Abstract
Vaccinia virus (VACV) is the cause of bovine vaccinia (BV), an emerging zoonotic disease that affects dairy cows and milkers. Some chemical disinfectants have been used on farms affected by BV to disinfect cow teats and milkers' hands. To date, there is no information about the efficacy of disinfectants against VACV. Therefore, this study aimed to assess the virucidal activity of some active disinfectants commonly used in the field. Sodium hypochlorite, quaternary ammonium combined with chlorhexidine, and quaternary ammonium combined with glutaraldehyde were effective in inactivating the virus at all concentrations tested. Iodine and quaternary ammonium as the only active component were partially effective. The presence of bovine feces as organic matter and light decreased the effectiveness of sodium hypochlorite. These results show that an appropriated disinfection and asepsis of teats and hands may be helpful in the control and prevention of BV and other infections with VACV.
Introduction
Bovine vaccinia (BV) is an emerging zoonosis caused by Vaccinia virus (VACV), an orthopoxvirus. The disease is characterized by the appearance of lesions on the teats of cows and on the hands of milkers. This disease has serious veterinary, public health, and economic effects. In Brazil, VACV has been consistently identified as the causative agent of several outbreaks of BV in many states since the late 1990s.1–7 In addition to cattle and humans, in Brazil, VACV has also been isolated and/or identified in other species such as rodents and monkeys.8,9
One of the measures used to control and prevent this disease within the herd is the use of disinfectants and antiseptics on the teats and udders of cows. Both pre-dipping and post-dipping procedures are used, in addition to treating the hands of the milkers. The use of disinfectants reduces the viral load, which decreases the risk of disease transmission to humans and animals, thus decreasing the number of cases.6 Because it is easy to buy and handle, inexpensive, and has low toxicity to living tissue, bleach, whose active ingredient is sodium hypochlorite, is the most widely used disinfectant in the field. It is common to use a solution prepared from one part household bleach to three parts water to disinfect human hands and the teats and udders of dairy cows.
In addition to bleach, other disinfectants such as iodine, quaternary ammonium, and chlorhexidine have also been used on farms affected by the disease. Despite being a widespread practice, there are no data about the sensitivity of VACV to different disinfectants. The objective of this study was to evaluate the sensitivity of VACV to different disinfectants commonly used in the field. The titer of active chlorine in a 1:4 dilution of bleach subjected to conditions commonly encountered in the field, such as light and the presence of organic matter (bovine feces), was also examined.
Materials and methods
Cells.
Vero cells were obtained from the American Type Culture Collection (Manassas, VA) (catalog no. CCL-81). Cells were grown as described by Abrahão and others.10
Virus.
Vaccinia virus Guarani (VACV-GP2), which was isolated from lesions on the teats of a cow from an outbreak of BV in Guarani, Minas Gerais, Brazil in 2001, was used. It was previously characterized by using serologic and molecular procedures as VACV.3
Titration.
Assays were conducted to determine the titers of viral stocks and virus controls during the tests with disinfectants. They were also used to calculate reduction in the viral titer (original titer – final titer) in tests using disinfectants. All titration procedures were performed as described by Abrahão and others10 and Campos and Kroon.11
Calculation of reduction in viral titer.
Reduction in viral titers in response to disinfectants was represented as a percentage and a log10 value. The percentage of reduction in viral titer was calculated by using the formula12 % Reduction in viral titer = T before – T after × 100, where T before is the viral titer (plaque-forming units [PFU]/mL) in the no disinfectant virus control and T after is the average viral titer (PFU/mL) of eight samples after treatment with disinfectant. The SD was also calculated. The logarithmic reduction was calculated by using the formula Log reduction = T before (log10) – T after (log10).
Disinfectants.
Commercial disinfectants with the following active ingredients were tested: chlorine, polyvinylpyrrolidone, iodine, quaternary ammonium (benzalkonium chloride), glutaraldehyde, and chlorhexidine gluconate. The tested concentrations were recommended by the manufacturers on product labels according to its use as a disinfectant or an antiseptic (Table 1). All disinfectants were tested for 1, 5, and 30 minutes. Disinfectants and theirs recommended dilutions were selected if they contained active ingredients commonly used for disinfection of the teats during pre-dipping and post-dipping or for disinfection of surfaces in the dairy stables. The selected disinfectants, with the exception of the glutaraldehyde-based disinfectant, are also antiseptics if used at the dilutions recommended by the manufacturer.
Table 1.
Disinfectants used, active ingredients, and directions for use according to the manufacturer for inactivation of vaccinia virus*
| Product | Active ingredient | % Active ingredient in product | Recommended dilution | Indications | % Concentration of active ingredient in test |
|---|---|---|---|---|---|
| 1 | Active chlorine | 2 to 2.5 w/v | 1:4 | Dilution recommended for antisepsis of teats and milker's hands | 0.5 to 0.625 v/v active chlorine |
| 2 | Iodophor | 11.25 w/v | 1:250 | Disinfection and washing of udders of cows and milkers hands | 0.045 v/v iodophor |
| 1:1,200 | Washing and sterilization of equipment, tanks, cans, milk tanks, slaughterhouses and meatpackers | 0.009 v/v iodophor | |||
| 1:2,000 | Prevention of disease through drinking water of animals | 0.005 v/v iodophor | |||
| 3 | Quaternary ammonium (benzalkonium chloride) | 12.5 w/v | 1:500 | Disinfection of poultry environments such as warehouses, processing rooms of chickens and eggs, stables, milking parlors, hutches, pens, kennels and other animal housing | 0.025 v/v quaternary ammonium |
| 1:1,000 | External use in animals and disinfection of utensils, hands and equipment used for milking, artificial insemination and other uses at poultry industry, livestock, fish farms, and kennels | 0.0125 v/v quaternary ammonium | |||
| 4 | Quaternary ammonium | 30 w/v | 1:2000 | Spraying and disinfecting poultry litter, poultry, incubator chambers, trays, equipment in general, maternity, nursery, milking machines, udders, milker's hands, footbath | 0.015 v/v quaternary ammonium |
| 1:3000 | Disinfection in general (uniform, walls and floors), washing and asepsis of wounds and interdigital dermatitis | 0.01 v/v quaternary ammonium | |||
| 5 | Chlorhexidine gluconate | 0.2 w/v | 1:20 | Disinfection of instruments, machinery and agricultural and veterinary facilities | 0.01 v/v chlorhexidine gluconate |
| Quaternary ammonium | 0.5 p/v | 0.025 v/v quaternary ammonium | |||
| 6 | Glutaraldehyde | 10.625 v/v | 1:1,500 | Disinfection of equipment, tools, environment of dairy industry, fish and meat | 0.007 v/v |
| Quaternary ammonium (benzalkonium chloride) | 15 v/v | 0.01 v/v quaternary ammonium |
w/v = weight/volume; v/v = volume/volume; p/v = parts/volume.
Testing the effectiveness of disinfectants.
The protocol of the European Committee for Standardization (no. EN 14675, 2006) was used to determine the virucidal activity of disinfectants and antiseptics used in veterinary medicine.13 This test includes variables that simulate the conditions of product application in the field, such as the use of hard water to perform the dilutions of the disinfectants.
Briefly, eight parts of each disinfectant to be tested at the dilutions described in Table 1 and one part of hard water were added to viral suspensions containing 106 PFU/mL. At this stage, the concentration of disinfectant in the test was 1.25 times higher than recommended by the manufacturer to compensate for the dilution of the disinfectant during the test (80%). The mixture was kept at room temperature for 1, 5, and 30 minutes. After each period, each virus and disinfectant reaction was diluted 100-fold (10−2 dilution) in minimal essential medium and kept on ice until completion of the remaining dilutions up to 10−4. An aliquot of 300 μL of each dilution was inoculated into VACV-infected Vero cell monolayers in six-well plates. For each test, a negative control was performed by replacing the disinfectant with phosphate-buffered saline (PBS). The plates were incubated at 37°C and after 60 hours, the cells were fixed and stained with crystal violet solution. Evaluation of the effectiveness of the disinfectant was performed by comparing the viral titer in the control well with those in the test wells after the incubation period. Each concentration of disinfectant was tested in eight replicates. The disinfectant was considered effective if the viral titer was reduced by at least 4 log10 or 99.99% on viral titer reduction.13
The control of viral titers was also examined in the presence of PBS at the previously determined disinfectant concentrations. After 30 minutes, serial dilutions of PBS were made in minimal essential medium from 10−1 to 10−4. An aliquot (300 μL) of each dilution was inoculated into each well of the cell culture plate, and viral titers were determined as described.
Preparation of hard water.
Hard water, a solution of MgCl2, CaCl2, and NaHCO3, was prepared according to protocol EN14675: 2006.13 Water hardness was measured by the amount of CaCO3 in grams/liter or parts per million. In this test, the water contained CaCO3 at a concentration of 300 mg/L.
Control of cytotoxicity.
This test was designed to evaluate the possible cytotoxic effect of the disinfectants at the recommended dilutions. It was conducted as described,13 except for the replacement of the virus by PBS.
Determination of active chlorine in a 1:4 bleach dilution.
The amount of chlorine present in the 1:4 solution of bleach used in the tests was determined by using the iodometric method.14 Legislation defines the percentage of active chlorine in commercial bleach to range between 2.0% and 2.5%, with an acceptable range between 1.75% and 2.75% weight/weight.15 Although bleach labels indicate 2.0–2.5% active chlorine, this amount can be altered by the storage time. However, for the solution used in this test, it was expected that the concentrations would range from 0.5% to 0.625% active chlorine in the dilute solution, corresponding to 0.25% of the total concentration specified on the label.
Titration of active chlorine subjected to light and presence of organic matter.
The titrations were determined according to a published protocol.15 The test was repeated three times, and the mean and SD of concentrations of chlorine was determined in each titration. The titrations were performed at 48 hours (T1), 96 hours (T2), and 144 hours (T3) at the same time of the day.
To assess the influence of light and evaporation on the concentration of active chlorine, different storage containers were used. The solution was stored in an open 10-liter bucket or three properly closed two-liter bottles made of ethyl polyethylene (PET bottles). One bottle was green, one was transparent, and one was covered with aluminum foil before exposure to the environment.
Aliquots of the contaminated bleach stock solution were inoculated with 1%, 5%, 10%, and 20% solutions of bovine feces to evaluate the influence of organic matter in the concentration of active chlorine. These samples were stored at 4°C, after which titration of active chlorine was performed as described above.
Statistical design.
Each disinfectant was tested for two factors (concentration and time of action). Eight samples were taken for every possible combination further analyzed for each concentration of disinfectant. Thus, for all experiments, in addition to testing the main effect of the factors (concentration and time of action) and the interaction between them, the consistency of the replicates was also evaluated.
Analysis of the significance of the experiment was performed by using a generalized linear model composed of factors of interest (concentration and time of action). The main goal was to test the significance of these factors in reducing viral load and the possible effect of the interaction between them. The significance cutoff value was P < 0.05. The effect of the eight samples of each possible combination was tested. The alpha level of significance assumed for this analysis was 0.05 (5%).
Results
Sensitivity of VACV-GP2 to iodophor-based disinfectants.
Disinfectant 2, an iodophor-based compound with iodine as the active ingredient, did not reduce viral titers by at least 4 log10 (or 99.99%) at concentrations of 0.005% and 0.009%. However, at a concentration of 0.045% iodine, it reduced the viral titer by 100% after 1, 5, or 30 minutes (Figure 1). The initial viral titer was 2.9 × 105 PFU/mL.
Figure 1.
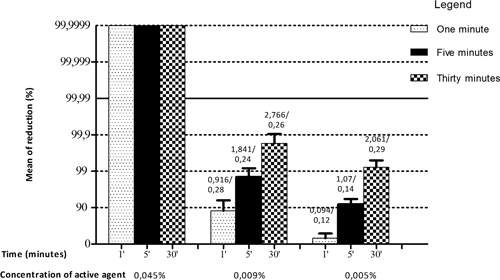
Sensitivity of Vaccinia virus VACV-GP2 to disinfectant 2. Shown are reduction of % mean and SD VACV-GP2 titer after treatment with disinfectant 2 at concentrations of 0.045%, 0.009%, and 0.005% of active agent. From a reduction of 99.99%, effectiveness of concentration of active ingredients was evaluated. Values above the bars represent average reduction of eight samples in log10/SD.
Sensitivity of VACV-GP2 to quaternary ammonium compound-based disinfectants.
Disinfectant 3, a 0.0125% quaternary ammonium solution, reduced the virus titer by 4 log10 (99.99%) only after 30 minutes of incubation. However, at a concentration of 0.025%, it reduced the viral titer by at least 4 log10 (99.99%) after 5 minutes (Figure 2). The initial virus titer was 2.1 × 106 PFU/mL.
Figure 2.
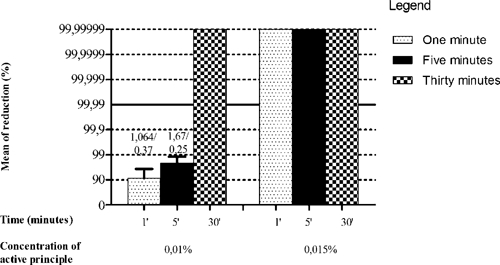
Sensitivity of Vaccinia virus VACV-GP2 to disinfectant 3. Shown are reduction of % mean and SD VACV-GP2 titer after treatment with disinfectant 3 at concentrations of 0.0125% and 0.025% of the active ingredient. From the reduction of 99.99%, effectiveness of concentration of active ingredients was evaluated. Values above the bars represent average reduction of eight samples in log10/SD.
Disinfectant 4 was a 0.015% quaternary ammonium solution that was effective against the virus at all times tested. At a concentration of 0.01%, this disinfectant reduced the viral titer by 100% after the maximum time tested (30 minutes). At a concentration of 0.01%, length of time of the treatment influenced the disinfectant effectiveness, and there was a progressively increased reduction in viral titer as the time of incubation increased. However, the maximal decrease was only achieved after 30 minutes (Figure 3).
Figure 3.
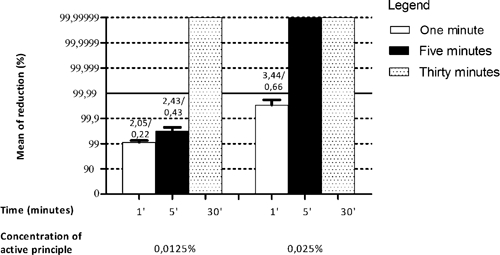
Sensitivity of Vaccinia virus VACV-GP2 to disinfectant 4. Shown are reduction of % mean and SD VACV-GP2 titer after treatment with disinfectant 4 at concentrations of 0.01% and 0.015% of the active ingredient. From the reduction of 99.99%, effectiveness of concentration of active ingredients was evaluated. Values above the bars represent average reduction of eight samples in log10/SD.
Sensitivity of VACV-GP2 to a chlorhexidine gluconate and quaternary ammonium–based disinfectant.
Disinfectant 5, which contained 0.01% chlorhexidine and 0.025% quaternary ammonium, was completely effective against the virus at 1, 5 and 30 minutes.
Sensitivity of VACV to a glutaraldehyde and quaternary ammonium–based disinfectant.
Disinfectant 6, which contained 0.007% glutaraldehyde and 0.01% quaternary ammonium, was effective against VACV at 1, 5, and 30 minutes. The initial titer of the virus was 5.61 × 105 PFU/mL.
Sensitivity of VACV-GP2 to bleach at a 1:4 dilution.
Bleach at 1:4 dilution (0.636% active chlorine) was able to completely reduce the viral titer after 1, 5, and 30 minutes of treatment. No cytotoxic effect was observed on the cell monolayer in control wells. The initial titer of the virus was 5.61 × 105 PFU/mL.
Concentration of active chlorine in a bleach solution in the presence of light.
The average value of three chlorine measurements in a 1:4 solution of bleach through the iodometric method was 0.636% volume/volume (SD = 0.075). If one considers the dilution factor of the bleach, this concentration should be between 0.5% and 0.625%. However, the amount of chlorine measured was higher than the minimum recommended.15
The iodometric titration showed that the concentration of active chlorine in the bleach solution in the bottles and bucket decreased after 48 hours of exposure (T1) even without the presence of organic matter, as shown in Figure 4. However, the bleach solution kept in the transparent PET bottles showed a greater decrease compared with the other containers. After 96 hours (T2 and T3), the comparative decrease in the concentration of active chlorine in the solution kept at the green and transparent PET bottles became more evident than at T1. The concentration of active chlorine in the bucket had a slight decrease.
Figure 4.
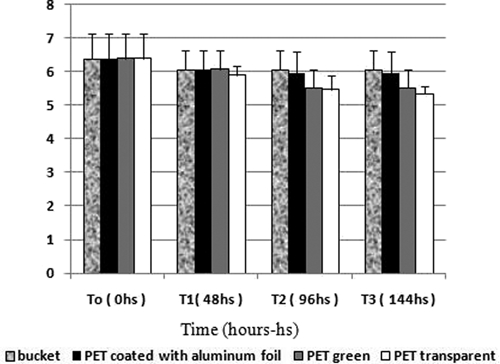
Concentration of active chlorine in a solution of sodium hypochlorite (bleach) in the presence of light.
Concentration of active chlorine in a bleach solution in the presence of organic matter (bovine feces).
Iodometric titration of the bleach solution in the presence of 1%, 5%, 10%, and 20% organic matter (bovine feces) demonstrated a decrease in the levels of active chlorine at the moment of the dilution (T0). However, at feces concentrations of 5%, 10%, and 20%, the reduction of active chlorine was more intense (Figure 5). Analysis over time (T1, T2, and T3) showed that the decrease in active chlorine was progressive and was time and concentration related.
Figure 5.
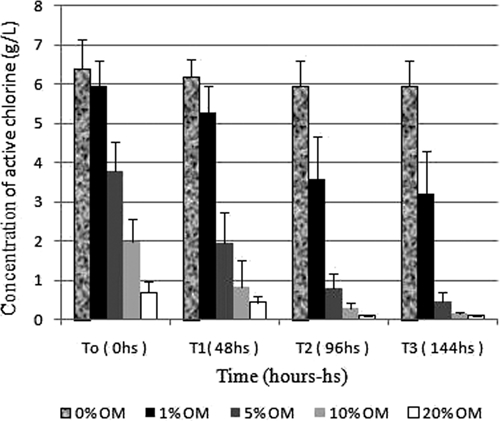
Concentration of active chlorine in a solution of sodium hypochlorite (bleach) in the presence of organic matter (bovine feces). OM = organic matter.
As shown in Figures 4 and 5, the reduction in the amount of active chlorine in the prepared solutions was significantly higher in the presence of organic matter compared with solutions exposed to light or the environment during the same period. Moreover, the higher the concentration of feces and the longer the exposure time, the greater the decrease in chlorine (Figure 5). All solutions shown in Figure 4 maintained levels of active chlorine up to 5 g/L during the seven-day test period. As shown in Figure 5, the solution that contained 1% feces maintained a sufficient amount of active chlorine for only 24 hours. The other concentrations of feces reduced the levels of active chlorine to less than 5 g/L at the time of the dilution (T0).
Discussion
In this study, disinfectant 1, whose active ingredient was sodium hypochlorite at the 1:4 dilution commonly used in the field, was able to prevent viral multiplication after 1-, 5-, and 30-minute exposures. This bleach dilution, containing approximately 5,000 parts per million or 0.5% active chlorine, has been used to control BV in the field. This concentration is sufficient to inactivate most enveloped viruses, including VACV.16 Although the concentration of chlorine in the bleach solution used in this experiment was somewhat higher than that prescribed by law, this average reflects only one batch from one particular brand of bleach. However, the 1:4 dilution can still be safely used as a hand or teat antiseptic to control the spread of BV.
Tanabe and Hotta evaluated the effectiveness of disinfectants against smallpox virus (orthopoxvirus).17 Among the tested disinfectants, sodium hypochlorite was the most effective, reducing 100% of the initial viral titer at the concentration of 0.1%. The hypochlorite acts against viruses by disrupting viral capsid and nucleic acid.18
Disinfectant 2, which contained 0.045% iodine as the active ingredient, was effective against VACV-GP2, and this concentration is recommended by the manufacturer for use as a hand or cow udder antiseptic (Table 1). However, use of Disinfectant 2, which at a concentration of 0.009% is recommended for use in disinfection of tanks and other dairy milking equipment, was not effective in inactivating the virus and therefore should not be used for this purpose.
The Centers for Disease Control and Prevention (Atlanta, GA) recommends using 0.0075% iodine for 10 minutes to disinfect surfaces that contain smallpox virus. However, this concentration should be increased when the surfaces vary or if higher disinfection is desired, such as in hospital environments.19 In this study, 0.009% iodine, which is higher than the concentration recommended by the Centers for Disease Control and Prevention, was not effective in the inactivation of VACV-GP2.
Disinfectant 3, which contained 0.025% quaternary ammonium, was not effective against VACV after a one-minute incubation. However, the same concentration of quaternary ammonium was able to reduce the viral titer by at least 4 log10 of the initial titer after 5 minutes and 30 minutes. Tanabe and Hotta evaluated the effectiveness of disinfectants against the smallpox virus in cell cultures.17 They found that 0.001% and 0.025% quaternary ammonium were not effective disinfectants at 1, 3, 5, and 10 minutes. In the present study, 0.025% quaternary ammonium was not effective against VACV after a one-minute incubation.
Disinfectant 4, which contained 0.015% quaternary ammonium, reduced the viral titer by at least 4 log10 at all times tested. However, the 0.025% concentration of the same active component in disinfectant 3, which is higher than that in disinfectant 4, was not effective. The fact that better results were obtained with disinfectant 4 at lower concentrations of active ingredients may be attributed to the addition of a surfactant in its formulation (polyethylene oxide). Although this agent has no virucidal action, it is a nonionic surfactant with detergent properties.
Disinfectant 5, which contained 0.01% chlorhexidine and 0.025% quaternary ammonium, was completely effective against the virus at 1, 5, and 30 minutes. McDonnell and Russell reported that chlorhexidine is more efficient against enveloped viruses than against non-enveloped viruses, and it works by disrupting the lipid chains of the viral envelope.16 Although the 0.05% concentration tested in this experiment is recommended by the manufacturer for cleaning agricultural machinery and facilities, it can also be used for disinfection of teats because this concentration is not toxic to tissues. Jones highlights the ability of 0.55% chlorhexidine to form a protective barrier on the teats, which protects them more effectively than treatment with 1% iodophor.20
Disinfectant 6, which contained 0.007% glutaraldehyde and 0.01% quaternary ammonium, was effective against VACV at all tested times. Testing the efficiency of 2% glutaraldehyde against VACV in dermal wounds in rabbits, Schümann and Grossgebauer found that it was effective only when the scabs were treated for 90 minutes with the disinfectant at this high concentration.21 Although its use is restricted to disinfecting surfaces and footbaths, and it cannot be directly used on living tissues, glutaraldehyde is recommended because of its broad spectrum of action, which includes enveloped and non-enveloped viruses.16
In the present study, there was a higher reduction in the concentration of active chlorine in sodium hypochlorite solutions in PET bottles (transparent and green), which enables greater light penetration (Figure 4). The mechanism of reduction of active chlorine in bleach solutions by ultraviolet light has not yet been elucidated.18
The presence of organic matter, especially in higher concentrations and for longer exposure times, tended to reduce the concentration of potentially active chlorine (Figure 5). Organic matter may decrease the effectiveness of the hypochlorite solution because of the reaction of chlorine with nitrogen compounds to form chloramines.18
Bleach is a cheap and easy to acquire disinfectant. Moreover, it can be used for either the disinfection of facilities and equipment, or asepsis of cows' teats and milkers' hands. Furthermore, when used at the proper dilution, it does not leave toxic products in the milk and is also known to be efficient in controlling other diseases such as mastitis. For these reasons, it is a popular disinfectant used by farmers. However, on farms, is it is common to see inadequate storage of bleach, which could cause a decrease in its germicidal effectiveness. Other commercial products in addition to the bleach can also be used as chlorine sources. However, the different initial concentrations of commercial chlorine on the market make it difficult to recommend a standard dilution for use as an antiseptic or disinfectant for surfaces and milking utensils. The use of a higher concentration of chlorine can lead to lesions on the teats of the cows and on the hands of the milkers, making them ultimately more susceptible to VACV infection.
The results of the present study show that hypochlorite and quaternary ammonium combined with chlorhexidine or glutaraldehyde could be considered for use as recommended products for the control of BV because of the susceptibility of VACV to these disinfectants. Among the disinfectants tested, sodium hypochlorite is the cheapest, but to be effective, it is recommended to be stored in opaque containers, protected from sunlight and discarded whenever contamination with organic matter occurs.
The use of effective disinfectants against VACV, as shown in this study, represents an important step in the control and prevention of BV and other infections with VACV. These disinfectants may also be used by health professionals for disinfection when dealing with VACV-infected patients. They may also be used to avoid laboratory-acquired infections because VACV is a common virus in laboratory-based research.
ACKNOWLEDGMENTS
We thank Grazielle Gallinari and colleagues from the Laboratorio de Pesquisa em Virologia Animal, Escola de Veterinaria, and colleagues from Laboratorio de Virus, Instituto de Ciencias Biologicas at the Universidade Federal de Minas Gerais for excellent technical support.
Footnotes
Financial support: This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais, (FAPEMIG), and Pro-Reitoria de Pesquisa/ Universidade Federal de Minas Gerais. E. G. Kroon and Z. I. P. Lobato were supported by fellowships from CNPq I. S. Rehfeld was supported by a fellowship from CNPq/Ministério de Agricultura, and M. I. M. C. Guedes was supported by a fellowship from FAPEMIG.
Authors' addresses: Tércia Moreira Ludolfo de Oliveira, Izabelle Silva Rehfeld, Maria Isabel Maldonado Coelho Guedes, and Zélia Inês Portela Lobato, Laboratório de Pesquisa em Virologia Animal, Departamento de Medicina Veterinária Preventiva, Escola de Veterinária, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, E-mails: tercialud@gmail.com, izarehfeld@gmail.com, mariaisabel.guedes@gmail.com, and ziplobato@gmail.com. Jaqueline Maria Siqueira Ferreira, Laboratorio de Microbiologia, Universidade Federal de Sao Joao Del-Rei, Campus Centro-Oeste Dona Lindu, Divinopolis, Brazil, E-mail: jackmaria4@gmail.com. Erna Geessien Kroon, Laboratório de Vírus, Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, E-mail: kroone@icb.ufmg.br.
References
- 1.Damaso CA, Esposito JE, Condit RC, Moussatché N. An emergent poxvirus from humans and cattle in Rio de Janeiro state: Cantagalo virus may from Brazilian smallpox vaccine. Virology. 2000;27:439–449. doi: 10.1006/viro.2000.0603. [DOI] [PubMed] [Google Scholar]
- 2.Schatzmayr HG, Lemos ER, Mazur C, Schubach A, Majerowicz S, Rozental T, Schubach TM, Bustamante MC, Barth OM. Detection of poxvirus in cattle associated with human cases in state of Rio de Janeiro: preliminary report. Mem Inst Oswaldo Cruz. 2000;95:625–627. doi: 10.1590/s0074-02762000000500007. [DOI] [PubMed] [Google Scholar]
- 3.De Souza Trindade G, da Fonseca FG, Marques JT, Nogueira ML, Mendes LC, Borges AS, Peiró JR, Pituco EM, Ferreira PC, Kroon EG. Aracatuba virus: a vaccinia like virus associated with infection in humans and cattle. Emerg Infect Dis. 2003;9:155–160. doi: 10.3201/eid0902.020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagasse-Sugahara TK, Kiesielius JJ, Ueda-Ito M, Curti SP, Figueiredo CA, Cruz AS. Human vaccinia-like virus outbreaks in Sao Paulo and Goias States, Brazil: virus detection, isolation, and identification. Rev Inst Med Trop Sao Paulo. 2004;46:315–322. doi: 10.1590/s0036-46652004000600004. [DOI] [PubMed] [Google Scholar]
- 5.Leite JA, Drumond BP, Trindade GS, Lobato ZI, da Fonseca FG, dos Santos Junior J, Madureira MC, Guedes MI, Ferreira JM, Bonjardim CA, Ferreira PC, Kroon EG. Passatempo virus, a vaccinia virus strain. Brazil. Emerg Infect Dis. 2005;11:1935–1938. doi: 10.3201/eid1112.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobato ZI, Trindade GS, Frois MC, Ribeiro EB, Dias GR, Teixeira BM, Lima FA, Almeida GM, Kroon EG. Surto de varíola bovina causada pelo vírus Vaccinia na região da Zona da Mata Mineira. Arquivo Brasileiro Med Veterinaria Zootecnia. 2005;57:423–429. [Google Scholar]
- 7.de Souza Trindade G, Drumond BP, Guedes MI, Leite JA, Mota BE, Campos MA, Da Fonseca FG, Nogueira ML, Lobato ZI, Bonjardim CA, Ferreira PC, Kroon EG. Zoonotic vaccinia virus infection in Brazil: clinical description and implications for health professionals. J Clin Microbiol. 2007;45:1370–1372. doi: 10.1128/JCM.00920-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrahão JS, Guedes MI, Trindade GS, Fonseca FG, Campos RK, Mota BF, Lobato ZI, Silva-Fernandes AT, Rodrigues GO, Lima LS, Ferreira PC, Bonjardim CA, Kroon EG. One more piece in the VACV ecological puzzle: could peridomestic rodents be the link between wildlife and bovine vaccinia outbreaks in Brazil? PLoS ONE. 2009;4:7428. doi: 10.1371/journal.pone.0007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrahão JS, Silva-Fernandes AT, Lima LS, Campos RK, Guedes MI, Cota MM, Assis FL, Borges IA, Souza-Júnior MF, Lobato ZI, Bonjardim CA, Trindade GS, Ferreira PC, Kroon EG. Vaccinia virus infection in monkeys, Brazilian Amazon. Emerg Infect Dis. 2010;16:976–979. doi: 10.3201/eid1606.091187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrahão JS, Trindade GS, Ferreira JM, Campos RK, Bonjardim CA, Ferreira PC, Kroon EG. Long-lasting stability of vaccinia virus strains in murine feces: implications for virus circulation and environmental maintenance. Arch Virol. 2009;154:1551–1553. doi: 10.1007/s00705-009-0470-1. [DOI] [PubMed] [Google Scholar]
- 11.Campos MA, Kroon EG. Critical period for irreversible block of vaccinia virus replication. Rev Microbiol. 1993;24:104–110. [Google Scholar]
- 12.Malik YS, Maherchandani S, Goyal SM. Comparative efficacy of ethanol and isopropanol against feline calicivirus, a norovirus surrogate. Am J Infect Control. 2006;34:31–35. doi: 10.1016/j.ajic.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 13.European Committee for Standardization . Disinfectants and Antiseptics: Quantitative Suspension Test for Evaluating the Virucidal Activity of Disinfectants and Antiseptics Used in Veterinary Medicine—Test Method and Prerequisites (Phase 2, Step 1) Paris: CEN EN14675; 2006. [Google Scholar]
- 14.Oliveira JM, Silva LR. Resposta Técnica Produzida pelo Serviço Brasileiro de Respostas Técnicas/SBRT. SENAI: Rio Grande do Sul; 2006. 2006. http://www.sbrt.ibict.br (Informações Sobre a Determinação do Teor de Cloro Ativo em Água Sanitária ou Alvejantes). Available at. Accessed January 20, 2008. [Google Scholar]
- 15.ANVISA (Agência Nacional de Vigilância Sanitária) Portaria no. 89, de 25 de Agosto de 1994. Determina Que o Registro dos Produtos Saneantes Domissanitários “Água Sanitária” e “Alvejante” Categoria Congênere a Detergente Alvejante e Desinfetante para Uso Geral Seja Procedido de Acordo com as Normas Regulamentares Anexas a Presente. Diário Oficial da União, [Brasília, DF], 26 de Agosto de 1994. Brasil: 1994. http://elegis.anvisa.gov.br/leisref/public/showAct.php?id=329 Available at. Accessed October 23, 2007. [Google Scholar]
- 16.McDonnel G, Russell D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanabe I, Hota S. Effect of disinfectants on Variola virus in cell culture. Appl Environ Microbiol. 1976;32:209–211. doi: 10.1128/aem.32.2.209-212.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block SS. Disinfection, Sterilization and Preservation. Fifth edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Guide F: Environmental Control of Smallpox Virus—Response Plan [s.l.] 2003. http://www.bt.cdc.gov/agent/smallpox/response-plan/files/guide-f.doc Available at. Accessed: August 2, 2008. [Google Scholar]
- 20.Jones GM. Milking Practices Recommended to Assure Milk Quality and to Prevent Mastitis. Dairy Science—Virginia Cooperative Extension, 404–227. 1998. http://www.ext.vt.edu/pubs/dairy/404-227/404-227.pdf Available at. Accessed September 18, 2008. [Google Scholar]
- 21.Schümann K, Grossgebauer K. Experiments on disinfection of vaccinia virus embedded in scabs and/or at the hand [in German] Zentralbl Bakteriol Orig B. 1977;164:45–63. [PubMed] [Google Scholar]


