Abstract
Ehrlichia chaffeensis causes human monocytic ehrlichiosis, and Anaplasma phagocytophilum causes human granulocytic anaplasmosis. These related tick-borne rickettsial organisms can cause severe and fatal illness. During 2000–2007, the reported incidence rate of E. chaffeensis increased from 0.80 to 3.0 cases/million persons/year. The case-fatality rate was 1.9%, and the hospitalization rate was 49%. During 2000–2007, the reported incidence of A. phagocytophilum increased from 1.4 to 3.0 cases/million persons/year. The case-fatality rate was 0.6%, and the hospitalization rate was 36%. Rates among female patients were lower than among male patients for ehrlichiosis (rate ratio = 0.68) and anaplasmosis (rate ratio = 0.70). Most (80%) ehrlichiosis and anaplasmosis cases met only a probable case definition, although, use of a polymerase chain reaction to confirm infections increased during 2000–2007. Heightened reporting of these diseases will likely continue with improving recognition, changing surveillance practices, and appropriate application of diagnostic assays.
Introduction
Ehrlichiosis and anaplasmosis are tick-borne illnesses caused by obligate intracellular bacteria of the genera Ehrlichia and Anaplasma, respectively. Although suspected cases were previously reported, the first case of human monocytic ehrlichiosis was documented in a patient at Fort Chaffee, Arkansas in 1991, and the causative agent was named Ehrlichia chaffeensis.1–3 Human granulocytic infections by Anaplasma were first reported in patients from Wisconsin and Minnesota in 1994.4–6 Originally classified under the genus Ehrlichia, the causative agent of human granulocytic ehrlichiosis was later determined to be the same agent as Ehrlichia phagocytophila and Ehrlichia equi. All three agents were reclassified under the species Anaplasma phagocytophilum in 2001.7 Ehrlichia ewingii was first identified as an agent of human disease from four residents of Missouri.8
Clinical presentations of ehrlichiosis and anaplasmosis are similar and nonspecific.9 Symptoms frequently include fever, chills, headache, myalgia, and nausea. Up to 30% of patients and 60% of children infected with E. chaffeensis have a rash; a rash is less commonly reported in A. phagocytophilum patients.10,11 Laboratory findings associated with ehrlichiosis and anaplasmosis include leukopenia, thrombocytopenia, elevated serum aminotransferase levels, and elevated creatinine levels.9–11 Patients treated with tetracycline typically recover quickly, and surveillance reports have indicated that the overall case-fatality rate was low.12,13 Complications can be severe and include adult respiratory distress syndrome, disseminated intravascular coagulopathy, central nervous system involvement, and renal failure.9,12
Human cases are associated with exposure to ticks. The Lone Star tick, Amblyomma americanum, maintains the enzootic cycle of E. chaffeensis primarily among white-tailed deer (Odocoileus virginianus).14–16 The intersection of the vector and the reservoir of E. chaffeensis occurs largely in the southeastern and south-central United States.17,18 Ehrlichia ewingii may be maintained in a similar enzootic cycle, with deer and domestic dogs proposed as possible reservoir species.19–21 Small mammals are the primary reservoir for A. phagocytophilum, and the vectors Ixodes scapularis (black-legged or deer tick) and Ixodes pacificus (western black-legged tick) maintain A. phagocytophilum prevalence primarily in the northeastern, the upper midwestern, and the Pacific coast regions.16,17,22,23 Previous surveillance summaries found most cases reported within these areas.12,13
In 2000, the Council of State and Territorial Epidemiologists (CSTE) published surveillance case definitions for infection by E. chaffeensis and A. phagocytophilum.24 A third reportable category for Undetermined, Unspecified, or Other Agent (UUOA) captured cases where available laboratory evidence was insufficient to specify the causative agent and cases where the causative agent was specified as an agent other than E. chaffeensis or A. phagocytophilum. These three surveillance case definitions were used until January 2008, when the CSTE implemented new case definitions.25 We summarize ehrlichiosis and anaplasmosis surveillance data reported to the Centers for Disease Control and Prevention (CDC) during 2000–2007.
Methods
National surveillance systems.
Individual state and territory health departments report surveillance data to CDC through the National Electronic Telecommunications System for Surveillance (NETSS) and through manually completed Case Report Forms (CRFs). NETSS captures demographics such as county of residence, sex, race group, Hispanic ethnicity, and age. These data are used to calculate reported incidence rates across demographics and time. Cases are reported as either probable or confirmed by the State or Local Public Health Departments. Reports from California do not differentiate between probable and confirmed cases.
The CRFs capture additional data on clinical presentation, clinical course, laboratory data, and patient outcome. These data from CRFs are used to calculate descriptive statistics supplementing the NETSS data. The quality of the data from both systems varies by state and locality. Clinicians must make accurate diagnoses, request appropriate laboratory assays, and report sufficient information before a case may be classified according to the CSTE definitions.
The data presented in this report differ from the data presented in the Morbidity and Mortality Weekly Report (MMWR) annual summaries; the MMWR lists cases by report date whereas this analysis uses date of onset when available. Also, the reportable category UUOA was not published in the MMWR during 2000–2004. However, these reported cases are included in this analysis.
Analytical and statistical methods.
Confirmed and probable cases reported through NETSS with an event date during 2000–2007 were used as the numerator for calculating reported incidence rates. Reported incidence was calculated as a rate per million persons per year (PY) by using population estimates from the United States Census Bureau for each county and for both sexes.26,27 Because of the large proportion of cases with missing race and ethnicity information in the NETSS data, comparisons of rates between races and ethnic groups were omitted. Cases reported through the CRFs were included in this analysis if the onset date was during 2000–2007 and the cases met confirmed or probable case definition. All analyses were performed using SAS® software.28 Because these results were calculated from all reported cases, the sampling fraction was one, and statistical tests of significance and confidence intervals were not included.
Reporting states.
States that did not consider the disease reportable for a given year were excluded from that year's analysis. During 2000–2007, E. chaffeensis and A. phagocytophilum were not reportable through NETSS for Alaska (2000–2001, 2005–2007), Colorado (2000–2003, 2005, 2007), Washington, DC (2000, 2003–2005, 2007), Hawaii (2000, 2006–2007), Iowa (2005–2007), Idaho (2000–2001, 2005–2007), Illinois (2000), Louisiana (2000–2001, 2003–2005), Maryland (2000–2001), Mississippi (2001, 2007), Montana (2000–2001, 2006–2007), North Dakota (2000–2005, 2007), New Mexico (2000–2001, 2007), Nevada (2000, 2005, 2007), Oregon (2000), Pennsylvania (2003–2004), Vermont (2000), and Washington (2006–2007).
During 2000, the UUOA category was reportable through NETSS only in New York. During 2001–2007, this category was not reportable for Alaska (2001, 2005–2007), Colorado (2001–2003, 2005, 2007), Washington, DC (2003–2006, 2007), Hawaii (2006–2007), Iowa (2005–2007), Idaho (2001, 2005–2007), Louisiana (2003–2005), Maryland (2001), Mississippi (2001, 2007), Montana (2001, 2006–2007), North Dakota (2001–2005, 2007), Nebraska (2007), New Jersey (2005–2006), New Mexico (2001, 2007), Nevada (2005, 2007), Pennsylvania (2003–2004), and Washington (2006–2007).
Results
Ehrlichia chaffeensis.
During 2000–2007, a total of 3,126 cases of E. chaffeensis were reported through NETSS (Table 1). The national reported incidence rate was 1.4 cases per million PY. Reported incidence rates by state (Table 2) and county (Figure 1A) indicate endemic disease in southeastern and south-central United States, especially in Central and Atlantic census regions. Reported incidence increased during the study period from 0.80 cases per million PY during 2000 to 3.0 cases per million PY during 2007 (Figure 2).
Table 1.
Demographics profiles and case classification for Ehrlichia chaffeensis, Anaplasma phagocytophilum, and undetermined, unknown or other agent (UUOA) as reported to the National Electronic Telecommunications System for Surveillance (NETSS) and Case Report Forms (CRFs), United States, 2000–2007
| NETSS | CRFs | |||||
|---|---|---|---|---|---|---|
| E. chaffeensis, n = 3,126, no. (%) | A. phagocytophilum, n = 4,271, no. (%) | UUOA, n = 824, no. (%) | E. chaffeensis, n = 1,206, no. (%) | A. phagocytophilum, n = 2,040, no. (%) | UUOA, n = 656, no. (%) | |
| Case classification | 3,122 (99.9) | 4,267 (99.9) | 814 (98.8) | |||
| Confirmed | 875 (28.0) | 1,307 (30.6) | 261 (31.1) | 240 (19.9) | 437 (21.4) | 113 (17.2) |
| Probable | 2,247 (72.0) | 2,960 (69.4) | 553 (67.9) | 966 (80.1) | 1,603 (78.6) | 543 (82.8) |
| Sex | 3,100 (99.2) | 4,123 (96.5) | 823 (99.9) | 1,189 (98.6) | 1,994 (97.7) | 656 (100) |
| Male | 1,820 (58.7) | 2,396 (58.1) | 475 (57.7) | 717 (60.3) | 1,205 (60.4) | 374 (57) |
| Female | 1,280 (41.3) | 1,727 (41.9) | 348 (42.3) | 472 (39.7) | 789 (39.6) | 282 (43) |
| Race | 2,340 (74.9) | 2,267 (53.1) | 711 (86.3) | 1,004 (83.3) | 1,084 (53.1) | 609 (92.8) |
| White | 2,201 (94.1) | 2,200 (97) | 682 (95.9) | 920 (91.6) | 1,051 (97) | 590 (96.9) |
| Black | 77 (3.3) | 29 (1.3) | 17 (2.4) | 31 (3.1) | 10 (0.9) | 7 (1.1) |
| American Indian* | 57 (2.4) | 21 (0.9) | 12 (1.7) | 53 (5.3) | 17 (1.6) | 5 (0.8) |
| Asian† | 5 (0.2) | 17 (0.7) | 0 (0) | 0 (0) | 6 (0.6) | 7 (1.1) |
| Ethnicity | 2,060 (65.9) | 1,598 (37.4) | 673 (81.7) | 899 (74.5) | 909 (44.6) | 585 (89.2) |
| Hispanic | 64 (3.1) | 59 (3.7) | 44 (6.5) | 19 (2.1) | 17 (1.9) | 35 (6) |
| Non-Hispanic | 1,996 (96.9) | 1,539 (96.3) | 629 (93.5) | 880 (97.9) | 892 (98.1) | 550 (94) |
| Age (years) | ||||||
| Mean | 50.1 | 52.2 | 50.5 | 46.8 | 49.4 | 50.4 |
| Median | 52 | 54 | 54 | 51 | 53 | 54 |
Or Alaskan Native.
Or Pacific Islander.
Table 2.
Cases and incidence rates for Ehrlichia chaffeensis, Anaplasma phagocytophilum, and undetermined, unknown, or other agent (UUOA) as reported to the National Electronic Telecommunications System for Surveillance, United States, 2000–2007
| Region | E. chaffeensis | A. phagocytophilum | UUOA | |||
|---|---|---|---|---|---|---|
| No. cases | Rate* | No. cases | Rate* | No. cases | Rate* | |
| New England | 167 | 1.5 | 1,006 | 8.9 | 26 | 0.3 |
| Connecticut | 2 | 0.1 | 362 | 13.1 | – | – |
| Massachusetts | 80 | 1.6 | 333 | 6.5 | 2 | < 0.1 |
| Maine | 9 | 0.9 | 31 | 3.0 | 2 | 0.2 |
| New Hampshire | 8 | 0.8 | 4 | 0.4 | 1 | 0.1 |
| Rhode Island | 67 | 7.9 | 273 | 32.1 | 21 | 2.8 |
| Vermont | 1 | 0.2 | 3 | 0.7 | – | – |
| Mid Atlantic | 650 | 2.2 | 1,351 | 4.6 | 34 | 0.1 |
| New Jersey | 222 | 3.2 | 152 | 2.2 | 1 | < 0.1 |
| New York | 424 | 2.8 | 1,189 | 7.7 | 17 | 0.1 |
| Pennsylvania | 4 | 0.1 | 10 | 0.1 | 16 | 0.3 |
| West north central | 641 | 4.5 | 1,303 | 9.1 | 63 | 0.5 |
| Iowa | – | – | 1 | 0.1 | – | – |
| Kansas | 7 | 0.3 | 3 | 0.1 | 1 | 0.1 |
| Minnesota | 109 | 2.7 | 1,223 | 30.2 | 1 | < 0.1 |
| Missouri | 523 | 11.4 | 71 | 1.5 | 60 | 1.5 |
| North Dakota | – | – | – | – | – | – |
| Nebraska | 2 | 0.1 | 5 | 0.4 | 1 | 0.1 |
| South Dakota | – | – | – | – | – | – |
| East north central | 117 | 0.3 | 392 | 1.1 | 479 | 1.5 |
| Illinois | 77 | 0.9 | 18 | 0.2 | 28 | 0.3 |
| Indiana | 11 | 0.2 | 2 | < 0.1 | 1 | < 0.1 |
| Michigan | 3 | < 0.1 | 3 | < 0.1 | 2 | < 0.1 |
| Ohio | 16 | 0.2 | 9 | 0.1 | 1 | < 0.1 |
| Wisconsin | 10 | 0.2 | 360 | 8.2 | 447 | 11.6 |
| South Atlantic | 708 | 1.7 | 121 | 0.3 | 112 | 0.3 |
| Washington, DC | – | – | – | – | – | – |
| Delaware | 38 | 5.8 | 24 | 3.6 | – | – |
| Florida | 61 | 0.4 | 13 | 0.1 | – | – |
| Georgia | 78 | 1.1 | 7 | 0.1 | 1 | < 0.1 |
| Maryland | 229 | 6.9 | 33 | 1.0 | 59 | 1.8 |
| North Carolina | 237 | 3.5 | 24 | 0.4 | 12 | 0.2 |
| South Carolina | 17 | 0.5 | 10 | 0.3 | 7 | 0.2 |
| Virginia | 45 | 0.8 | 10 | 0.2 | 33 | 0.6 |
| West Virginia | 3 | 0.2 | – | – | – | – |
| East south central | 259 | 1.9 | 25 | 0.2 | 27 | 0.2 |
| Alabama | 22 | 0.6 | 11 | 0.3 | 2 | 0.1 |
| Kentucky | 24 | 0.7 | 1 | < 0.1 | 3 | 0.1 |
| Mississippi | – | – | – | – | – | – |
| Tennessee | 213 | 4.5 | 13 | 0.3 | 22 | 0.5 |
| West south central | 577 | 2.4 | 64 | 0.3 | 72 | 0.3 |
| Arkansas | 225 | 10.3 | 18 | 0.8 | 17 | 0.9 |
| Louisiana | 1 | 0.1 | – | – | 1 | 0.1 |
| Oklahoma | 338 | 12 | 44 | 1.6 | 1 | < 0.1 |
| Texas | 13 | 0.1 | 2 | < 0.1 | 53 | 0.3 |
| Mountain | 3 | < 0.1 | 2 | < 0.1 | 1 | < 0.1 |
| Arizona | 1 | < 0.1 | 1 | < 0.1 | – | – |
| Colorado | – | – | – | – | – | – |
| Idaho | – | – | – | – | – | – |
| Montana | – | – | – | – | – | – |
| New Mexico | 1 | 0.1 | – | – | – | – |
| Nevada | 1 | 0.1 | 1 | 0.1 | – | – |
| Utah | – | – | – | – | – | – |
| Wyoming | – | – | – | – | 1 | 0.3 |
| Pacific | 4 | < 0.1 | 7 | < 0.1 | 10 | < 0.1 |
| Alaska | – | – | – | – | – | – |
| California | 4 | < 0.1 | 4 | < 0.1 | 10 | < 0.1 |
| Hawaii | – | – | – | – | – | – |
| Oregon | – | – | 3 | 0.1 | – | – |
| Washington | – | – | – | – | – | – |
Incidence rate per million persons per year.
Figure 1.
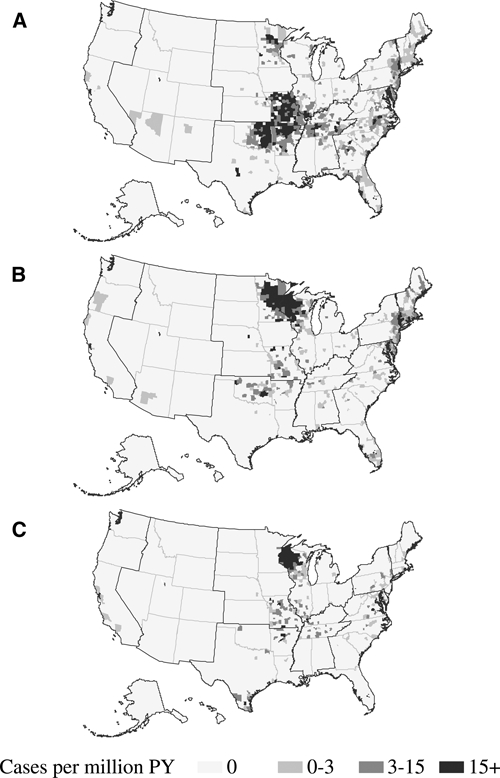
County level incidence rates of A, Ehrlichia chaffeensis (n = 3,116), B, Anaplasma phagocytophilum (n = 4,116), and C, undetermined, unspecified, or other agent (n = 819) as reported to the National Electronic Telecommunications System for Surveillance, United States, 2000–2007. Incidence rates are cases per million persons per year.
Figure 2.
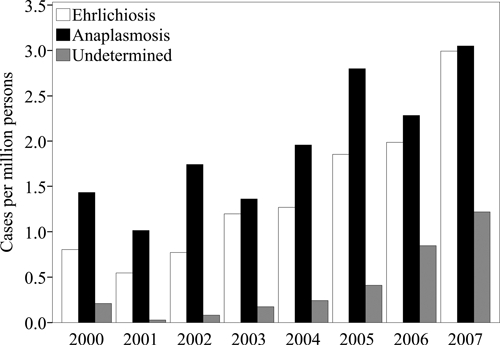
Annual incidence rates of Ehrlichia chaffeensis (n = 3,126), Anaplasma phagocytophilum (n = 4,271), and undetermined, unspecified, or other agent (n = 824) as reported to the National Electronic Telecommunications System for Surveillance, United States, 2000–2007. Incidence rates are cases per million persons per year.
Reported rates among female patients (rate ratio [RR] = 0.68) were lower than among male patients. Reported incidence rates increased with age (Figure 3), and rates among persons 60–69 years of age (RR = 2.4) were the highest compared with the entire population. Reported cases were primarily among persons of white race and non-Hispanic ethnicity (Table 1). Of the 2,365 reported cases with a date for onset of symptoms (Figure 4), reporting peaked in the summer months from June through August (n = 1,458, 62%), and the fewest cases were reported during the winter months of December, January, or February (n = 75, 3.2%).
Figure 3.
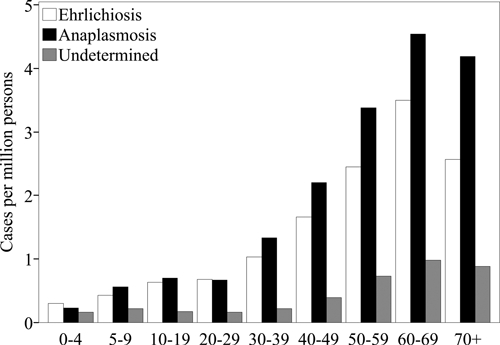
Incidence rates by age group of Ehrlichia chaffeensis (n = 3,103), Anaplasma phagocytophilum (n = 4,135), and undetermined, unspecified, or other agent (n = 782) as reported to the National Electronic Telecommunications System for Surveillance, United States, 2000–2007. Incidence rates are cases per million persons per year.
Figure 4.
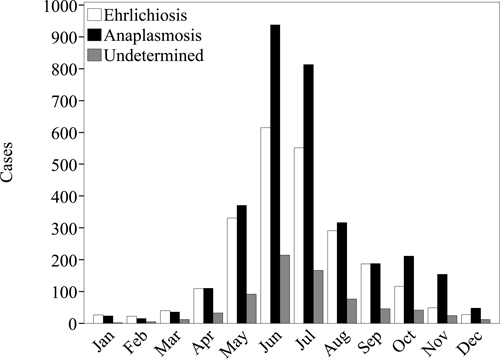
Case counts by month of onset of Ehrlichia chaffeensis (n = 2,365), Anaplasma phagocytophilum (n = 3,224), and undetermined, unspecified, or other agent (n = 723) as reported to the National Electronic Telecommunications System for Surveillance, United States, 2000–2007.
During the same study period 1,206 cases of E. chaffeensis were reported to CDC via CRFs (Table 1), of which 240 (20%) were classified as confirmed cases. Among cases reporting hospitalization status (Table 3), 570 (49%) were hospitalized during the course of illness. A higher proportion was hospitalized among persons 60–69 years of age (54%) and persons ≥ 70 years of age (71%) than among other age groups (Figure 5). Of the cases reporting clinical outcome (Table 3), there were 19 fatal cases (1.9%). Case-fatality rates were higher among persons 5–9 years of age (3.7%), 60–69 years of age (2.5%) and ≥ 70 years of age (3.5%) than among other age groups. The median time between onset and death was 11 days (range = 7–32 days). There were 113 cases (9.2%) for which life-threatening complications were reported (Table 4).
Table 3.
Hospitalization and outcome for cases of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and undetermined, unknown, or other agent (UUOA) as reported through Case Report Forms, United States, 2000–2007
| Characteristic | E. chaffeensis, n = 1,206, no. (%) | A. phagocytophilum, n = 2,040, no. (%) | UUOA, n = 656, no. (%) |
|---|---|---|---|
| Reporting | |||
| Hospitalization | 1,173 (97.3) | 1,907 (93.5) | 638 (97.3) |
| Hospitalized | 570 (48.6) | 687 (36.0) | 225 (35.3) |
| Confirmed | 169 (29.6) | 163 (23.7) | 51 (22.7) |
| Probable | 401 (70.4) | 524 (76.3) | 174 (77.3) |
| Not hospitalized | 603 (51.4) | 1220 (64.0) | 413 (64.7) |
| Confirmed | 63 (10.4) | 218 (17.9) | 55 (13.3) |
| Probable | 540 (89.6) | 1,002 (82.1) | 358 (86.7) |
| Reporting | |||
| Outcome | 1,027 (85.2) | 1,921 (94.2) | 642 (97.9) |
| Died | 19 (1.9) | 11 (0.6) | 3 (0.5) |
| Confirmed | 8 (42.1) | 2 (18.2) | 0 (0) |
| Probable | 11 (57.9) | 9 (81.8) | 3 (100) |
| Survived | 1,008 (98.1) | 1,910 (99.4) | 639 (99.5) |
| Confirmed | 215 (21.3) | 380 (19.9) | 109 (17.1) |
| Probable | 793 (78.7) | 1,530 (80.1) | 530 (82.9) |
Figure 5.
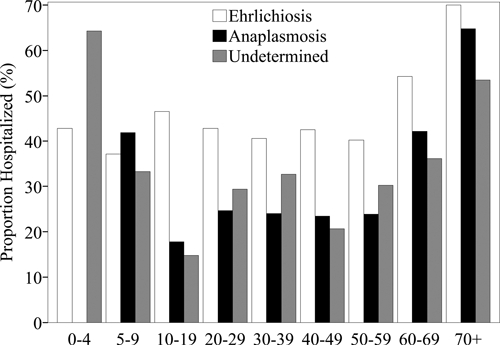
Proportion of cases hospitalized by age group of Ehrlichia chaffeensis (n = 1,141), Anaplasma phagocytophilum (n = 1,827), and undetermined, unspecified, or other agent (n = 630) as reported through Case Report Forms, United States, 2000–2007.
Table 4.
Reports of life-threatening complications during the clinical course of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and undetermined, unknown, or other agent (UUOA) as reported through Case Report Forms, United States, 2000–2007*
| Complication | E. chaffeensis, n = 113, no. (%) | A. phagocytophilum, n = 60, no. (%) | UUOA, n = 51, no. (%) |
|---|---|---|---|
| ARDS† | 20 (18.2) | 8 (13.3) | 7 (13.7) |
| DIC‡ | 14 (12.7) | 2 (3.3) | 4 (7.8) |
| Meningitis/encephalitis | 32 (29.1) | 5 (8.3) | 14 (27.5) |
| Renal failure | 34 (30.9) | 12 (20) | 14 (27.5) |
| Other | 48 (43.6) | 37 (61.7) | 27 (52.9) |
Percentages are based on the proportion of patients reported with any life-threatening illness. Overall reports of life-threatening illness were low, and details regarding how a specific diagnosis (such as DIC or ARDS) was made were not acquired. Data include confirmed and probable cases.
ARDS = adult respiratory distress syndrome.
DIC = disseminated intravascular coagulopathy.
Among those reporting immune status (n = 742), 89 cases (12%) reported immunosuppressive conditions, including 17 cases (2.3%) with cancer, 12 cases (1.6%) with diabetes, 8 cases (1%) with arthritis, and 5 cases (0.7%) with a history of organ transplantation. The median age of those with an immunosuppressive condition was 58 years, and the median age among immunocompetent persons was 53 years. The clinical course was worse for immunosuppressed cases relative to immunocompetent cases when the outcomes hospitalization (RR = 1.8), a life-threatening complication (RR = 2.5), and death (RR = 3.7) were used.
Most E. chaffeensis cases were diagnosed by using indirect immunofluorescence assays (IFAs) specific for IgG (n = 880, 73%). However, only a small number of these serologic diagnoses (n = 97, 11%) demonstrated seroconversion and were classified as confirmed cases. A positive, acute-phase serologic result was the only supporting laboratory evidence for 876 cases (73%). A total of 131 cases of E. chaffeensis cases (10.9%) were confirmed by polymerase chain reaction (PCR). The proportion of cases confirmed by PCR increased from less than 10% during 2000–2005 to 20% in 2007 (Figure 6A).
Figure 6.
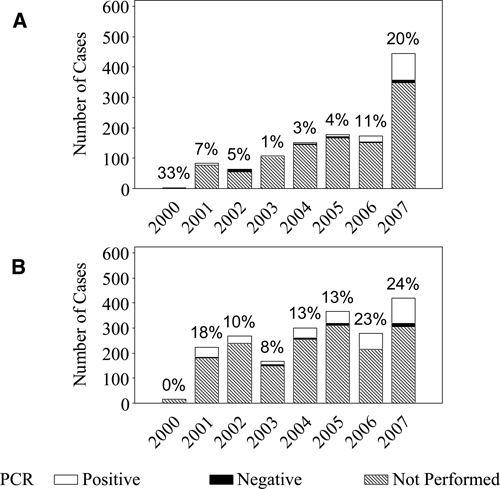
Number of annual cases of A, Ehrlichia chaffeensis (n = 1,191) and B, Anaplasma phagocytophilum (n = 2,035), reported through Case Report Forms (CRFs), United States, 2000–2007. The annual number of cases are subdivided into polymerase chain reaction (PCR)–positive cases, PCR-negative cases, and cases in which PCR was not performed. The annotation above each vertical bar is the annual percent of reported cases diagnosed by using PCR. Because the Centers for Disease Control and Prevention began collecting CRFs in 2000, few cases were reported in 2000.
Anaplasma phagocytophilum.
During 2000–2007, a total of 4,271 cases of A. phagocytophilum were reported through NETSS (Table 1). The national reported incidence rate was 2.0 cases per million PY. Reported incidence rates by state (Table 2) and county (Figure 1B) identified endemic disease transmission in the New England, mid Atlantic, and Western north central census regions. Reported incidence increased from 1.4 cases per million PY during 2000 to 3.0 cases per million during 2007 (Figure 2). Reported rates among female patients (RR = 0.70) were lower than among male patients. Reported incidence rates increased with age (Figure 3), and the highest rate was among persons 60–69 years of age (RR = 2.4). Most cases were reported as white race group and non-Hispanic ethnicity (Table 1). Of the 3,224 cases with a reported onset date (Figure 4), the largest number of cases (n = 2,068, 64%) were during the summer months of June through August, and 86 cases of anaplasmosis (2.7%) reported an onset during the winter months of December, January, or February.
During the study period 2,040 cases of A. phagocytophilum were reported to CDC via CRFs meeting the CSTE case definition (Table 1); 437 cases (21%) met the CSTE case definition for confirmed anaplasmosis. Of those reporting hospitalization statuses (Table 3), 687 cases (36%) were hospitalized. A higher proportion of cases were hospitalized among persons ≥ 70 years of age (65%), followed by persons 60–69 years of age (42%) and 5–9 years of age (42%) (Figure 5). Of the cases reporting a clinical outcome (Table 3), there were 11 fatal cases (0.6%). Case-fatality rates were highest among persons 20–39 years of age (1.2%). The median time between onset and death was 8.5 days (range = 2–36 days). Of those cases reporting complications, 60 (3.0%) reported developing life-threatening complications (Table 4).
Of the cases reporting immune status (n = 1,943), 79 (6.5%) reported immunosuppressive conditions, including 15 cases (0.77%) with cancer, 9 cases with diabetes (0.5%), 5 cases (0.2%) with arthritis, and 4 cases (0.2%) with asplenia. The median age among those reporting an immunosuppressive condition was 65.5 years, and the median age among immunocompetent persons was 54 years. The clinical course was worse for immunosuppressed cases relative to immunocompetent cases when the outcomes hospitalization (RR = 1.9), a life-threatening complication (RR = 2.8), and death (RR = 3.9) were used.
A total of 1,080 cases (53%) relied on a single positive serologic test result for supporting laboratory evidence. Most A. phagocytophilum cases were reported with only IFA IgG (n = 1,068, 52%) as supporting laboratory evidence. However, only a small number of these serologic diagnoses (n = 89, 8.3%) demonstrated seroconversion on IFA and were classified as confirmed cases. A small proportion of A. phagocytophilum cases (n = 334, 16%) were confirmed by means of PCR. However, the proportion of PCR-positive cases increased in 2006 and 2007 (Figure 6B).
Undetermined, unspecified, or other agent.
During 2000–2007 a total of 824 cases of UUOA were reported through the NETSS (Table 1). Although this category included possible reports of E. ewingii during the study period, this category was most frequently used by states to report cases for which the causative agent could not be definitively identified or for which a conclusive assay was not available. The national reported incidence of UUOA cases was 0.42 cases per million PY. Reported incidence rates of UUOA were highest in states where A. phagocytophilum was endemic during the same time period (Table 2, Figure 1C). Reporting under this category increased from 0.21 cases per million PY during 2000 up to 1.2 cases per million PY during 2007 (Figure 2). Reported incidence rates for the UUOA category increased with age similar to those for ehrlichiosis and anaplasmosis (Figure 3). Reported rates among female patients (RR = 0.71) were lower than among male patients. Cases were predominantly reported for the white race group and non-Hispanic ethnicity (Table 1). Of the 723 cases with reported onset dates (Figure 4), 456 cases (63%) became symptomatic during the summer months from June through August.
A total of 656 cases of UUOA were reported to CDC via CRFs (Table 1), including 113 (17%) that met a confirmed case definition. Among these cases of UUOA, nine cases (1%) of E. ewingii were reported from Missouri (n = 8) and Minnesota (n = 1). The single E. ewingii case reported from Minnesota was later determined to be a species of Ehrlichia distinct from E. ewingii and E. chaffeensis (McFadden JW and others, International Conference of Emerging Infectious Diseases, 2010). Among UUOA cases reporting hospitalization status (Table 3), 225 cases (35%) were hospitalized. The proportion hospitalized was highest among persons < 5 year of age (64%) and ≥ 70 years of age (53%) (Figure 5). Of the cases reporting clinical outcome (Table 3), there were 3 fatal cases (0.5%); all fatal cases were in adults ≥ 40 years of age. The median time between onset and death was 6 days (range = 5–7 days). Life-threatening complications were reported for 51 cases (7.8%) (Table 4).
Among those with reported immune status (n = 525), 71 cases (14%) reported some immunosuppressive condition, including 10 cases (1.9%) with diabetes, 8 cases (2%) with cancer, 5 cases (1%) with arthritis, and 5 cases (1%) with chronic obstructive pulmonary disease. The hospitalization RR comparing immunosuppressed cases and immunocompetent cases was 1.9, and the RR of life-threatening complications was 2.8. There were no deaths reported among immunosuppressed cases. A total of 278 cases (42%) were diagnosed by using only a single, positive laboratory serologic titer determined by IFA.
Discussion
Geography plays an important role in the epidemiology of ehrlichiosis and anaplasmosis infections. During 2000–2007, the Ozark Mountains and the Eastern Seaboard regions reported high incidence rates of E. chaffeensis infection. Similarly, the Northern Midwest and the Northern Atlantic Seaboard regions reported high incidence rates of A. phagocytophilum infection. These spatial trends are consistent with the epizoology of these pathogens, which are transmitted by distinct tick species with defined geographic ranges and host preferences.16 National surveillance for these diseases is important for several reasons: assessment of the overall burden of tick-borne disease as an important public health problem, detection of the emergence of novel related pathogens in unexpected areas, and monitoring for changes in human disease risk with the possible expansion of tick populations.
The annual incidence of reported ehrlichiosis and anaplasmosis increased during 2000–2007. The reported incidence of other tick-borne diseases, including Rocky Mountain spotted fever (RMSF) and Lyme disease, also increased during the same period, leading to speculation about possible ecologic or climatic changes resulting in expanding vector or reservoir ranges.14,29,30 However, numerous other factors likely influenced these observations. These factors include novel diagnostic assays, revised case definitions, increased physician awareness and reporting, and improved state and local surveillance for tick-borne diseases.30 Specifically, case definition changes in 2000 and 2001 and the publication of national guidelines for the diagnosis of tick-borne rickettsial diseases in 2006 may have broadly increased awareness and case recognition.16,24,25 During the study period, PCR of whole blood became more widely available as a sensitive and specific tool to aid the early diagnosis of ehrlichiosis and anaplasmosis (Holzbauer S and others, International Conference on Emerging Infectious Diseases, 2010). These changes may have improved reporting of cases through national surveillance systems.
Ehrlichiosis and anaplasmosis contribute substantially to the overall burden of tick-borne rickettsial diseases in the United States. The combined reported incidence rate for ehrlichiosis, anaplasmosis, and UUOA during 2000–2007 was more than 7.2 per million PY, which is similar to the reported incidence of RMSF in recent years.29 The hospitalization rate was high among reported cases of ehrlichiosis (49%) and anaplasmosis (36%) and was disproportionately greater among the elderly. During 2000–2007 the reported number of fatal outcomes associated with ehrlichiosis and anaplasmosis (n = 43) was similar to that reported for RMSF (n = 35), which has traditionally been considered a disease with a high potential for fatal outcome.29 Past studies have suggested that infections with E. chaffeensis are fatal 3% of the time, and fatal outcomes for infections with A. phagocytophilum are less frequently reported.12,13 Risk factors for severe or fatal outcome include immune compromise and older age: several accounts of ehrlichiosis and anaplasmosis among prior organ allograft recipients have been reported.31–36 In the current surveillance report, 9 patients reported by CRF had a history of organ transplant, and 239 reported other conditions associated with immune compromise, including cancer, diabetes, and arthritis.
Although not specifically captured as a risk factor in the current surveillance system, at least two anaplasmosis infections transmitted through blood transfusion have been reported, suggesting that blood product recipients are a group at potential risk for infection.37,38 Finally, delayed administration of doxycycline treatment is a significant risk factor for severe and fatal outcome in tick-borne rickettsial diseases.39 Although antibiotic administration information was not obtained as part of this study, we expect severe or fatal outcomes among patients for whom doxycycline treatment was initiated early in the course of illness to be rare.
The reported incidence of UUOA was 0.45 cases per million PY, and most cases reported under UUOA were cases of ehrlichiosis or anaplasmosis where the causative species was not differentiated. During the current surveillance period, 70% of UUOA cases were reported from Wisconsin, a state where A. phagocytophilum is endemic but E. chaffeensis and E. ewingii are rarely confirmed. Furthermore, the overall use of the UUOA reporting category in much of the country increased during the studied period. A likely explanation for this increase in use of the category UUOA is physician difficulty in ordering and interpreting appropriate tests to diagnose A. phagocytophilum. In 2001, the name human granulocytic ehrlichiosis shifted to human anaplamosis when the organism Ehrlichia phagocytophila was renamed Anaplasma phagocytophilum.7,16 We believe this name change led to confusion among healthcare providers about which diagnostic test to request in a given geographic area.
In areas where ehrlichiosis and anaplasmosis are endemic, discerning the etiologic agent requires testing for both. In these geographic areas, cases are ideally reported under UUOA when only a single test is ordered, although the stringency with which this recommendation has been applied during the current study period is not clear. In geographic areas where one agent predominates, lack of testing for the less common agent should not necessarily require a report of UUOA. Further confusing this issue, however, is the fact that a new Ehrlichia agent was identified in Wisconsin and Minnesota in 2009 (McFadden JW, International Conference on Emerging Infectious Disease 2010). The new agent appears similar to E. muris and may cross-react with assays for E. chaffeensis (CDC, unpublished data). The impact of this new agent on national surveillance programs is unknown, and recommended diagnostic testing strategies beyond a differential PCR have not yet been established.
Another factor complicating surveillance for these organisms is cross-reactivity among E. chaffeensis, E. ewingii, and A. phagocytophilum on serologic assays, making it difficult to interpret the results from inappropriate testing. Wisconsin used the UUOA category to report these poorly defined cases during 2000–2007 (Johnson D, Wisconsin Department of Health, unpublished data). Using an E. chaffeensis serologic test to diagnose the cause of illness in a patient residing in an A. phagocytophilum-endemic area increases the chance of false-negative results. Serologic cross-reactivity to E. chaffeensis can occur in up to half of A. phagocytophilum cases.40 Thus, consistent use of incorrect tests at the provider level will result in a substantial number of missed diagnoses and underestimate the true burden of disease.
Acquisition of appropriate documentation to confirm cases in accordance with the surveillance case definitions remains problematic for rickettsial diseases. Because of its availability and reliability, IFA is considered the gold standard for testing. However, its usefulness is limited unless paired serum samples are tested and an increase in titer (seroconversion) is documented. Patient convalescent-phase samples are not often obtained, yielding at best a probable case definition. In the current study, 2,234 reported ehrlichiosis, anaplasmosis, and UUOA cases (57%) were diagnosed as probable cases using only a single positive serum test result. Because antibodies may remain elevated for months or even years after infection, probable cases represent a group of patients for whom a definitive diagnosis of ehrlichiosis or anaplasmosis is less assured because past exposures can be identified as current cases. Further complicating the validity of a single serum sample is the fact that most cases may lack detectable antibody in the first 7–10 days of illness.41 Given the continued reliance of physicians in the United States on single serum samples for diagnosis (in this case, most reported cases were diagnosed by using a single serum sample), the true number of ehrlichiosis and anaplasmosis reports is significantly underestimated.
In this report, hospitalized and fatal cases are more likely to be reported as confirmed than less severe cases. We believe cases without a prompt convalescence offer more opportunity and motivation for acquiring diagnostic evidence. Case status lies on the causal pathway between severity of disease and clinical course. Although the variable is designed to capture the strength of supporting laboratory evidence, case status also captures the severity of disease.
Reported incidence rates for ehrlichiosis and anaplasmosis generally increased with age during the study period (Figure 3). Although this finding may reflect the true relationship between age and incidence, this increase may also be the result of an artifact of surveillance. The increasing seropositivity with age may be due to diagnosis of other nonspecific febrile illnesses as tick-borne rickettsial disease among older patients relative to younger patients.42–44 Conversely, the impact of immune status on the clinical course of illness indicates the importance of host factors, and age may also play an important role in the clinical course.
In this report, almost all cases reporting race and ethnicity were white and non-Hispanic (Table 1). However, a representative cross-sectional study found no association between race and ethnicity with E. chaffeensis seropositivity.44 Because the surveillance case definitions require supporting laboratory evidence, these results may be confounded by access to and use of diagnostic services.24,45,46 Similarly, missing data and misclassification of race and ethnicity in these surveillance data may significantly bias the results.47 Because these results relied on passive surveillance methods, the rate estimates presented here represent a minimum incidence. Similarly, these reports relied on patients, physicians, laboratories, State Public Health Departments, and the CDC for accurate reporting, and misclassification and missing data can lead to information bias of an unknown magnitude.
These results summarize the surveillance data reported using the CSTE case definitions during 2000–2007. New case definitions were implemented in 2008 for E. chaffeensis, A. phagocytophilum, and E. ewingii that explicitly name the etiologic agent should help minimize the confusion from prior taxonomic changes.25 However, surveillance trends may change again in response to these new case criteria. For future iterations of case definitions, specifying stricter requirements on the timing of acute-phase and convalescent-phase serologic analysis for laboratory confirmation of a case would help improve the quality of data obtained. We anticipate continually evolving case definitions will be needed to better define our understanding, recognition, and the reporting of these emerging tick-borne diseases.
ACKNOWLEDGMENTS
This project was possible because of the efforts from the National Center for Health Statistics, the National Center for Public Health Informatics, our partners at the State and Local Health Departments, and clinicians and laboratorians around the country. We are also grateful to Lindsey Pool for her continued assistance. Thanks to Arianne Folkema for her careful reading of our methods.
Footnotes
Financial support: This study was supported by the Oak Ridge Institute for Science and Education, the United States Department of Energy, and the Centers for Disease Control and Prevention.
Authors' address: F. Scott Dahlgren, Eric J. Mandel, John W. Krebs, Robert F. Massung, and Jennifer H. McQuiston, Division of Vectorborne Infectious Diseases, National Center for Enteric, Zoonotic, and Infectious Disease, Centers for Disease Control and Prevention, Atlanta, GA.
References
- 1.Maeda K, Markowitz N, Hawley RC, Ristic M, Cox D, Mcdade JE. Human infection with Ehrlichia canis, a leukocytic Rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 2.Dawson JE, Anderson BE, Fishbein DB, Sanchez JL, Goldsmith CS, Wilson KH, Duntley CW. Isolation and characterization of an Ehrlichia species from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BE, Dawson JE, Jones DC, Wilson KH. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumler JS, Bakken JS, Eckman MR, Vanetta LL, Chen SM, Walker DH. Human granulocytic ehrlichiosis: a new, potentially fatal tick-borne infection diagnosed by peripheral blood smear and PCR. Lab Invest. 1994;70:A126–A126. [Google Scholar]
- 5.Bakken JS, Dumler JS, Chen SM, Eckman MR, Vanetta LL, Walker DH. Human granulocytic ehrlichiosis in the upper midwest United States: a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 6.Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 8.Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikihisa Y, Unver A, Gaudreault-Keener R, Manian FA, Liddell AM, Schmulewitz N, Storch GA. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 9.Walker DH, Dumler JS. In: Douglas and Bennett's Principles and Practice of Infectious Diseases. Sixth Edition. Mandell GL, Bennet JE, Dolin R, editors. Volume 2. New York: Elsevier; 2005. pp. 2310–2318. (Ehrlichia chaffeensis (human monocytic ehrlichiosis), Anaplasma phagocytophilum (human granulocytic anaplasmosis), and other ehrlichieae). Mandell. [Google Scholar]
- 10.Bakken JS, Krueth J, Wilson-Nordskog C, Tilden RL, Asanovich K, Dumler JS. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 11.Eng TR, Harkess JR, Fishbein DB, Dawson JE, Greene CN, Redus MA, Satalowich FT. Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. JAMA. 1990;264:2251–2258. [PubMed] [Google Scholar]
- 12.Demma LJ, Holman RC, McQuiston JH, Krebs JW, Swerdlow DL. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001–2002. Am J Trop Med Hyg. 2005;73:400–409. [PubMed] [Google Scholar]
- 13.McQuiston JH, Paddock CD, Holman RC, Childs JE. The human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–642. doi: 10.3201/eid0505.990504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paddock CD, Yabsley MJ. Ecological havoc, the rise of White-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr Top Microbiol. 2007;315:289–324. doi: 10.1007/978-3-540-70962-6_12. [DOI] [PubMed] [Google Scholar]
- 15.Childs JE, Paddock CD. The Ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis – United States. MMWR Recomm Rep. 2006;55:1–27. [PubMed] [Google Scholar]
- 17.Walker DH, Barbour AG, Oliver JH, Lane RS, Dumler JS, Dennis DT, Persing DH, Azad AF, McSweegan E. Emerging bacterial zoonotic and vector-borne diseases. Ecological and epidemiological factors. JAMA. 1996;275:463–469. [PubMed] [Google Scholar]
- 18.Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, Dasch GA. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J Med Entomol. 2006;43:1261–1268. doi: 10.1603/0022-2585(2006)43[1261:poebar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Yabsley MJ, Varela AS, Tate CM, Dugan VG, Stallknecht DE, Little SE, Davidson WR. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus) Emerg Infect Dis. 2002;8:668–671. doi: 10.3201/eid0807.020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson BE, Greene CE, Jones DC, Dawson JE. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol. 1992;42:299–302. doi: 10.1099/00207713-42-2-299. [DOI] [PubMed] [Google Scholar]
- 21.Steiert JG, Gilfoy F. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector Borne Zoonotic Dis. 2002;2:53–60. doi: 10.1089/153036602321131841. [DOI] [PubMed] [Google Scholar]
- 22.Walls JJ, Greig B, Neitzel DF, Dumler JS. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:853–855. doi: 10.1128/jcm.35.4.853-855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and in Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- 24.Counsel of State and Territorial Epidemiologists Position Statements 2000 ID-03: Changes in the Case Definition for Human Ehrlichiosis, and Addition of a New Ehrlichiosis Category as a Condition Placed under Surveillance According to the National Public Health Surveillance System (NPHSS) 2000. http://www.cste.org/ps/2000/2000-id-03.htm Available at. Accessed December 8, 2009.
- 25.Counsel of State and Territorial Epidemiologists Position Statements 2007 ID-03: Revision of the National Surveillance Case Definition for Ehrlichiosis (Ehrlichiosis/Anaplasmosis) 2007. http://www.cste.org/ps/2007ps/2007psfinal/id/07-id-03.pdf Available at. Accessed December 8, 2009.
- 26.U.S. Census Bureau County Population, Population Change and Estimated Components of Population Change: April 1, 2000 to July 1, 2008. 2008. http://www.census.gov/popest/counties/files/CO-EST2008-ALLDATA.csv Available at. Accessed December 15, 2009.
- 27.U.S. Census Bureau Annual State Resident Population Estimates for 6 Race Groups (5 Race Alone Groups and One Group with Two or more Race Groups) by Age, Sex, and Hispanic Origin: April 1, 2000 to July 1, 2008. 2008. http://www.census.gov/popest/states/asrh/files/SC-EST2008-alldata6-ALL.csv Available at. Accessed November 20, 2009.
- 28.SAS Institute . SAS System for Windows [computer program] Cary, NC: SAS Institute; 2008. Version 9.2. [Google Scholar]
- 29.Openshaw JJ, Swerdlow DL, Krebs JW, Holman RC, Mandel E, Harvey A, Haberling D, Massung RF, McQuiston JH. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg. 2010;83:174–182. doi: 10.4269/ajtmh.2010.09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease – United States, 1992–2006. MMWR Surveill Summ. 2008;57:1–9. [PubMed] [Google Scholar]
- 31.Antony SJ, Dummer JS, Hunter E. Human ehrlichiosis in a liver transplant recipient. Transplantation. 1995;60:879–881. [PubMed] [Google Scholar]
- 32.Thomas LD, Hongo I, Bloch KC, Tang YW, Dummer S. Human ehrlichiosis in transplant recipients. Am J Transplant. 2007;7:1641–1647. doi: 10.1111/j.1600-6143.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 33.Tan HP, Dumler JS, Maley WR, Klein AS, Burdick JF, Poordad FF, Thuluvath PJ, Markowitz JS. Human monocytic ehrlichiosis: an emerging pathogen in transplantation. Transplantation. 2001;71:1678–1680. doi: 10.1097/00007890-200106150-00030. [DOI] [PubMed] [Google Scholar]
- 34.Adachi JA, Grimm EM, Johnson P, Uthman M, Kaplan B, Rakita RM. Human granulocytic ehrlichiosis in a renal transplant patient: case report and review of the literature. Transplantation. 1997;64:1139–1142. doi: 10.1097/00007890-199710270-00010. [DOI] [PubMed] [Google Scholar]
- 35.Trofe J, Reddy KS, Stratta RJ, Flax SD, Somerville KT, Alloway RR, Egidi MF, Shokouh-Amiri MH, Gaber AO. Human granulocytic ehrlichiosis in pancreas transplant recipients. Transpl Infect Dis. 2001;3:34–39. doi: 10.1034/j.1399-3062.2001.003001034.x. [DOI] [PubMed] [Google Scholar]
- 36.Sadikot R, Shaver MJ, Reeves WB. Ehrlichia chaffeensis in a renal transplant recipient. Am J Nephrol. 1999;19:674–676. doi: 10.1159/000013540. [DOI] [PubMed] [Google Scholar]
- 37.Eastlund T, Persing D, Mathiesen D, Kim D, Bieging J, McCann P, Heller G, Raynovic S. Human granulocytic ehrlichiosis after red cell transfusion. Transfusion. 1999;39:117s. [Google Scholar]
- 38.Centers for Disease Control and Prevention Anaplasma phagocytophilum transmitted through blood transfusion – Minnesota, 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1145–1148. [PubMed] [Google Scholar]
- 39.Holman RC, Paddock CD, Curns AT, Krebs JW, McQuiston JH, Childs JE. Analysis of risk factors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis. 2001;184:1437–1444. doi: 10.1086/324372. [DOI] [PubMed] [Google Scholar]
- 40.Comer JA, Nicholson WL, Olson JG, Childs JE. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J Clin Microbiol. 1999;37:558–564. doi: 10.1128/jcm.37.3.558-564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Childs JE, Sumner JW, Nicholson WL, Massung RF, Standaert SM, Paddock CD. Outcome of diagnostic tests using samples from patients with culture-proven human monocytic ehrlichiosis: implications for surveillance. J Clin Microbiol. 1999;37:2997–3000. doi: 10.1128/jcm.37.9.2997-3000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakken JS, Goellner P, Van Etten M, Boyle DZ, Swonger OL, Mattson S, Krueth J, Tilden RL, Asanovich K, Walls J, Dumler JS. Seroprevalence of human granulocytic ehrlichiosis among permanent residents of northwestern Wisconsin. Clin Infect Dis. 1998;27:1491–1496. doi: 10.1086/515048. [DOI] [PubMed] [Google Scholar]
- 43.Aguero-Rosenfeld ME, Donnarumma L, Zentmaier L, Jacob J, Frey M, Noto R, Carbonaro CA, Wormser GP. Seroprevalence of antibodies that react with Anaplasma phagocytophila, the agent of human granulocytic ehrlichiosis, in different populations in Westchester County, New York. J Clin Microbiol. 2002;40:2612–2615. doi: 10.1128/JCM.40.7.2612-2615.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall GS, Jacobs RF, Schutze GE, Paxton H, Buckingham SC, DeVincenzo JP, Jackson MA, San Joaquin VH, Standaert SM, Woods CR, Stu T-BIC. Ehrlichia chaffeensis seroprevalenee among children in the southeast and south-central regions of the United States. Arch Pediatr Adolesc Med. 2002;156:166–170. doi: 10.1001/archpedi.156.2.166. [DOI] [PubMed] [Google Scholar]
- 45.Escarce JJ, Epstein KR, Colby DC, Schwartz JS. Racial differences in the elderly's use of medical procedures and diagnostic tests. Am J Public Health. 1993;83:948–954. doi: 10.2105/ajph.83.7.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lieu TA, Newacheck PW, McManus MA. Race, ethnicity, and access to ambulatory care among US adolescents. Am J Public Health. 1993;83:960–965. doi: 10.2105/ajph.83.7.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hahn RA. The state of federal health statistics on racial and ethnic groups. JAMA. 1992;267:268–271. [PubMed] [Google Scholar]


