Abstract
Leishmaniasis is considered an emerging opportunistic disease in human immunodeficiency virus (HIV)–infected patients who have considerably variable clinical presentation. We report a patient with visceral leishmaniasis who had unexpected clinical aspects (atypical cutaneous lesions appearing after long-term evidence of visceral parasites). The patient had hepatoesplenomegaly in the absence of fever, but was otherwise generally healthy. The HIV viral load was low despite severe immunossupression (low lymphocyte proliferation and low level of interferon-γ, concomitant with a high lymphocyte activation status). Surprisingly, two Leishmania strains were isolated from his bone marrow (typical L. infantum sequence MON-1, type A) and skin (L. donovani MON-2 sequence); this second strain had not been previously identified in Brazil. The association of visceral leishmaniasis and HIV/acquired immunodeficiency syndrome is a largely unknown disease, particularly in areas in which leishmaniasis is not endemic. Such atypical cases indicate that this disease can be undiagnosed in clinical settings.
Introduction
Visceral leishmaniasis (VL) is endemic to different regions of Brazil and is, similar to that in other parts of the world, predominantly a childhood disease. In adult cases, co-infection with human immunodeficiency virus (HIV) emerges as an important predisposing factor for reactivation.1 Fever, hepatosplenomegaly, and weight loss are the main symptoms of VL, and the clinical picture is similar in patients infected with HIV and those not infected with HIV. However, certain features are more frequently described for patients co-infected with Leishmania and HIV. These features include several relapses after appropriate therapy, atypical parasite locations, and skin involvement.2
Cutaneous lesions may occur before or after visceral infection and show a considerably variable dermatologic aspect that includes macules,3 papules,4 nodules,5 or ulcers.6 Leishmaniasis in immunocompromised patients appears in advanced stages of HIV infection and shows CD4+ T cell counts less than 200 cells/mm3 in most patients.7 Cutaneous lesions in a persons co-infected with VL and HIV may occur by dissemination after an external infection2,8 or reactivation after a latent infection.
We report a severely immunocompromised patient with cutaneous lesions that appeared after a long time of visceral disease. Because Leishmania parasites could be observed in blood smears of patients co-infected with VL and HIV2,8 we hypothesized that cutaneous lesions in our patient were the result of hematogenic dissemination of visceral disease. Such a hypothesis was strengthened by histologic appearance of cutaneous lesions; Leishmania parasites were observed in deep dermis under normal-appearing epidermis and papillary-medial dermis (Figure 1). A follow-up study of the immunologic state of the patient was conducted up to one year after treatment for leishmaniasis has ended.
Figure 1.
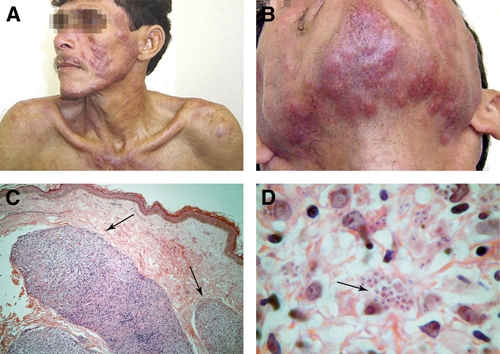
Clinical aspects of lesions on the face and chest (A) and chin (B) of the patient. Also shown are compact inflammatory infiltrate on deep dermis under normal epidermis (C) and numerous amastigotes on an inflammatory infiltrate (D).
Case Report
Written informed consent was obtained from the patient for publication of this case report. A 39-year-old man from Brazil, originally from Rio Grande do Norte and a resident of the Rio de Janeiro State, was diagnosed with pulmonary tuberculosis and HIV infection in 1998. The patient had traveled throughout different countries in South America but had never traveled outside South America. Treatment for tuberculosis and antiretroviral therapy (azidothymidine and lamivudine [AZT/3TC]) were administered.
In 2002, highly active antiretroviral therapy was initiated. This treatment was composed of two nucleoside analogs, reverse transcriptase inhibitors (lamivudine and tenofovir) and protease inhibitors (lopinavir and ritonavir). After this treatment, the patient reported mild abdominal pain, occasional nausea, and gastric fullness. A physical examination showed hepatosplenomegaly, and a hepatic histopathologic analysis by using serologic tests identified infection with hepatitis C virus. The HIV viral load was < 80 copies/mL, as quantified by a nucleic acid sequence–based amplification assay (Organon Teknika, Durham, NC). However, CD4+ T cell counts were not available.
After one year of the above-mentioned treatment schedule for infection with HIV, the immunologic profile remained similar; it showed identical levels of viral load (< 80 copies/mL) and a CD4+ T cell count of 33 cells/mm3. In 2004, the abdominal volume was increased, and abdominal computerized tomography showed homogenous hepatosplenomegaly. At this time, the patient had pancytopenia, and levels of CD4+ T lymphocytes were maintained. The viral load, as evaluated elsewhere by the same HIV/RNA technique, showed a low level of plasmatic virus (540 copies/mL). In 2005, the patient had asymptomatic, erythematous lumps on the trunk and face, but despite low CD4+ T lymphocyte cell counts, HIV-1 RNA was not detected.
In 2006, the patient was admitted to the Instituto de Pesquisa Evandro Chagas/FIOCRUZ for evaluation of cutaneous lesions. On physical examination, the patient appeared emaciated and chronically ill. However, his vital signs were normal. The liver was palpable 8 cm below the costal edge, and the spleen was palpable at the umbilical level. There was no fever. Cervical, axillary, and inguinal lymphoadenopathy were detected. Cutaneous lesions, i.e., elevated, erythematous papules and plaques were noted on the trunk, cheeks, and chin (Figure 1A and B). Blood cell counts were as follows: erythrocytes, 2.9 × 106 cells/µL; leukocytes, 2.4 × 103 cells/µL; hemoglobin, 8.2 g/dL; and platelets, 12.6 × 104 cells/µL. The creatinine level was 3.3 mg/dL (291.7 µmol/L), and blood urea nitrogen level was 57 mg/dL (20.4 mmol/L).
Fragments of trunk lesions and bone marrow aspirate were submitted for histopathologic analysis, mycological culture, parasite isolation by using Novy-MacNeal-Nicolle culture medium, and polymerase chain reaction detection of Leishmania DNA. Histopathologic analysis of the skin showed normal epidermis. However, in the mid- and deep dermis, great masses of granulomatous inflammatory infiltrate and numerous amastigotes within macrophages and in the intercellular spaces were noted (Figure 1C and D). Histopathologic analysis of bone marrow showed numerous amastigotes. Culture in NNN medium was positive for Leishmania. Silver impregnation, Wade staining, and mycological culture were negative for fungal elements. Re-examination of the hepatic histopathologic sample from 2002 showed numerous amastigotes (Figure 2), indicating that visceral leishmaniasis had had a long-term clinical course (at least five years) before the skin dissemination.
Figure 2.
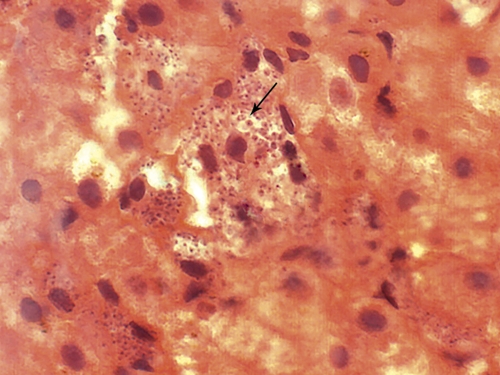
Histopathologic aspect of the liver of the patient, showing amastigote forms of Leishmania sp.
Results for restriction fragment length polymorphism assays specific for the hsp70 Leishmania9 gene were positive for the skin and bone marrow samples. Parasites from these samples were identified as L. donovani complex. Two Leishmania isolates were obtained and characterized by enzyme electrophoresis10: one from the cutaneous lesion (IOC/L2930-MHOM/BR/2007/JFF) and one from bone marrow (IOC/L3020-MHOM/BR/2007/JFF_BM). IOC/L2930 was characterized as L. infantum IOC/Z1. Strain IOC/L3020 differed from IOC/L2930 in the 6-phosphogluconate dehydrogenase profile. The internal transcribed spacer (ITS)–5.8S–ITS2 ribosomal DNA region of both strains was sequenced. IOC/L3020 has a typical L. infantum sequence (MON-1, type A),11 and IOC/L2930 had an L. donovani MON-2 sequence (Genbank accession numbers FN398343 and FN398344 for IOC/L3020 and IOC/L2930, respectively). Multilocus microsatellite typing12,13 confirmed this data.
When cutaneous leishmaniasis was diagnosed, Montenegro's skin test was negative, and results of immunofluorescent assay was positive at a dilution of 1/720. The CD4+ T absolute counts were < 50 cells/mm3, and the plasmatic viral load was undetectable as measured by bDNA technology (Versant® HIV RNA 3.0 assay; Siemens, Tarrytown, NY (Figure 3A). After a cumulative dose of 3 g of amphotericin B, cutaneous lesions were reduced to mild erythema. However, the hepatosplenomegaly persisted with no improvement. Bone marrow histopathologic analysis showed no parasites, and culture for Leishmania was also negative.
Figure 3.
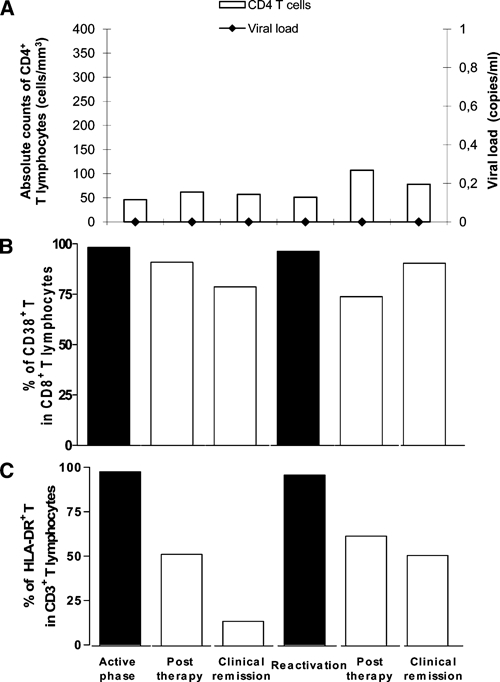
Analysis of the immune system commitment during the clinical follow-up of the patient co-infected with visceral leishmaniasis and human immunodeficiency virus (HIV). The patient was evaluated at the time of the first episode of visceral leishmaniasis (active phase), one month after antileishmanial therapy (post-therapy), during the period without signs or symptoms of leishmaniasis (clinical remission), and during relapses (reactivation). A, Absolute counts of circulating CD4+ T lymphocytes/mm3 and HIV viral load (RNA copies/mL). B, Population of CD8+ T lymphocytes expressing CD38 molecules. C, Population of CD3+ T lymphocytes expressing HLA-DR molecules.
Three months after anti-Leishmania therapy, the patient had fever and a new erythematous papule on the skin (Figure 4A). Histopathologic analysis showed a granulomatous reaction and several amastigotes. The patient was re-treated with pentavalent antimony at a dose of 20 mg of Sb+/kg/day for 3 weeks. Four weeks after the end of the antimonial therapy, only mild erythema and purpuric lesions on the legs were observed (Figure 4B). We considered several differential diagnoses for the purpuric lesions: papular-purpuric gloves and socks syndrome, B19 parvovirus infection, early manifestations of Kaposi's sarcoma, a coagulation defect linked to low platelet counts or lack of certain coagulation factors, or initial lesions of bacillary angiomatosis. However, the patient refused further investigations, and a precise diagnosis could not be ascertained.
Figure 4.
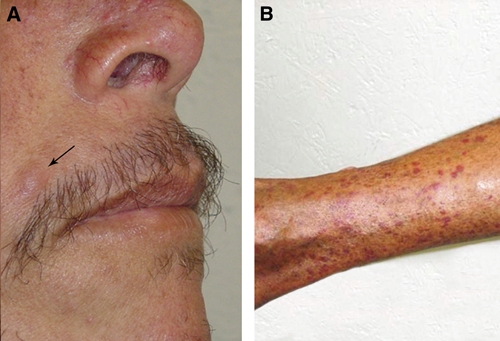
Clinical aspect of reactivation of cutaneous lesions on the face of the patient after treatment for visceral leishmaniasis (A) and purpuric lesions on a leg (B).
A qualitative analysis of cellular immune responses was performed during the clinical follow-up as described elsewhere.14,15 High levels of T cell activation were observed during the active phase of leishmaniasis (first episode and relapse) (Figure 3B), as assessed by the expression of CD38 in CD8+ T cells and HLA-DR in CD3+ T cells.16 The degree of activation decreased after therapy and was stably maintained during the remission phases, especially for HLA-DR (Figure 3C). Lymphocyte activation in response to Leishmania or HIV antigens (p-24 protein) in vitro was also assessed by lymphocyte proliferative response (LPR) assays and interferon-γ (IFN-γ) production.14 The LPR in response to parasite or viral antigens was negative (stimulation index < minimal limit of 2.5) at any time during follow-up, although the LPR was positive for the mitogen concanavalin A. Interferon-γ (790 pg/mL) was detected in response to leishmanial antigens during the first episode of leishmaniasis, but no difference was noted between cultures with and without stimuli. However, the IFN-γ production decreased progressively during clinical evolution, decreasing to levels of 15 pg/mL.
Discussion
Patients co-infected with VL and HIV/acquired immunodeficient syndrome (AIDS) may have distinct atypical skin involvement,2,17 such as cutaneous lesions. The pathogenesis of cutaneous lesions in an HIV-positive patient with VL is not clear18 since amastigotes can also be observed in different types of skin lesions of HIV patients, such as Kaposi's sarcoma, herpes infections, and bacillary angiomatosis,18–20 and in healthy skin.21 Classically, cutaneous lesions associated with VL represent the well-known post-kala-azar dermal leishmaniasis (PKDL)22 which is an aftermath of visceral disease and is seen only after cure of systemic manifestations. Cutaneous lesions of PKDL include hypochromic or erythematous macules and infiltrated plaques and nodules. Photosensitivity is present during PKDL, and there is a predominance of lesions on the face and upper thorax.22 However, some cases of African kala-azar have cutaneous lesions and parasites in lymph nodes and bone marrow, a clinical presentation known as para-kala-azar dermal leishmaniasis.23
In our patient, the distribution of cutaneous lesions and their visual aspect were compatible with that of classical PKDL. The patient also showed features of African para-kala-azar dermal leishmaniasis, i.e., concomitance of cutaneous and visceral active disease and parasites present in the liver, bone marrow and skin. Leishmania spp. can be isolated from blood.24,25 In the present case, based on the skin histopathologic analysis, which showed normal epidermis and great masses of infiltrate with many parasites in the deep and mid-dermis (Figure 1C and D), we hypothesized that hematologic dissemination could have occurred. Furthermore, the profound degree of immunodeficiency possibly facilitated survival of the parasite within macrophages.
The skin Leishmania isolate (IOC/L2930) was distinguished from the reference strain of Brazilian L. infantum (IOC/L579) and from the bone marrow isolate (IOC/L3020) by the mobility of 6-phosphogluconate dehydrogenase. This enzyme analysis suggested that the patient had a Leishmania mixed infection, which was confirmed by DNA sequence analysis. ITS rDNA sequence analysis and multilocus microsatellite typing indicated that the cutaneous lesion parasite was probably L. donovani MON-2, the most common zymodeme reported from India.26 To our knowledge, MON-2 has never been isolated from cutaneous lesions. However, this zymodeme is closely related to MON-18 and MON-37, both of which are associated with cutaneous leishmaniasis in the Old World. The immunologic status of the patient probably contributed to the clinical manifestations observed.
Until now, L. infantum IOC/Z1 (i.e., MON-1) was the only species from the L. donovani complex associated with leishmaniasis in Brazil. Identification of L. donovani in this patient suggests that this parasite is circulating in Brazil or in other VL-endemic areas in South America as the patient traveled across different countries in South America without causing disease or being identified. The immunocompromised status probably favored the pathologic damage caused by this species.
Visceral leishmaniasis and HIV/AIDS induce lymphocyte depletion, polyclonal activation, and cytokine disregulations, and showed a synergy in their immunodeficiency mechanisms that aggravates both diseases.27 This fact could explain the maintenance of CD4+ T lymphocyte counts < 100 cells/mm3 despite the administration of anti-leishmanial therapy and successful highly active antiretroviral therapy (undetectable viral load during follow-up).
Although immunologic discordance28 could have occurred in this patient, we cannot exclude that lymphopenia was a consequence of VL pathogenesis.29 It is intriguing that this patient was evaluated over a long period and showed a high Leishmania infection load plus severe immunosuppression without affecting the viral load.
This case of co-infection with VL and HIV with long-term evolution of liver parasites is surprising, given the known severity of VL in HIV/AIDS patients.1 The absence of fever in the patient and his good general clinical status could have contributed toward refuting the VL hypothesis. However, the newly revised guidelines in Brazil consider that hepatomegaly or splenomegaly with or without fever and cytopenia in AIDS patients must be investigated for VL.30 The patient had three unexpected features: cutaneous lesions after dissemination of long-term VL parasites, infection by two viscerotropic strains, one of them not previously identified in Brazil, and low HIV viral load despite the severe immunosuppression status. The association between VL and HIV/AIDS is a largely unknown disease entity, especially in areas in which leishmaniasis is not endemic. The present case suggests that many such cases may pass undiagnosed in clinical practice, and that greater clinical awareness is needed for accurate diagnosis.
ACKNOWLEDGMENTS
We thank Marcelo Alves Coelho Jr. (Photography and Documentation Sector, Instituto de Pesquisa Clínica Evandro Chagas) for help with the figures and R. Pellegrino for secretarial assistance.
Footnotes
Financial support: This study was supported by Programa Nacional de DST/AIDS, Ministério da Saúde (grant ED00095/2007); Instituto de Pesquisa Clínica Evandro Chagas; Instituto Oswaldo Cruz/FIOCRUZ (internal funds); Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro; Conselho Nacional de Desenvolvimento Científico e Tecnológico; and the European Union (LeishEpinetSA, INCO-CT2005-015407). Joanna R. Santos-Oliveira was supported by a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico. Alda M. Da-Cruz and Elisa Cupolillo were supported by fellowships from Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Disclosure: None of the authors have any potential financial conflicts of interest.
Authors' addresses: Joanna R. Santos-Oliveira, Alda M. Da-Cruz, Elisa Cupolillo, and Carmem B. W. Giacoia-Gripp, Instituto Oswaldo Cruz, FIOCRUZ, Rio de Janeiro, Brazil, E-mails: joannars@ioc.fiocruz.br, alda@ioc.fiocruz.br, ecupolillo@ioc.fiocruz.br, and carmembg@ioc.fiocruz.br. Lucy H. S. Pires, Secretaria Municipal de Saúde de Cabo Frio, São Cristovão, Cabo Frio, Brazil, E-mail: lucypires@ig.com.br. Katrin Kuhls, Institute of Microbiology and Hygiene, Charité University Medicine Berlin, Berlin, Germany, E-mail: katrin.kuhls@o2online.de. Manoel P. Oliveira-Neto, Instituto de Pesquisa Clínica Evandro Chagas, FIOCRUZ, Rio de Janeiro, Brazil, E-mail: manoel.paes@ipec.fiocruz.br.
References
- 1.Russo L, Laguna F, Lopez-Velez R, Medrano FJ, Rosenthal E, Cacopardo B, Nigro L. Visceral leishmaniasis in those infected with HIV: clinical aspects and other opportunistic infections. Ann Trop Med Parasitol. 2003;97((Suppl 1)):S99–S105. doi: 10.1179/000349803225002570. [DOI] [PubMed] [Google Scholar]
- 2.Ara M, Maillo C, Peón G, Clavel A, Cuesta J, Grasa MP, Carapeto FJ. Visceral leishmaniasis with cutaneous lesions in a patient infected with human immunodeficiency virus. Br J Dermatol. 1998;139:114–117. doi: 10.1046/j.1365-2133.1998.02326.x. [DOI] [PubMed] [Google Scholar]
- 3.Danden E, Peñas PF, Rios L, Jimenez M, Fraga J, Alvar J, García-Diez A. Leishmania presenting as dermatomyositis-like eruption in AIDS. J Am Acad Dermatol. 1996;35:316–319. doi: 10.1016/s0190-9622(96)90658-1. [DOI] [PubMed] [Google Scholar]
- 4.Botelho R, SanMartin O, Febrer MI. Leishmaniasis cutaneo-visceral: una nuova infección oportunista en pacientes infectados con HIV. Med Cut ILA. 1990;18:119–123. [PubMed] [Google Scholar]
- 5.Perrin C, Taillan B, Hofman P, Mondain V, Lefichoux Y, Michiels JF. Atypical cutaneous histopathological features of visceral leishmaniasis in acquired immunodeficiency syndrome. Am J Dermatopathol. 1995;17:145–150. doi: 10.1097/00000372-199504000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Colebunders R, Depraetera K, Verstraeten T, Lambert J, Hauben E, Van Marck E, Maurer T, Bañuls AL, Dujardin JC. Unusual cutaneous lesions in two patients with visceral leishmaniasis and HIV infection. J Am Acad Dermatol. 1999;41:847–850. doi: 10.1016/s0190-9622(99)70342-7. [DOI] [PubMed] [Google Scholar]
- 7.Mondain-Milton V, Toussaint-Gari M, Hofman P, Marty P, Carles M, De Salvador F, Miton F, Le Fichoux Y, Dellamonica P. Atypical leishmaniasis in a patient infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:663–665. doi: 10.1093/clinids/21.3.663. [DOI] [PubMed] [Google Scholar]
- 8.Calza L, D'Antuono A, Marinacci G, Manfredi R, Colangeli V, Passarini B, Orioli R, Varoli O, Chiodo F. Disseminated cutaneous leishmaniasis after visceral disease in a patient with AIDS. J Am Acad Dermatol. 2004;50:461–465. doi: 10.1016/j.jaad.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 9.da Silva LA, de Sousa Cdos S, da Graça GC, Porrozzi R, Cupolillo E. Sequence analysis and PCR-RFLP profiling of the hsp70 gene as a valuable tool for identifying Leishmania species associated with human leishmaniasis in Brazil. Infect Genet Evol. 2010;10:77–83. doi: 10.1016/j.meegid.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Cupolillo E, Grimaldi G, Jr, Momen H. A general classification of New World Leishmania using numerical zymotaxonomy. Am J Trop Med Hyg. 1994;50:296–311. doi: 10.4269/ajtmh.1994.50.296. [DOI] [PubMed] [Google Scholar]
- 11.Kuhls K, Mauricio IL, Pratlong F, Presber W, Schonian G. Analysis of ribosomal DNA internal transcribed spacer sequences of the Leishmania donovani complex. Microbes Infect. 2005;7:1224–1234. doi: 10.1016/j.micinf.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Ochsenreither S, Kuhls K, Schaar M, Presber W, Schonian G. Multilocus microsatellite typing as a new tool for discrimination of Leishmania infantum MON-1 strains. J Clin Microbiol. 2006;44:495–503. doi: 10.1128/JCM.44.2.495-503.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhls K, Keilonat L, Ochsenreither S, Schaar M, Schweynoch C, Presber W, Schonian G. Multilocus microsatellite typing (MLMT) reveals genetically isolated populations between and within the main endemic regions of visceral leishmaniasis. Microbes Infect. 2007;9:334–343. doi: 10.1016/j.micinf.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Da-Cruz AM, Mattos M, Oliveira-Neto MP, Coutinho Z, Machado ES, Coutinho SG. Cellular immune responses to Leishmania braziliensis in patients with AIDS-associated American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 2000;94:569–571. doi: 10.1016/s0035-9203(00)90090-7. [DOI] [PubMed] [Google Scholar]
- 15.Da-Cruz AM, Rodrigues AC, Mattos M, Oliveira-Neto MP, Sabbaga-Amato V, Posada MP, Lindoso JA, Goto H. Alterações imunopatológicas na co-infecção HIV-Leishmania. Rev Soc Bras Med Trop. 2006;39((Suppl 3)):75–79. [PubMed] [Google Scholar]
- 16.Rodrigues DS, Cunha RM, Kallas EG, Salomão R. Distribution of naive/effector CD4+ T lymphocytes and expression of CD38 on CD8+ T lymphocytes in AIDS patients with tuberculosis. Braz J Infect Dis. 2003;7:161–165. doi: 10.1590/s1413-86702003000200010. [DOI] [PubMed] [Google Scholar]
- 17.Pourahmad M, Hooshmand F, Rahiminejad M. Cutaneous leishmaniasis associated with visceral leishmaniasis in a case of acquired immunodeficiency syndrome (AIDS) Int J Dermatol. 2009;48:59–61. doi: 10.1111/j.1365-4632.2009.03870.x. [DOI] [PubMed] [Google Scholar]
- 18.Herrera E, Bosch RJ, Fernández F. The presence and significance of Leishmania in mucocutaneous biopsies from HIV+ patients with visceral leishmaniasis. Eur J Dermatol. 1996;6:501–504. [Google Scholar]
- 19.Barrio J, Lecona M, Cosin J, Olalquiaga FJ, Hernanz JM, Soto J. Leishmania infection occuring in herpes zoster lesions in a HIV patient. Br J Dermatol. 1996;134:16416–6. [PubMed] [Google Scholar]
- 20.Gallego MA, Aguilar A, Plaza S, Gomez JM, Burgos F, Agud JL, Marco J, García C. Kaposi sarcoma with an intense parasitization by Leishmania. Cutis. 1996;57:103–105. [PubMed] [Google Scholar]
- 21.Yebra M, Segovia J, Manzano L, Vargas JA, Bernaldo de Quirós L, Alvar J. Disseminated-to-skin kala-azar and the acquired immunodeficiency syndrome. Ann Intern Med. 1998;108:490–491. doi: 10.7326/0003-4819-108-3-490_2. [DOI] [PubMed] [Google Scholar]
- 22.Ramesh V, Singh R, Salotra P. Post-kala-azar dermal leishmaniasis: an appraisal. Trop Med Int Health. 2007;12:848–851. doi: 10.1111/j.1365-3156.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 23.Zijstra EE, Khalil EA, Kager PA, El-Hassan AM. Post-kala-azar dermal leishmaniasis in Sudan: clinical presentation and differential diagnosis. Br J Dermatol. 2000;143:136–143. doi: 10.1046/j.1365-2133.2000.03603.x. [DOI] [PubMed] [Google Scholar]
- 24.López-Velez R, Laguna F, Alvar J, Pérez-Molina JA, Molina R, Martinez P, Villarrubia J. Parasitic culture of buffy-coat for diagnosis of visceral leishmaniasis in human immunodeficiency virus-infected paients. J Clin Microbiol. 1995;33:937–939. doi: 10.1128/jcm.33.4.937-939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dereure J, Pratlong F, Reynes J, Basset D, Bastien P, Dedet JP. Haemoculture as a tool for diagnosing visceral leishmaniasis in HIV-negative and HIV-positive patients: interest for parasite identification. Bull World Health Organ. 1998;76:203–206. [PMC free article] [PubMed] [Google Scholar]
- 26.Pratlong F, Dereure J, Bucheton B, El-Saf S, Dessein A, Lanotte G, Dedet JP. Sudan: the possible original focus of visceral leishmaniasis. Parasitology. 2001;122:599–605. doi: 10.1017/s0031182001007867. [DOI] [PubMed] [Google Scholar]
- 27.Cacopardo B, Nigro L, Preiser W, Fama A, Satariano MI, Braner J, Celesia BM, Weber B, Russo R, Doerr HW. Prolonged Th2 cell activation and increased viral replication in HIV-Leishmania co-infected patients despite treatment. Trans R Soc Trop Med Hyg. 1996;90:434–435. doi: 10.1016/s0035-9203(96)90538-6. [DOI] [PubMed] [Google Scholar]
- 28.Spritzler J, Mildvan D, Russo A, Asthana D, Livnat D, Schock B, Kagan J, Landay A, Haas DW. Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:551–558. doi: 10.1086/376986. [DOI] [PubMed] [Google Scholar]
- 29.Saha S, Mondal S, Banerjee A, Ghose J, Bhowmick S, Ali N. Immune responses in kala-azar. Indian J Med Res. 2006;123:245–266. [PubMed] [Google Scholar]
- 30.Ministério da Saúde, Secretaria de Vigilância em Saúde, Brasil . Manual de Recomendações para Diagnóstico, Tratamento e Acompanhamento da Co-infecção Leishmania-HIV. Brasília: Programa Nacional de DST/HIV/AIDS; 2004. http://portal.saude.gov.br/portal/arquivos/pdf/manual_leish_hiv.pdf Available at. [Google Scholar]


