1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the varied combination of motor symptoms including tremor and bradykinesia (slowness of movement). As the disease progresses, motor symptoms worsen and medication side effects become more prevalent. As quality of life becomes increasingly compromised, patients may consider deep brain stimulation (DBS) surgery. Numerous studies show benefits of DBS of the subthalamic nucleus (STN) and the globus pallidus internus (GPi) in PD (Katayama et al., 2001; Obeso et al., 2001; Deuschl et al., 2006). Following DBS surgery, stimulation parameters are chosen to optimize patient motor symptoms while minimizing any side effects (Kumar, 2002; Hunka et al., 2005). Stimulation parameters include choice of contacts and polarity (cathodal versus anodal), amplitude, pulse width, and frequency (Volkmann et al., 2006). Depending on the institution, DBS programming may be performed by a variety of healthcare professionals, including movement disorder neurologists, neurosurgeons, fellows, occupational and physical therapists, and nurses (Hunka et al., 2005). Clinical rating scales, most commonly the Unified Parkinson’s Disease Rating Scale (UPDRS) (Goetz et al., 2007), are used to evaluate PD symptom improvement in response to different stimulation settings. Symptoms are rated on a 0 to 4 integer scale corresponding to normal, slight, mild, moderate, and severe. The UPDRS ratings are used to evaluate and adjust stimulation parameters (Lukhanina et al., 2000; Goetz et al., 2007). This subjective assessment can be highly dependent on the observer’s assessment and skill in evaluating these motor symptoms. Stimulation programming that utilizes an objective assessment method across multiple motor symptoms would allow for the selection of parameters based on clear quantitative measures rather than subjective assessments. Greater reliance on objective measures has the potential to improve motor outcomes while side effects are minimized.
Current methods of optimizing programming parameters require a continuous process that takes a significant amount of time and effort by both the clinician and patient. Programming sessions of DBS require more than twice the time of a typical PD evaluation by a movement disorder neurologist (Okun et al., 2005). Extended programming sessions result in patient fatigue and worsening symptoms, making programming even more difficult (Kumar, 2002; Hunka et al., 2005). It is estimated that programming a patient for the first year following implantation requires approximately 30 hours of clinical time (Hunka et al., 2005). Multiple visits lead to additional travel expenses for patients and can be particularly difficult for those who live in rural areas without adequate access to clinics specialized in DBS programming (Okun et al., 2005). While DBS has been effective in treating advanced PD, a more quantitative approach of programming could reduce the frequency and duration of programming sessions, reducing health care costs while improving patient outcomes.
The objective of this preliminary study was to evaluate the feasibility of an automated system to assist with motor symptom assessment during DBS programming. Two subjects diagnosed with PD underwent standard procedures for DBS outpatient programming while wearing a small, compact motion sensor. Data were collected as subjects completed motor tasks typically performed during DBS assessment. Quantitative variables of tremor and bradykinesia were used to create tuning maps, which correlated the severity of these motor symptoms as a function of stimulation parameters. A stimulation parameter estimation algorithm was developed to output optimal settings for individual symptoms and overall motor response across multiple symptoms.
2. Material and methods
Case studies were performed at the Center for Neurological Restoration at the Cleveland Clinic under the purview of both Cleveland Medical Devices Inc. and Cleveland Clinic Institutional Review Boards. All subjects provided informed consent.
2.1 Technology Overview
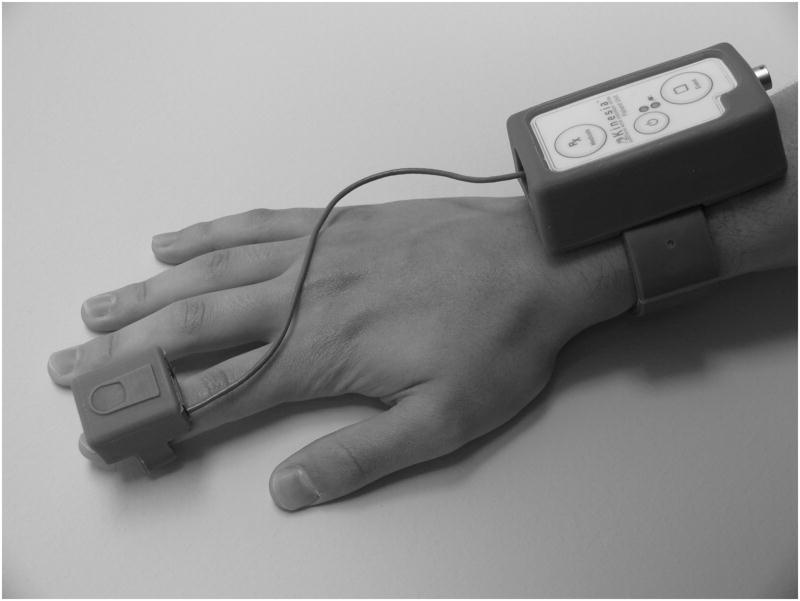
Kinesia™ (CleveMed, Cleveland, OH), a Food and Drug Administration cleared-to-market device, captures three dimensional motion data (Figure 1). The finger worn motion sensor contains three orthogonal accelerometers and three orthogonal gyroscopes to capture linear accelerations and angular velocities, respectively. Sensor data are sampled at 128 Hz and wirelessly transmitted to a computer in real time. A prototype software interface designed using LabVIEW 8.6 (National Instruments, Austin, TX) collects and saves to disk motion sensor, UPDRS-based motor task, and stimulation parameter data. Recorded DBS settings consisted of the positive and negative electrode contacts used for stimulation and stimulation voltage (volts), frequency (Hz), and pulse width (μsec).
Figure 1.
Kinesia Motion Capture Device. The unit consists of a finger-worn motion sensor and wrist-worn command module for data acquisition and wireless data transmission.
2.2 Data Collection Protocol
Two subjects were evaluated during their first outpatient programming session one month following DBS surgery. Both subjects presented primarily with moderate tremor (UPDRS rest tremor score 3) and with moderate bradykinesia (UPDRS finger tapping score 2 (subject 1) and 3 (subject 2)) during baseline assessment (off medication and off stimulation). Both received an electrode implant targeting the right STN and the contralateral upper extremity was assessed. Subjects were off antiparkinsonian medication overnight prior to the DBS programming session.
A trained nurse practitioner performed the programming procedure while Kinesia wirelessly transmitted kinematic data to the computer without obstructing hand movement or interfering with the standard DBS programming protocol. The sensor unit was placed over the most distal phalanx of the index finger (Figure 1). Each subject was instructed to perform two tasks from the UPDRS motor section III per stimulation setting. The tasks were determined by the clinician based on existing motor symptoms. Rest tremor (item #20) and finger tapping (item #23) were performed for twenty seconds each and used to evaluate symptom response to stimulation parameters during programming.
Each of the four monopolar electrode contact settings was assessed individually by incrementing stimulation voltage from 0 V until stimulation-induced side effects were elicited as determined by the programmer. These effects manifested as blurry vision, slurred speech, muscle contractions, etc. and subsided when the stimulator was turned off. To evaluate each contact, frequency was fixed at 130 Hz and pulse width at 60 μsec. Voltage was typically increased in 0.5 to 1.0 V increments and then reduced to 0.2 to 0.3 V once transient side effects occurred. On average, 2–3 minutes were given after each voltage increment before UPDRS motor tasks were performed. Subjects were given a rest period approximately twenty minutes before starting the next series of measurements along the subsequent contact.
2.3 Motor Symptom Features
Motion data collected during the DBS programming sessions were processed into quantitative variables that described motor symptom features. These features were plotted versus contact and voltage stimulation settings to provide a tuning map for assessing motor symptom severity in response to DBS settings.
2.3.1 Tremor
Kinematic data collected during rest tremor assessment were band pass filtered (2nd order Butterworth, 3 to 10 Hz). In a previous clinical study (Giuffrida et al., 2009), the log peak power of Kinesia motion sensor data demonstrated a high correlation to clinician scores for tremor tasks. This kinematic variable was processed from the motion sensor channel that exhibited the largest average signal amplitude.
2.3.2 Bradykinesia
While tremor improvement can be effectively characterized with a single variable, more complex analysis was required for bradykinesia assessment. Bradykinesia was evaluated from changes in amplitude and speed. Rhythm was another factor to consider; however, inconsistent movement can be reflected in amplitude or speed deficits. Therefore, kinematic angular velocity data collected during finger tapping were processed into two quantitative variables. Gyroscope sensor data were used to characterize the UPDRS finger tapping task since the finger naturally rotates about the second metacarpal joint and gyroscopes are designed to capture dynamic angular motions. First, kinematic signals were band pass filtered (2nd order Butterworth, 0.3 to 8 Hz). Log peak power of the angular velocity signal was selected to quantify tapping speed. Log peak power of the integrated angular velocity signal was selected to characterize tapping amplitude, the excursion angle of the index finger.
2.4 Tuning Maps
The tuning map provides a visual representation of motor symptom severity in response to different DBS parameters such as contact and voltage. Clinician-selected stimulation voltage and contact settings are displayed on the y and x axes, respectively. The values in the map represent the quantitative variable selected for a specific motor symptom and are color-coded based on a symptom severity range normalized between 0 and 1. The color range spans from white to black where white (value of 0) signifies the lowest symptom severity while black (value of 1) signifies the highest symptom severity achieved during a single DBS programming session. Normalization of data was only done when generating tuning maps. Individual tuning maps were created for rest tremor and finger tapping speed and amplitude.
2.5 Optimal Stimulation Parameter Estimation
An algorithm was designed to output the optimal stimulation contact and voltage combination for individual motor symptoms (tremor, bradykinesia speed, bradykinesia amplitude) as well as overall motor function across multiple symptoms. To minimize the effect of outlier values while still accurately capturing general motor response, a cubic trend fit of the severity variable was calculated separately along each contact at the voltage measurements for each symptom. Optimal stimulation settings for an individual symptom were assigned to the contact and voltage combination that yielded the largest percent improvement relative to baseline at 0 V.
A single set of stimulation settings for optimal motor response across the three symptoms tested was calculated using weights between 0 and 1 assigned to each individual symptom proportional to its motor response at optimal stimulation settings. More responsive motor symptoms corresponded to weight values closer to 1. Tuning map data were multiplied by their respective weighting constants and summed to give an overall weighted tuning map. Optimal stimulation settings for overall motor response were calculated as performed for individual symptoms.
3. Results
3.1 Case Study 1
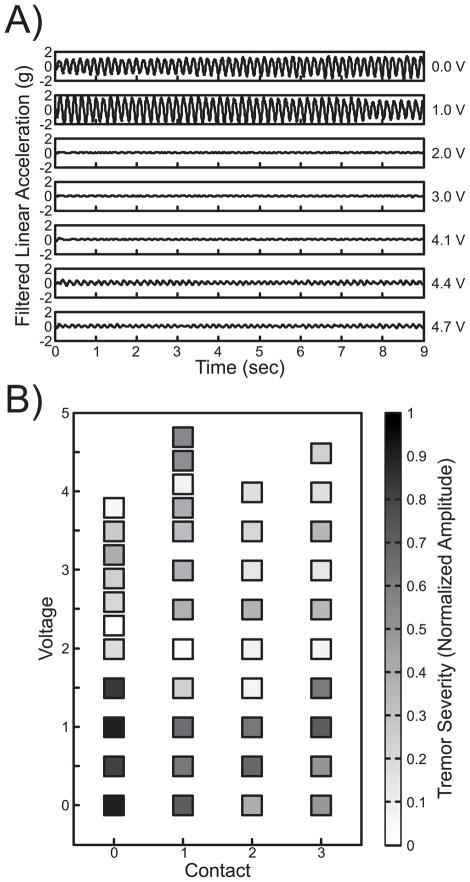
Subject 1 presented primarily with moderate tremor and bradykinesia during baseline motor assessment. Figure 2A shows example rest tremor motion data collected at a range of voltages for monopolar contact 1. Tremor activity decreases at 2.0 V and begins to return at 4.4 V. The normalized log peak power was then used to generate a tuning map. The dark colors at low voltages corresponding to high tremor acceleration transition to lighter colors as symptoms improved and darkened again at higher voltages (Figure 2B). The parameter estimation algorithm generated the largest percent improvement at 3.2 V on monopolar contact 0 as an optimal setting for rest tremor.
Figure 2.
Case Study 1 Tremor Summary. A) Filtered kinematic data collected during the rest tremor task for each clinician-selected voltage across contact 1. B) Clinician-selected contact and voltage settings are displayed on the x and y axes, respectively, with corresponding color-coded 0 to 1 motor symptom severity in terms of normalized tremor amplitude for each data point.
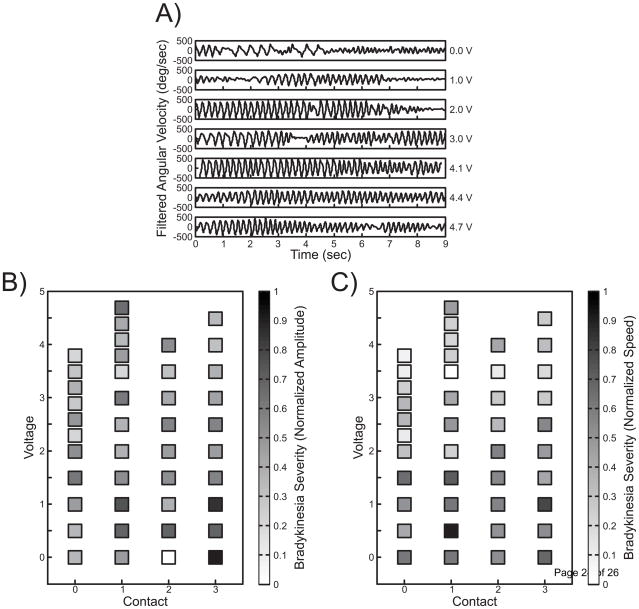
In contrast to tremor, finger tapping is a voluntary movement that may be impacted by several variables including speed and amplitude. Figure 3A shows a single channel of angular velocity for the finger tapping task across the same stimulation voltages and contact settings used for rest tremor. Signals at 0 V and 1 V demonstrate inconsistent finger tapping compared to higher voltages. Tuning maps plotted across stimulation settings for that subject were generated for the amplitude and speed (Figure 3B–C). The parameter estimation algorithm generated the largest percent improvement at 4.5 V on monopolar contact 3 (amplitude) and 3.8 V on monopolar contact 1 (speed).
Figure 3.
Case Study 1 Bradykinesia Summary. A) Filtered kinematic data collected during the finger tapping task for each clinician-selected voltage across contact 1. Clinician-selected contact and voltage settings are displayed on the x and y axes, respectively, with corresponding color-coded 0 to 1 motor symptom severity in terms of normalized finger tapping (B) amplitude and (C) speed for each data point.
Overall motor symptom response to stimulation was calculated using symptom weights for tremor (0.64) and bradykinesia amplitude (0.18) and speed (0.18). The parameter estimation algorithm generated the largest percent improvement at 3.5 V on monopolar contact 0.
3.2 Case Study 2
Subject 2 presented primarily with both moderate tremor and bradykinesia during baseline motor assessment. Figure 4A shows rest tremor amplitude was reduced when monopolar contact 0 was stimulated at 2.0 V. The parameter estimation algorithm generated the largest percent improvement at 2.0 V on monopolar contact 1 as an optimal setting for rest tremor (Figure 4B).
Figure 4.
Case Study 2 Tremor Summary. A) Filtered kinematic data collected during the rest tremor task for each clinician-selected voltage across contact 0. B) Clinician-selected contact and voltage settings are displayed on the x and y axes, respectively, with corresponding color-coded 0 to 1 motor symptom severity in terms of normalized tremor amplitude for each data point.
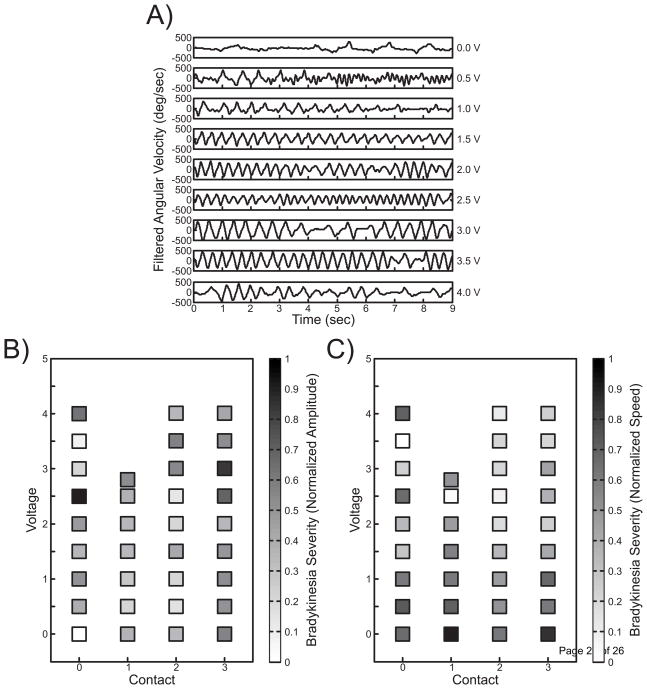
Subject 2 also demonstrated improvement in bradykinesia. Figure 5A shows a single channel of angular velocity for the finger tapping task across the same stimulation voltages and contact setting used for rest tremor. At 0 V finger tapping has a very low speed, except about four taps beginning half way through the task. As the voltage was increased, the subject increased tapping speed, and regularity improved. When the voltage was increased to 4.0 V, symptom impairment returned. The speed tuning map (Figure 5C) shows a gradual but distinct reduction in symptom severity across multiple contacts. The parameter estimation algorithm generated the largest percent improvement at 4.0 V on monopolar contact 3 (amplitude) and 4.0 V on monopolar contact 3 (speed).
Figure 5.
Case Study 2 Bradykinesia Summary. A) Filtered kinematic data collected during the finger tapping task for each clinician-selected voltage across contact 0. Clinician-selected contact and voltage settings are displayed on the x and y axes, respectively, with corresponding color-coded 0 to 1 motor symptom severity in terms of normalized finger tapping (B) amplitude and (C) speed for each data point.
Overall motor symptom response to stimulation was calculated from symptom weights for tremor (0.73) and bradykinesia amplitude (0.11) and speed (0.16). The parameter estimation algorithm generated the largest improvement at 3.0 V on monopolar contact 0.
4. Discussion
The Kinesia system successfully quantified motor symptom severity during DBS outpatient programming sessions. Quantitative motion features were selected to characterize rest tremor and bradykinesia during the UPDRS finger tapping task. Tuning maps summarized a full spectrum of voltage and contact combinations and corresponding severity values in a clear and organized manner. The stimulation parameter estimation algorithm generated optimal DBS settings to demonstrate differential motor response to stimulation. This technology utilized in conjunction with traditional clinical assessment may provide a tool to quickly and accurately tailor stimulation effectiveness for individual motor symptoms as well as minimize impairment across multiple motor signs.
4.1 Motor Symptom Stimulation Response
Parkinson’s disease manifests as several different motor symptoms including tremor and bradykinesia. While some individuals may exhibit a single dominant motor symptom, most exhibit a range of severities across multiple symptoms. Furthermore, different treatments may not affect all motor symptoms equally and some symptoms may be more difficult to assess than others. For example, it is difficult to visually rate all aspects of bradykinesia with a single measure for the finger tapping task, especially when comparing the case of low amplitude/high speed versus high amplitude/low speed. A recent pilot study characterized bradykinesia tasks not only by the standard UPDRS single severity score, but also developed a modified scoring system to evaluate multiple movement components (Espay et al., 2009). Results indicated levodopa improved finger tapping speed more than amplitude, suggesting assessing bradykinesia across multiple quantitative components may provide additional insight into treatment response. It is not well known why motor response varies for medication; however, part of the explanation may lie in the complex neural circuitry impacted by PD which spans across multiple brain regions including the motor cortex, basal ganglia, subthalamic nucleus, thalamus, and brainstem regions (Carr, 2002; Hallett, 2003; Schnitzler and Gross, 2005). While not fully understood, it is clear that symptoms vary across patients and may respond differentially to levodopa use.
Similar observations can be made for surgical interventions such as DBS. In this study, both subjects exhibited moderate baseline tremor and bradykinesia; however, stimulation response varied. First, stimulation-induced motor side effects were more predominant at higher voltages for subject 1 when comparing tremor tuning maps. Second, while tremor in both case studies exhibited a sudden drop in symptom severity, finger tapping impairment reduced gradually and continued to improve at higher voltages than tremor. Third, subject 2 symptom weights were larger for tremor and smaller for bradykinesia amplitude and speed than subject 1.
Understanding the neural mechanisms of stimulation in PD is still very much in its infancy. However, there are differences between tremor and bradykinesia that may account for the differential response to stimulation. Parkinsonian models have been developed that show reduced excitatory activity leading from the motor cortex to the spinal cord (Jahanshahi et al., 1995; Wichmann and DeLong, 1996; Sabatini et al., 2000). Stimulation may help regulate the abnormal excitatory and inhibitory pathways between the basal ganglia and motor cortex caused by dopaminergic cell loss in PD. Therefore, one may speculate that as stimulation voltage, or volume of tissue activated, is gradually increased, more dopaminergic cell loss is compensated by the stimulation. Tremor severity, on the other hand, is not correlated to L-Dopa uptake on PET imaging and may not be explained by dopaminergic cell loss alone (Fishman, 2008). Low-frequency oscillatory signals near 5 Hz in the STN (Levy et al., 2000), thalamus (Lenz et al., 1994), GPi (Hurtado et al., 1999), and motor cortex (Timmermann et al., 2003) have been associated with Parkinsonian tremor. Therefore, one may speculate that there exists a stimulation threshold required to cancel out the underlying tremor frequency.
Suggested final stimulation settings for the two case studies provided further support for the varied response of motor symptoms to different DBS settings. The parameter estimation algorithm output stimulation tremor settings at lower voltages and different contacts than for bradykinesia. Both subjects demonstrated continued improvement in finger tapping performance at higher voltages than with tremor. As a result, increasing stimulation voltage as demonstrated with the overall motor response would achieve optimal benefit across both symptoms. By calculating the overall motor response, we were able to achieve a single contact and voltage setting that factored in stimulation effectiveness (weights) for individual symptoms.
While the parameter estimation algorithm successfully demonstrated differential response of motor symptoms, several additional considerations could be made for potential clinical implementation. First, when calculating optimal stimulation settings, other variables may be explored to define symptoms weights including patient-perceived importance or off stimulation severity. Second, the recommended voltage may need to be decreased if the patient experiences stimulation-induced side effects at that setting. Also, stimulator battery life may be improved at a lower voltage output. The parameter estimation algorithm could calculate new settings if a lower voltage was still able to achieve comparable symptom benefit, for example within 10% of the lowest severity. Third, compliance criteria could be defined to automatically accept or reject a motor task measurement by processing motion sensor kinematic data in real time. This algorithm feature could correct outlier symptom severity values on tuning maps and assist ‘non-expert’ programmers in recognizing motor tasks performed incorrectly and recommend that a task assessment be repeated or excluded.
4.2 Programming Efficiency and Patient Access
During the two case studies, the standard protocol called for recorded stimulation settings (contact configuration, voltage, frequency, and pulse width) and corresponding UPDRS scores to be hand-written. Depending on the number of motor tasks selected and the size of the stimulation voltage increments, the programming flow sheet often required several pages, making it increasingly difficult to visually assess optimal stimulation outcome for multiple motor symptoms across such a large number of variables, particularly if these variables responded differently to stimulation parameters. Tuning maps could provide a powerful tool to standardize DBS programming and more quickly determine the optimal combination of stimulation parameters that may achieve the maximal motor improvement across multiple motor symptoms.
Significant healthcare costs are associated with DBS procedures. In 2006, the cost of unilateral DBS surgery, including equipment, facility costs, and physician time was estimated around $40,000 plus $10,000 to 20,000 for each stimulator battery replacement (Fraix et al., 2006). Although surgical costs are primarily fixed, the time and subsequent costs associated with outpatient programming sessions may be addressed by developing an automated and objective assessment system. Deep brain stimulation adjustment can cost over $1000 per appointment (Pereira et al., 2008) and geographic disparities play a major role in additional travel costs. A recent study tracked 34 PD subjects over three years for 100 general follow up visits. Results estimated that approximately 1500 travel hours, over 60,000 travel miles, and $37,000 in travel and lodging costs were accrued (Samii et al., 2006). Improving the DBS tools available to clinicians may reduce the duration and frequency of programming sessions.
While there are several highly experienced and trained DBS programming clinics that may match optimization results achieved with a quantitative assessment system, some socioeconomic and geographic disparate patient populations may not have access to these expert centers. Therefore, the development of an automated motor symptom assessment system for programming DBS patients could improve access and efficacy of this procedure by allowing one to transfer the knowledgebase of expert programming sites to DBS programs that may not have this expertise. In addition, even skilled centers may benefit from this technology to potentially reduce the time and frequency of programming sessions.
Furthermore, development of home monitoring capabilities using Kinesia motion capture technology and symptom severity scoring algorithms may provide additional insight into motor response to stimulation. This adjunct diagnostic tool may have several advantages over current in-clinic testing. First, the system could capture not only motor response of individual assessments, but also motor fluctuations throughout the day from the DBS and antiparkinsonian medicine interaction. Second, a significant limitation of current programming methods is the varied response time shown across PD motor symptoms. Studies have demonstrated that once subthalamic DBS was turned off, tremor severity worsened within minutes while other symptoms such as bradykinesia required up to an hour and axial signs three to four hours, significantly longer than a typical programming session (Temperli et al., 2003). Therefore, modifying current programming methods by incorporating ambulatory assessments may allow clinicians to capture the full effect of stimulation setting adjustments to achieve optimal DBS settings without extending in-clinic programming time. Finally, compliance criteria as discussed in section 4.1 could be used to ensure that unsupervised patients at home are performing the instructed motor tasks correctly.
Acknowledgments
The authors would like to thank Dustin A. Heldman, PhD, for his thoughtful comments on the manuscript. This work was supported by the National Institutes of Health, National Institute on Aging, 1R44AG033520-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature references
- Carr J. Tremor in Parkinson’s disease. Parkinsonism Relat Disord. 2002;8:223–34. doi: 10.1016/s1353-8020(01)00037-2. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, Daniels C, Deutschlander A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Beaton DE, Morgante F, Gunraj CA, Lang AE, Chen R. Impairments of speed and amplitude of movement in Parkinson’s disease: a pilot study. Mov Disord. 2009;24:1001–8. doi: 10.1002/mds.22480. [DOI] [PubMed] [Google Scholar]
- Fishman PS. Paradoxical aspects of parkinsonian tremor. Mov Disord. 2008;23:168–73. doi: 10.1002/mds.21736. [DOI] [PubMed] [Google Scholar]
- Fraix V, Houeto JL, Lagrange C, Le Pen C, Krystkowiak P, Guehl D, Ardouin C, Welter ML, Maurel F, Defebvre L, Rougier A, Benabid AL, Mesnage V, Ligier M, Blond S, Burbaud P, Bioulac B, Destee A, Cornu P, Pollak P. Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:443–9. doi: 10.1136/jnnp.2005.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida JP, Riley DE, Maddux BN, Heldman DA. Clinically deployable Kinesia technology for automated tremor assessment. Mov Disord. 2009;24:723–30. doi: 10.1002/mds.22445. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–7. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- Hallett M. Parkinson revisited: pathophysiology of motor signs. Adv Neurol. 2003;91:19–28. [PubMed] [Google Scholar]
- Hunka K, Suchowersky O, Wood S, Derwent L, Kiss ZH. Nursing time to program and assess deep brain stimulators in movement disorder patients. J Neurosci Nurs. 2005;37:204–10. doi: 10.1097/01376517-200508000-00006. [DOI] [PubMed] [Google Scholar]
- Hurtado JM, Gray CM, Tamas LB, Sigvardt KA. Dynamics of tremor-related oscillations in the human globus pallidus: a single case study. Proc Natl Acad Sci U S A. 1999;96:1674–9. doi: 10.1073/pnas.96.4.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118 ( Pt 4):913–33. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Kasai M, Oshima H, Fukaya C, Yamamoto T, Ogawa K, Mizutani T. Subthalamic nucleus stimulation for Parkinson disease: benefits observed in levodopa-intolerant patients. J Neurosurg. 2001;95:213–21. doi: 10.3171/jns.2001.95.2.0213. [DOI] [PubMed] [Google Scholar]
- Kumar R. Methods for programming and patient management with deep brain stimulation of the globus pallidus for the treatment of advanced Parkinson’s disease and dystonia. Mov Disord. 2002;17 (Suppl 3):S198–207. doi: 10.1002/mds.10164. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Martin RL, Tasker RR, Dostrovsky JO, Lenz YE. Single unit analysis of the human ventral thalamic nuclear group. Tremor-related activity in functionally identified cells. Brain. 1994;117 ( Pt 3):531–43. doi: 10.1093/brain/117.3.531. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20:7766–75. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukhanina EP, Kapoustina MT, Karaban IN. A quantitative surface electromyogram analysis for diagnosis and therapy control in Parkinson’s disease. Parkinsonism Relat Disord. 2000;6:77–86. doi: 10.1016/s1353-8020(99)00052-8. [DOI] [PubMed] [Google Scholar]
- Obeso J, Olanow C, Rodriguez-Oroz M, Krack P, Kumar R, Lang A. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–63. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, Foote KD. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005;62:1250–5. doi: 10.1001/archneur.62.8.noc40425. [DOI] [PubMed] [Google Scholar]
- Pereira E, Nandi D, Aziz T. Deep Brain Stimulation: an underused panacea? Advances in Clinical Neuroscience and Rehabilitation. 2008:8. [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000;123 ( Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Samii A, Ryan-Dykes P, Tsukuda RA, Zink C, Franks R, Nichol WP. Telemedicine for delivery of health care in Parkinson’s disease. J Telemed Telecare. 2006;12:16–8. doi: 10.1258/135763306775321371. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–96. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease. Mov Disord. 2006;21 (Suppl 14):S284–9. doi: 10.1002/mds.20961. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–8. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]