Abstract
The effectiveness of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine (IPTp-SP) against malaria and anemia is unclear because of the spread of SP-resistant Plasmodium falciparum. This study evaluates the effectiveness of IPTp-SP among pregnant women attending the antenatal clinic at Korle-Bu Teaching Hospital in Accra, Ghana. A cross-sectional study comparing malaria and anemia prevalence among pregnant women using IPTp-SP with non-IPTp-SP users was conducted during June–August 2009. A total of 363 pregnant women (202 of IPTp users and 161 non-IPTp users) were recruited. A total of 15.3% of IPTp users had malaria compared with 44.7% of non-IPTp users (P < 0.001). A total of 58.4% of non-IPTp users were anemic compared with 22.8% of IPTp users (P < 0.001). When we controlled for other variables, the difference in the prevalence of malaria (odds ratio = 0.18, 95% confidence interval = 0.08–0.37) and anemia (odds ratio = 0.20, 95% confidence interval = 0.12–0.34) remained significant. The recommended IPTp-SP regimen is useful in preventing malaria and anemia among pregnant women in Ghana.
Introduction
An estimated 243 million cases of malaria occurred in 2008 globally, most of which were reported in sub-Saharan African children less than five years of age and resulted in 863,000 deaths.1 Malaria in pregnancy is an immense public health problem that affects approximately 50 million women per year in malaria-endemic areas.2 Pregnant women, especially primigravidae and secundigravidae, are particularly vulnerable to malaria than non-pregnant women from the same area.3 Maternal anemia and low birth weight babies (LBW) are two important consequences of malaria in pregnancy.4 The etiology of anemia in pregnancy is often multi-factorial with causes ranging from nutritional deficiency of iron, folate, vitamin A or other nutrients, hemoglobinopathies (sickle cell disease), and infection with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) or parasites, such as hookworm and Plasmodium.5–7 However, in sub-Saharan Africa, malaria is a major contributory cause of anemia, especially among primigravidae living in holoendemic areas or perennially exposed to malaria.6 Malaria-associated anemia puts pregnant women at greater risk of other morbidities including placental abruption, placenta previa, premature labor, and maternal death.8 In addition, LBW babies are at an increased risk for early childhood mortality.
In Ghana, among pregnant women, malaria accounts for 13.8% of outpatient department (OPD) attendance, 10.6% of admissions, and 9.4% of deaths.9 Malaria prevention in pregnancy with chemoprophylaxis is associated with reduced incidence of malaria episodes, higher mean maternal hemoglobin levels, and reduced incidence of LBW babies.10–12 However, chemoprophylaxis has some potential drawbacks such as poor compliance, high cost, and impairment of natural immunity to malaria.10 The previous anti-malarial drug policy in Ghana promoted chloroquine chemoprophylaxis during pregnancy and six weeks post-partum.11 However, compliance was low (11.6%).9 This low compliance rate reduced effectiveness of malaria prevention among this group.9
As a result, Ghana adopted the current World Health Organization (WHO) recommended standard of care for malaria in malaria-endemic regions, which is intermittent preventive treatment of malaria in pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP).13 IPTp-SP has been shown to reduce malaria episodes, malaria-related anemia, and incidence of LBW.14–17 In addition, IPTp-SP is attractive because of its single-dose therapy, which lends itself to supervised administration and ensures compliance.
Although this preventive strategy has been implemented in some hospitals in Ghana such as Korle-Bu Teaching Hospital (KBTH), there has been little assessment of its effectiveness in preventing maternal malaria and malaria-associated anemia in Africa.18 In addition, resistance to SP is increasing in West Africa and thus the effectiveness of IPTp-SP has been questioned.19,20 Furthermore, SP treatment failure has been observed in 36% of children and approximately 30% of pregnant women in central Ghana.21 Nevertheless, the frequency of the dihydrofolate reductase triple mutation among Plasmodium falciparum isolates, which confers resistance to SP has been observed in 73% of pregnant women in other parts of the country.22 However, recent meta-analysis indicated no marked decrease in the efficacy of the IPTp regimen among women in areas with treatment-failure rates of 3–39% among children.23 Examining possible correlation between IPTp-SP use and decreased prevalence of maternal malaria and malaria-associated anemia despite increased resistance may provide insight into its effectiveness in controlling malaria and anemia in pregnancy. In this study, the effectiveness of IPTp-SP in preventing maternal malaria and malaria-associated anemia among pregnant women attending antenatal clinic (ANC) at KBTH was assessed.
Materials and Methods
Study area and population.
This study was conducted at KBTH, which is located in Accra, Ghana. The hospital, which is the leading teaching hospital in Ghana, serves as the ultimate referral institution for patients from diverse population all over the country and functions as the premier teaching hospital for the Ghana Medical School. Malaria is the number one cause of morbidity in the country, accounting for approximately 38% of all OPD attendance, 36% of all admissions, and 33.4% of all mortality in children less than five years of age.24 The groups affected most by malaria in the country are children less than five years of age and pregnant women, who constitute 20% and 4%, respectively, of the general population.25 The current study determined whether pregnant women attending prenatal clinic at KBTH who have taken IPTp-SP have low prevalence of malaria and malaria-related anemia compared with those who have not used the IPTp.
Recruitment of participants.
The study was designed to assess the effectiveness of IPTp-SP in pregnant women attending the ANC at KBTH during June–August 2009. The pregnant women were recruited into the study from the prenatal outpatient clinic based in the KBTH after providing informed consent according to the standards of Morehouse School of Medicine's Institutional Review Board and the Ghana Health Service ethical review committee. The selection criteria for recruitment included pregnant women with a gestational age of ≥ 26 weeks (third trimester), ≥ 18 years of age, who have received at least one dose of IPTp-SP during the first two trimesters of the pregnancy. Women with severe pregnancy complications such as hemorrhages, sepsis, and infections other than malaria, as well as women taking malaria prophylaxis other than IPTp-SP during the first two trimesters, were excluded. The women were categorized based on use of IPTp-SP before visiting the clinic.
Data collection methods.
The women were screened for malaria by microscopic evaluation of thick blood films stained with Giemsa and classified as either positive or negative for malaria. Because the reliability of Giemsa-stained thick blood films depends on the quality of laboratory preparation, staining, and examining techniques, at least 10 fields were read from each slide and three independent or separate microscopists were selected to review each slide to enhance accuracy and prevent errors. Discordant results were excluded from the study. Hemoglobin (Hb) levels of each participant were determined by using the Sysmex KX-21 Hematology Analyzer (Sysmex Corporation, Kobe Japan), and the numerical values were recorded. The participants were also classified according to their Hb levels as anemic (Hb < 11.0 g/dL), severely anemic (Hb < 7.0 g/dL), and non-anemic (Hb > 11.0 g/dL) according to WHO recommendations.2,26
Information was obtained by using an interviewer-administered structured questionnaire written in English that was administered by two local interviewers and one of the principal investigators in the language the respondents understood better. The questionnaire comprised of questions on socio-demographic characteristics, obstetric history, IPTp use, knowledge of malaria and anemia prevention, and food or folate supplement to determine the effect of these factors on malaria and anemia. The participants were also asked the type of preventive measures or methods they use against malaria. The HIV, sickle cell, glucose-6-phosphate dehydrogenase (G6PD) deficiency status, IPTp use of the participants, and helminth infections were determined by reviewing their antenatal cards or records. In terms of education, the women were classified as primary where they have completed ≤ 9 years of education, secondary where they had completed senior secondary school comprising 10–12 years of education, and tertiary where participants had completed university education or higher (≥ 13 years).
Statistical analysis.
All data entry, management, and basic statistics were conducted using SPSS statistical software version 17.0 for Windows (SPSS Inc., Chicago, IL) and transferred to Stata version 9.2 (Stata Corp., College Station, TX) for logistic regression analysis. The basic demographic factors, chart extraction characteristics, effect of IPTp-SP and malaria prevention method on malaria, and effect of IPTp-SP and anemia prevention knowledge on anemia among the women were compared for differences by using the Pearson chi-square test and adjusting for the Yates correction for continuity where appropriate. For instances in which there were too few persons per cell for the Pearson chi-square test to be used, Fisher exact test was used to compare discrete outcomes. One-way analysis of variance on ranks was used to determine the significance of the difference in anemia and severe anemia among IPTp users and non-IPTp users. Associations between the outcome variables, malaria and different categories of anemia, were determined by using bivariate analysis. To assess the effect of IPTp-SP on malaria and malaria-associated anemia, we constructed a logistic regression model and controlled for possible confounding effects such as age, parity, gravidity, level of education, sickle cell status, G6PD, HIV status, helminth infection, employment, marital status, family size, anemia prevention knowledge, and malaria prevention method used. The model fit was assessed for robustness by using the log-likelihood ratio test and model estimates were compared at P < 0.001. In the logistic regression model, age, parity, marital status, and family size were treated as covariate and other variables were treated as categorical variables. The odds ratio (OR) and 95% confidence interval (CI) were obtained by using logistic regression in multivariate analyses. In addition, the OR for malaria and malaria-related anemia was calculated for women using IPTp-SP and those who were not using IPTp-SP. Statistical significance was set at P ≤ 0.05.
Results
Study population.
A total of 381 women, which represented approximately 23% of the total women attending the ANC during June–August 2009 were recruited and 18 were excluded from the study because of incomplete questionnaire or data. A total of 363 women with complete questionnaires participated in the study analysis. The mean age of all women was 33.2 years (range = 18–48 years). There was a significant difference in mean age of the women using IPTp-SP (33.8 years) and those not using IPTp, (32.4 years; P = 0.013). Most women in the study had had a primary education, and more women using IPTp had attained secondary level education (31.7% versus 29.8%) and tertiary level education (25.7% versus 6.8%), a factor that may influence health-seeking behavior (Table 1). Approximately 90% of the women were employed; however, 70.5% of the women were in the low-income category (Table 1). Most women not using IPTp (80.1%) were in the low-income category compared with 62.9% of those using IPTp (P < 0.001) (Table 1). Eighty-seven percent of the IPTp users had a family size ≤ 4 persons compared with 83.2% non-IPTp users (P = 0.371) (Table 1).
Table 1.
Socio-demographic characteristics of pregnant women receiving antenatal care at Korle-Bu Teaching Hospital, Accra, Ghana, June–August 2009*
| Socio-demographic characteristic | IPTp-SP users (n = 202) | Non-IPTp-SP users (n = 161) | P |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 33.8 ± 5.2 | 32.4 ± 5.8 | 0.013 |
| 18–24 | 4 (1.9%) | 13 (8.1%) | 0.013 |
| 25–29 | 46 (22.8%) | 46 (28.6%) | |
| 30–34 | 46 (22.8%) | 36 (22.4%) | |
| ≥ 35 | 106 (52.5%) | 66 (40.9%) | |
| Marital status | |||
| Single | 5 (2.5%) | 7 (4.3%) | 0.486 |
| Married | 197 (97.5%) | 154 (95.7%) | |
| Education | |||
| No education | 29 (14.4%) | 41 (25.5%) | < 0.001 |
| Primary | 57 (28.2%) | 61 (37.9%) | |
| Secondary | 64 (31.7%) | 48 (29.8%) | |
| Tertiary | 52 (25.7%) | 11 (6.8%) | |
| Employment grade | |||
| Professional | 28 (13.9%) | 10 (6.2%) | 0.004 |
| Clerical | 59 (29.2%) | 35 (21.7%) | |
| Skilled | 68 (33.7%) | 57 (35.4%) | |
| Unskilled | 45 (22.3%) | 51 (31.7%) | |
| Unemployed | 2 (0.9%) | 8 (5.0%) | |
| Socioeconomic status | |||
| Low income | 127 (62.9%) | 129 (80.1%) | < 0.001 |
| Middle income | 75 (37.1%) | 32 (19.9%) | |
| Family size | |||
| ≤ 4 | 176 (87.1%) | 134 (83.2%) | 0.371 |
| > 4 | 26 (12.9%) | 27 (16.8%) |
IPTp-SP = intermittent preventive treatment with sulfadoxine-pyrimethamine; SD = standard deviation. Pearson chi-square test adjusting for the Yates correction for continuity where appropriate was used to compare for statistical differences among the pregnant women. For instances in which there were too few persons per cell for the Pearson chi-square test to be used, Fisher exact test was used to compare discrete outcomes. Differences between group means were assessed by using analysis of variance. Statistical significance was set at P ≤ 0.05.
Data from medical records or antenatal cards showed that 342 (94.2%) of 363 women were in their third trimester and approximately 43% were primigravidae or secundigravidae (Table 2). Most of the women had normal G6PD and were negative for sickle cell status (93.1% and 90.3%, respectively). Most (334, 92.0%) of the women were negative for HIV and approximately 341 (94%) had no helminth infections. However, 8 (4%) of 202 women using IPTp had roundworm infections compared with 4 (3%) of 161 women not using IPTp (P = 0.032) (Table 2). Helminth co-infection with malaria was observed in 5 (3.1%) of 161 women not using IPTp (Table 2). There were no statistically significant differences between the two groups in terms of their marital status, gestation, gravidity, parity, family size, sickle cell status, G6PD status, and HIV status (P > 0.05).
Table 2.
Health information and obstetric history of pregnant women receiving antenatal care at Korle-Bu Teaching Hospital, Accra, Ghana, June–August 2009*
| Variable | IPTp-SP users (n = 202) | Non-IPTp-SP users (n = 161) | P |
|---|---|---|---|
| Gestation | |||
| Second trimester | 10 (5.0%) | 11 (6.8%) | 0.591 |
| Third trimester | 192 (95.0%) | 150 (93.2%) | |
| Gravidae | |||
| Primigravidae | 23 (11.4%) | 27 (16.8%) | 0.311 |
| Secundigravidae | 59 (29.2%) | 47 (29.2%) | |
| Gravidae ≥ 3 | 120 (59.4%) | 87 (54.0%) | |
| Parity | |||
| 0 | 34 (16.8%) | 28 (17.4%) | 0.767 |
| 1 | 67 (33.2%) | 58 (36.0%) | |
| 2 | 71 (35.1%) | 57 (35.4%) | |
| ≥ 3 | 30 (14.9%) | 18 (11.2%) | |
| Sickle cell status | |||
| Positive | 16 (7.9%) | 12 (7.5%) | 0.344 |
| Negative | 184 (91.1%) | 144 (89.4%) | |
| Not known | 2 (1.0%) | 5 (3.1%) | |
| G6PD | |||
| Normal | 191 (94.5%) | 147 (91.3%) | 0.453 |
| Abnormal | 7 (3.5%) | 8 (5.0%) | |
| Not known | 4 (2.0%) | 6 (3.7%) | |
| HIV status | |||
| Positive | 7 (3.4%) | 6 (3.7%) | 0.887 |
| Negative | 187 (92.6%) | 147 (91.3%) | |
| Not known | 8 (4.0%) | 8 (5.0%) | |
| Helminth infection | |||
| Roundworm | 8 (4.0%) | 4 (2.5%) | < 0.032 |
| Malaria co-infection | – | 5 (3.1%) | |
| None | 194 (96.0%) | 152 (94.4%) |
IPTp-SP = intermittent preventive treatment with sulfadoxine-pyrimethamine; G6PD = glucose-6-phosphate dehydrogenase; HIV, human immunodeficiency virus. Pearson chi-square test adjusting for the Yates correction for continuity where appropriate were used to compare for statistical differences among the pregnant women. For instances in which there were too few persons per cell for the Pearson chi-square test to be used, Fisher exact test was used to compare discrete outcomes. Statistical significance was set at P ≤ 0.05.
Effect of IPTp on malaria.
Thick blood smears were prepared from all the 363 women at recruitment. One hundred three (28.4%) women were positive for malaria parasites: 31 (15.3%) of 202 using IPTp and 72 (44.7%) of 161 not using IPTp (P < 0.001). The effect of the use of malaria prevention method (insecticide-treated nets [ITNs] or indoor residual spraying [IRS]) on malaria among the women was determined by asking the participant what preventive method they use. There was no significant difference in ITN use among the IPTp users (7.0%, 14 of 202) and non-IPTp users (5.6%, 9 of 161) (P = 0.657) (Table 3). However, 13.4% of IPTp users not using ITNs had malaria compared with 42.2% of non-IPTp users not using ITNs (P < 0.0001) (Table 3). In addition, 6.4% of IPTp users not using IRS had malaria compared with 20.5% of non-IPTp users not using IRS (P < 0.0001) (Table 3).
Table 3.
Malaria prevention methods used and effect of intermittent preventive treatment with sulfadoxine-pyrimethamine on malaria in pregnant women receiving antenatal care at Korle-Bu Teaching Hospital, Accra, Ghana, June–August 2009*
| Variable | IPTp-SP users (n = 202) | Non-IPTp-SP users (n = 161) | P | |||
|---|---|---|---|---|---|---|
| Malaria+ | Malaria– | Malaria+ | Malaria– | |||
| ITN use | Yes | 4 (2.0%) | 10 (5.0%) | 4 (2.5%) | 5 (3.1%) | 0.657 |
| No | 27 (13.4%) | 161 (79.7%) | 68 (42.2%) | 84 (52.2%) | < 0.0001 | |
| IRS use | Yes | 18 (8.9%) | 98 (48.5%) | 39 (24.2%) | 53 (32.9%) | < 0.0001 |
| No | 13 (6.4%) | 75 (37.1%) | 33 (20.5%) | 36 (22.4%) | < 0.0001 | |
IPTp-SP = intermittent preventive treatment with sulfadoxine-pyrimethamine; ITN = insecticide-treated net; IRS = indoor residual spraying. Pearson chi-square test adjusting for the Yates correction for continuity where appropriate were used to compare for statistical differences among the pregnant women. For instances in which there were too few persons per cell for the Pearson chi-square test to be used, Fisher exact test was used to compare discrete outcomes. Statistical significance was set at P ≤ 0.05.
Logistic regression analysis was used to assess the effect of IPTp on malaria infection. The effect of age, gestation, marital status, parity, and other variables was controlled. Data indicate that after controlling for possible confounding factors, the statistically significant difference in the effect of the IPTp in preventing malaria in pregnancy still remained (P < 0.001). However, only the study status (i.e., IPTp use) and educational status were shown to be statistically significant as an explanatory variable for malaria in pregnancy (Table 4). The chance of malaria infection among pregnant women using IPTp was 18% that of women not using IPTp during pregnancy (OR = 0.18, 95% CI = 0.08–0.37, P < 0.0001). The chance of malaria infection among educated (secondary or tertiary) pregnant women with at least secondary education was 48% that of women with no education (OR = 0.48, 95% CI = 0.28–0.81, P < 0.006) (Table 4).
Table 4.
Logistic regression analyses of contributory factors for malaria among pregnant women receiving antenatal care at Korle-Bu Teaching Hospital, Accra, Ghana, June–August 2009*
| Variable | Odds ratio | 95% CI | P |
|---|---|---|---|
| Age | 1.00 | 0.94–1.07 | 0.917 |
| Marital status | 2.03 | 0.41–10.11 | 0.389 |
| Gestation | 0.52 | 0.19–1.41 | 0.198 |
| Parity | 1.08 | 0.68–1.72 | 0.752 |
| Primigravidae | 1.31 | 0.38–4.54 | 0.676 |
| Secundigravidae | 1.08 | 0.47–2.48 | 0.865 |
| Education | 0.48 | 0.28–0.81 | 0.006 |
| Employment grade | 2.04 | 0.42–9.91 | 0.377 |
| Family size | 0.93 | 0.37–2.30 | 0.867 |
| IPTp-SP Use | 0.18 | 0.08–0.37 | < 0.0001 |
| IPTp-SP × primigravidae | 3.37 | 0.79–14.83 | 0.102 |
| IPTp-SP × secundigravidae | 1.52 | 0.46–4.98 | 0.489 |
| Sickle cell positive | 0.72 | 0.28–1.88 | 0.507 |
| Abnormal G6PD | 2.64 | 0.98–7.15 | 0.056 |
| HIV positive | 1.24 | 0.48–3.20 | 0.655 |
| ITN use | 1.38 | 0.49–3.84 | 0.544 |
| IRS use | 0.93 | 0.54–1.59 | 0.780 |
CI = confidence interval; IPTp-SP = intermittent preventive treatment with sulfadoxine-pyrimethamine; G6PD = glucose-6-phosphate dehydrogenase; HIV = human immunodeficiency virus; ITN = insecticide-treated net; IRS = indoor residual spraying. The odds ratio and 95% CI were obtained with logistic regression in multivariate analyses adjusted for age, parity, and other variables in the study. Statistical significance was set at P ≤ 0.05. Model I was used for the logistic regression analysis. In the logistic regression model, age, parity, marital status, and family size were treated as covariate and other variables were treated as categorical variables. The reference for primigravidae and secundigravidae was gravidae ≥ 3, that for IPTp-SP was pregnant women not using IPTp, and that for education was women with no education. Values in bold are statistically significant.
Effect of IPTp on anemia.
Blood samples for Hb analyses were obtained from 363 pregnant women at recruitment. Women using IPTp had significantly higher mean Hb levels (11.6 g/dL) than women not using IPTp (9.8 g/dL) (P < 0.001) (Table 5). The Hb level ranged from 6.1 to 14.8 g/dL for IPTp users and from 5.0 to 13.0 g/dL for non-IPTp users (Figure 1). The proportion of anemic women not using IPTp was significantly higher (58.4%, 94 of 161) than in women using IPTp (22.8%, 46 of 202) (P < 0.001) (Table 5). The proportion of severely anemic women was also significantly higher among women not using IPTp (12.4%, 20 of 161) than among women using IPTp (3.5%, 7 of 202) (P < 0.001) (Table 5).
Table 5.
Anemia prevention knowledge and effect of intermittent preventive treatment with sulfadoxine-pyrimethamine on anemia in pregnant women receiving antenatal care at Korle-Bu Teaching Hospital, Accra, Ghana, June–August 2009*
| Variable | IPTp-SP users (n = 202) | Non-IPTp-SP users (n = 161) | P |
|---|---|---|---|
| Hemoglobin, g/dL | |||
| Mean ± SD | 11.6 ± 1.6 | 9.7 ± 1.8 | < 0.001 |
| Anemia (< 11.0) | 46 (22.8%) | 94 (58.4%) | < 0.001 |
| Severe anemia (< 7.0) | 7 (3.5%) | 20 (12.4%) | < 0.001 |
| ≥ 11.0 | 149 (73.7%) | 47 (29.2%) | < 0.001 |
| Anemia prevention knowledge | |||
| Food supplement | 49 (24.2%) | 38 (23.6%) | 0.030 |
| Adequate nutrient | 148 (73.3%) | 109 (67.7%) | |
| None | 5 (2.5%) | 14 (8.7%) | |
| Food supplement | |||
| Yes | 168 (83.2%) | 134 (83.2%) | 0.900 |
| No | 34 (16.8%) | 27 (16.8%) | |
| Food allergies | |||
| Yes | 4 (2.0%) | 3 (1.9%) | 0.761 |
| No | 198 (98.0%) | 158 (98.1%) |
IPTp-SP, intermittent preventive treatment with sulfadoxine-pyrimethamine; SD = standard deviation. Pearson chi-square test adjusting for the Yates correction for continuity where appropriate were used to compare for statistical differences among the pregnant women. For instances in which there were too few persons per cell for the Pearson chi-square test to be used, Fisher exact test was used to compare discrete outcomes. Differences between group means were assessed by using analysis of variance and statistical significance was set at P ≤ 0.05.
Figure 1.
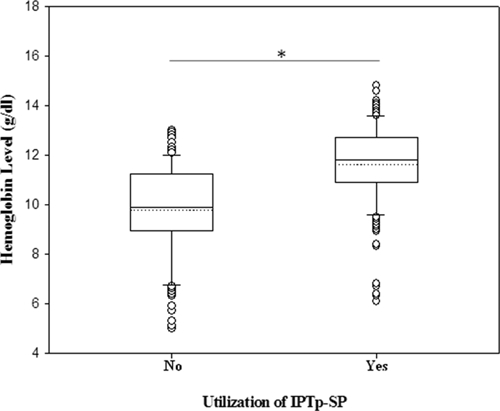
Hemoglobin levels among intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine (IPTp-SP) and non IPTp-SP groups of pregnant women receiving antenatal care at Korle-Bu Teaching Hospital, Accra, Ghana. Differences between group means and median were assessed by using analysis of variance. Statistical significance was set at P ≤ 0.05. Box plots represent median of hemoglobin levels (g/dL) and 25th and 75th percentiles. Outliers are shown as points outside the 10th and 90th percentile bars. Dotted line represents the mean and * denotes P < 0.05.
When the possible confounding effect of age, marital status, gestation, parity, food supplement, and other variables was controlled by using multivariate logistic regression, the effect of IPTp in prevention of anemia and severe anemia remained statistically significant (P < 0.001). Malaria infection and IPTp were the only variables shown to be statistically significant as explanatory variables for anemia and severe anemia in pregnancy. In addition, sickle cell status was also statistically significant as an explanatory variable for anemia in pregnancy (OR = 2.35, 95% CI = 1.12–6.01, P < 0.035). The chance of anemia among pregnant women using IPTp was 20% that of women not using IPTp (OR = 0.20, 95% CI = 0.12–0.34, P < 0.0001), and the chance of severe anemia was 17% that of women not using IPTp (OR = 0.17, 95% CI = 0.06–0.49, P < 0.0001). Interestingly, the chances of anemia and severe anemia among pregnant women with malaria infection were approximately five-fold (OR = 5.08, 95% CI = 2.72–9.49, P < 0.0001) and 10-fold (OR = 9.92, 95% CI = 3.58–27.48, P < 0.0001) higher, respectively, compared with those in women with no malaria infection (Table 6).
Table 6.
Logistic regression analyses of contributory factors for anemia among the pregnant women receiving antenatal care at Korle-Bu Teaching Hospital, Accra, Ghana, June–August 2009*
| Variables | Anemia | Severe anemia | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| Age | 0.96 | 0.90–1.03 | 0.240 | 0.99 | 0.88–1.11 | 0.991 |
| Marital status | 1.82 | 0.41–7.98 | 0.420 | 4.53 | 0.29–69.21 | 0.277 |
| Gestation | 1.60 | 0.51–5.00 | 0.423 | 2.76 | 0.26–29.63 | 0.402 |
| Parity | 1.12 | 0.65–1.94 | 0.673 | 1.07 | 0.42–2.71 | 0.887 |
| Primigravidae | 1.15 | 0.34–3.75 | 0.816 | 7.02 | 0.99–49.72 | 0.051 |
| Secundigravidae | 1.31 | 0.63−2.73 | 0.465 | 2.46 | 0.61–9.92 | 0.205 |
| Education | 0.97 | 0.66–1.43 | 0.881 | 0.84 | 0.40–1.79 | 0.659 |
| Employment grade | 0.95 | 0.66–1.37 | 0.789 | 1.02 | 0.52–2.00 | 0.947 |
| Family size | 0.92 | 0.65–1.30 | 0.624 | 0.98 | 0.64–1.49 | 0.926 |
| Sickle cell positive | 2.35 | 1.12–6.01 | 0.035 | 1.70 | 0.29–9.49 | 0.570 |
| HIV positive | 0.60 | 0.22–1.66 | 0.324 | 0.61 | 0.09–4.14 | 0.615 |
| Abnormal G6PD | 0.38 | 0.10–1.39 | 0.142 | 1.87 | 0.37–9.62 | 0.452 |
| Helminth infection | 1.06 | 0.28–4.03 | 0.935 | 1.37 | 0.17–11.40 | 0.769 |
| Malaria infection | 5.08 | 2.72–9.49 | < 0.0001 | 9.92 | 3.58–27.48 | < 0.0001 |
| IPTp-SP use | 0.20 | 0.12–0.34 | < 0.0001 | 0.17 | 0.06–0.49 | 0.001 |
| Food supplement | 1.00 | 0.50–2.00 | 0.996 | 0.68 | 0.21–2.21 | 0.523 |
| ITN use | 0.99 | 0.33–2.97 | 0.576 | 0.38 | 0.04–3.94 | 0.419 |
| IRS use | 1.17 | 0.67–2.05 | 0.576 | 0.54 | 0.20–1.46 | 0.223 |
| Anemia prevention knowledge | 0.70 | 0.21–2.35 | 0.557 | 1.70 | 0.10–28.36 | 0.711 |
CI = confidence interval; HIV = human immunodeficiency virus; G6PD = glucose-6-phosphate dehydrogenase; IPTp-SP, intermittent preventive treatment with sulfadoxine-pyrimethamine; ITN, insecticide-treated net; IRS = indoor residual spraying. The odds ratio and 95% CI were obtained with logistic regression in multivariate analyses adjusted for age, parity, and other variables in the study. Statistical significance was set at P ≤ 0.05. Model I was used for the logistic regression analysis. In the logistic regression model, age, parity, marital status, and family size were treated as covariate and other variables were treated as categorical variables. The reference for primigravidae and secundigravidae was gravidae ≥ 3, that for IPTp-SP was pregnant women not using IPTp, and that for education was women with no education. Values in bold are statistically significant.
Discussion
Malaria is the leading cause of illness and death in Ghana and it impacts negatively on different demographic and socio-economic groups.25 It contributes to the relatively high maternal mortality in the country, which accounts for 11% of mortality in pregnant women.25 Although anemia remains a major challenge in sub-Sahara Africa, malaria is an important contributor to maternal and perinatal morbidity and mortality in these countries.10 The WHO currently recommends that each pregnant woman should receive IPTp-SP at each ANC visit after quickening, which in practice leads to two or three doses during the course of the pregnancy.2 This recommendation is based on reports of beneficial effects of IPTp-SP in preventing maternal malaria and improving pregnancy outcome in studies conducted in Africa.10,15,17,18,27,28 This policy has been adopted by most countries in Africa, but implementation has been suboptimal.29 Furthermore, the spread of SP-resistant P. falciparum reinforces the importance of investigating the ongoing effectiveness of IPTp-SP.19,20,23,30,31 This study assessed whether pregnant women attending the ANC at KBTH who had used IPTp-SP had low prevalence of malaria and anemia or severe anemia compared with those who had not used IPTp-SP.
Results showed that pregnant women who used IPTp-SP had a statistically lower prevalence or rate of malaria, anemia, and severe anemia than those who did not use IPTp-SP. Only 15% of the women using IPTp were positive for malaria infection compared with approximately 45% of the women not using IPTp. The lower prevalence may be partly caused by the frequency of ITN and IRS use among the participants. However, controlling the effect of the variables in the study by using logistic regression analysis, ITN and IRS use did not influence the outcome of malaria infection. The ITNs represent a powerful tool for preventing malaria transmitted by mosquitoes residing indoors at night.32,33 However, recent evidence suggests that some mosquito populations avoid contact with ITNs by either feeding predominately outdoors or in the early part of the evening when individuals are not under the nets.32,34,35 Such behavioral changes may reduce the level of personal protection conferred by ITNs.34,35
Although ITNs may be ineffective against outdoor-biting mosquitoes,35,36 ITNs continue to play an integral part of malaria vector control.32 Personal protective measures such as spatial repellents should be considered as a supplement to ITNs to protect against indoor (outside nets) and outdoor bites in the morning or early evening.32,37 The ITNs have been shown to be effective in the control of malaria in pregnant women.38,39 However, their use among pregnant women in the study was low (6.3%). This finding may be caused by lack of access or compliance, the cost of acquiring ITNs, or by the fact that this cohort of participants prefer IRS over ITNs. Furthermore, a higher proportion of non-IPTp-SP users not using ITNs (42.2% versus 13.4%) or IRS (20.5% versus 6.4%) had malaria when compared with IPTp-SP users (P < 0.0001), which suggests that IPTp-SP is effective in reducing the prevalence of malaria.
Although the prevalence of malaria among IPTp users could be caused by re-infection between doses of SP, drug resistance may have played a part in this observation because high levels of resistance to SP has been reported.40–42 Furthermore, a high prevalence rate (73%) of the gene encoding the dihydrofolate reductase triple mutation among P. falciparum that confers resistance to SP has been reported in pregnant women in Ghana.22 In addition, SP treatment failure has been observed in approximately 30% of pregnant women in Ghana.21 Nevertheless, supplementation with high dosages of folate during pregnancy may compromise the efficacy of IPTp-SP.29 Sulfadoxine-pyrimethamine works through its inhibition of folate synthesis, and studies have linked high host folate levels with reduced SP efficacy.43 Because of folate synthesis inhibition by SP, folate supplementation has been recommended for all pregnant women in Africa to reduce the risk of fetal neural tube defects.29 In addition, because most (83.2%) of the women in this study used food supplements containing folate (Table 5), the efficacy of IPTp-SP may be reduced and may result in vulnerability to malaria infection. However, the result indicates that the deleterious effects of malaria during pregnancy can be substantially reduced using IPTp-SP in pregnant women.
Prevalence of malaria infections is high in primigravidae from malaria-endemic communities with different levels of transmission.44 Additionally, the presence of malaria parasites in the peripheral blood of clinically symptomatic pregnant women is an indication of on-going placental infection.45 Other studies have observed a strong correlation between placental infection and peripheral infection at the beginning and at the end of pregnancy.46–48 These studies suggest that peripheral parasitemia at any stage of the pregnancy was significantly correlated with placental infection at delivery.46–48 Several studies have shown that peripheral and placental parasitemia decrease with increasing parity among pregnant women.44,49 It has been observed that pregnant women, especially primigravidae and secundigravidae, are more susceptible to malaria infections.46 In a community-based study in Ghana, Ofori and others44 reported that malaria infections were most common among primigravidae and secundigravidae, suggesting that any measure to protect pregnant women against malaria should be prioritized to first and second pregnancies.44 A common explanation is that pregnancy is associated with a decrease in immunity, which is more pronounced in primigravidae than in multigravidae.46 The decreasing susceptibility to pregnancy-associated malaria with increasing parity is reflected in the acquisition of antibodies specific for parasite variant antigens expressed on the surface of infected erythrocytes.46,50 Although there is increasing evidence that prevalence of malaria infections is parity-related and primigravidae was the most at-risk group during pregnancy,44,51–55 in the current study, parity or primigravidae did not show any association with malaria infection (Table 4). This finding may have been caused by the failure of small sample size to show any association between parity or gravidity and malaria infection. Interestingly, with the exception of IPTp-SP use, education was a factor that showed an association with malaria (Table 4). This finding indicate that education may influence health-seeking behavior; educated women may be more proactive in taking measures to prevent malaria than uneducated women. However, in this study, use of malaria prevention methods such as ITNs or indoor spraying did not show any association with malaria infection. Nevertheless, several studies have shown ITNs to reduce malaria morbidity and mortality.23,38,56,57
Anemia is a well-recognized consequence of malaria. Although maternal anemia is multi-factorial, malaria is known to contribute significantly to its occurrence in pregnancy.55,59–61 The beneficial effect of IPTp-SP on reduction of the prevalence of maternal anemia will improve pregnancy outcome. The lower rate of anemia and severe anemia observed among pregnant women using IPTp (Table 5) was consistent with the findings reported earlier in Malawi, where the use of SP was associated with a higher maternal hemoglobin level.28 The results of our study were consistent with studies in Kenya,17 Nigeria,10 and other parts of Africa, which demonstrates that IPTp is effective in reducing the risk of anemia among pregnant women.14,15,62–64 However, these studies compared IPTp-SP with weekly chloroquine prophylaxis10,14,64 or placebo and case management,15,17,62–64 and our approach was a cross-sectional study that compared IPTp-SP users with non-users. In addition, amid growing concerns of chloroquine resistance and the approximately 11% of pregnant women adhering to the policy of using chloroquine to prevent malaria in pregnancy, Ghana adopted IPTp-SP as the only nationally recommended drug for management of malaria in pregnancy in 2003.25 Despite this limitation, IPTp-SP was highly effective in reducing maternal malaria-associated anemia in pregnancy (Table 5).
The contribution of malaria infection to anemia in pregnant women is one of the reasons for preventing malaria during pregnancy.65 Although other conditions such as helminth infections, HIV, and sickle cell disease may contribute to the incidence of anemia among populations in sub-Saharan Africa, controlling for these confounding effects in our study did not influence the outcome of anemia among the participants. However, malaria was the only contributory factor for anemia and severe anemia among pregnant women (Table 6). The impact of malaria infections on anemia might vary with different malaria endemicity levels and therefore with the level of pre-pregnancy–acquired malaria immunity.66 Malaria-associated anemia may be caused by an increase in the density of parasitemia rather than mere presence of parasite infection.67 However, the current study did not measure parasite density in the peripheral blood to determine its association with anemia. Furthermore, parasite density measured from peripheral blood does not reflect degree of sequestration in placenta. Evidence that IPTp-SP reduces maternal malaria-associated anemia has been shown in several studies in sub-Saharan Africa10,15,17,27. Nevertheless, our study indicates that IPTp-SP use was the only factor that provides protection against anemia or severe anemia among the pregnant women (Table 6).
The results from this study demonstrate the effectiveness of IPTp-SP in reducing the prevalence of malaria and the development of malaria-related anemia or severe anemia in pregnancy in a malaria-endemic area. These findings provide an evidence-based template on which policymakers and health workers may develop interventions to reduce maternal mortality caused by malaria and malaria-associated anemia in pregnancy. The simple regimen of SP and its relatively low incidence of side effects make it highly acceptable to pregnant women and health workers. In addition, studies from Africa have reported high prevalence rates of congenital malaria ranging from 4.9% to 46.7%.68–77 Furthermore, a recent study reported that mothers using anti-malarial prophylaxis during pregnancy have fewer babies with congenital malaria compared with mothers who are not using anti-malarial prophylaxis.78 This finding suggests that the use of SP may not only prevent maternal morbidity and mortality caused by malaria and malaria-associated anemia in pregnancy but also prevent gestational and placental malaria, thereby reducing the incidence of congenital malaria. Interestingly, a previous study in Ghana reported approximately 36% of asymptomatic malaria (presence of malaria parasite without symptoms) among pregnant women.79 Similarly, up to 82.4% of asymptomatic infection has been reported in other parts of Africa,55,80 suggesting that most malaria infection among pregnant women go undiagnosed. Thus, the SP regimen will be useful in decreasing the prevalence of asymptomatic malaria among pregnant women in malaria-endemic areas. Although there is a small risk of adverse reactions to sulfa-containing drugs,81,82 in malaria-endemic area with associated high level of malaria attributable anemia in pregnancy with its implications for negative pregnancy outcomes, the benefits of this regimen far outweighs this potential risk.10 Much work still needs to be done to improve coverage of SP so that all pregnant women receive at least two doses of IPTp. Furthermore, strategies need to be developed to distribute IPTp-SP to the various regions and rural communities to reach pregnant women who do not attend ANC.
The main limitation of this study was the lack of measurements of pregnancy outcomes such as LBW and placental parasitemia, which are sensitive indicators of malaria in pregnancy. The study was cross-sectional and the data did not include follow-up. Measurement of these parameters could have given insight into the effect of the IPTp-SP on the prevalence of LBW and placenta parasitemia at KBTH. However, several studies have indicated that IPTp-SP is efficient in reducing placental parasitemia and prevention of LBW.27,28,59,83–85 Although the study recruited more than 300 women, these women only account for less than 10% of the total number of women attending ANC annually. In addition, facility-based surveys as tools to evaluate changing malaria epidemiology have been critiqued in a recent report.86 Because the study used hospital-based convenience sampling, we cannot claim that these results are representative of changing malaria epidemiology in the whole community. However, by using one facility and the same approach to assess all variables throughout the study,87 we believe that the study provides insight into malaria control and prevention.
We recommend that further studies be conducted to assess the efficacy of SP in pregnancy in the clinical setting where LBW and placental parasitemia is measured and to evaluate other drug combinations for malaria prevention in pregnancy. Additionally, there is a need to evaluate and monitor the effectiveness of SP for IPTp because resistance to this drug is increasing. A search for drugs that are more efficacious or a combination of such is therefore necessary. Because folate supplementation has been recommended for all pregnant women in Africa to reduce the risk of fetal neural tube defects, its influence on the efficacy of SP need to be evaluated because high doses inhibit SP efficacy. In addition, this was a clinic-based study, a community-based study would have captured IPTp use and knowledge on malaria and anemia prevention among women who have not been exposed to health facilities, especially antenatal clinics.
Importantly, use of IPTp-SP is a useful and practical strategy to reduce risk of malaria and malaria-related anemia and asymptomatic malaria among pregnant women living in malaria-endemic areas such as Ghana. This goal may be attained by increasing the proportion of pregnant women receiving IPTp-SP through focused antenatal care services and information campaigns. In the present study, women with higher educational status were more likely to take preventive measures against malaria compared with less educated women. This finding means that health education programs for pregnant women should be intensified among women with low educational status.
ACKNOWLEDGMENTS
We thank the pregnant women attending antenatal care at Korle-Bu Teaching Hospital for participating in this study, the nurses of Obstetrics and Gynecology Department of Korle-Bu Teaching Hospital, the laboratory staff of Korle-Bu Teaching Hospital's Child Health Department, the staff of Ghana Ministry of Health, Reproductive and Child Health Department, and the staff of Ghana National Malaria Control Program for their assistance. The support of Center for Disease Diagnosis and Research in Ghana is acknowledged.
Footnotes
Financial support: This study was supported by the Centers for Disease Control and Prevention “Reproductive Epidemiological” grant to the Master of Public Health Program, Morehouse School of Medicine; the Minority International Health Disparities Research Training Program at Howard University; National Institutes of Health grants NIH-RCMI (RR03034), NIHNIGM- MBRS (SO6GM08248), and NIH-FIC (R21TW006804-01).
Authors' addresses: Nana O. Wilson and Jonathan K. Stiles, Department of Microbiology, Biochemistry and Immunology, Morehouse School of Medicine, HG Room 350 720 Westview Drive SW, Atlanta, GA 30310, E-mails: nwilson@msm.edu and jstiles@msm.edu. Fatou K. Ceesay, Patricia Rodney, and Yassa Ndjakani, Department of Community Health and Preventive Medicine, Morehouse School of Medicine, 720 Westview Drive SW, Atlanta, GA 30310, E-mails: fceesay@msm.edu, prodney@msm.edu, and yndjakani@msm.edu. Samuel A. Obed, Department of Obstetrics and Gynecology, University of Ghana Medical School, Korle-Bu, Accra, Ghana, E-mail: obedamenyi@yahoo.com. Andrew A. Adjei and Richard K. Gyasi, Department of Pathology, University of Ghana Medical School, Korle-Bu, Accra, Ghana, E-mails: andrewanthonyadjei@yahoo.com and rkg539us@yahoo.com. Winston A. Anderson, Department of Biology, Howard University, Just Hall, 415 College St NW, Washington, DC 20059, E-mail: wanderson@howard.edu. Naomi W. Lucchi, Malaria Branch, Centers for Disease Control and Prevention, Atlanta, GA 30333, E-mail: frd9@cdc.gov.
References
- 1.World Health Organization The World Malaria Report 2009. 2010. http://whqlibdc.who.int/publications/2009/9789241563901_eng.pdf Available at. Accessed February 20, 2010.
- 2.World Health Organization . A Strategic Framework for Malaria Prevention and Control during Pregnancy in the African Region. Brazzaville: World Health Organization Regional Office for Africa.AFR/MAL/04/01; 2004. http://www.cdc.gov/Malaria/pdf/strategic_framework_mip_04.pdf Available at. Accessed May 25, 2009. [Google Scholar]
- 3.Brabin B. Malaria in pregnancy: current issues. Afr Health. 1997;19:19–20. [PubMed] [Google Scholar]
- 4.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh K, Ghosh K. Pathogenesis of anemia in malaria: a concise review. Parasitol Res. 2007;101:1463–1469. doi: 10.1007/s00436-007-0742-1. [DOI] [PubMed] [Google Scholar]
- 6.Savage EJ, Msyamboza K, Gies S, D'Alessandro U, Brabin BJ. Maternal anemia as an indicator for monitoring malaria control in pregnancy in sub-Saharan Africa. BJOG. 2007;114:1222–1231. doi: 10.1111/j.1471-0528.2007.01420.x. [DOI] [PubMed] [Google Scholar]
- 7.Verhoeff FH, Brabin BJ, Chimsuku L, Kazembe P, Broadhead RL. An analysis of the determinants of anemia in pregnant women in rural Malawi: a basis for action. Ann Trop Med Parasitol. 1999;93:119–133. doi: 10.1080/00034989958609. [DOI] [PubMed] [Google Scholar]
- 8.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 9.Gomez P, Kinzie B. Basic Maternal and Newborn Care: Section Two: Antenatal Care. 2002. http://www.whiteribbonalliance.org/Resources/Documents/Basic%20Maternal%20and%20Newborn%20Care.pdf Available at. Accessed May 27, 2009.
- 10.Asa OO, Onayade AA, Fatusi AO, Ijadunola KT, Abiona TC. Efficacy of intermittent preventive treatment of malaria with sulphadoxine-pyrimethamine in preventing anemia in pregnancy among Nigerian women. Matern Child Health J. 2008;12:692–698. doi: 10.1007/s10995-008-0319-3. [DOI] [PubMed] [Google Scholar]
- 11.Geelhoed DW, Visser LE, Addae V, Asare K, Schagen van Leeuwen JH, van Roosmalen J. Malaria prophylaxis and the reduction of anemia at childbirth. Int J Gynaecol Obstet. 2001;74:133–138. doi: 10.1016/s0020-7292(01)00419-2. [DOI] [PubMed] [Google Scholar]
- 12.Mutabingwa TK, Malle LN, de Geus A, Oosting J. Malaria chemosuppression in pregnancy. II. Its effect on maternal hemoglobin levels, placental malaria and birth weight. Trop Geogr Med. 1993;45:49–55. [PubMed] [Google Scholar]
- 13.World Health Organization, UNICEF Africa Malaria Report. WHO/CDS/MAL/2003.1093. 2003. http://www.rollbackmalaria.org/amd2003/amr2003/pdf/ Available at. Accessed May 25, 2009.
- 14.Kayentao K, Kodio M, Newman RD, Maiga H, Doumtabe D, Ongoiba A, Coulibaly D, Keita AS, Maiga B, Mungai M, Parise ME, Doumbo O. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis. 2005;191:109–116. doi: 10.1086/426400. [DOI] [PubMed] [Google Scholar]
- 15.Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, Muga R, Oloo AJ, Steketee RW. Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg. 1998;59:813–822. doi: 10.4269/ajtmh.1998.59.813. [DOI] [PubMed] [Google Scholar]
- 16.Salihu HM, Naik EG, Tchuinguem G, Bosny JP, Dagne G. Weekly chloroquine prophylaxis and the effect on maternal hemoglobin status at delivery. Trop Med Int Health. 2002;7:29–34. doi: 10.1046/j.1365-3156.2002.00824.x. [DOI] [PubMed] [Google Scholar]
- 17.Shulman CE, Dorman EK, Cutts F, Kawuondo K, Bulmer JN, Peshu N, Marsh K. Intermittent sulphadoxine-pyrimethamine to prevent severe anemia secondary to malaria in pregnancy: a randomized placebo-controlled trial. Lancet. 1999;353:632–636. doi: 10.1016/s0140-6736(98)07318-8. [DOI] [PubMed] [Google Scholar]
- 18.van Eijk AM, Blokland IE, Slutsker L, Odhiambo F, Ayisi JG, Bles HM, Rosen DH, Adazu K, Lindblade KA. Use of intermittent preventive treatment for malaria in pregnancy in a rural area of western Kenya with high coverage of insecticide-treated bed nets. Trop Med In Health. 2005;10:1134–1140. doi: 10.1111/j.1365-3156.2005.01497.x. [DOI] [PubMed] [Google Scholar]
- 19.Mockenhaupt FP, Teun BJ, Eggelte TA, Schreiber J, Ehrhardt S, Wassilew N, Otchwemah RN, Sauerwein RW, Bienzle U. Plasmodium falciparum dhfr but not dhps mutations associated with sulphadoxine-pyrimethamine treatment failure and gametocyte carriage in northern Ghana. Trop Med Int Health. 2005;10:901–908. doi: 10.1111/j.1365-3156.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 20.Newman RD, Parise ME, Slutsker L, Nahlen B, Steketee RW. Safety, efficacy and determinants of effectiveness of antimalarial drugs during pregnancy: implications for prevention programmes in Plasmodium falciparum-endemic sub-Saharan Africa. Trop Med Int Health. 2003;8:488–506. doi: 10.1046/j.1365-3156.2003.01066.x. [DOI] [PubMed] [Google Scholar]
- 21.Tagbor H, Bruce J, Ord R, Randall A, Browne E, Greenwood B, Chandramohan D. Comparison of the therapeutic efficacy of chloroquine and sulphadoxine-pyrimethamine in children and pregnant women. Trop Med Int Health. 2007;12:1288–1297. doi: 10.1111/j.1365-3156.2007.01927.x. [DOI] [PubMed] [Google Scholar]
- 22.Mockenhaupt FP, Bedu-Addo G, Eggelte TA, Hommerich L, Holmberg V, von Oertzen C, Bienzle U. Rapid increase in the prevalence of sulfadoxine-pyrimethamine resistance among Plasmodium falciparum isolated from pregnant women in Ghana. J Infect Dis. 2008;198:1545–1549. doi: 10.1086/592455. [DOI] [PubMed] [Google Scholar]
- 23.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 24.Ghana Health Service Ghana Health Service 2007 Annual Report. 2009. http://www.ghanahealthservice.org/includes/upload/publications/GHS%202007%20Annual%20Report.pdf Available at. Accessed July 13, 2009.
- 25.Ghana National Malaria Control Program Malaria Operational Plan for Ghana 2009–2015. 2008. http://www.fightingmalaria.gov/countries/mops/ghana_mop-fy09.pdf Available at. Accessed November 29, 2009.
- 26.World Health Organization Worldwide Prevalence of Anemia 1993–2005: WHO Global Database on Anemia. 2008. http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf Available at. Accessed May 25, 2009.
- 27.Gies S, Coulibaly SO, Ouattara FT, D'Alessandro U. Individual efficacy of intermittent preventive treatment with sulfadoxine-pyrimethamine in primi- and secundigravidae in rural Burkina Faso: impact on parasitemia, anemia and birth weight. Trop Med Int Health. 2009;14:174–182. doi: 10.1111/j.1365-3156.2008.02215.x. [DOI] [PubMed] [Google Scholar]
- 28.Rogerson SJ, Chaluluka E, Kanjala M, Mkundika P, Mhango C, Molyneux ME. Intermittent sulfadoxine-pyrimethamine in pregnancy: effectiveness against malaria morbidity in Blantyre, Malawi, in 1997–99. Trans R Soc Trop Med Hyg. 2000;94:549–553. doi: 10.1016/s0035-9203(00)90083-x. [DOI] [PubMed] [Google Scholar]
- 29.Parikh S, Rosenthal PJ. Intermittent preventive therapy for malaria in pregnancy: is sulfadoxine-pyrimethamine the right drug? Clin Pharmacol Ther. 2010;87:160–162. doi: 10.1038/clpt.2009.284. [DOI] [PubMed] [Google Scholar]
- 30.Nkhoma S, Molyneux M, Ward S. Molecular surveillance for drug-resistant Plasmodium falciparum malaria in Malawi. Acta Trop. 2007;102:138–142. doi: 10.1016/j.actatropica.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Plowe CV, Kublin JG, Dzinjalamala FK, Kamwendo DS, Mukadam RA, Chimpeni P, Molyneux ME, Taylor TE. Sustained clinical efficacy of sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Malawi after 10 years as first line treatment: five year prospective study. BMJ. 2004;328:545. doi: 10.1136/bmj.37977.653750.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Govella NJ, Okumu FO, Killeen GF. Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am J Trop Med Hyg. 2010;82:415–419. doi: 10.4269/ajtmh.2010.09-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Geissbuhler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, Mtasiwa D, Mshinda H, Fillinger U, Lindsay SW, Kannady K, de Castro MC, Tanner M, Killeen GF. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malar J. 2007;6:126. doi: 10.1186/1475-2875-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pates H, Curtis C. Mosquito behavior and vector control. Annu Rev Entomol. 2005;50:53–70. doi: 10.1146/annurev.ento.50.071803.130439. [DOI] [PubMed] [Google Scholar]
- 36.Oyewole IO, Awolola TS. Impact of urbanisation on bionomics and distribution of malaria vectors in Lagos, southwestern Nigeria. J Vector Borne Dis. 2006;43:173–178. [PubMed] [Google Scholar]
- 37.Pates HV, Line JD, Keto AJ, Miller JE. Personal protection against mosquitoes in Dar es Salaam, Tanzania, by using a kerosene oil lamp to vaporize transfluthrin. Med Vet Entomol. 2002;16:277–284. doi: 10.1046/j.1365-2915.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- 38.D'Alessandro U, Langerock P, Bennett S, Francis N, Cham K, Greenwood BM. The impact of a national impregnated bed net programme on the outcome of pregnancy in primigravidae in The Gambia. Trans R Soc Trop Med Hyg. 1996;90:487–492. doi: 10.1016/s0035-9203(96)90289-8. [DOI] [PubMed] [Google Scholar]
- 39.ter Kuile FO, Terlouw DJ, Phillips-Howard PA, Hawley WA, Friedman JF, Kariuki SK, Shi YP, Kolczak MS, Lal AA, Vulule JM, Nahlen BL. Reduction of malaria during pregnancy by permethrin-treated bed nets in an area of intense perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 2003;68:50–60. [PubMed] [Google Scholar]
- 40.Bakyaita N, Dorsey G, Yeka A, Banek K, Staedke SG, Kamya MR, Talisuna A, Kironde F, Nsobya S, Kilian A, Reingold A, Rosenthal PJ, Wabwire-Mangen F. Sulfadoxine-pyrimethamine plus chloroquine or amodiaquine for uncomplicated falciparum malaria: a randomized, multisite trial to guide national policy in Uganda. Am J Trop Med Hyg. 2005;72:573–580. [PubMed] [Google Scholar]
- 41.Dorsey G, Dokomajilar C, Kiggundu M, Staedke SG, Kamya MR, Rosenthal PJ. Principal role of dihydropteroate synthase mutations in mediating resistance to sulfadoxine-pyrimethamine in single-drug and combination therapy of uncomplicated malaria in Uganda. Am J Trop Med Hyg. 2004;71:758–763. [PubMed] [Google Scholar]
- 42.Talisuna AO, Langi P, Bakyaita N, Egwang T, Mutabingwa TK, Watkins W, Van ME, D'Alessandro U. Intensity of malaria transmission, antimalarial-drug use and resistance in Uganda: what is the relationship between these three factors? Trans R Soc Trop Med Hyg. 2002;96:310–317. doi: 10.1016/s0035-9203(02)90108-2. [DOI] [PubMed] [Google Scholar]
- 43.van Eijk AM, Ouma PO, Williamson J, ter Kuile FO, Parise M, Otieno K, Hamel MJ, Ayisi JG, Kariuki S, Kager PA, Slutsker L. Plasma folate level and high-dose folate supplementation predict sulfadoxine-pyrimethamine treatment failure in pregnant women in western Kenya who have uncomplicated malaria. J Infect Dis. 2008;198:1550–1553. doi: 10.1086/592715. [DOI] [PubMed] [Google Scholar]
- 44.Ofori M, Ansah E, Agyepong I, Ofori-Adjei D, Hviid L, Akanmori B. Pregnancy-associated malaria in a rural community of Ghana. Ghana Med J. 2009;43:13–18. [PMC free article] [PubMed] [Google Scholar]
- 45.Ofori MF, Staalsoe T, Bam V, Lundquist M, David KP, Browne EN, Akanmori BD, Hviid L. Expression of variant surface antigens by Plasmodium falciparum parasites in the peripheral blood of clinically immune pregnant women indicates ongoing placental infection. Infect Immun. 2003;71:1584–1586. doi: 10.1128/IAI.71.3.1584-1586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cottrell G, Mary JY, Barro D, Cot M. Is malarial placental infection related to peripheral infection at any time of pregnancy? Am J Trop Med Hyg. 2005;73:1112–1118. [PubMed] [Google Scholar]
- 47.McGready R, Davison BB, Stepniewska K, Cho T, Shee H, Brockman A, Udomsangpetch R, Looareesuwan S, White NJ, Meshnick SR, Nosten F. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg. 2004;70:398–407. [PubMed] [Google Scholar]
- 48.Watkinson M, Rushton DI. Plasmodial pigmentation of placenta and outcome of pregnancy in West African mothers. Br Med J (Clin Res Ed) 1983;287:251–254. doi: 10.1136/bmj.287.6387.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shulman CE, Dorman EK. Importance and prevention of malaria in pregnancy. Trans R Soc Trop Med Hyg. 2003;97:30–35. doi: 10.1016/s0035-9203(03)90012-5. [DOI] [PubMed] [Google Scholar]
- 50.Staalsoe T, Megnekou R, Fievet N, Ricke CH, Zornig HD, Leke R, Taylor DW, Deloron P, Hviid L. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Infect Dis. 2001;184:618–626. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- 51.Espinoza E, Hidalgo L, Chedraui P. The effect of malarial infection on maternal-fetal outcome in Ecuador. J Matern Fetal Neonatal Med. 2005;18:101–105. doi: 10.1080/147670500231989. [DOI] [PubMed] [Google Scholar]
- 52.Leenstra T, Phillips-Howard PA, Kariuki SK, Hawley WA, Alaii JA, Rosen DH, Oloo AJ, Nahlen BL, Kager PA, ter Kuile FO. Permethrin-treated bed nets in the prevention of malaria and anemia in adolescent schoolgirls in western Kenya. Am J Trop Med Hyg. 2003;68:86–93. [PubMed] [Google Scholar]
- 53.Marques PX, Saute F, Pinto VV, Cardoso S, Pinto J, Alonso PL, Rosario VE, Arez AP. Plasmodium species mixed infections in two areas of Manhica district, Mozambique. Int J Biol Sci. 2005;1:96–102. doi: 10.7150/ijbs.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogerson SJ, van den Broek NR, Chaluluka E, Qongwane C, Mhango CG, Molyneux ME. Malaria and anemia in antenatal women in Blantyre, Malawi: a twelve-month survey. Am J Trop Med Hyg. 2000;62:335–340. doi: 10.4269/ajtmh.2000.62.335. [DOI] [PubMed] [Google Scholar]
- 55.Walker-Abbey A, Djokam RR, Eno A, Leke RF, Titanji VP, Fogako J, Sama G, Thuita LH, Beardslee E, Snounou G, Zhou A, Taylor DW. Malaria in pregnant Cameroonian women: the effect of age and gravidity on submicroscopic and mixed-species infections and multiple parasite genotypes. Am J Trop Med Hyg. 2005;72:229–235. [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention Malaria. 2009. http://www.cdc.gov/malaria/biology/index.htm Available at. Accessed November 25, 2009.
- 57.Dolan G, ter Kuile FO, Jacoutot V, White NJ, Luxemburger C, Malankirii L, Chongsuphajaisiddhi T, Nosten F. Bed nets for the prevention of malaria and anemia in pregnancy. Trans R Soc Trop Med Hyg. 1993;87:620–626. doi: 10.1016/0035-9203(93)90262-o. [DOI] [PubMed] [Google Scholar]
- 58.Marchant T, Schellenberg JA, Edgar T, Nathan R, Abdulla S, Mukasa O, Mponda H, Lengeler C. Socially marketed insecticide-treated nets improve malaria and anemia in pregnancy in southern Tanzania. Trop Med Int Health. 2002;7:149–158. doi: 10.1046/j.1365-3156.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 59.Falade CO, Yusuf BO, Fadero FF, Mokuolu OA, Hamer DH, Salako LA. Intermittent preventive treatment with sulphadoxine-pyrimethamine is effective in preventing maternal and placental malaria in Ibadan, south-western Nigeria. Malar J. 2007;6:88. doi: 10.1186/1475-2875-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mockenhaupt FP, Ulmen U, von Gaertner C, Bedu-Addo G, Bienzle U. Diagnosis of placental malaria. J Clin Microbiol. 2002;40:306–308. doi: 10.1128/JCM.40.1.306-308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41:1370–1374. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mbaye A, Richardson K, Balajo B, Dunyo S, Shulman C, Milligan P, Greenwood B, Walraven G. A randomized, placebo-controlled trial of intermittent preventive treatment with sulphadoxine-pyrimethamine in Gambian multigravidae. Trop Med Int Health. 2006;11:992–1002. doi: 10.1111/j.1365-3156.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 63.Njagi JK, Magnussen P, Estambale B, Ouma J, Mugo B. Prevention of anemia in pregnancy using insecticide-treated bednets and sulfadoxine-pyrimethamine in a highly malarious area of Kenya: a randomized controlled trial. Trans R Soc Trop Med Hyg. 2003;97:277–282. doi: 10.1016/s0035-9203(03)90141-6. [DOI] [PubMed] [Google Scholar]
- 64.Schultz LJ, Steketee RW, Macheso A, Kazembe P, Chitsulo L, Wirima JJ. The efficacy of antimalarial regimens containing sulfadoxine-pyrimethamine and/or chloroquine in preventing peripheral and placental Plasmodium falciparum infection among pregnant women in Malawi. Am J Trop Med Hyg. 1994;51:515–522. doi: 10.4269/ajtmh.1994.51.515. [DOI] [PubMed] [Google Scholar]
- 65.Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am J Trop Med Hyg. 2001;64:36–44. doi: 10.4269/ajtmh.2001.64.36. [DOI] [PubMed] [Google Scholar]
- 66.Saute F, Menendez C, Mayor A, Aponte J, Gomez-Olive X, Dgedge M, Alonso P. Malaria in pregnancy in rural Mozambique: the role of parity, submicroscopic and multiple Plasmodium falciparum infections. Trop Med Int Health. 2002;7:19–28. doi: 10.1046/j.1365-3156.2002.00831.x. [DOI] [PubMed] [Google Scholar]
- 67.Anchang-Kimbi JK, Achidi EA, Nkegoum B, Sverremark-Ekstrom E, Troye-Blomberg M. Diagnostic comparison of malaria infection in peripheral blood, placental blood and placental biopsies in Cameroonian parturient women. Malar J. 2009;8:126. doi: 10.1186/1475-2875-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akindele JA, Sowunmi A, Abohweyere AE. Congenital malaria in a hyperendemic area: a preliminary study. Ann Trop Paediatr. 1993;13:273–276. doi: 10.1080/02724936.1993.11747658. [DOI] [PubMed] [Google Scholar]
- 69.Balaka B, Agbere AD, Bonkoungou P, Kessie K, Assimadi K, Agbo K. Congenital malarial disease due to Plasmodium falciparum in high-infection-risk newborn [in French] Arch Pediatr. 2000;7:243–248. doi: 10.1016/s0929-693x(00)88739-4. [DOI] [PubMed] [Google Scholar]
- 70.Djibo A, Cenac A. Congenital malaria. Parasitological and serological studies in Niamey (Niger) [in French] Sante. 2000;10:183–187. [PubMed] [Google Scholar]
- 71.Egwunyenga OA, Ajayi JA, Popova-Duhlinska DD, Nmorsi OP. Malaria infection of the cord and birthweights in Nigerians. Cent Afr J Med. 1996;42:265–268. [PubMed] [Google Scholar]
- 72.Fischer PR. Congenital malaria: an African survey. Clin Pediatr (Phila) 1997;36:411–413. doi: 10.1177/000992289703600706. [DOI] [PubMed] [Google Scholar]
- 73.Larkin GL, Thuma PE. Congenital malaria in a hyperendemic area. Am J Trop Med Hyg. 1991;45:587–592. doi: 10.4269/ajtmh.1991.45.587. [DOI] [PubMed] [Google Scholar]
- 74.Menendez C, Mayor A. Congenital malaria: the least known consequence of malaria in pregnancy. Semin Fetal Neonatal Med. 2007;12:207–213. doi: 10.1016/j.siny.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 75.Mukhtar MY, Lesi FE, Iroha EU, Egri-Okwaji MT, Mafe AG. Congenital malaria among inborn babies at a tertiary centre in Lagos, Nigeria. J Trop Pediatr. 2006;52:19–23. doi: 10.1093/tropej/fmi044. [DOI] [PubMed] [Google Scholar]
- 76.Ndyomugyenyi R, Magnussen P. Chloroquine prophylaxis, iron/folic-acid supplementation or case management of malaria attacks in primigravidae in western Uganda: effects on congenital malaria and infant hemoglobin concentrations. Ann Trop Med Parasitol. 2000;94:759–768. doi: 10.1080/00034980020015189. [DOI] [PubMed] [Google Scholar]
- 77.Obiajunwa PO, Owa JA, Adeodu OO. Prevalence of congenital malaria in Ile-ife, Nigeria. J Trop Pediatr. 2005;51:219–222. doi: 10.1093/tropej/fmi003. [DOI] [PubMed] [Google Scholar]
- 78.Sotimehin SA, Runsewe-Abiodun TI, Oladapo OT, Njokanma OF, Olanrewaju DM. Possible risk factors for congenital malaria at a tertiary care hospital in Sagamu, Ogun State, south-west Nigeria. J Trop Pediatr. 2008;54:313–320. doi: 10.1093/tropej/fmn016. [DOI] [PubMed] [Google Scholar]
- 79.Yatich NJ, Yi J, Agbenyega T, Turpin A, Rayner JC, Stiles JK, Ellis WO, Funkhouser E, Ehiri JE, Williams JH, Jolly PE. Malaria and intestinal helminth co-infection among pregnant women in Ghana: prevalence and risk factors. Am J Trop Med Hyg. 2009;80:896–901. [PubMed] [Google Scholar]
- 80.Bouyou-Akotet MK, Ionete-Collard DE, Mabika-Manfoumbi M, Kendjo E, Matsiegui PB, Mavoungou E, Kombila M. Prevalence of Plasmodium falciparum infection in pregnant women in Gabon. Malar J. 2003;2:18. doi: 10.1186/1475-2875-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Czeizel AE, Rockenbauer M, Sorensen HT, Olsen J. The teratogenic risk of trimethoprim-sulfonamides: a population based case-control study. Reprod Toxicol. 2001;15:637–646. doi: 10.1016/s0890-6238(01)00178-2. [DOI] [PubMed] [Google Scholar]
- 82.Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343:1608–1614. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 83.Hommerich L, von Oertzen C, Bedu-Addo G, Holmberg V, Acquah PA, Eggelte TA, Bienzle U, Mockenhaupt FP. Decline of placental malaria in southern Ghana after the implementation of intermittent preventive treatment in pregnancy. Malar J. 2007;6:144. doi: 10.1186/1475-2875-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mbonye AK, Bygbjerg I, Magnussen P. Intermittent preventive treatment of malaria in pregnancy: a community-based delivery system and its effect on parasitemia, anemia and low birth weight in Uganda. Int J Infect Dis. 2008;12:22–29. doi: 10.1016/j.ijid.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 85.Mbonye AK, Bygbjerg IC, Magnussen P. Intermittent preventive treatment of malaria in pregnancy: a new delivery system and its effect on maternal health and pregnancy outcomes in Uganda. Bull World Health Organ. 2008;86:93–100. doi: 10.2471/BLT.07.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rowe AK, Kachur SP, Yoon SS, Lynch M, Slutsker L, Steketee RW. Caution is required when using health facility-based data to evaluate the health impact of malaria control efforts in Africa. Malar J. 2009;8:209. doi: 10.1186/1475-2875-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng G, Simpson JA, Chaluluka E, Molyneux ME, Rogerson SJ. Decreasing burden of malaria in pregnancy in Malawian women and its relationship to use of intermittent preventive therapy or bed nets. PLoS ONE. 2010;5:e12012. doi: 10.1371/journal.pone.0012012. [DOI] [PMC free article] [PubMed] [Google Scholar]


