Abstract
In 2004 an aggressive plan was instituted aiming to achieve nationwide transmission control of schistosomiasis by 2015. Here, we report a longitudinal study on the control of schistosomiasis in Anhui province, China. Using a mathematical model, we compared the effects of different control strategies implemented in the study area. During the 5-year study period, a 60.8% reduction in human prevalence was observed from 2005 (7.95%) to 2009 (3.1%), and snail infection decreased from 0.063% in 2005 to zero in 2009. Results of the model agree well with the first 3-year field observations and suggest continuous decrease in human infections in the last 2 years, whereas the last 2-year field observations indicated that human infections appeared to be stable even with continuous control. Our findings showed that the integrated control strategy was effective, and we speculated that other factors besides bovines might contribute to the local transmission of the disease.
Introduction
Schistosomiasis, one of the world's most prevalent infections, is draining the economic and social development in much of the tropics.1 Worldwide ~200 million people are infected, whereas an estimated 779 million people are at risk of infections.1,2 Recent reassessment of schistosomiasis-related disability indicates that the actual burden of schistosomiasis is substantially greater than previously appreciated (e.g., 2–15% versus 0.5% disability weight assigned to schistosomiasis).3 Schistosomiasis japonica, which is caused by infection with Schistosoma japonicum, is a zoonosis of major public health importance in China, with ~730,000 people and 100,000 bovines infected.4–6 The praziquantel-based morbidity control has been central to China's national schistosomiasis control program since the 1990s, especially during the 10-year World Bank Loan Project period between1992 and 2001.7,8 Although both prevalence and intensity of S. japonicum infection in the endemic areas have decreased dramatically to a relatively low level, it is difficult for this measure alone to go beyond to eliminate the transmission of S. japonicum in China.8–12
In 2004, together with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), tuberculosis, and hepatitis B, schistosomiasis was placed by the Chinese government on the top priority list for the control of communicable diseases in China, and the two control targets were then established for the national schistosomiasis control program—to reduce human infection rates in all endemic counties to below 5% by 2008, and then to < 1% by 2015.13 To reach these targets, a comprehensive national strategy has been developed to aim at reducing the roles of bovines and humans as sources of infection for snails, and have been implemented by the Chinese government in many endemic regions of China.13,14 In this study, we carried out a longitudinal study to observe the effect of the integrated control approach on S. japonicum transmission in the reach of the Qiupu River in Guichi district, Anhui province, China. We compared the impacts of different interventions on the transmission dynamics using the data collected from the longitudinal survey and interpreted these effects using a mathematical model. Here, we report findings coming out of the study.
Material and Methods
Study area.
The study area is located along the reach of the Qiupu River in Guichi district, Anhui province, China. The Qiupu River is a southern tributary of the Yangtze River, and its reach is historically one of the most serious endemic areas of S. japonica in China. Oncomelania hupensis, the sole intermediate host snail of S. japonicum, are distributed in the bottomlands of Qiupu River. These bottomlands are natural pastures for bovines. There are ~32,000 total residents and 5.7 km2 of the snail-infected area in the study region. A longitudinal survey was conducted in 12 randomly selected natural villages (i.e., a hamlet, which is a small group of dwellings under an administrative village that typically consists of natural villages), and a school (the only school serving these natural villages) between 2005 and 2009 (Figure 1).
Figure 1.
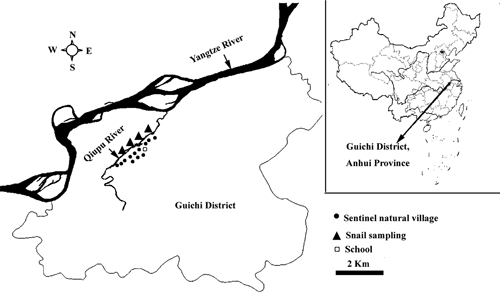
The study site in Guichi district, Anhui province, China.
Control measures.
During the study period, an integrated control strategy aiming at reducing the transmission of S. japonicum from bovines and humans to snails had been instituted and implemented in the reach of Qiupu River, Guichi district, Anhui province.14 Specifically, these interventions included that 1) all bovines were removed from the study area (e.g., some farm bovines were replaced with tractors, or transferred to non-endemic regions; some bovines were killed) in February, 2006; 2) all residents 5 to 65 years of age in the study area, except those who received direct fecal examination from the 12 natural villages and the school, were examined annually using indirect hemagglutination assay (IHA)15–17 in autumn, and all IHA positives were subjected to fecal examination (e.g., Kato-Katz egg count) to confirm infection. Residents with positive egg counts were treated with a single oral dose of praziquantel (40 mg/kg of body weight) as recommended by the World Health Organization (WHO)18; 3) improved sanitation facilities(e.g., sanitary lavatories and piped water were installed; 4) a health education program was implemented among residents focusing on avoidance of snail-infected areas and associated river water; 5) modifying snail microhabitat through agricultural practices (e.g., modifying crop structures in snail-inhabited areas to reduce water use) (Figure 2).
Figure 2.
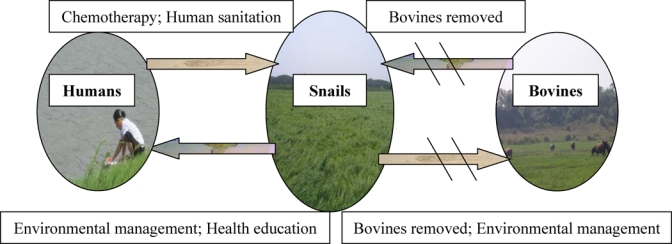
A diagrammatic sketch of schistosomiasis japonica transmission model and control approaches in the Qiupu River drainage system. The oblique lines show the transmission between bovine and snails were blocked through the removal of bovines.
Fecal samples and examinations.
In the autumn surveys in the 12 natural villages and the school, all eligible participants 5 to 65 years of age were asked to produce a fecal specimen and the minimum requirement for the amount of the sample was 50 grams. The samples were delivered to the local schistosomiasis station. Within 24 hours of sample collection, the samples were examined microscopically using the quantitative Kato-Katz thick smear technique (KK, three slides).19 Stool samples from residents were evaluated for the presence of S. japonicum eggs by the KK method. For each sample, three slides (41.7 mg/smear, with a total sample weight of 125 mg) were prepared and read within 1–12 hours of their initial preparation by two experienced technicians who were unaware of the subject's infection status. Schistosoma japonicum egg counts were expressed in eggs/gram of stool (EPG). Residents with a positive fecal examination were treated with a single dose of praziquantel (40 mg/kg).18
Fecal samples were also collected from all buffaloes in the study area in the autumn of 2005 and the infection status for S. japonicum was examined using the miracidial hatching test and a detailed procedure was described elsewhere.20
Written informed consent was obtained from all adult participants and from the parents or legal guardians of children. Ethical approval for the study was obtained from village (local government), country (anti-schistosomiasis station), and provincial (schistosomiasis headquarters) authorities, and was endorsed by the Ethical Review Committee of School of Public Health, Fudan University.
Snail survey.
The snail survey was performed yearly in four major grasslands relevant to the 12 study villages in October from 2005 to 2009 (Figure 1). This was conducted by using the Chinese traditional method of random quadrant sampling (0.11 m2-sized frames, 20-m apart between frames).7,21 All snails within the square frames were collected and brought to the laboratory. These collected snails were then counted and crushed to examine microscopically for schistosome infections.
Mathematical model.
Following the basic modeling framework (e.g., two-host model) by Barbour22 and an extended model by Williams and others23 allowing for the mammalian host heterogeneity characteristic of the S. japonicum life cycle, we used a similar framework to assess the long-term impacts of the aforementioned integrated national strategies on human S. japonicum transmission. This model is composed of three sets of differential equations, which track rates of change in prevalence in the two definitive hosts (humans and bovines) and in oncomelanid snails over time. The equations and associated parameters were described in detail elsewhere.22,23 Control measures in the study villages were explicitly integrated in the model, which was solved numerically to predict human prevalence of infections. The numerical simulation was solved using Matlab software (The MathWorks, Inc., Natick, MA).
Control measures, model parameters, and assumptions.
On the basis of the current control measures implemented in China, three scenarios of integrated control strategies were specifically considered in the models13,14; strategy A, coupling synchronous treatment with praziquantel for humans and bovines, which was carried out before the initiation of our study and continued to be implemented during the study period in our study region; strategy B, implemented in our study area, consisting of an integrated interventions as illustrated in Figure 2; and strategy C, presently carried out in other regions of China, including measures illustrated in Figure 2 except for environmental management. Similar to assumptions made by Williams and others,23 we assume that the schistosome lifespan is 4 years for humans, 1.5 years for bovines, and 6 months for snails. Other model variables were estimated from the data of the 5-year longitudinal survey (e.g., density of the definitive hosts).
The “observed” prevalence of infection among humans used in the model were adjusted according to the sensitivity (65%) and specificity (100%) of the KK method, respectively16,24; because specificity = 100%, the “adjusted” prevalence = the “observed” prevalence/sensitivity. The unit of snail density (snails/0.11 m2) was converted into snails/m2 when it was used in the model. The coverage of selected human treatment with praziquantel is assumed to be 45% (i.e., 45% of infected humans were treated with praziquantel) and the drug efficacy is assumed to be 85% (e.g., 85% of treated infected humans are cured).16,23,24 A 20% reduction in human water contact and 30% coverage of sanitation facilities were assumed from the sanitation improvement. A reduction in 35% snail habitats was assumed to manage agricultural practice with 40% reduction in density of snails.
Statistical analysis.
Data were compiled in Microsoft Excel 2003 (Microsoft Corp., Redmond, WA). Analyses were performed using the SPSS Statistical Package (SPSS Inc., Chicago, IL, 2007). Egg counts were transformed to EPG and geometric mean intensities of the infected individuals were calculated by using the log-transformed egg counts. Confidence intervals (CI) were calculated using standard formulas based on the binomial distribution (prevalence) and the lognormal distribution (intensity). Presence of bovine infections was determined by a positive miracidial hatching test result. The prevalence of infection of snails was calculated as the proportion of infected snails, and density of infected snails as the number of infected snails/0.11 m2.
Human prevalence and infection intensity were analyzed using a generalized linear model,12 and the base model included year (from 2005 to 2009), sex, and age. Densities of infected snails and live snails were analyzed by using a generalized linear model (with the actual number of infected snails or living snails/0.11 m2 as the dependent variable) with a log link and negative binomial distribution. Snail infection prevalence was analyzed by the χ2 test.12 Spearman correlation was used to analyze the association between the density and prevalence of snails.
Results
Livestock treatment.
Before the intervention in the autumn of 2005, prevalence of infection of buffaloes (1,131) was 12.5% (95% CI = 10.5–14.4%) in the study area. All these bovines were removed away from the reach of the Qiupu River in February 2006. Since the intervention, no buffaloes have been pastured in the bottomlands (snail habitats) of Qiupu River.
Prevalence of infection by age, sex, and year for the population.
Prevalence of infection by age and year is shown in Figure 3; clearly, human infection in the study area increased with age, showing a monotonic increase in the first 3 years. Most infected residents were 30 years of age and older, although individuals younger than 30 years of age were infrequently infected, especially when the infection rate was low.
Figure 3.
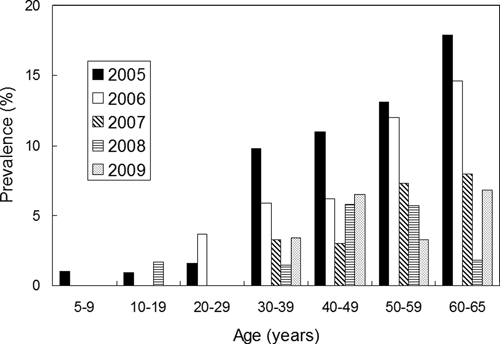
Human prevalence of infection by age and year.
Prevalence of infection by sex and year is shown in Figure 4. Infections were higher in males than in females before 2007, and the trend reversed after 2007, and infections in females began to rise after a 3-year continuous decrease. Nevertheless, there was no significant difference between male and female prevalence during the study period (P > 0.05).
Figure 4.
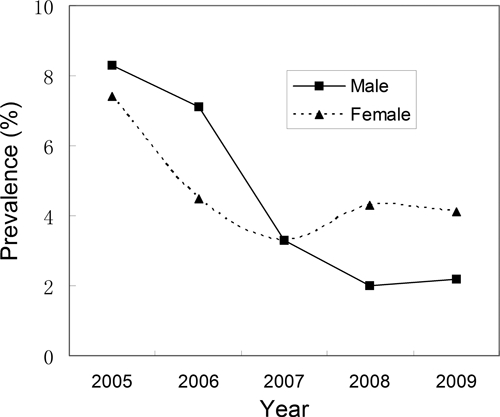
Human prevalence of infection by sex and year.
Human prevalence and intensity of infection.
The prevalence of S. japonicum infection, intensity of infection, mean age, and gender distribution of residents are summarized in Table 1. Mean age of residents detected rose year after year except for 2009, but these differences were not significant (P > 0.05). There were also no significant differences in infections between males and females during the study period (P > 0.05). The infection prevalence decreased significantly from 7.9% in 2005 to 3.1% in 2009, and declined by 60.8% (Wald χ2 = 31.03, P = 0.000). The geometric mean intensity of infection in the positives decreased gradually from 32.8 EPG in 2005 to 18.4 EPG in 2009, and declined by 43.9% (Wald χ2 = 202.51, P = 0.000).
Table 1.
Prevalence and intensity of Schistosoma japonicum infection from 2005 to 2009 years*
| Year | No. examined | Mean age (year) | Sex ratio (F/M) | Prevalence (%) | Adjusted prevalence (%)† | Mean EPG in the positives |
|---|---|---|---|---|---|---|
| 2005 | 798 | 34.0 (32.8–35.7) | 0.900 | 7.9 (6.0–9.8) | 12.2 (9.9–14.4) | 32.8 (26.1–41.2) |
| 2006 | 797 | 35.0 (33.7–36.2) | 1.028 | 5.8 (4.2–7.4) | 8.9 (6.9–10.9) | 21.1 (16.6–26.9) |
| 2007 | 792 | 36.8 (35.6–38.0) | 1.005 | 3.3 (2.1–4.5) | 5.1 (3.5–6.6) | 19.0 (13.3–27.2) |
| 2008 | 701 | 36.9 (35.6–38.2) | 0.997 | 3.1 (1.8–4.4) | 4.8 (3.2–6.3) | 17.4 (10.7–26.9) |
| 2009 | 797 | 35.1 (33.8–36.4) | 0.943 | 3.1 (1.8–4.3) | 4.8 (3.3–6.2) | 18.4 (13.4–25.4) |
EPG = eggs/gram of stool.
Prevalence was adjusted according to the sensitivity of 65% and specificity of 100% of the Kato-Katz method (95% confidence interval [CI] in parentheses).
Snail density and infection.
The snail infection (e.g., proportion of infection) decreased from 0.063% in 2005 to 0.00% in 2009 (χ2 = 14.6, P = 0.005). Densities of living snails and infected snails decreased over the study period (Wald χ2 = 305.33, P = 0.000 for living snails; Wald χ2 = 15.08, P = 0.000 for infected snails) except for 2006. There were significant correlations between the density of infected snails and the density of living snails (r = 0.975, P = 0.005), and prevalence (r = 1), and there was also a significant correlation between the density of living snails and prevalence (r = 0.975, P = 0.005) (Table 2).
Table 2.
Infection prevalence and density of infected snail Oncomelania hupensis from 2005 to 2009 years
| Year | Sample size | Density of living snails (living snail/0.11 m2) | Mortality of snails (%) | Density of infected snails (infected snail/0.11 m2) | Prevalence (%) |
|---|---|---|---|---|---|
| 2005 | 858 | 29.35 ± 1.253 | 1.33 | 0.019 ± 0.0045 | 0.063 |
| 2006 | 1063 | 4.05 ± 0.237 | 46.59 | 0.000 ± 0.0000 | 0.000 |
| 2007 | 779 | 19.93 ± 1.040 | 2.69 | 0.004 ± 0.0022 | 0.019 |
| 2008 | 808 | 13.90 ± 0.530 | 6.32 | 0.001 ± 0.0012 | 0.009 |
| 2009 | 903 | 8.61 ± 0.457 | 3.57 | 0.000 ± 0.0000 | 0.000 |
Model prediction.
Figure 5 shows a comparison of modeled human prevalence of infections, superimposed with field observations and adjusted human prevalence infections, over the 10-year period under the three control strategies. Overall modeling results from scenario B (e.g., actually implemented in our study area) shows a good agreement with the adjusted field observations (Figure 5). The human infections showed a sharp drop for the first year from 2005 to 2007, and then remarkably slowed down since 2007 with no change from 2008 to 2009, even with continuing interventions (Table 1), whereas our model prediction for Scenario B shows a continuous reduction (Figure 5). Not surprisingly, control strategies under scenarios B and C appear to have better control effects than that under Scenario A. The modeling results indicate that, under Strategy B, human prevalence infection will decrease to below 1% by 2010, and a similar goal can also be achieved 7 years later under Strategy C. If only chemotherapy strategy is implemented in both humans and bovines, human infection will remain around 3% even after 10 years of interventions.
Figure 5.
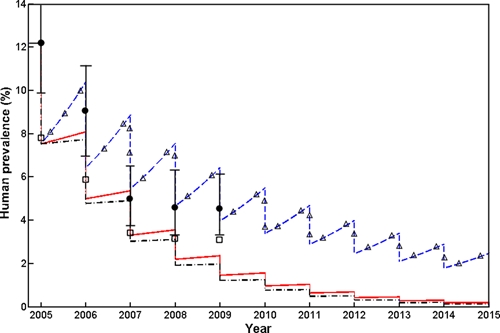
Effects of the integrated strategy on human prevalence. Three control scenarios are modeled here, scenario A (Δ - - Δ) represented concurrent chemotherapy of humans and bovines; Scenario B (_ . _. _.) was the strategy implemented in our study area including human chemotherapy, bovine removal, improvement in sanitation, health education, and environmental modification); Scenario C (_________) represented intervention programs implemented in other areas of China, which included human chemotherapy, bovine removal, improvement in sanitation, and health education. The hollow quadrates are actual “observed” human prevalence; the black dots (with 95% confidence interval [CI]) are the values of actual “observed” human prevalence adjusted according to the sensitivity (65%) and specificity (100%) of the Kato-Katz (KK) method.
Discussion
This 5-year longitudinal study showed that, under the integrated interventions, S. japonicum infections in humans and snails rapidly decreased in the first 2 years of interventions. The human prevalence and infection intensity declined by 60.8% and 43.9%, respectively, and no infected snails were found at the end of the study. These results were similar to the results obtained from all of Guichi district, where the human infection decreased by 68.1% from 2005 to 2008.14 Our results were also similar to findings from a study from two pilot villages of Jinxian County, Jiangxi province, where the same intervention strategies were implemented—the human infections declined by more than 75%, and the snail infection declined by 93–100%.13 The modeling results showed that the effects of control strategies B and C were apparently better than that of the A control approach, suggesting that the integrated control strategy implemented in our study area was effective, in agreement with a recent report based on the study in the same endemic region.13
There are no available uncontrolled villages for comparison in our study areas; the two national targets were to reduce the human infection rate to below 5% by 2008, and then to < 1% by 2015.13 In addition, it is incredibly costly to continue to survey communities over time. To offset such limitations, we used the modeling framework by Williams and others.23 to integrate the field data to explore the effects of three control strategies. The dynamic model was developed to model the transmission dynamics of schistosomiasis in China and had the flexibility to explore the control measures.25 The validity of this model is supported by some trial results.12,23,25 In the model, we integrated the epidemiological information obtained from our study area and modeled the effects of chemotherapy on human and bovines only on the transmission of the parasite in humans. The modeling result suggested that, with the continuing annual chemotherapy intervention strategy, human infections remain higher than 3% even after 10 years of non-stop interventions. This result was consistent with our previous findings in which the chemotherapy-based approach could only reduce the human infection rate to 1–6% but was unable to eliminate the transmission of the parasite.8 This result also further demonstrated the usefulness of mathematical models that could be used to inform and evaluate treatment, and even to assess treatment coverage in a community according to the epidemiological information from the community. Overall, the model's predictions were in agreement with our field observations (e.g., adjusted observations) for the first 2 years. Here, the model was used to examine the importance of bovine removal, which might be very difficult in many settings, for schistosomiasis transmission25; modeling results indicated a continuing decrease for the last 2 years' simulation, whereas the field observations showed stabilized human infections. The control effects achieved in the villages of our study were also not as good as that reported from the two pilot villages where the C control approach was implemented—the human infection rapidly decreased to below 1% within the same timeframe of intervention with the reduction by more than 75% compared with the beginning of the intervention.13
Our findings suggested that, in addition to humans and bovines, there might be other factors contributing to the transmission in our study villages that were not captured in this study. Bovines have been reported as a key reservoir for S. japonicum in the lake region and removal of bovines would significantly help to reduce human infections with regular chemotherapy12,25; nevertheless, the findings from our study reflected something different from this approach. Indeed, the zoonotic nature of S. japonicum renders control particularly challenging. It is estimated that 45 species of mammal are infected with S. japonicum in China.26 Relatively high rates of S. japonicum infection were observed in dogs and cats in the marshland regions of Anhui Province, together with a relatively high transmission index (eggs/day) estimate, and these estimates indicated that the role of dogs and cats should not be ignored in the marshland regions.27 Potentially important implications arise for current control criteria of S. japonicum in China,28 which is essentially based on S. japonicum infection in bovines and humans, rather than any current concern over other less agriculturally important livestock.27 However, further studies are needed to understand the importance of such animals in the transmission of the parasite. Moreover, the dilemma in the detection of S. japonicum infection in humans still remains.16,17,24 The KK method, a standard method for the detection and quantification of egg burdens in humans,19 has very poor sensitivity, especially when the intensity of infection is very low.16,17 In our study, the infection intensity in humans gradually reduced along with the decrease in human infection rate. Thus, humans with very mild infections, which become increasingly common as chemotherapy results in generally lower worm burdens in China, are often not detected during the community-wide surveys and therefore go untreated.8,16,17 In addition, population movement is very common in China,29 therefore the coverage of human treatment with praziquantel was lower, and that was supported by our model, in which only the coverage of 45% for infected humans was used. Other factors, such as ecologic changes in the environment, population movement, praziquantel treatment failures, and frequent flooding, may complicate the efforts to control schistosomiasis in our study region13,21; it is unclear whether transmission could be maintained by these previously mentioned factors in the absence of bovines in the marshland areas. To address this question further investigation is needed, and the long-term effectiveness and feasibility of the national integrated strategy should be also further evaluated.25
The assumptions embedded in our models were made on the basis of previous studies16,17,23,24,29 and the field information obtained from our study area. For example, the coverage of treatment with praziquantel was assumed to be 45% because of the low sensitivity (~65%) of the KK method16,17 and population migration (~30% total population)29; a reduction in snail density was assumed to be 40% according to Table 2. These assumptions were relevant in our study, but could possibly be adjusted for other settings to help these communities plan their own specific strategies tailored to local situations to maximize control effects.
Infections were higher in males than in females before 2007, and then the trend reversed after 2007. This gender “crossing” of prevalence indicated that the pattern of infection with S. japonicum might be changed because of bovine removal. However, further work is certainly needed to address this question.
The reduction in the rates and intensities of S. japonicum infection in humans was consistent with the reduction in the densities and rates of S. japonicum infection in snails, except for 2006. In 2006, the grasslands of our study area were burned before the snail survey. The fire resulted in a considerable reduction in the density of living snails and a considerable rise in the mortality of snails, and no infected snails were found. However, the effect of the fire on snails was not long term, and the density of living snails rapidly increased in the next year. Our findings suggested the environmental management was very effective for snail control, and could result in a persistent reduction in the density of living snails. However, the environmental management is limited by the floods. The environmental management is unprompted, and if the floods frequently occur and result in a bad harvest, this management will be stopped. In addition, the environmental management is not implemented in all of the snail habitats of our study areas, only in some suitable places for farm crop growth. No infected snails were found in 2009, this might be caused by the low sensitivity of the microscopy-based method, and with this microscopy method it is very difficult to find early infected snails.30 Recently, molecular tools such as polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) have shown capability of detecting the potential infection in the snails with early sporocysts, and show promise for monitoring the early infection rate in snails.30 To assess the long-term effectiveness of the national integrated strategy and maintain the success of this strategy, it may be necessary to use these new molecular tools (PCR or LAMP) to monitor snails in the future.
ACKNOWLEDGMENTS
We are very grateful to the staff at Guichi Anti-Schistosomiasis Station for their kind collaboration and making the field data available. We very much appreciate reviewers' comments and suggestions.
Footnotes
Financial support: This work received financial support from the National Natural Science Foundation of China (no. 30590374), the National High Technology Research and Development Program of China (no. 2006AA02Z402), the National S&T Major Program (grant no. 2008ZX10004-011), and Shanghai Leading Academic Discipline Project (project no. B118). SL is thankful for the support from the Public Health Preparedness for Infectious Disease (PHPID) of the Ohio State University.
Authors' addresses: Yi-Biao Zhou, Zhi-Jie Zhang, Jian-Guo Wei, Gen-Ming Zhao, and Qing-Wu Jiang, Department of Epidemiology, School of Public Health, Fudan University, Shanghai, People's Republic of China, and Key Laboratory of Public Health Safety, Fudan University, Ministry of Education, China, E-mails: z_yibiao@hotmail.com, epistat@gmail.com, jgwei@fudan.edu.cn, gmzhao@shmu.edu.cn, and jiangqw@fudan.edu.cn. Song Liang and Chris Rea, College of Public Health, The Ohio State University, Columbus, OH, E-mails: ehs.liang@gmail.com and chrislrea@gmail.com. Geng-Xin Chen and Zong-Gui He, Guichi Anti-Schistosomiasis Station, Anhui, China, E-mails: cgx5611@163.com and gcxfz@yahoo.com.cn.
Reprint requests: Yi-biao Zhou, Department of Epidemiology, School of Public Health, Fudan University, 138 Yi Xue Yuan Road, Shanghai 200032, People's Republic of China, E-mail: z_yibiao@hotmail.com.
References
- 1.Utzinger J, Bergquist R, Shu-Hua X, Singer BH, Tanner M. Sustainable schistosomiasis control–the way forward. Lancet. 2003;362:1932–1934. doi: 10.1016/S0140-6736(03)14968-9. [DOI] [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 3.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 4.Ross AG, Sleigh AC, Li Y, Davis GM, Williams GM, Jiang Z, Feng Z, McManus DP. Schistosomiasis in the People's Republic of China: prospects and challenges for the 21st Century. Clin Microbiol Rev. 2001;14:270–295. doi: 10.1128/CMR.14.2.270-295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou XN, Guo JG, Wu XH, Jiang QW, Zheng J, Dang H, Wang XH, Xu J, Zhu HQ, Wu GL, Li YS, Xu XJ, Chen HG, Wang TP, Zhu YC, Qiu DC, Dong XQ, Zhao GM, Zhang SJ, Zhao NQ, Xia G, Wang LY, Zhang SQ, Lin DD, Chen MG, Hao Y. Epidemiology of schistosomiasis in the People's Republic of China, 2004. Emerg Infect Dis. 2007;13:1470–1476. doi: 10.3201/eid1310.061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utzinger J, Zhou XN, Chen MG, Bergquist R. Conquering schistosomiasis in China: the long march. Acta Trop. 2005;96:69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 7.World Bank Loan Program Completion Report on Infectious and Endemic Disease Control Project . Schistosomiasis Control Component (1992–2001) Beijing, People's Republic of China: Department of Diseases Control and Foreign Loan Office, Ministry of Health, People's Republic of China; 2002. [Google Scholar]
- 8.Zhou YB, Zhao GM, Jiang QW. Effects of the praziquantel-based control of schistosomiasis japonica in China. Ann Trop Med Parasitol. 2007;101:695–703. doi: 10.1179/136485907X241488. [DOI] [PubMed] [Google Scholar]
- 9.Yuan HC, Guo JG, Bergquist R, Tanner M, Chen XY, Wang HZ. The 1992–1999 World Bank schistosomiasis research initiative in China: outcome and perspectives. Parasitol Int. 2000;49:195–207. doi: 10.1016/s1383-5769(00)00045-3. [DOI] [PubMed] [Google Scholar]
- 10.Yuan HC, Jiang QW, Zhao GM, He N. Achievements of schistosomiasis control in China. Mem Inst Oswaldo Cruz. 2002;97((Suppl 1)):187–189. doi: 10.1590/s0074-02762002000900036. [DOI] [PubMed] [Google Scholar]
- 11.Jiang QW, Wang LY, Guo JG, Chen MG, Zhou XN, Engels D. Morbidity control of schistosomiasis in China. Acta Trop. 2002;82:115–125. doi: 10.1016/s0001-706x(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 12.Guo JG, Li YS, Gray DJ, Ning A, Hu GH, Chen HG, Davis GM, Sleigh AC, Feng Z, McManus DP, Williams GM. A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People's Republic of China. Am J Trop Med Hyg. 2006;74:335–341. [PubMed] [Google Scholar]
- 13.Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, Wu XH, Wang XH, Wang LY, Xia G, Hao Y, Chin DP, Zhou XN. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 14.Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, Zhu SP, Zhang ZH, Steinmann P, Yang GJ, Wang SP, Wu ZD, Wang LY, Hao Y, Bergquist R, Utzinger J, Zhou XN. China's new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health. 2009;14:1475–1483. doi: 10.1111/j.1365-3156.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- 15.Department of Disease Control, Ministry of Health, China . Handbook of Schistosomiasis Control. Third edition. Shanghai: Shanghai Press on Science and Technology; 2000. Diagnosis of schistosomiasis. [Google Scholar]
- 16.Zhou YB, Yang MX, Wang QZ, Zhao GM, Wei JG, Peng WX, Jiang QW. Field comparison of immunodiagnostic and parasitological techniques for the detection of schistosomiasis japonica in the People's Republic of China. Am J Trop Med Hyg. 2007;76:1138–1143. [PubMed] [Google Scholar]
- 17.Zhou YB, Yang MX, Tao P, Jiang QL, Zhao GM, Wei JG, Jiang QW. A longitudinal study of comparison of the Kato-Katz technique and indirect hemagglutination assay (IHA) for the detection of schistosomiasis japonica in China, 2001–2006. Acta Trop. 2007;107:251–254. doi: 10.1016/j.actatropica.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . WHO Preventive Chemotherapy in Human Helminthiasis. Geneva: WHO; 2006. [Google Scholar]
- 19.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 20.Chen MG. Schistosomiasis control program in the People's Republic of China: a review. Southeast Asian J Trop Med Public Health. 1989;20:511–517. [PubMed] [Google Scholar]
- 21.Gray DJ, Williams GM, Li YS, Chen HG, Li RS, Forsyth SJ, Barnett AG, Guo JG, Feng Z, McManus DP. A cluster-randomized bovine intervention trial against Schistosoma japonicum in the People's Republic of China: design and baseline results. Am J Trop Med Hyg. 2007;77:866–874. [PMC free article] [PubMed] [Google Scholar]
- 22.Barbour AD. Modeling the transmission of schistosomiasis: an introductory view. Am J Trop Med Hyg. 1996;55((Suppl)):135–143. doi: 10.4269/ajtmh.1996.55.135. [DOI] [PubMed] [Google Scholar]
- 23.Williams GM, Sleigh AC, Li Y, Feng Z, Davis GM, Chen H, Ross AG, Bergquist R, McManus DP. Mathematical modeling of schistosomiasis japonica: comparison of control strategies in the People's Republic of China. Acta Trop. 2002;82:253–262. doi: 10.1016/s0001-706x(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 24.Yu JM, de Vlas SJ, Jiang QW, Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int. 2007;56:45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Gray DJ, Williams GM, Li Y, McManus DP. Transmission dynamics of Schistosoma japonicum in the lakes and marshlands of China. PLoS ONE. 2008;3:e4058. doi: 10.1371/journal.pone.0004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TP, Vang Johansen M, Zhang SQ, Wang FF, Wu WD, Zhang GH, Pan XP, Ju Y, Ørnbjerg N. Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui province, China. Acta Trop. 2005;96:198–204. doi: 10.1016/j.actatropica.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Lu DB, Wang TP, Rudge JW, Donnelly CA, Fang GR, Webster JP. Contrasting reservoirs for Schistosoma japonicum between marshland and hilly regions in Anhui, China–a two-year longitudinal parasitological survey. Parasitology. 2010;137:99–110. doi: 10.1017/S003118200999103X. [DOI] [PubMed] [Google Scholar]
- 28.Liang S, Yang C, Zhong B, Qiu D. Re-emerging schistosomiasis in hilly and mountainous areas of Sichuan, China. Bull World Health Organ. 2006;84:139–144. doi: 10.2471/blt.05.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen GX, Wang MS, Han SM. Analysis of schistosomiasis japonica epidemiology of migrant population in the endemic region of Guichi district. Chin J Schisto Control. 2001;13:102–103. [Google Scholar]
- 30.Kumagai T, Furushima-Shimogawara R, Ohmae H, Wang TP, Lu SH, Chen R, Wen LY, Ohta N. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) Assay. Am J Trop Med Hyg. 2010;83:542–548. doi: 10.4269/ajtmh.2010.10-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]


