prion induction frequencies were low and the detection of true prion isolates was challenging due to the intrinsic instability of newly arising prions and the high proportion of Ade+ mutants, we introduced into strain BY241 the additional centromeric plasmid pVTG12, expressing a Ure2N–GFP fusion under control of the native URE2 promoter. Expression of Ure2N–GFP at low levels does not induce or cure [URE3] (Edskes et al. 1999), but enables testing for the prion by fluorescent microscopy analysis: Ure2N–GFP shows diffuse cytoplasmic staining in nonprion cells and one or two foci in prion-containing cells due to co-aggregation with prion aggregates. Using this additional test, we confirmed that most of the medium or large size colonies on −Ade plates were indeed [URE3] and an excess of Hsp104p did not significantly affect spontaneous [URE3] formation. In addition, we show for the first time that spontaneous [URE3] formation is clearly [PIN+] dependent, similar to the induced [URE3] formation by an excess of Ure2p (Table S1).
TABLE 7.
Spontaneous induction of [URE3] and [URE3alb]
| Yeast strains | Ade+ colonies/107 cells |
| BY241 [PIN+] + control | 22 ± 8 |
| BY241 [PIN+] + hcHSP104 | 27 ± 5 |
| DK174 [PIN+] + control | 19 ± 2 |
| DK174 [PIN+] + hcHSP104 | 20 ± 5 |
| BY302 [PIN+] + control | 1 ± 1 |
| BY302 [PIN+] + hcHSP104 | 3 ± 2 |
Yeast strains were transformed with either the control vector or the HSP104 overexpression plasmid. A total of 107 cells were spread on –Ade plates to detect and compare prion induction frequencies. Numbers are averages of three independent induction experiments.
Persistent epigenetic effect of overproduced Hsp104p on [URE3alb] prion induction:
The ability of Hsp104p to stimulate prion formation only in the presence of preformed amyloid seeds ([PIN+] factor) indicates that Hsp104p may work directly on the Rnq1p aggregates of the [PIN+] prion, making them more suited for priming Ure2p amyloid formation. To check this hypothesis, we introduced the high-copy plasmid with HSP104 into yeast strain BY302, bearing the PGAL–Ure2alb plasmid. After growth for 30 generations, the plasmid with HSP104 was lost (see materials and methods) and cells were grown further for 20 generations to ensure that the level of Hsp104p returned to normal (Figure 3, A and B). Then the culture was split; one portion of cells was washed with galactose–raffinose medium and [URE3alb] was induced as usual (2 days on galactose–raffinose medium followed by spreading on −Ade plates), whereas another part was grown for 20 more generations in dextrose before induction. For controls, the same strain that either had the HSP104 overexpression plasmid or never received the HSP104 plasmid were used for [URE3alb] induction without the 20- or 40-generation delay. Finally, cells that were grown for 20 + 20 generations after the HSP104 plasmid was lost were also subjected to [URE3alb] induction. The results of all induction experiments were analyzed and compared (Figure 3C). Remarkably, the presence of overproduced Hsp104p for a limited time creates long-lasting conditions for increased prion generation, indicating that preincubation with an excess of Hsp104p may modify the intrinsic [PIN+] factor. The full stimulatory effect of Hsp104p requires the presence of an excess of Hsp104p during prion induction. Note that a similar experiment on the S. cerevisiae [URE3] would be very challenging since the stimulatory effect of Hsp104p on [URE3] is much smaller.
DISCUSSION
The primary physiological function of Hsp104 is to disaggregate proteins inactivated by heat or other stresses; Hsp104 is a true “heat shock protein” essential for induced resistance to heat shock but otherwise dispensable (Sanchez and Lindquist 1990; Parsell et al. 1994). There is conflicting evidence about whether Hsp104p by itself can interact with and fragment yeast prion aggregates in vitro (Inoue et al. 2004; Shorter and Lindquist 2004, 2006; Krzewska et al. 2007), but little doubt that, in concert with Hsp70s, Hsp40s and possibly other components, this is its major role in vivo (Paushkin et al. 1996; Ness et al. 2002; Kryndushkin et al. 2003; Satpute-Krishnan et al. 2007). Here, we show for the first time that Hsp104p can increase de novo [URE3] and [URE3alb] prion generation. Moreover, we tested other chaperones that were known to interact with prions (including members of Hsp70 and Hsp40 members and their cofactors) in both induction and curing assays. Interestingly, the effects on the [URE3alb] prion were particularly striking. In the past, similar prions formed by homologous proteins from different species were used successfully to reveal additional chaperone players involved in prion propagation (Kryndushkin et al. 2002). Such genetic systems can be attractive models for studying cellular interactions of prions when they are more sensitive to perturbations in the cellular environment compared to conventional prions.
Chaperones are the main system for correcting protein folding, and they are induced by many stressful conditions, including the presence of the [URE3] and [PSI+] prions (Jung et al. 2000; Schwimmer and Masison 2002). Nonetheless, prions can propagate stably during cell growth. Both induction and propagation of prions are tightly dependent on cellular chaperones. By analogy with viruses, whose characteristics are shaped by their hosts, yeast prions are shaped and selected by the cellular chaperone environment.
Remarkably, in some cases we observed that the same factor may both enhance de novo prion generation and destabilize existing prion variants. For example, many variants of [URE3] are mildly destabilized by Hsp104p overproduction and at the same time we found that overproduction of Hsp104p caused overall increase in [URE3] induction levels. Similarly, existing [URE3alb] variants can be destabilized by overexpression of either Ydj1p or Sse1p; however, the same overexpression resulted in an overall increase in [URE3alb] colonies. This observation can be explained by the dual role of chaperones in the replication cycle of amyloid-based prions. Stable prion propagation requires mechanisms for fragmentation of prion fibers, which is mediated by cellular chaperones. Insufficient fragmentation will cause the formation of large prion polymers with limited seeding capacity and impaired ability to transmit to daughter yeast cells. We assume that overproduction of Hsp104p causes the remodeling of amyloid aggregates generated during prion induction. It eliminates conformations that are especially sensitive to the chaperone network and accumulate variants that can be fragmented but not destroyed, thus resulting in an overall increase in the numbers of prion-positive yeast colonies. Chaperones of the Hsp70 and Hsp40 families work together with Hsp104p and may cause similar effects. One prediction from the above explanation is that the spectrum of arising prion variants will be narrower when Hsp104p is overproduced during prion induction. However, it is extremely difficult to prove this hypothesis experimentally. The majority of de novo arising variants are extremely unstable and there are no methods available to measure the distinctions among them. Our observation of selective decrease of weak [PSI+] variants generated during excess of Hsp104p is consistent with this idea.
Since several members of the Hsp70 and Hsp40 families can cooperate with Hsp104p, it is important to understand the substrate specificity of its action. Recent studies have proposed that the yeast prion propagation cycle involves a Sis1p-dependent delivery of prion substrates to Hsp104p (Higurashi et al. 2008; Tipton et al. 2008). Our results support the hypothesis of cooperation between Sis1p and Hsp104p during yeast prion maintenance. Sis1p can modulate the effects of Hsp104p on both prion induction and prion curing in a prion-specific manner. In contrast, another major yeast Hsp40 member, Ydj1p, acts differently, indicating the specificity of Sis1p action.
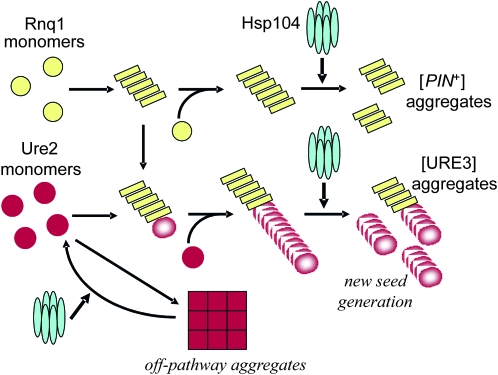
What is the mechanism of Hsp104p’s stimulatory effect on prion induction? On the basis of its known cellular function, Hsp104p may act in several different ways. It may induce the initial nucleation event assembling Ure2p monomers or small oligomers into amyloid seeds; a similar mechanism was proposed on the basis of in vitro studies (Shorter and Lindquist 2004, 2006). Alternatively, Hsp104p may act on other stages of the prion assembly process by remodeling existing prion aggregates or by preventing off-pathway aggregation of overproduced prion protein. We tried to distinguish between these possibilities using a genetic approach. First, we can detect a stimulatory effect of Hsp104p only in [PIN+] strains, indicating that preformed amyloid seeds are required (Tables 3 and 4). Second, we showed that the presence of overproduced Hsp104p for a limited time period generates persistent conditions for increased prion induction (Figure 3). Presumably, Hsp104p modifies the [PIN+] variant by fragmenting and/or reshaping Rnq1 amyloid seeds, making them more suited for priming Ure2p amyloid formation. Finally, the excess of Hsp104p influences only prion formation induced by overproduction of the prion protein, but has no effect on prions generated from endogenous prion protein (Table 7), suggesting the involvement of Hsp104p in disassembly of nonprion aggregates of the overproduced prion protein that drives the aggregation toward the amyloid pathway (Figure 4).
Figure 4.—
A model explaining the stimulatory effect of Hsp104p on prion induction. Hsp104p fragments Rnq1 amyloid and possibly Ure2 amyloid, creating new prion seeds. By modifying Rnq1 amyloid, Hsp104p may select out a different [PIN+] variant. In addition, it disassembles amorphous Ure2 aggregates, driving the aggregation toward the amyloid pathway.
The efficient disaggregation activity of Hsp104p raises hopes that Hsp104p might hold therapeutic potential for antagonizing several devastating neurodegenerative amyloid disorders, including Alzheimer, Huntington, and Parkinson diseases. Surprisingly, no metazoan homolog or analog of Hsp104p has been identified; such unique disaggregation activity is restricted to bacteria, fungi, and plants. Several attempts were made to introduce the well-characterized yeast HSP104 gene in rodent models of neurodegenerative disorders (Vacher et al. 2005; Perrin et al. 2007; Arimon et al. 2008; Lo Bianco et al. 2008). These reports showed that Hsp104 was able to reduce aggregation rates for various amyloids and even prolong the lifespan of a Huntington disease mouse model by ∼20% (Vacher et al. 2005). Transgenic mice expressing HSP104 appear to be normal, indicating that Hsp104p does not interfere with mammalian development (Vacher et al. 2005). However, our study reveals an additional potential complication for this approach, namely that overproduction of Hsp104 may generate new prion variants or that partial disassembly of amyloid aggregates by Hsp104p could generate new amyloid seeds that might be self-propagating. Remodeling of amyloid aggregates by Hsp104p might make them more transmissible to the nearest tissue and result in faster spread of a disease state. Further studies are required to assess potential dangers of Hsp104p expression in the mammalian brain. It would be of interest to test whether overproduced Hsp104p can increase the production of self-propagating species made from disease-associated proteins in cell culture models of neurodegenerative disorders.
Acknowledgments
We thank D. Masison [National Institutes of Health (NIH), Bethesda, MD] and M. D. Ter-Avanesyan (Cardiology Research Center, Moscow, Russia) for plasmids and strains; D. M. Cyr (University of North Carolina, Chapel Hill, NC) and P. Needham (University of Pittsburgh, Pittsburgh, PA) for kindly sharing antibodies; K. O’Connell (NIH) for help with microscopy; and members of our lab for a critical reading of the paper. This research was supported by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
LITERATURE CITED
- Aguzzi A., Baumann F., Bremer J., 2008. The prion’s elusive reason for being. Annu. Rev. Neurosci. 31: 439–477 [DOI] [PubMed] [Google Scholar]
- Allen K. D., Wegrzyn R. D., Chernova T. A., Muller S., Newnam G. P., et al. , 2005. Hsp70 chaperones as modulators of prion life cycle: novel effects of Ssa and Ssb on the Saccharomyces cerevisiae prion [PSI+]. Genetics 169: 1227–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K. D., Chernova T. A., Tennant E. P., Wilkinson K. D., Chernoff Y. O., 2007. Effects of ubiquitin system alterations on the formation and loss of a yeast prion. J. Biol. Chem. 282: 3004–3013 [DOI] [PubMed] [Google Scholar]
- Arimon M., Grimminger V., Sanz F., Lashuel H. A., 2008. Hsp104 targets multiple intermediates on the amyloid pathway and suppresses the seeding capacity of Abeta fibrils and protofibrils. J. Mol. Biol. 384: 1157–1173 [DOI] [PubMed] [Google Scholar]
- Bailleul P. A., Newnam G. P., Steenbergen J. N., Chernoff Y. O., 1999. Genetic study of interactions between the cytoskeletal assembly protein Sla1 and prion - forming domain of the release factor Sup35 (eRF3) in Saccharomyces cerevisiae. Genetics 153: 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul-Winslett P. A., Newnam G. P., Wegrzyn R. D., Chernoff Y. O., 2000. An antiprion effect of the anticytoskeletal drug latrunculin A in yeast. Gene Expr. 9: 145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxa U., Ross P. D., Wickner R. B., Steven A. C., 2004. The N-terminal prion domain of Ure2p converts from an unfolded to a thermally resistant conformation. J. Mol. Biol. 339: 259–264 [DOI] [PubMed] [Google Scholar]
- Bessen R. A., Marsh R. F., 1992. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J. Virol. 66: 2096–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchsenius A. S., Muller S., Newnam G. P., Inge-Vechtomov S. G., Chernoff Y. O., 2006. Prion variant maintained only at high levels of the Hsp104 disaggregase. Curr. Genet. 49: 21–29 [DOI] [PubMed] [Google Scholar]
- Brachmann A., Baxa U., Wickner R. B., 2005. Prion generation in vitro: amyloid of Ure2p is infectious. EMBO J. 24: 3082–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann A., Toombs J. A., Ross E. D., 2006. Reporter assay systems for [URE3] detection and analysis. Methods 39: 35–42 [DOI] [PubMed] [Google Scholar]
- Bradley M. E., Edskes H. K., Hong J. Y., Wickner R. B., Liebman S. W., 2002. Interactions among prions and prion “strains” in yeast. Proc. Natl. Acad. Sci. USA 99(Suppl. 4): 16392–16399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. E., 2003. TSE strain variation: an investigation into prion disease diversity. Br. Med. Bull. 66: 99–108 [DOI] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J., Bessen R. A., 1998. Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J. Biol. Chem. 273: 32230–32235 [DOI] [PubMed] [Google Scholar]
- Caughey B., Baron G. S., Chesebro B., Jeffrey M., 2009. Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu. Rev. Bioch. 78: 177–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff Y. O., Lindquist S. L., Ono B.-I., Inge-Vechtomov S. G., Liebman S. W., 1995. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268: 880–884 [DOI] [PubMed] [Google Scholar]
- Chernoff Y. O., Newnam G. P., Kumar J., Allen K., Zink A. D., 1999. Evidence for a protein mutator in yeast: role of the Hsp70-related chaperone Ssb in formation, stability and toxicity of the [PSI+] prion. Mol. Cell. Biol. 19: 8103–8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J., Clarke A. R., 2007. A general model of prion strains and their pathogenicity. Science 318: 930–936 [DOI] [PubMed] [Google Scholar]
- Cox B. S., 1965. PSI, a cytoplasmic suppressor of super-suppressor in yeast. Heredity 20: 505–521 [Google Scholar]
- Cox B. S., Byrne L. J., Tuite M. F., 2007. Prion stability. Prion 1: 170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch I. L., Chernoff Y. O., Kushnirov V. V., Inge-Vechtomov S. G., Liebman S. W., 1996. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics 144: 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch I. L., Bradley M. E., Zhou P., Chernoff Y. O., Liebman S. W., 1997. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics 147: 507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch I. L., Bradley M. E., Hong J. Y., Liebman S. W., 2001. Prions affect the appearance of other prions: the story of [PIN]. Cell 106: 171–182 [DOI] [PubMed] [Google Scholar]
- Edskes H. K., Gray V. T., Wickner R. B., 1999. The [URE3] prion is an aggregated form of Ure2p that can be cured by overexpression of Ure2p fragments. Proc. Natl. Acad. Sci. USA 96: 1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes H. K., McCann L. M., Hebert A. M., Wickner R. B., 2009. Prion variants and species barriers among Saccharomyces Ure2 proteins. Genetics 181: 1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes H. K., Engel A., McCann L. M., Brachmann A., Tsai H.-F., et al. , 2011. Prion-forming ability of Ure2 of yeasts is not evolutionarily conserved. Genetics 188: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q., Park K.-W., Du Z., Morano K. A., Li L., 2007. The role of Sse1 in the de novo formation and variant determination of the [PSI+] prion. Genetics 177: 1583–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover J. R., Kowal A. S., Shirmer E. C., Patino M. M., Liu J.-J., et al. , 1997. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89: 811–819 [DOI] [PubMed] [Google Scholar]
- Haslberger T., Bukau B., Mogk A., 2010. Towards a unifying mechanism for the ClpB/Hsp104-mediated protein disaggregation and prion propagation. Biochem. Cell. Biol. 88: 63–75 [DOI] [PubMed] [Google Scholar]
- Higurashi T., Hines J. K., Sahi C., Aron R., Craig E. A., 2008. Specificity of the J-protein Sis1 in the propagation of 3 yeast prions. Proc. Natl. Acad. Sci. USA 105: 16596–16601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Taguchi H., Kishimoto A., Yoshida M., 2004. Hsp104 binds to yeast Sup35 prion fiber but needs other factor(s) to sever it. J. Biol. Chem. 279: 52319–52323 [DOI] [PubMed] [Google Scholar]
- Jones G., Song Y., Chung S., Masison D. C., 2004. Propagation of yeast [PSI+] prion impaired by factors that regulate Hsp70 substrate binding. Mol. Cell. Biol. 24: 3928–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G., Jones G., Wegrzyn R. D., Masison D. C., 2000. A role for cytosolic Hsp70 in yeast [PSI+] prion propagation and [PSI+] as a cellular stress. Genetics 156: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.-Y., 2001. Supporting the structural basis of prion strains: induction and identification of [PSI] variants. J. Mol. Biol. 307: 1247–1260 [DOI] [PubMed] [Google Scholar]
- King C.-Y., Diaz-Avalos R., 2004. Protein-only transmission of three yeast prion strains. Nature 428: 319–323 [DOI] [PubMed] [Google Scholar]
- King C.-Y., Tittmann P., Gross H., Gebert R., Aebi M., et al. , 1997. Prion-inducing domain 2–114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc. Natl. Acad. Sci. USA 94: 6618–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryndushkin D., Wickner R. B., 2007. Nucleotide exchange factors for Hsp70s are required for [URE3] prion propagation in Saccharomyces cerevisiae. Mol. Biol. Cell 18: 2149–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryndushkin D., Smirnov V. N., Ter-Avanesyan M. D., Kushnirov V. V., 2002. Increased expression of Hsp40 chaperones, transcriptional factors, and ribosomal protein Rpp0 can cure yeast prions. J. Biol. Chem. 277: 23702–23708 [DOI] [PubMed] [Google Scholar]
- Kryndushkin D. S., Alexandrov I. M., Ter-Avanesyan M. D., Kushnirov V. V., 2003. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J. Biol. Chem. 278: 49636–49643 [DOI] [PubMed] [Google Scholar]
- Kryndushkin D., Shewmaker F., Wickner R. B., 2008. Curing of the [URE3] prion by Btn2p, a Batten disease-related protein. EMBO J. 27: 2725–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewska J., Melki R., 2006. Molecular chaperones and the assembly of the prion Sup35p, an in vitro study. EMBO J. 25: 822–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewska J., Tanaka M., Burston S. G., Melki R., 2007. Biochemical and functional analysis of the assembly of full-length Sup35p and its prion-forming domain. J. Biol. Chem. 282: 1679–1686 [DOI] [PubMed] [Google Scholar]
- Kushnirov V. V., Kryndushkin D., Boguta M., Smirnov V. N., Ter-Avanesyan M. D., 2000. Chaperones that cure yeast artificial [PSI+] and their prion-specific effects. Curr. Biol. 10: 1443–1446 [DOI] [PubMed] [Google Scholar]
- Lacroute F., 1971. Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. J. Bacteriol. 106: 519–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C., Shorter J., Regulier E., Lashuel H., Iwatsubo T., et al. , 2008. Hsp104 antagonizes alpha-synuclein aggregation and reduces dopaminergic degeneration in a rat model of Parkinson disease. J. Clin. Invest. 118: 3087–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama H., Edskes H. K., Wickner R. B., 2000. [URE3] prion propagation in Saccharomyces cerevisiae: requirement for chaperone Hsp104 and curing by overexpressed chaperone Ydj1p. Mol. Cell. Biol. 20: 8916–8922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayashiki T., Kurtzman C. P., Edskes H. K., Wickner R. B., 2005. Yeast prions [URE3] and [PSI+] are diseases. Proc. Natl. Acad. Sci. USA 102: 10575–10580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness F., Ferreira P., Cox B. S., Tuite M. F., 2002. Guanidine hydrochloride inhibits the generation of prion “seeds” but not prion protein aggregation in yeast. Mol. Cell. Biol. 22: 5593–5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnam G. P., Wegrzyn R. D., Lindquist S. L., Chernoff Y. O., 1999. Antagonistic interactions between yeast chaperones Hsp104 and Hsp70 in prion curing. Mol. Cell. Biol. 19: 1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osherovich L. Z., Weissman J. S., 2001. Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI+] prion. Cell 106: 183–194 [DOI] [PubMed] [Google Scholar]
- Parsell D. A., Kowal A. S., Singer M. A., Lindquist S., 1994. Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372: 475–478 [DOI] [PubMed] [Google Scholar]
- Patel B. K., Liebman S. W., 2007. “Prion proof” for [PIN+]: infection with in vitro-made amyloid aggregates of Rnq1p-(132–405) induces [PIN+]. J. Mol. Biol. 365: 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paushkin S. V., Kushnirov V. V., Smirnov V. N., Ter-Avanesyan M. D., 1996. Propagation of the yeast prion-like [psi+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 15: 3127–3134 [PMC free article] [PubMed] [Google Scholar]
- Paushkin S. V., Kushnirov V. V., Smirnov V. N., Ter-Avanesyan M. D., 1997. In vitro propagation of the prion-like state of yeast Sup35 protein. Science 277: 381–383 [DOI] [PubMed] [Google Scholar]
- Perrin V., Regulier E., Abbas-Terki T., Hassiq R., Brouillet E., et al. , 2007. Neuroprotection by Hsp104 and Hsp27 in lentiviral-based rat models of Huntington’s disease. Mol. Ther. 15: 903–911 [DOI] [PubMed] [Google Scholar]
- Reidy M., Masison D. C., 2010. Sti1 regulation of Hsp70 and Hsp90 is critical for curing of Saccharomyces cerevisiae [PSI+] prions by Hsp104. Mol. Cell. Biol. 30: 3542–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. T., Moriyama H., Wickner R. B., 2004. [URE3] prion propagation is abolished by a mutation of the primary cytosolic Hsp70 of budding yeast. Yeast 21: 107–117 [DOI] [PubMed] [Google Scholar]
- Romanova N. V., Chernoff Y. O., 2009. Hsp104 and prion propagation. Protein Pept. Lett. 16: 598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V., Lindquist S. L., 1990. HSP104 required for induced thermotolerance. Science 248: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Satpute-Krishnan P., Langseth S. X., Sero T. R., 2007. Hsp104-dependent remodeling of prion complexes mediates protein-only inheritance. PLoS Biol. 5: e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe S. J., 2007. A short history of small s: a prion of the fungus Podospora anserina. Prion 1: 110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savistchenko J., Krzewska J., Fay N., Melki R., 2008. Molecular chaperones and the assembly of the prion Ure2p in vitro. J. Biol. Chem. 283: 15732–15739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumpberger M., Prusiner S. B., Herskowitz I., 2001. Induction of distinct [URE3] yeast prion strains. Mol. Cell. Biol. 21: 7035–7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer C., Masison D. C., 2002. Antagonistic interactions between yeast [PSI+] and [URE3] prions and curing of [URE3] by Hsp70 protein chaperone Ssa1p but not by Ssa2p. Mol. Cell. Biol. 22: 3590–3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Masison D. C., 2008. Functionally redundant isoforms of a yeast Hsp70 chaperone subfamily have different antiprion effects. Genetics 179: 1301–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Masison D. C., 2009. Hsp70 structure, function, regulation and influence on yeast prions. Prot. Pep. Lett. 16: 571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Stanley R. F., Masison D. C., 2009. Curing of yeast [URE3] prion by the Hsp40 chaperone Ydj1p is mediated by Hsp70. Genetics 181: 129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., 1991. Getting started with yeast, pp. 3–21 Guide to Yeast Genetics and Molecular Biology, edited by Guthrie C., Fink G. R. Academic Press, San Diego [Google Scholar]
- Shorter J., Lindquist S., 2004. Hsp104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science 304: 1793–1797 [DOI] [PubMed] [Google Scholar]
- Shorter J., Lindquist S., 2006. Destruction or potentiation of different prions catalyzed by similar Hsp104 remodeling actiivities. Mol. Cell 23: 425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J., Lindquist S., 2008. Hsp104, Hsp70 and Hsp40 interplay regulate formation, growth and elimination of Sup35 prions. EMBO J. 27: 2712–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer N., Lindquist S., 2000. Rnq1: an epigenetic modifier of protein function in yeast. Mol. Cell 5: 163–172 [DOI] [PubMed] [Google Scholar]
- Sondheimer N., Lopez N., Craig E. A., Lindquist S., 2001. The role of Sis1 in the maintenance of the [RNQ+] prion. EMBO J. 20: 2435–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Chien P., Naber N., Cooke R., Weissman J. S., 2004. Conformational variations in an infectious protein determine prion strain differences. Nature 428: 323–328 [DOI] [PubMed] [Google Scholar]
- Taylor K. L., Cheng N., Williams R. W., Steven A. C., Wickner R. B., 1999. Prion domain initiation of amyloid formation in vitro from native Ure2p. Science 283: 1339–1343 [DOI] [PubMed] [Google Scholar]
- Tipton K. A., Verges K. J., Weissman J. S., 2008. In vivo monitoring of the prion replication cycle reveals a critical role for Sis1 in delivering substrates to Hsp104. Mol. Cell 32: 584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama B. H., Kelly M. J., Gross J. D., Weissman J. S., 2007. The structural basis of yeast prion strain variants. Nature 449: 233–237 [DOI] [PubMed] [Google Scholar]
- Turoscy V., Cooper T. G., 1987. Ureidosuccinate is transported by the allantoate transport system in Saccharomyces cerevisiae. J. Bacteriol. 169: 2598–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher C., Garcia-Oroz L., Rubinsztein D. C., 2005. Overexpression of yeast hsp104 reduces polyglutamine aggregation and prolongs survival of a transgenic mouse model of Huntington’s disease. Hum. Mol. Genet. 14: 3425–3433 [DOI] [PubMed] [Google Scholar]
- Wickner R. B., 1994. [URE3] as an altered URE2 protein: evidence for a prion analog in S. cerevisiae. Science 264: 566–569 [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Shewmaker F., Edskes H., Kryndushkin D., Nemecek J., et al. , 2010. Prion amyloid structure explains templating: how proteins can be genes. FEMS Yeast Res. 10: 980–991 [DOI] [PMC free article] [PubMed] [Google Scholar]