Abstract
Opportunistic parasitic infections such as leishmaniasis are common in human immunodeficiency virus (HIV)-infected patients and are usually acquired several days after initial diagnosis of HIV infection. Here, we report on a patient who presented with diffuse cutaneous leishmaniasis (DCL) caused by Leishmania tropica as the first and only clinical manifestation of HIV infection. To the best of our knowledge, this is the first case that illustrates that DCL could be the first clinical indicator of HIV infection. Cutaneous leishmaniasis (CL) and DCL are becoming frequent opportunistic infections in HIV-infected individuals throughout the world. To date, all documented cases of CL and HIV coinfections have been reported in patients who were known cases of HIV and who subsequently developed CL. In this report, we present a case that illustrates that DCL could be the first clinical indicator of HIV infection.
Case Report
A 32-year-old housewife, resident of Bikaner, Rajasthan, India, presented to the dermatology outpatient department of Sardar Patel Medical College with multiple intensely itchy oval to round papulo-nodular lesions of variable size scattered over her limbs (Figure 1), face, and trunk for the last 7 months. History revealed that the lesions first appeared over her limbs and then, gradually spread to her whole body without affecting mucous membranes. New lesions kept on appearing, whereas older lesions gradually increased in size; none of the lesions ulcerated. She never traveled to endemic areas of Kala azar and leprosy. General and systemic examination of the patient did not reveal any abnormality. The laboratory investigations showed anemia (Hb = 8.5%) and lymphopenia (18%). All other investigations, including liver and renal function tests, chest X-ray, and ultrasonography (USG) of the abdomen, were found to be normal. Histopathology of the skin lesions revealed heavy infiltrate of macrophages infected with Leishmania, which was identified as L. tropica by restriction fragment-length polymorphism (RFLP) polymerase chain reaction (PCR)1 (Figure 2). Montenegro test for leishmaniasis was not available, but Mantoux test for tuberculosis (TB) was negative. Human immunodeficiency virus (HIV) infection was suspected, because the patient presented with atypical, generalized, intensely pruritic lesions of cutaneous leishmaniasis (CL); this was confirmed using three different enzyme-linked immunosorbent assay (ELISA) test kits (Tridot, TMB Micro-ELISA, and Combs AIDS; J Mitra & Co., New Delhi, India). Further investigations showed that the CD4 + T cells count was 44 cells/mm3 and that the viral load was 165,400 copies/mL. Highly active antiretroviral therapy (HAART; Zidovudine, Lamivudine, and Efaviranz, Ranbaxy, India) was instituted immediately, and systemic antileishmanial treatment was started simultaneously with intramuscular injections of 20 mg/kg per day of sodium stibogluconate (SSG; Albert David, Kolkotta, India) for 21 days. The patient did not improve clinically, and new lesions containing parasites continued to appear 12 weeks after completion of SSG treatment. The patient refused a second course of intramuscular SSG and hence, was started on Rifampicin (1,200 mg/day) along with HAART. Unfortunately, patient was lost for further follow-up.
Figure 1.
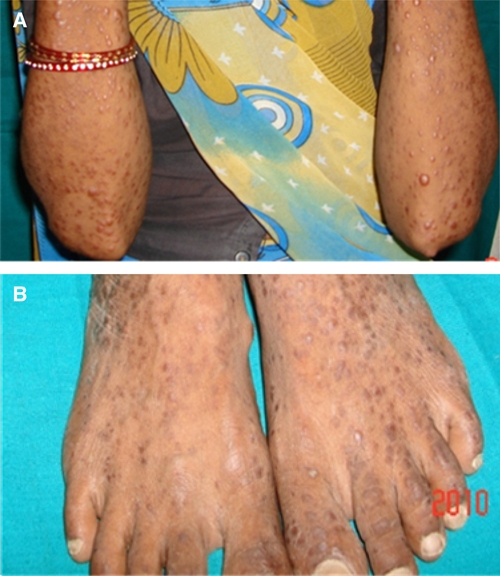
Papulo-nodular lesions on the upper (A) and lower extremities (B) in an HIV-infected patient suffering from DCL.
Figure 2.
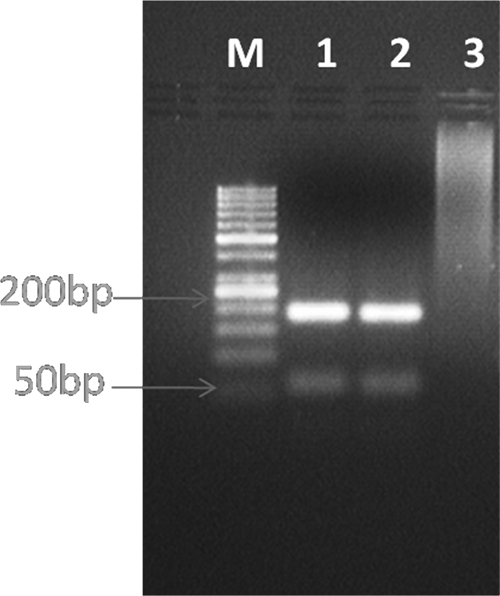
The restriction analysis pattern of 1 PCR product digested with HaeIII. Lane M = DNA ladder (50 bp). Lane 1 = DNA from skin biopsy of the patient. Lane 2 = DNA from L. tropica WR664 (MHOM/SU/74/K27; positive control). Lane 3 = DNA from a skin biopsy of a healthy person (negative control).
Discussion
Infections caused by protozoan Leishmania represent a wide range of diseases, including CL, mucocutaneous leishmaniasis (ML), and visceral leishmaniasis (VL). Incidence of infections caused by Leishmania in HIV-infected patients is increasing throughout the world. Although VL is the most frequent form of Leishmania infection seen in HIV-infected patients, CL and HIV coinfections are also becoming frequent in these individuals.
Diffuse cutaneous leishmaniasis (DCL) is a rare form of Leishmania infection that has been reported in patients infected with L. aethiopica and L. mexicana amazonensis.2 In immune-suppressed patients, lesions of CL may become diffuse to involve extensive areas of skin, resulting in a condition known as DCL. DCL clinically manifests as multiple widespread non-tender papulo-nodular skin lesions full of parasites, which seldom ulcerate, respond poorly to standard antimonial treatment, and show frequent realapses.3 Weinstock and others4 have also reported a single case of DCL caused by L. tropica in an immunocompetent patient, and this case was associated with impaired production of cytokines.4 DCL is becoming increasingly frequent in HIV-infected patients worldwide.5–8 Several Leishmania species, including L. major, L. braziliensis braziliensis, and L. infantum, have been identified as causative agents of DCL in HIV-infected patients.9–12 Almost all patients with DCL and HIV coinfection are individuals who develop DCL as a secondary opportunistic infection after diagnosis of HIV infection. Our case was unusual for several reasons. First, our patient presented with progressive DCL of 7-months duration without typical clinical signs of HIV infection. Second, to best of our knowledge, this is the first case of DCL caused by L. tropica in an HIV-infected patient. Third, in this patient, there was no visceralization or mucosal spread of L. tropica infection, despite low CD4 + T cell counts. This is surprising given that L. tropica has been shown to visceralize even in immunocompetent individuals. Fourth, this patient did not respond to SSG, which may be because of drug resistance or very low CD4 + counts.13 Finally, our patient presented with intensely pruritic skin lesions, which have never been reported in DCL caused by other species of Leishmania. Collectively, our case illustrates that L. tropica can cause intensely pruritic, diffuse CL without involvement of mucous membranes and systemic organs in HIV-infected patients and that DCL could be the first clinical indicator of HIV infection. Therefore, we propose that all patients presenting with DCL should be screened for HIV infection.
Footnotes
Authors' addresses: Kanika Khandelwal, Ram Awatar Bumb, and Rajesh Dutt Mehta, Department of Dermatology, Sardar Patel Medical College, Bikaner, Rajasthan, India, E-mails: drkanikakhandelwal@gmail.com, dr_bumb@rediffmail.com, and mehtarddr@yahoo.co.in. Himanshu Kaushal and Poonam Salotra, Institute of Pathology, ICMR, New Delhi, India, E-mails: hkaushal@yahoo.co.in and psalotra@vsnl.net. Claudio Lezama-Davila and Abhay R. Satoskar, Departments of Pathology and Microbiology, Ohio State University, Columbus, OH, E-mails: lezama-davila.1@osu.edu and abhay.satoskar@osumc.edu.
References
- 1.Kumar R, Bumb RA, Ansari NA, Mehta RD, Salotra P. Cutaneous Leishmaniasis caused by Leishmania tropica in Bikaner, India: parasite identification and characterization using molecular and immunologic tools. Am J Trop Med Hyg. 2007;76:896–901. [PubMed] [Google Scholar]
- 2.Suzanne A, Grevelink MD, Lerner EA. Leishmaniasis. J Am Acad Dermatol. 1996;34:257–272. doi: 10.1016/s0190-9622(96)80121-6. [DOI] [PubMed] [Google Scholar]
- 3.Vega-Lopez F, Hay RJ. In: Rook's Text Book of Dermatology. 8th ed. Burns T, Breathnach S, Cox N, Griffiths C, editors. West Sussex, United Kingdom: Wiley-Blackwell; 2010. pp. 32–43. (Parasitic worms and protozoa). [Google Scholar]
- 4.Weinstock C, Knobloch J, Schultheis W, Northoff H. Impaired production of cytokines in a case of human leishmaniasis. Clin Infect Dis. 1997;25:1334–1339. doi: 10.1086/516123. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary RG, Bilimoria FE, Katare SK. Diffuse cutaneous leishmaniasis: co-infection with human immunodeficiency virus (HIV) Indian J Dermatol Venereol Leprol. 2008;74:641–643. doi: 10.4103/0378-6323.45111. [DOI] [PubMed] [Google Scholar]
- 6.Mehta V, Balachandran C, Rao R, Dil SK, Indusri L. Diffuse cutaneous leishmaniasis in HIV. Dermatol Online J. 2009;15(9) http://dermatology.cdlib.org Available at. Accessed April 15, 2009. [PubMed] [Google Scholar]
- 7.Niamba P, Traore A, Goumbri-Lompo O, Labreze C, Traore-Barro F, Bonkoungou M, Iiboudo L, Gauller L, Soudre RE. Cutaneous leishmaniasis in an HIV patient in Ouagadougou: clinical and therapeutic aspects. Ann Dermatol Venereol. 2006;133:537–542. doi: 10.1016/s0151-9638(06)70958-9. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf SM, Uloko AE, Adamu HA, Iliyasu G, Mohammad AM. Disseminated cutaneous leishmaniasis in HIV positive patient—a case report. Niger J Med. 2010;19:112–114. doi: 10.4314/njm.v19i1.52501. [DOI] [PubMed] [Google Scholar]
- 9.Barro-Traore F, Preney L, Traore A, Darie H, Tapsoba P, Bassole A, Sawadogo S, Niamba P, Grosshans E, Geniaux M. Cutaneous leishmaniasis due to Leishmania major involving bone marrow in an AIDS patient in Burkino Faso. Ann Dermatol Venereol. 2008;135:380–383. doi: 10.1016/j.annder.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Machado ES, Braga Mda P, Da Cruz AM, Coutinho SG, Vieira AR, Rutowitsch MS, Cuzzi-Maya T, Grimaldi junior G, Menezes JA. Disseminated American muco-cutaneous leishmaniasis caused by Leishmania braziliensis braziliensis in a patient with AIDS: a case report. Mem Inst Oswaldo Cruz. 1992;87:487–492. doi: 10.1590/s0074-02761992000400005. [DOI] [PubMed] [Google Scholar]
- 11.Durand I, Beylot-Barry M, Weill FX, Doutre MS, Beylot C. Disseminated cutaneous leishmaniasis revealing human immunodeficiency viral infection. Ann Dermatol Venereol. 1998;125:268–270. [PubMed] [Google Scholar]
- 12.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, Gradoni L, Horst RT, Lopez-Velez R, Moreno J. The relation between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft SL, Shyam S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


