Abstract
Cerebral cortical activity is heavily influenced by interactions with the basal ganglia. These interactions occur via cortico-basal ganglia-thalamo-cortical loops. The putamen is one of the major sites of cortical input into basal ganglia loops and is frequently activated during pain. This activity has been typically associated with the processing of pain-related motor responses. However, the potential contribution of putamen to the processing of sensory aspects of pain remains poorly characterized. In order to more directly determine if the putamen can contribute to sensory aspects of pain, nine individuals with lesions involving the putamen underwent both psychophysical and functional imaging assessment of perceived pain and pain-related brain activation. These individuals exhibited intact tactile thresholds, but reduced heat pain sensitivity and widespread reductions in pain-related cortical activity in comparison with 14 age-matched healthy subjects. Using magnetic resonance imaging to assess structural connectivity in healthy subjects, we show that portions of the putamen activated during pain are connected not only with cortical regions involved in sensory-motor processing, but also regions involved in attention, memory and affect. Such a framework may allow cognitive information to flow from these brain areas to the putamen where it may be used to influence how nociceptive information is processed. Taken together, these findings indicate that the putamen and the basal ganglia may contribute importantly to the shaping of an individual subjective sensory experience by utilizing internal cognitive information to influence activity of large areas of the cerebral cortex.
Keywords: basal ganglia, functional MRI, pain, stroke, selection
Introduction
The putamen, together with the caudate nucleus, makes up the striatum, a major site of cortical and subcortical input into the basal ganglia (Alexander et al., 1990). Although the putamen is frequently activated during pain (Jones et al., 1991; Coghill et al., 1999; Peyron et al., 2000; Bingel et al., 2004), its role during pain has often been assumed to be related to motor processing (Jones et al., 1991; Coghill et al., 1994). This role would be consistent with the traditional view of the basal ganglia as structures closely associated with movement (Albin et al., 1989; Alexander et al., 1990).
Several lines of evidence, however, indirectly suggest that the putamen may contribute to the processing of sensory aspects of pain. The striatum is rich in opioid receptors and contains nociceptive neurons responsive to graded noxious stimuli (Chudler and Dong, 1995; Chudler, 1998; Koyama et al., 2000; Sprenger et al., 2005; Baumgartner et al., 2006). Nociceptive neurons in lamina V of the spinal cord project directly to globus pallidus, a structure that is intimately tied to the basal ganglia circuitry and the striatum (Braz et al., 2005). Additionally, striatal activation and striatal dopamine D2 receptor activity are correlated with individual variability in pain, pain modulation and several chronic pain syndromes (Hagelberg et al., 2002, 2003a, b, 2004; Pertovaara et al., 2004; Apkarian et al., 2005; Becerra et al., 2006; Scott et al., 2006; Wood et al., 2007; Geha et al., 2008). Furthermore, patients with Parkinson’s disease have increased heat pain sensitivity (Djaldetti et al., 2004), while patients with various chronic pain syndromes such as fibromyalgia, chronic vulvar pain or chronic low back pain display structural changes in striatal grey matter (Schmidt-Wilcke et al., 2006, 2007; Schweinhardt et al., 2008). However, since motor responses and/or motor control demands elicited by pain may covary with perceived intensity, it has remained difficult to conclusively determine whether the putamen also contributes to the processing of sensory aspects of pain (Willer, 1977; Piche et al., 2010). Moreover, the mechanisms by which the putamen and related basal ganglia circuitry contribute to the construction of an experience of sensory features of pain remain to be determined.
To test the hypothesis that the putamen contributes to sensory aspects of pain, we recruited nine patients with lesions involving the putamen. These individuals underwent both psychophysical evaluation and functional MRI to determine if damage to the putamen disrupts sensory aspects of pain and reduces pain-related brain activation. The putamen is connected to numerous cerebral cortical and subcortical regions, but to date, the brain networks associated with nociceptive processing in the putamen remain poorly characterized. Thus, the structural connectivity of regions of the putamen activated during pain was also explored in healthy control subjects to determine how nociceptive information may access the putamen.
Materials and methods
Subjects
Fourteen normal healthy adults were recruited to participate in the study. There were eight females and six males (age 46–75 years; mean 59 years). Out of the 14 subjects, 13 participated in the imaging session. One female participant did not participate in the brain imaging session due to claustrophobia. In addition, to examine how brain lesions affecting the putamen may alter pain perception, sensory thresholds and pattern of brain activations, a group of nine stroke patients, seven males and two females (age 46–71 years; mean 59 years) were also recruited. The proportion of males and females was not significantly different between groups (Fisher’s exact test, P = 0.20). All patients suffered from left ischaemic strokes with lesions encompassing the putamen (Fig. 1 and Supplementary Table 1). All subjects underwent the same experimental protocols. All gave written, informed consent acknowledging that: (i) they would experience experimental painful stimuli; (ii) all methods and procedures were clearly explained; and (iii) they were free to withdraw from the experiment at any time without prejudice. All procedures were approved by the Institutional Review Board of Wake Forest University School of Medicine.
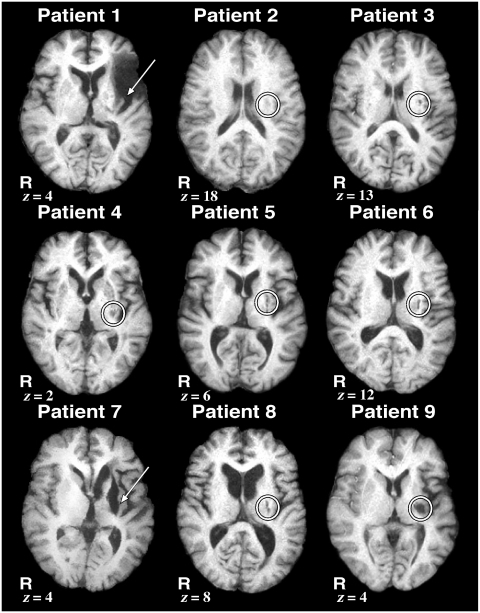
Figure 1.
High-resolution structural MRI images showing the extent of the lesions in each patient. All nine patients had ischaemic strokes with lesions encompassing the left putamen, indicated by an arrow or circle. Slice location is given as millimetres above the anterior–posterior commissure line in standard space in the z-axis. R = right.
Psychophysical data collection
Subjects rated pain using a 15-cm plastic visual analogue scale that has been widely used to assess pain because of ease of use while providing quantifiable measurements of pain intensity and pain unpleasantness [Parisian Novelty Co. (Price et al., 1994)]. The minimum was anchored with ‘no pain sensation’ or ‘not at all unpleasant’, while the maximum was anchored with ‘most intense pain imaginable’ or ‘most unpleasant imaginable’. Using an audio analogy, subjects were instructed to distinguish between pain intensity and pain unpleasantness (Price et al., 1989). All thermal stimuli used for suprathreshold stimulation were applied to the posterior aspect of the lower leg via a 16 × 16-mm2 Peltier device (Medoc TSA II) secured with a Velcro strap. Baseline temperature was maintained at 35°C, and stimulus temperatures were delivered with rise and fall rates of 6°C/s. During a psychophysical training session, subjects rated 32 noxious heat stimuli (35 and 43–49°C, 5 s duration) using the visual analogue scale in order to gain experience rating pain. These ratings are not reported further.
Next, subjects provided post-stimulus pain intensity and pain unpleasantness visual analogue scale ratings of 18 graded 5-s duration noxious heat stimuli of three different temperatures (35, 45 or 50°C) delivered in a pseudo-random fashion on each leg. To minimize sensitization, habituation or hyperalgesia, all trials were separated by a minimum of 30 s and were administered on previously unstimulated skin sites (Pedersen and Kehlet, 1998a, b).
Finally, in order to ensure that subjects could tolerate the stimuli used during the MRI session, subjects also rated long-duration noxious stimuli during the training session. These long-duration noxious stimuli were delivered using a block design (49°C, 30 s off to 30 s on, 5 cycles) with baseline temperature of 35°C. Continuous ratings of this stimulus series have been previously shown to remain largely stable from one stimulus epoch to the next (Koyama et al., 2003). A total of four series [two during stimulation of the left (unaffected/ipsilesional) side, two during stimulation of the right (affected/contralesional) side, alternating between sides] were given. At the end of each series, the subjects were asked to provide overall pain intensity and unpleasantness visual analogue scale ratings. These data are not reported further.
Quantitative testing of sensory thresholds
Tactile thresholds
To quantitatively assess tactile thresholds of the healthy subjects and deficits of areas that may be affected by the lesions in patients, von Frey filaments were used to examine the ventral forearms and dorsal calves bilaterally using the method of constant stimuli. Minimum filament size required for subjects to consistently (greater than ∼75%) detect touch for each of the areas was recorded. Each body area was tested a variable number of times as the threshold was successively approximated with different von Frey filaments.
Thermal thresholds
Heat pain, cold pain, warm detection and innocuous cool detection thresholds were determined by the method of limits. For each of the four modalities, a 32 × 32-mm2 thermode was applied to the right and left dorsal calf. For warm detection and heat pain thresholds, the temperature was increased at 1°C/s from 35°C to 50°C. Subjects were then asked to indicate either the point at which the baseline temperature transitioned to a warm sensation (warm detection) or when a non-painful warm sensation changed to a painful heat sensation (heat pain) by pressing a button. For innocuous cool and cold pain thresholds, temperature was decreased at 1°C/s from 35°C to 0°C. Subjects were subsequently asked to indicate the point at which the baseline temperature transitioned to a cool sensation (innocuous cool) or when a non-painful cool sensation transitioned to a painful cold sensation (cold pain). For each modality, the test was repeated successively six times and the mean threshold temperature was calculated. To minimize sensitization, habituation or hyperalgesia, all trials were separated by a minimum of 30 s and were performed on previously unstimulated skin sites (Pedersen and Kehlet, 1998a, b).
Functional imaging
The functional MRI session consisted of eight series [four during stimulation of the left (unaffected/ipsilesional) side, four during stimulation of the right (affected/contralesional) side, alternating between sides]. Long-duration noxious stimuli were delivered using a block design (49°C, 30 s off to 30 s on, 5 cycles) with a baseline temperature of 35°C. At the end of each functional MRI series, the subjects were asked to provide overall pain intensity and unpleasantness visual analogue scale ratings.
Image acquisition and processing
Functional MRI data were acquired on a 1.5 T General Electric Twin-Speed LX Scanner with a birdcage quadrature head coil (General Electric Medical Systems). For functional imaging, blood oxygenation level-dependent images of the entire brain were acquired continuously by using single-shot echoplanar imaging [echo time = 40 ms; repetition time = 2 s; 28 × 5-mm thick slices; in-plane resolution 3.72 × 3.75 mm; flip angle = 90°; no slice gap] (Ogawa et al., 1990). Each functional MRI series consisted of 165 volumes and lasted 350 s with 20 s equilibration time at the beginning of each series. During each acquisition series, subjects were requested to close their eyes. High-resolution structural scans were acquired using a 3D spoiled gradient-echo (3D inversion spoiled gradient-recalled acquisition in a steady state) sequence (inversion time = 600 ms; repetition time = 9.1 ms; flip angle = 20°; echo time = 1.98 ms; matrix 256 × 196; slice thickness 1.5 mm with no gap between sections; 124 sections; in-plane resolution 0.9375 × 0.9375 mm; field of view = 24 cm). In addition, to help visualize the extent of the lesion, high-resolution fast spin echo images (repetition time = 4200 ms; flip angle = 90°; echo time = 85 ms; matrix 256 × 256; slice thickness 5 mm with no gap between slices; 26 slices; in-plane resolution 0.85937 × 0.85938 mm; field of view = 22 cm) and high-resolution axial fluid-attenuated inversion recovery (inversion time = 2000 ms; repetition time = 8000 ms; flip angle = 90°; echo time = 120 ms; matrix 256 × 256; slice thickness 5 mm with no gap between slices; 26 slices; in-plane resolution 0.85937 × 0.85938 mm; field of view = 22 cm) were also acquired.
The functional image analysis package FSL [Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (Centre for FMRIB, University of Oxford)] was used for image processing and statistical analysis. The functional data were movement corrected, spatially smoothed by 5-mm full-width at half-maximum with a 3D isotropic Gaussian kernel, and temporally filtered by a non-linear high-pass filter with a cut-off period of 90 s. Each functional image was scaled by its mean global intensity (intensity normalization). Next, each subject’s functional images were registered to their structural data using a seven-parameter linear 3D transformation and transformed into standard stereotaxic space (as defined by the Montreal Neurological Institute) using a 12-parameter linear 3D transformation (Tailarach and Tournoux, 1988; Jenkinson et al., 2002). Visual inspection of an animated time series confirmed that spatial transformations during registration and movement correction were successfully accomplished.
Diffusion tensor image acquisition
To minimize the imaging distortion associated with eddy currents, diffusion tensor image data were acquired using a dual spin-echo single shot echo-planar imaging (Reese et al., 2003). The diffusion-weighted images were acquired along 25 isotropically distributed directions with a b-value of 1000 s/mm2 and a voxel size of 0.9375 × 0.9375 mm (repetition time = 11000 ms; echo time = 79.3 ms; 128 × 128 matrix; slice thickness 3 mm with no gap between sections; 45 sections; field of view = 24 cm).
Statistical analysis of psychophysical data
To examine the effects of putamen lesions on pain, we compared pain intensity and unpleasantness ratings between left (unaffected/ipsilesional) and right (affected/contralesional) sides using ANOVA (JMP software; SAS Institute). To determine if lesioned subjects exhibited different pain sensitivity during brief graded noxious stimuli (35, 45 and 50°C, 5 s), three-factor ANOVAs were used to examine the effects of body side, stimulus temperature and lesion on pain intensity and unpleasantness ratings. For long-duration noxious stimulation during functional MRI (49°C, 30 s off to 30 s on, 5 cycles), we used two-factor ANOVAs to determine if lesioned patients exhibited different sensitivity than healthy subjects. In addition, three-factor ANOVAs were used to examine the effects of body sides, body location (arm versus leg) and lesion on tactile thresholds, while two-factor ANOVAs were used to examine the effects of body side and lesion on thermal thresholds. In addition, regression analyses were used to assess the potential relationship between time after lesion and thermal thresholds, as well as side-to-side differences in heat pain sensitivity.
Lesion volume characterization
In order to better understand the relationship between sensory deficits and lesion volume and location, each patient’s lesion was identified in a semi-automated fashion. Segmentation of each patient’s lesion was performed manually using automated tissue class segmentation (FSL FAST software) as a guide for the visualization of lesions. Each patient’s high-resolution structural images (inversion spoiled gradient-recalled acquisition in a steady state, fast spin echo and fluid-attenuated inversion recovery) were used as inputs for multi-channel automated tissue class segmentation with four classes of tissues (grey matter, white matter, CSF and damaged tissue) specified as outputs of the segmentation (Zhang et al., 2001). To generate a lesion mask for each patient, segmentation outputs containing damaged tissues as well as other classes of tissues were overlaid on top of structural images to help with the visualization of the affected region. Subsequently, a lesion mask for each patient was manually drawn using FSLView. Additionally, since five patients had parts of the thalamus affected by the lesion, we also sought to determine if there was a relationship between the volume of the thalamus that was lesioned and pain ratings. The volume of lesions affecting the thalamus in these five patients were determined using Harvard–Oxford Atlas as a guide (Frazier et al., 2005; Desikan et al., 2006; Makris et al., 2006; Goldstein et al., 2007).
The lesion masks for each patient were registered to standard space and summed across individuals to generate a single-lesion location frequency map for display. In each individual subject, total lesion volume as well as thalamic lesion volume were expressed in relationship to standard stereotaxic space to compensate for individual differences in brain size. Next, lesion volume (in cubic millimetres in standard space), thalamic lesion volume (in cubic millimetres in standard space) and percent difference in pain intensity ratings between body sides for each patient {[(visual analogue scale left-visual analogue scale right)/visual analogue scale left] × 100} were log transformed. The relationships between these log-transformed values were then assessed by regression analysis.
Statistical analysis of regional signal changes within the brain
Pain-related activations were examined using simple boxcar functions. The regressor was convolved with a gamma-variate model of the haemodynamic response (delay, 6 s; SD, 3 s) and its temporal derivative, and was temporally filtered with the same parameters as the functional MRI data. For each individual, first-level fixed-effects general linear modelling analyses were used to identify pain-related brain activation associated with the modelled haemodynamic response function (Woolrich et al., 2001). Subsequently, second-level fixed-effects analyses were performed across the multiple series acquired for each subject. Next, random-effects third-level analyses were performed to assess activation across individuals for each group. In addition, direct contrast comparisons of pain-related brain activations between healthy subjects versus patients were done using fixed-effects analyses. These analyses were performed at third-level using results of second-level analyses as inputs. This allows us to determine which brain areas exhibited statistically significant greater activation in healthy subjects when compared with patients during painful stimulation. Z (Gaussianized T/F)-statistic images were thresholded using clusters determined by z > 2.3 and a (corrected) cluster significance threshold of P < 0.05 (Worsley et al., 1992).
Seed masks for structural connectivity
In order to analyse the structural connectivity of regions of the putamen activated during pain, seed masks were first generated. This was accomplished by binarizing both contralateral and ipsilateral regions of the putamen activated during both left and right-sided noxious stimulation in healthy subjects. Thus, four putamen seed masks were generated: (i) left putamen during stimulation of left body side; (ii) right putamen during stimulation of left body side; (iii) left putamen during stimulation of right body side; and (iv) right putamen during stimulation of right body side.
Statistical analysis of probabilistic tractography
To determine the structural connectivity of regions of the putamen activated during pain, probabilistic tractography was performed on diffusor tensor imaging data as described previously (Behrens et al., 2003b; Johansen-Berg et al., 2004, 2005). Probabilistic tractography utilizes the anisotropic diffusion of water in white matter. Since water diffuses along the direction of the white matter tract without crossing the myelin sheaths, the orientation of diffusion can provide useful insights into the orientation of white matter fibres. The orientation of maximal diffusion is called the principal diffusion direction. A probability density function for the uncertainty of the principal diffusion direction was computed using Bayes’ rule based on the parameters given in the data (Behrens et al., 2003b). Markov Chain Monte Carlo sampling was then used to identify possible tract directions based on the probability density functions. Multiple sampling of the data set produced connectivity distributions, i.e. the possible tracts that could occur from a particular brain region. This technique allows us to examine the directions and connections of each brain region (Hadjipavlou et al., 2006).
Probabilistic tractography provides quantification of the likelihood of connectivity between the seed mask and other brain areas. An image generated by probabilistic tractography shows the tract path between the seed and other connected brain areas. The difference between outputs of the different sample sizes converged with higher sample sizes. The sample size determines the number of individual pathways (or samples) that are drawn through the probability distributions on principle fibre direction. Convergence is reached with high number of samples (>5000) (Behrens et al., 2003b). However, a sample size of 10 000 was determined to be sufficiently large for connectivity analyses and tract image generation (Behrens et al., 2003b; Hadjipavlou et al., 2006). Because we wanted to explore all possible brain areas that were connected to our designated seed masks, we employed a whole brain search approach. Accordingly, no target regions were entered into the tractography analysis. For each of the 13 normal subjects, probabilistic tractography was used to determine which brain areas were anatomically connected to each of the four putamen seed masks. Four tract images (one for each of the four putamen seed masks) were generated for each individual. The quantification of tract pathways was performed in native diffusion space for each subject. The output of this analysis was transformed into standard stereotaxic space. These standard space images were then binarized and summed across individuals to generate a frequency map for each seed mask. This frequency map shows brain areas that were anatomically connected with each of the seed masks across individuals (Hadjipavlou et al., 2006). The colour scale of the map enabled easy visualization of the number of subjects, ranging from 1–13, that display each connection.
Additionally, since some patients had parts of the internal capsule and thalamic projections to primary somatosensory cortex affected by the lesion, we also sought to examine the integrity of these white matter projections to primary somatosensory cortex by diffusion tensor imaging probabilistic tractography. To ensure that psychophysical as well as functional differences in brain activation were not due to deafferentation of thalamic projections to primary somatosensory cortex, we performed probabilistic tractography for each of the lesion patients using a seed mask of the left primary somatosensory cortex derived from the Harvard–Oxford Atlas (Frazier et al., 2005; Desikan et al., 2006; Makris et al., 2006; Goldstein et al., 2007). The outputs of the tractography were then binarized and summed across individuals to generate a frequency map for display.
Results
Neurological findings of patients with putamen lesions
Nine ischaemic stroke patients, seven males and two females, (age 46–71 years; mean 59 years) with lesions affecting the left putamen participated in the study (Figs 1, 5A and Supplementary Table 1). Subjects were tested from 2 weeks to 15 years (mean time: 4 years) after their stroke. Sensory examinations were normal throughout position and vibration, although there were mild decreases in touch on the right (affected/contralesional) side in some patients (Supplementary Table 1). These were well within the range of normal variability of the healthy subjects. Motor testing revealed that most patients exhibited a degree of motor deficits on the right (affected/contralesional) side, including weakness of right upper and/or lower extremities (Supplementary Table 1). These findings are characteristic of lesions involving the putamen and the basal ganglia. In all patients, there was no aphasia or impaired cognition, no central post-stroke pain and no signs of hemineglect.
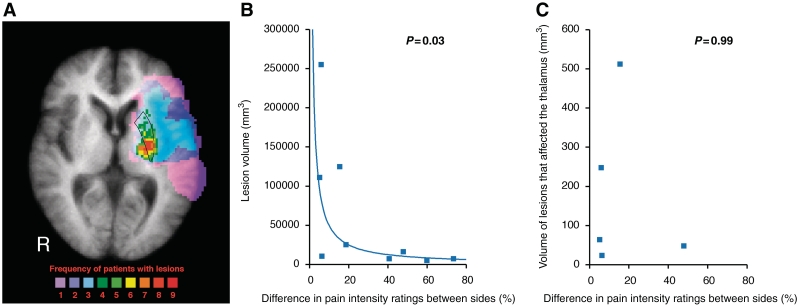
Figure 5.
Focal lesions specific to the putamen are effective in producing large differences in pain ratings between sides in patients. Voxels in colour represent the number of patients with lesions at a given locus (displayed on a structural MRI that has been averaged across all lesion patients, A). The patients’ lesions were concentrated within the left putamen (outlined in black) with a few subjects showing larger, more extensive lesions. The difference in pain intensity ratings between sides is inversely logarithmically related to lesion volume (B). However, in the five subjects where the lesion encroached into the thalamus, there was no relationship between the difference in pain intensity ratings between sides and volume of thalamus that was affected by the lesion (C). R = right.
Quantitative assessment of tactile and thermal thresholds
Quantitative assessment of tactile thresholds with von Frey filaments revealed that the tactile thresholds of patients were not significantly different from healthy subjects [group: F(1,21) = 1.0, P = 0.33; body sides: F(1,21) = 1.2, P = 0.28; group × body sides interaction: F = 1.7, P = 0.21], although in both groups, the arms were more sensitive than the legs [body location: F(1,21) = 12.2, P < 0.01; group × body location interaction: F(1,21) = 0.0, P = 0.96] (Fig. 2A and B).
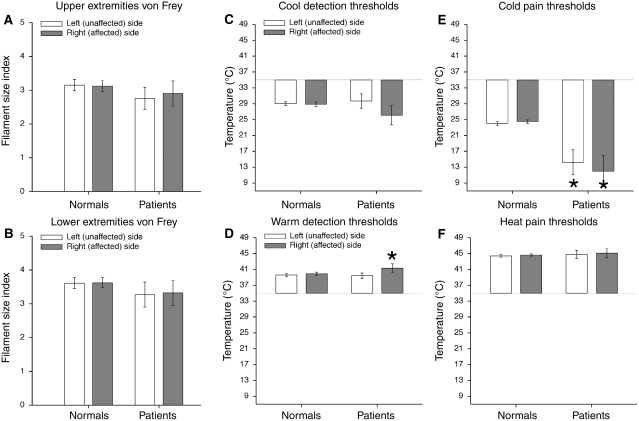
Figure 2.
Tactile and thermal thresholds (mean ± SEM). Tactile threshold of patients with putamen lesions were not significantly different from healthy controls (normals) (A, B). Patients with putamen lesions displayed some disturbances in cold pain thresholds and warm detection thresholds while exhibiting normal heat pain and innocuous cool detection thresholds (C–F). Cool detection thresholds on both the left and right sides of patients were in the range of those of the healthy subjects (C). However, cold pain thresholds of patients were significantly lower than those of the healthy subjects on both sides (P < 0.01) (E). In addition, patients had higher warmth detection thresholds on their right (affected/contralesional) sides than did healthy control subjects (P = 0.05) (D). The left (unaffected/ipsilesional) and right (affected/contralesional) heat pain thresholds of patients appear completely normal and symmetric (F). Asterisk denotes statistical significance (P < 0.05).
In patients, cool detection thresholds on both the left and right sides were in the range of those of the healthy subjects (Fig. 2C). There was no main effect of group or sides [group: F(1,16) = 0.9, P = 0.35; body sides: F(1,16) = 2.6; P = 0.12] nor was there a group × body side interaction [F(1,16) = 2.1, P = 0.16].
Cold pain thresholds of both sides of patients were significantly lower than those of the healthy subjects [group: F(1,15) = 12.8, P < 0.01] (Fig. 2E). In addition, there was no statistically reliable side-to-side difference in cold pain thresholds of patients when compared with healthy subjects [group × body sides interaction: F(1,15) = 0.6, P = 0.44], nor was there a significant main effect of stimulation side [body sides: F(1,15) = 0.1, P = 0.79]. This suggests that putamen lesions may be unique in disrupting cold pain sensations bilaterally.
Patients had higher warmth detection thresholds on their right (affected/contralesional) sides than did healthy subjects [group × body sides interaction: F(1,17) = 4.7, P = 0.05] (Fig. 2D). There was a significant main effect of side of stimulation on innocuous warm detection thresholds [body sides: F(1,17) = 8.1, P = 0.01]; however, there was no main effect of group [group: F(1,17) = 0.5, P = 0.50].
The left (unaffected/ipsilesional) and right (affected/contralesional) heat pain thresholds of patients appear completely normal and symmetric (Fig. 2F). There were no significant main effects of group and sides and no detectable side-to-side difference in patients when compared with healthy subjects [group: F(1,17) = 0.2, P = 0.66; body sides: F(1,17) = 0.4, P = 0.55; group × body side interaction: F(1,17) = 0.0, P = 0.91].
These results suggest that putamen lesions disrupt cold pain thresholds and warm detection thresholds while leaving tactile and other temperature thresholds intact. Thus, patients’ ability to experience and provide pain ratings for noxious heat stimuli was likely minimally affected by the lesions. Moreover, the differences seen among various thermal threshold modalities are unlikely to be due to impairments of reaction time since not all modalities were affected. Heat pain thresholds of the patients, for example, are virtually the same as (and not significantly different from) those of the healthy subjects. In addition, we found no statistically significant relationship between thermal thresholds and time after stroke or thalamic lesion volume (all P > 0.16, all P > 0.13, respectively). This suggests that our results are likely due to lesions confined to the putamen and not confounded by recovery time or thalamic involvement.
Short-duration pain ratings of patients with putamen lesions
Patients were able to evaluate brief noxious stimuli of graded intensities (35, 45 and 50°C, 5 s) applied to the posterior aspect of the lower legs on both left (unaffected/ipsilesional) and right (affected/contralesional) sides (Fig. 3). Both patients and healthy subjects exhibited monotonic increases in visual analogue scale ratings of pain intensity and unpleasantness as stimulus temperature increased [pain intensity: F(1,21) = 79.3, P < 0.01; pain unpleasantness: F(1,21) = 52.7, P < 0.01]. However, patients exhibited significantly lower pain intensity visual analogue scale ratings during stimulation of the right (affected/contralesional) side than those of left (unaffected/ipsilesional) side when compared with healthy subjects [body sides × group interaction: pain intensity F(1,21) = 8.6, P < 0.01] (Fig. 3A and B). Additionally, there was a strong trend towards side-to-side differences in pain unpleasantness ratings between the patients and healthy subjects [body sides × group interaction: pain unpleasantness: F(1,21) = 4.2, P = 0.052] (Fig. 3C and D). There was no significant main effect of body side and group on pain intensity and pain unpleasantness, although the effect of sides on pain unpleasantness ratings showed a strong trend [sides—pain intensity: F(1,21) = 1.1 P = 0.30; pain unpleasantness: F(1,21) = 4.1, P = 0.06; group—pain intensity: F(1,21) = 0.0, P = 0.85; pain unpleasantness: F(1,21) = 0.0, P = 0.87] (Fig. 3). These results indicate that putamen lesions may result in decreased pain sensitivity to brief graded noxious stimuli on the right (affected/contralesional) body side when compared with healthy subjects.
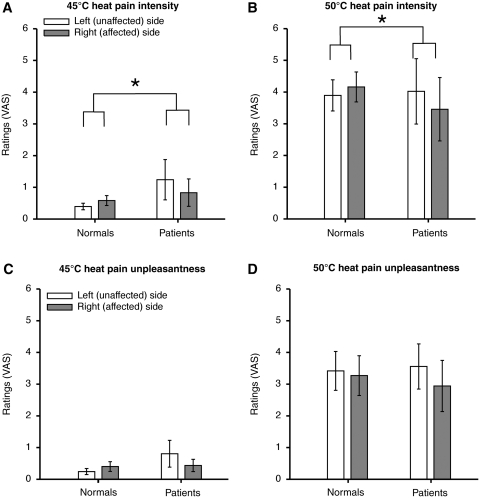
Figure 3.
Pain intensity and unpleasantness visual analogue scale (VAS) ratings during the graded noxious stimulation at 45 and 50°C (mean ± SEM). Patients retained the ability to discriminate noxious stimuli of graded intensities applied to the posterior aspect of the lower legs on both left (unaffected/ipsilesional) and right (affected/contralesional) sides. However, patients exhibited significantly lower visual analogue scale ratings of pain intensity during stimulation of the right (affected/contralesional) side than those of left (unaffected/ipsilesional) side when compared with healthy subjects (normals) (P < 0.01) (A, B). Although side-to-side differences in pain unpleasantness ratings between the patients and healthy subjects did not reach statistical significance, there was a strong trend towards a difference (P = 0.052) (C, D). Asterisk denotes statistical significance (P < 0.05). Please note that since visual analogue scale ratings of 35°C were zero, these values were not displayed on the bar graphs.
Long-duration pain ratings of patients with putamen lesions
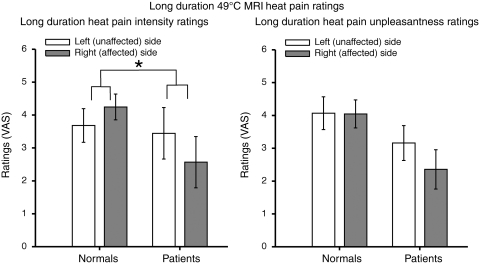
During long-duration noxious stimulation (30 s off, 30 s on, 5 cycles, 49°C) in the functional MRI session, patients exhibited significantly lower visual analogue scale ratings of pain intensity during stimulation of the right (affected/contralesional) side than those of the left (unaffected/ipsilesional) side when compared with healthy subjects [body sides × group interaction: pain intensity: F(1,20) = 9.5, P = 0.01] (Fig. 4). Although, side-to-side differences of pain unpleasantness ratings between patients and healthy subjects did not reach statistical significance, there was a strong trend towards a difference [body sides × group interaction: pain unpleasantness: F(1,20) = 3.5, P = 0.08] (Fig. 4). There were no significant main effects of body sides on pain intensity and unpleasantness ratings, although the effect of sides on pain intensity ratings showed a strong trend [pain intensity: F(1,21) = 3.9, P = 0.06; pain unpleasantness: F(1,21) = 0.5, P = 0.51]. Similarly, there were no significant main effects of group on pain intensity and unpleasantness ratings, but the effect of group on pain unpleasantness ratings showed a strong trend [pain intensity: F(1,20) = 1.4, P = 0.25; pain unpleasantness: F(1,20) = 3.4, P = 0.08] (Fig. 4). These results suggest that unilateral lesions of the putamen may decrease pain sensitivity to both brief and prolonged noxious stimulation on the right (affected/contralesional) body side when compared with healthy subjects.
Figure 4.
Pain intensity and unpleasantness visual analogue scale (VAS) ratings during long duration noxious stimulation (mean ± SEM). Putamen lesions produced decreased pain sensitivity to long duration noxious stimuli on the right (affected/contralesional) body side in patients when compared with healthy subjects. Patients exhibited significantly lower visual analogue scale ratings of pain intensity during stimulation of the right (affected/contralesional) side than those of the left (unaffected/ipsilesional) side when compared with healthy subjects (P = 0.0058). Although, side-to-side differences of pain unpleasantness ratings between patients and healthy subjects did not reach statistical significance, there was a strong trend towards a difference (P = 0.0762). Asterisk denotes statistical significance (P < 0.05).
Lesion volume and difference in pain ratings between sides
Patients’ lesions were centred in the left putamen with a few subjects showing larger, more extensive lesions (Figs 1 and 5A). Patients with smaller, focal lesions of the putamen exhibited greater differences in pain intensity ratings between sides, whereas patients with larger, more extensive lesions exhibited smaller differences in pain ratings between sides (r2 = 0.53, P = 0.02) (Fig. 5B). Additionally, in the five subjects with lesions that encroached on the thalamus, there was no statistically significant relationship between volume of the thalamus that was damaged and differences in pain intensity ratings between sides (r2 = 0.00, P = 0.99) (Fig. 5C). Thus, it appears that focal lesions specific to the putamen are effective in producing unilateral disruption of nociceptive processing. There was also no statistically significant relationship between time after stroke and differences in pain intensity ratings between sides (P = 0.44).
Putamen and other pain-related brain activation in healthy subjects
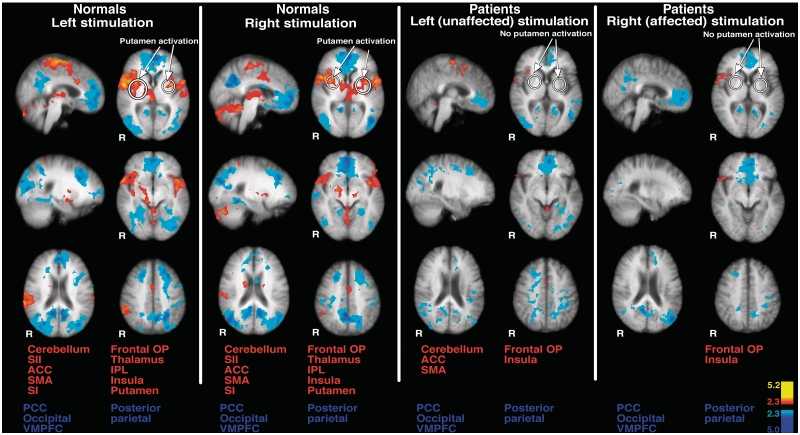
During long-duration noxious stimulation (49°C, 30 s off to 30 s on, 5 cycles) of the lower leg of the left side in healthy subjects, brain activation was identified within the anterior cingulate cortex, supplementary motor area, secondary somatosensory cortex, insula, primary somatosensory cortex, frontal operculum, thalamus, putamen and cerebellum (Fig. 6 and Supplementary Table 2). Stimulation of the right lower leg produced similar patterns of brain activation (Fig. 6 and Supplementary Table 2). This pattern of brain activation is consistent with normal brain activation during pain (Coghill et al., 1994, 2001; Peyron et al., 2000; Oshiro et al., 2007). While putamen activation was detected bilaterally during stimulation of each body side individually, the patterns and degree of ipsilateral and contralateral putamen activation differed somewhat (Figs 6, 8A and Supplementary Table 2). Regardless of side of stimulation, the ipsilateral putamen activation appears to be smaller when compared with that of contralateral putamen (Figs 6 and 8A).
Figure 6.
Pain-related brain activation in healthy subjects and patients. During long duration noxious stimulation of either body side in the healthy subjects (normals), brain activation was identified within the anterior cingulate cortex, supplementary motor area, secondary somatosensory cortex, insula, SI, frontal operculum, thalamus, putamen and cerebellum (left). Putamen activation was detected bilaterally during stimulation of either body side. Additionally, activation was identified within anterior areas of the thalamus during painful stimulation of either body side. In contrast, during painful stimulation of the left (unaffected/ipsilesional) body side in patients, pain-induced brain activation was identified within the anterior cingulate cortex, insula, frontal operculum, supplementary motor area and cerebellum (right). However, no secondary somatosensory cortex, thalamic or putamen activation was observed. Stimulation of the right (affected/contralesional) leg in patients activated the insula and frontal operculum. In general, brain activation during stimulation of the right (affected/contralesional) side was less pronounced than that during stimulation of the left (unaffected/ipsilesional) side in patients. In addition, pain-related brain activation during stimulation of either side in patients is less robust than that of healthy subjects. Anatomical images are the average of the structural MRIs of the healthy subjects and patients, respectively. ACC = anterior cingulate cortex; IPL = inferior parietal lobule; OP = operculum; PCC = posterior cingulate cortex; R = right; SI = primary somatosensory cortex; SMA = supplementary motor area; VMPFC = ventromedial prefrontal cortex.
Figure 8.
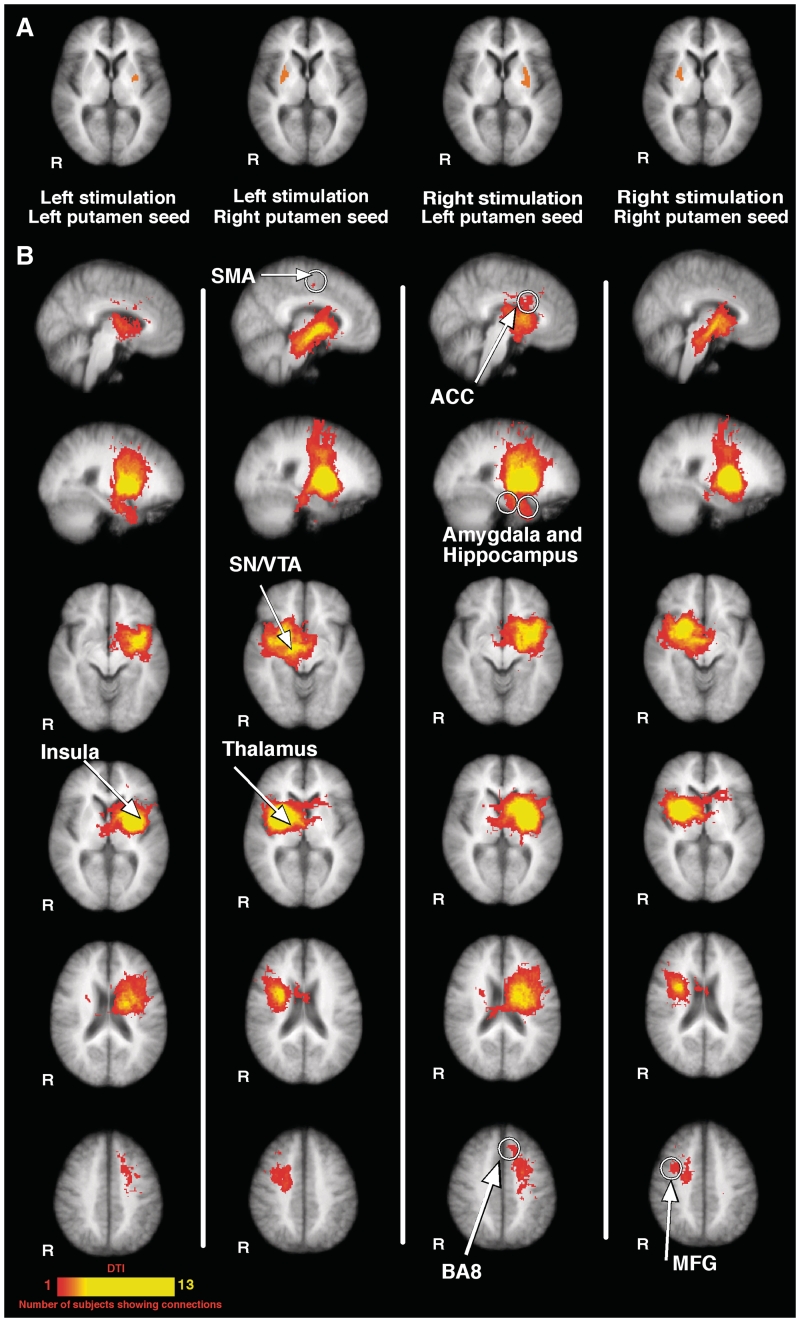
Putamen activation and structural connectivity of the putamen. The top row shows putamen activation during pain (A). The patterns of ipsilateral and contralateral putamen activation differed substantially regardless of side of stimulation. The ipsilateral putamen activation appears to be smaller when compared with that of contralateral putamen activation. These four putamen activation loci were used as seeds for structural connectivity analyses. Each of the four panels separated by white vertical lines displays structural (diffusion tensor imaging) connections of the corresponding seed mask (B). Structurally connected brain areas identified included: (i) nociceptive processing areas including anterior cingulate cortex, insula and thalamus; (ii) attention-related areas including middle frontal gyrus, BA8, anterior cingulate cortex and supplementary motor area; and (iii) memory processing areas including the amygdala and hippocampus. In addition, the substantia nigra/ventral tegmental area in the midbrain was also found to be structurally connected with the putamen. Note that the significance colour bar denotes the number of subjects displaying corresponding structural connections in the diffusion tensor image. Anatomical images are the average of the structural MRIs of the normal controls. ACC = anterior cingulate cortex; middle frontal gyrus = MFG; R = right; SI = primary somatosensory cortex; SMA = supplementary motor area; SN/VTA = substantia nigra/ventral tegmental area.
Additionally, during painful stimulation of either body side, activation was identified within anterior areas of the thalamus that have a high probability of having connections with the prefrontal cortex according to the Oxford Thalamic Connectivity Atlas (Behrens et al., 2003a, b) (Fig. 6 and Supplementary Table 2). Although correlating the thalamic activation loci to specific thalamic nuclei is difficult, these activated regions occur in areas consistent with the medial dorsal, ventral lateral and ventral anterior nuclei of the thalamus (Tailarach and Tournoux, 1988; Lancaster et al., 2000, 2007) (Fig. 6 and Supplementary Table 2).
Pain-related brain activation in patients with putamen lesions
During painful stimulation of the left (unaffected/ipsilesional) body side, pain-induced brain activation was identified within the anterior cingulate cortex, insula, frontal operculum, supplementary motor area and cerebellum (Fig. 6 and Supplementary Table 2). However, in contrast to that seen in normal subjects, no secondary somatosensory cortex, thalamic or putamen activation was observed. Stimulation of the right (affected/contralesional) leg activated the insula and frontal operculum (Fig. 6 and Supplementary Table 2). In general, brain activation during stimulation of the right (affected/contralesional) side was less robust than that during stimulation of the left (unaffected/ipsilesional) side. Compared with stimulation of the left (unaffected/ipsilesional) side, there was no detectable anterior cingulate cortex activation during stimulation of the right (affected/contralesional) side (Fig. 6 and Supplementary Table 2). This may be attributable to reduced pain experienced by the patients during stimulation of their right (affected/contralesional) side.
Direct comparison of pain-related brain activation between healthy subjects and patients
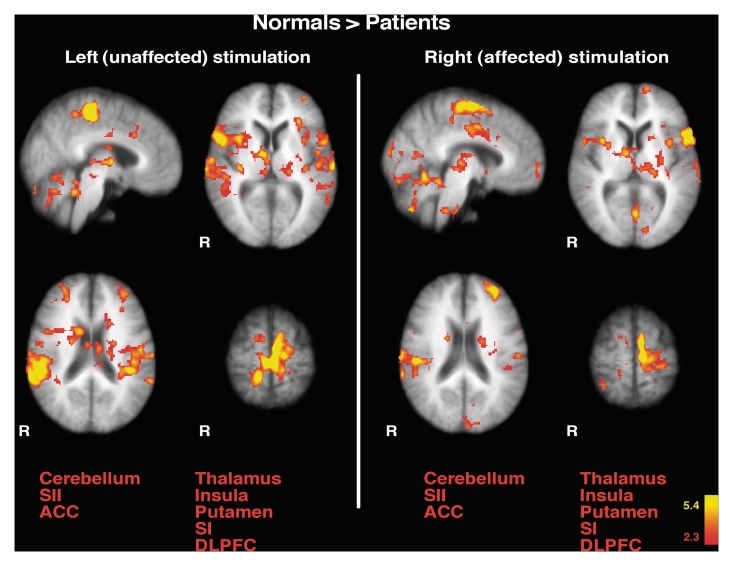
When compared with healthy subjects, patients exhibited less activation in a number of areas involved in nociceptive processing including secondary somatosensory cortex, primary somatosensory cortex, anterior cingulate cortex, insula, thalamus, cerebellum, dorsolateral prefrontal cortex, and putamen during stimulation of both right (affected/contralesional) and left (unaffected/ipsilesional) sides (Fig. 7). These findings indicate that while the psychophysical differences were only observed unilaterally, unilateral lesions of the putamen may produce a generalized, diffuse pattern of altered pain-related brain activity that is manifested bilaterally (Figs 3, 4, 6 and 7).
Figure 7.
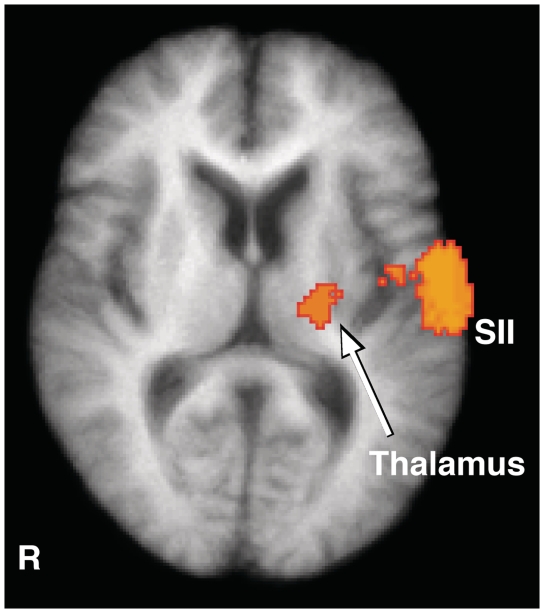
Direct comparison of pain-related brain activation between healthy subjects and patients. The activated areas represent regions where healthy subjects (normals) exhibited greater pain-related brain activation than patients (healthy subjects > patients). When compared with healthy subjects, patients exhibited less activation in a number of areas involved in nociceptive processing including secondary somatosensory cortex, SI, anterior cingulate cortex, insula, thalamus, cerebellum, dorsolateral prefrontal cortex, and putamen during stimulation of both affected (right) and unaffected sides (left). Anatomical images are the average of the structural MRIs of the healthy controls. ACC = anterior cingulate cortex; R = right; SI = primary somatosensory cortex; SII = secondary somatosensory cortex; DLPFC = dorsolateral prefrontal cortex.
Structural connectivity of the putamen
Probabilistic tractography analyses were performed in healthy subjects on each of the four putamen seed masks (two for each side of stimulation, one ipsilateral, one contralateral) (Fig. 8A and B). Tractography is a useful tool in identifying the anatomical framework that may support functional connections within a neural network and can provide useful insights into understanding the dynamics of an identified brain network. These analyses revealed that similar brain areas were structurally connected with each of the four putamen seed masks, suggesting that a similar brain network is associated with both contralateral and ipsilateral regions of the putamen activated during pain. Structurally connected brain areas identified included: (i) nociceptive processing areas including anterior cingulate cortex, insula and thalamus; (ii) attention- and premotor-related areas including middle frontal gyrus, Brodmann area 8 (BA8), anterior cingulate cortex and supplementary motor area; and (iii) memory processing areas including the amygdala and hippocampus (Fig. 8B). Additionally, structural connections were also identified in the ventral midbrain region. Although correlating these structurally connected midbrain areas to specific loci is difficult, these structurally connected regions occurred in areas consistent with the substantia nigra/ventral tegmental area (Fig. 8B) (Tailarach and Tournoux, 1988; Lancaster et al., 2000, 2007).
Effect of lesions on thalamo-cortical connectivity in patients
The putamen is in close proximity to the internal capsule, a white matter region that contains the connections between the somatosensory thalamus and the primary somatosensory cortex. Since disruption of thalamic projections in this white matter region would potentially confound interpretation of sensory changes associated with damage affecting the putamen, we performed an additional probabilistic tractography analysis in the lesion patients in order to assess the integrity of these connections. Using a seed mask in the primary somatosensory cortex, these analyses revealed that seven out of nine patients had connections between the thalamus and primary somatosensory cortex that were sufficiently intact to be detected with diffusion tensor imaging (Fig. 9). These connections were focused on a ventral/lateral region of the thalamus that, when viewed on the Tailarach atlas, was consistent with the location of the ventral posterolateral nucleus. The two patients in whom thalamic connections could not be detected were Patients 1 and 7, and they had the largest lesions and small side-to-side differences in heat pain sensitivity. In addition to the thalamic connections, connections between the primary somatosensory cortex and the parietal operculum/secondary somatosensory cortex were also detected in the majority of the patients.
Figure 9.
Integrity of thalamo-cortical connectivity in putamen-lesioned patients. Connections between the thalamus and primary somatosensory cortex as assessed with diffusion tensor imaging probabilistic tractography analysis. Regions depicted in colour represent detectible connections in seven or more patients. These connections traversed the internal capsule and extended into the ventral/lateral region of the thalamus. Lateral to the thalamus, connections to the parietal operculum/secondary somatosensory cortex were also detected. The anatomical image is the average of the structural MRIs of the lesion patients. R = right SII = secondary somatosensory cortex.
Discussion
Pain is an intrinsically intrusive experience that conveys highly salient information relevant to the status of the organism and has clear sensory, affective and motoric dimensions (Price, 1999). One brain region, the putamen, is frequently activated during studies of pain. As part of the basal ganglia, this structure has been traditionally associated with motor functions; however, its potential contributions to pain have remained unclear. The present investigation now provides direct evidence that the putamen also contributes to sensory aspects of pain. Patients with putamen lesions exhibited decreased pain sensitivity and widespread decreases in pain-related brain activation. This broad pattern of decreased activity indicates that the putamen may influence the activity of numerous brain regions engaged in different dimensions of the pain experience, and accordingly, may be critically involved in the shaping of the pain experience. Consistent with these changes in brain activation associated with lesions of the putamen, data from structural connectivity analyses in healthy subjects support the idea that regions of the putamen activated during pain interact with numerous cortical regions. Bilateral portions of the putamen activated during pain were shown to be connected not only to brain areas involved in sensory–motor processes but also to regions that play important roles in attention, affect and memory. Taken together, these data provide multiple, converging lines of evidence that indicate that the basal ganglia, and specifically, circuits that involve the putamen may contribute importantly to the processes that transform nociceptive information to a subjectively available experience of sensory features of pain such as intensity.
Impact of damage to the putamen versus adjacent structures on sensory processing
Studies of patients with naturally occurring brain lesions are challenging because of variability introduced by the heterogeneous nature of the regions affected by the lesion. To minimize these concerns, extra caution was applied to recruit patients with a significant overlap of lesion location. In the present investigation, all nine lesion patients had damage that encompassed the posterior/dorsal regions of the left putamen. Although an overlapping portion of the putamen was damaged, the lesions varied substantially in size. However, regression analysis confirmed that individuals with the largest differences in pain sensitivity had lesions that were smaller and more specific to the putamen (Fig. 5B; P = 0.02). Although some of our subjects had lesions that extended beyond the confines of putamen to include parts of the thalamus, we found no relationship between volume of the thalamus affected by the lesion and side differences in pain ratings or thermal thresholds (P = 0.99, all P > 0.13, respectively). Similarly, some patients had lesions that could have potentially affected parts of the internal capsule and disrupted thalamic projections to primary somatosensory cortex. However, seven of nine patients with lesions had sufficiently intact connections between the thalamus and primary somatosensory cortex to be detected with diffusion tensor imaging. Taken together with the observation that patients had intact tactile thresholds, these findings provide strong evidence that the observed changes in pain in patients were primarily attributable to damage to the putamen rather than deafferentation that occurred due to damage of the thalamus or internal capsule. Thus, these findings are distinct from sensory changes that occur following lesions that affect primary somatosensory cortex and/or projections to primary somatosensory cortex (Knecht et al., 1996). Primary somatosensory cortex lesions produce pronounced tactile deficits while leaving pain and temperature sensation mostly intact.
Integration of cognitive information within the putamen and basal ganglia
The striatum, composed of the caudate nucleus and putamen, serves as the major input structure of the basal ganglia and is at the centre of cortico-basal ganglia-thalamo-cortical loops (Alexander and Crutcher, 1990; Parent and Hazrati, 1995). In this framework, information from various cortical, limbic and thalamic areas accesses the striatum via segregated parallel channels (Alexander and Crutcher, 1990). In the present investigation, regions of the putamen activated during pain were structurally connected to various brain areas involved in sensory and affective aspects of nociceptive processing, including the thalamus, insula and anterior cingulate cortex (Fig. 8B). Also, brain areas associated with memory such as the amygdala and hippocampus (Murray and Mishkin, 1983; Ploghaus et al., 2001; Smith et al., 2004; Marschner et al., 2008) as well as those involved in attention- and premotor-related processes (i.e. Brodmann area 8, middle frontal gyrus, anterior cingulate cortex and supplementary motor area) were structurally connected with regions of the putamen activated during pain (Posner et al., 1980; Mesulam, 1999; Peyron et al., 1999; Nobre, 2001; Knudsen, 2007; Corbetta et al., 2008).
Although cortico-basal ganglia-thalamo-cortical loops are largely segregated, a degree of convergence and interaction among information within various loops, both within the basal ganglia and extrinsic structures, are facilitated by overlapping cortico-striatal terminal arborizations as well as by diverging efferent projections from different parallel loops that overlap at each relay point of the pathway (i.e. striatum, output nuclei, thalamus and cortex) (Joel and Weiner, 1994; Groenewegen et al., 1999; Haber, 2003; Haber et al., 2006; Calzavara et al., 2007). In addition, striatal medium spiny projection neurons can also facilitate integration since they receive large numbers of projections from both the cerebral cortex and intrinsic basal ganglia neurons (Parent and Hazrati, 1995). This configuration allows the putamen, as part of the striatum, to be well positioned to integrate information from a wide array of regions of the cerebral cortex and can provide a cognitive context for information processing.
Selective engagement of cortical networks by the putamen and the basal ganglia
The striatum is known to regulate thalamic activity via a process of disinhibition (Chevalier and Deniau, 1990). The striatum sends inhibitory projections to the internal segment of the globus pallidus and substantia nigra pars reticula, which in turn send inhibitory projections to the thalamus. Because the globus pallidus and substantia nigra pars reticula have high spontaneous activity, their thalamic targets are normally inhibited (Delong et al., 1984). However, when the striatum is activated (as by excitatory cortico-striatal inputs), the output nuclei are phasically inhibited, allowing their thalamic targets to become active. This process of disinhibition is the fundamental way in which the basal ganglia modulate thalamic activity (Chevalier and Deniau, 1990) and, by virtue of excitatory thalamic projections, cortical areas. Consistent with this circuitry, putamen activation during pain was associated with activation in several thalamic regions including ventral lateral, ventral anterior and medial dorsal nuclei. These thalamic nuclei, in turn, project back to widespread cortical regions including frontal, parietal, insula and cingulate areas (Mufson and Mesulam, 1984; Vogt et al., 1987; Alexander and Crutcher, 1990; Alexander et al., 1990; Parent and Hazrati, 1995; Middleton and Strick, 2000). Thus, the putamen can shape activity in large areas of cortex via differentially modulating the levels of inhibition sent into the thalamus. Furthermore, sustained activity of cortical networks including parietal, frontal and cingulate regions during task performance is frequently accompanied by sustained activity within the putamen (Koski et al., 1999; Coull et al., 2000; Downar et al., 2003). Moreover, a reduction in putamen activation during a pharmacologically induced decreased state of consciousness has been linked to a decrease in responsiveness to noxious stimuli as well as decreased connectivity between putamen and other brain areas (Mhuircheartaigh et al., 2010). Taken together with its role in the integration of cognitive information, the putamen may contribute importantly to the selection of behaviourally relevant processes by enabling engagement of specific sets of cortical networks needed to accomplish the process (Redgrave et al., 1999; Balleine et al., 2007). In the case of pain, the putamen may be able to utilize the cognitive context to influence the selection of appropriate brain networks that are engaged during the inflow of afferent nociceptive information.
Biasing of selection by afferent nociceptive information
In addition to cortico-striatal inputs, the putamen also receives direct excitatory inputs from the midline and intralaminar thalamic nuclei (Mengual et al., 1999; Van der Werf et al., 2002) that serve as major recipients of direct spinal nociceptive afferents (Willis, 1985). Thus, ascending nociceptive inputs can have short-latency access to the putamen via these thalamic relays. Moreover, substantia nigra/ventral tegmental area was also structurally connected with regions of the putamen activated during pain. The substantia nigra pars compacta/ventral tegmental area receives direct connections from the parabrachial nucleus in the midbrain (Schneider, 1986; Vankova et al., 1992), which receives direct afferent nociceptive information from the spinal cord (Bernard and Besson, 1990; Craig, 1995; Klop et al., 2005). Dopamine can modulate the cortico-striatal information transfer via dopamine receptors on the striatal projection neurons (Parent and Hazrati, 1995). These findings suggest that the dopaminergic regions of the midbrain may be in a position to utilize nociceptive information to influence striatal activity by modulating the efficiency of cortico-striatal terminals. These modulatory mesostriatal dopaminergic projections, together with nociceptive input conveyed by thalamo-striatal projections from the intralaminar/midline thalamus, are well positioned to bias the selection process to allow incoming nociceptive information to outcompete ongoing cognitive processes. Similarly, there are nociceptive afferents that project to the globus pallidus. This also allows nociceptive information from the spinal cord to directly access a major nucleus in the basal ganglia (Braz et al., 2005). These circuitries may contribute substantially to the cognitive intrusiveness of pain.
Disruption of selection by lesions of the putamen
Lesions of the putamen may affect the engagement of appropriate brain networks that are normally responsible for processing nociceptive information during pain. Consistent with this postulate, patients with putamen lesions exhibited significantly lower pain ratings when compared with healthy subjects and exhibited diffuse and generalized reduction in pain-related brain activation (Figs 4 and 6). Nevertheless, pain ratings and pain-related brain activation were not completely abolished, suggesting that nociceptive information from the spinal cord can still reach brain areas necessary for the instantiation of the pain experience. However, a more potent engagement of cortical networks appears to require the contribution of the putamen and associated portions of the basal ganglia.
The selection of cortical networks by the basal ganglia needs to be coordinated across hemispheres in order to integrate bilateral cognitive input with afferent sensory information. A portion of the projections of the output nuclei of the basal ganglia to the thalamus are crossed, thereby allowing the putamen on one side to exert its influence on the cortex bilaterally (Parent and De Bellefeuille, 1982; Morgan et al., 1983; Parent et al., 1983; Francois et al., 1984). Moreover, nociceptive neurons in the putamen often have large and bilateral receptive fields (Chudler et al., 1993). Thus, both the left and right putamen will contribute to processing information from a unilateral stimulus. Presumably, loss of the putamen on one side could produce perceptual deficits and reduced cortical engagement to both contralateral as well as ipsilateral stimuli. Given that thresholds are more susceptible to disruption than responses to suprathreshold stimuli, relatively subtle effects, such as the observed altered cold pain thresholds, could occur bilaterally, while responses to suprathreshold stimuli would largely be disrupted only contralaterally. Nevertheless, nociceptive stimulation may engage many cortical processes (attention, affect, reflection and interpretation) that are secondary to the generation of a percept of pain intensity but instead contribute to the totality of the subjective experience of pain. Thus, the reduced brain activation during stimulation of the unaffected side may indicate that the experience of nociceptive information is not fully elaborated.
Given that the heat pain thresholds and tactile thresholds of putamen-lesioned patients were virtually normal when compared with healthy subjects, the reduced pain sensitivity of these individuals cannot be attributed merely to non-specific decreases in somatosensory capabilities following the stroke. In addition, changes in pain sensitivity are likely due to lesions specific to the putamen. Patients with focal lesions limited to the putamen exhibited greater differences in pain intensity ratings between sides than patients with the larger lesions that also encompassed other structures outside of the putamen (Fig. 5).
Limitations
As in all lesion studies, the damage that occurs is unique to each individual. As noted above, converging lines of evidence indicate that the altered processing of heat pain in lesion patients is likely due to damage of the putamen per se rather than to deafferentation resulting from damage to the thalamus or internal capsule. Given the challenges of recruiting non-pain patients for a study involving pain, sample sizes are typically relatively small. Accordingly, the ability to control for time after lesion and/or gender is limited by the need to obtain a sufficiently large number of subjects with appropriate lesions. However, no statistically significant relationships were found between time after lesion and pain ratings (P = 0.44) or thermal thresholds (all P > 0.16). In addition, gender distributions were not significantly different between groups (P = 0.20). Thus, these variables can be excluded as potential confounders.
In addition, the relatively small number of lesion subjects also influenced the choice of statistical treatment of between-group comparisons. To maximize the ability to detect differences in pain-related brain activation between the lesion patients and healthy subjects, fixed-effects analyses were employed in between-group analyses. These analyses are more powerful than random-effects analyses for relatively small numbers of subjects, but may be less generalizable to the general population.
Additional limitations of this study are intrinsic to diffusion tensor imaging connectivity analyses in general. These analyses do not allow us to define true fibre distribution and polarity of fibre tracts within a single-seed voxel and may have limited sensitivity to detect smaller fibre tracts (Behrens et al., 2003a). Nevertheless, the findings of connectivity analyses in the present investigation are consistent with those of previous anatomical studies (Mufson and Mesulam, 1984; Vogt et al., 1987; Alexander and Crutcher, 1990; Alexander et al., 1990; Parent and Hazrati, 1995; Middleton and Strick, 2000).
Finally, the experience of pain encompasses multiple sensory aspects, including not only intensity, but also location, quality and duration. The present investigation examined but one sensory dimension—intensity; future investigations will be needed to clarify the role of this structure and related circuitry of the basal ganglia in other sensory aspects of pain.
Conclusion
The present data provide multiple lines of evidence indicating that the putamen is involved in sensory components of pain-related processes and that this role may extend far beyond its well-known involvement in motor processes. The putamen and associated neural networks may contribute importantly to neural processes that are involved in influencing and determining the behavioural relevance and saliency of incoming nociceptive information. These processes are determined not only by the strength of the sensory input, but also by other external environmental and internal cognitive information unique to each individual. Thus, these processes contribute importantly to the effectiveness by which nociceptive input is elaborated into an experience of pain.
Supplementary material
Supplementary material is available at Brain online.
Funding
This study is supported and funded by the National Institutes of Health (R01 NS 39426).
Supplementary Material
Acknowledgements
The authors thank FMRIB Image Analysis Group, Oxford University for the FSL analysis software and Stephania Jordan for her help in collecting data for the experiment.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–71. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, "prefrontal" and "limbic" functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, et al. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30:692–9. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–57. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003a;6:750–7. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003b;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–90. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bingel U, Glascher J, Weiller C, Buchel C. Somatotopic representation of nociceptive information in the putamen: an event-related functional MRI study. Cereb Cortex. 2004;14:1340–5. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel "pain" pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–93. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Calzavara R, Mailly P, Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24 c: an anatomical substrate for cognition to action. Eur J Neurosci. 2007;26:2005–24. doi: 10.1111/j.1460-9568.2007.05825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–80. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Chudler EH. Response properties of neurons in the caudate-putamen and globus pallidus to noxious and non-noxious thermal stimulation in anesthetized rats. Brain Res. 1998;812:283–8. doi: 10.1016/s0006-8993(98)00971-8. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60:3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Sugiyama K, Dong WK. Nociceptive responses in the neostriatum and globus pallidus of the anesthetized rat. J Neurophysiol. 1993;69:1890–903. doi: 10.1152/jn.1993.69.6.1890. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. J Neurophysiol. 2001;85:2602–12. doi: 10.1152/jn.2001.85.6.2602. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–43. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14:4095–108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Buchel C, Nobre AC. Orienting attention in time: behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38:808–19. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol. 1995;361:225–48. doi: 10.1002/cne.903610204. [DOI] [PubMed] [Google Scholar]
- Delong MR, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT, Alexander GE. Functional organization of the basal ganglia: contributions of single-cell recording studies. Ciba Found Symp. 1984;107:64–82. doi: 10.1002/9780470720882.ch5. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D. Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology. 2004;62:2171–5. doi: 10.1212/01.wnl.0000130455.38550.9d. [DOI] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage. 2003;20:1540–51. doi: 10.1016/s1053-8119(03)00407-5. [DOI] [PubMed] [Google Scholar]
- Francois C, Percheron G, Yelnik J. Localization of nigrostriatal, nigrothalamic and nigrotectal neurons in ventricular coordinates in macaques. Neuroscience. 1984;13:61–76. doi: 10.1016/0306-4522(84)90259-8. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–65. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Wang X, Harden RN, Paice JA, Apkarian AV. Brain dynamics for perception of tactile allodynia (touch-induced pain) in postherpetic neuralgia. Pain. 2008;138:641–56. doi: 10.1016/j.pain.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Makris N, Ahern T, O’Brien LM, Caviness VS, Jr, et al. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry. 2007;61:935–45. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Galis-de Graaf Y, Smeets WJ. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat. 1999;16:167–85. doi: 10.1016/s0891-0618(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–30. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–76. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain. 2006;123:169–78. doi: 10.1016/j.pain.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Aalto S, Rinne JO, Scheinin H, Taiminen T, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003a;106:43–8. doi: 10.1016/s0304-3959(03)00275-6. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003b;101:149–54. doi: 10.1016/s0304-3959(02)00323-8. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Jaaskelainen SK, Martikainen IK, Mansikka H, Forssell H, Scheinin H, et al. Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol. 2004;500:187–92. doi: 10.1016/j.ejphar.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Martikainen IK, Mansikka H, Hinkka S, Nagren K, Hietala J, et al. Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain. 2002;99:273–9. doi: 10.1016/s0304-3959(02)00121-5. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–79. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA. 2004;101:13335–40. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–9. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Biol Sci. 1991;244:39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- Klop EM, Mouton LJ, Hulsebosch R, Boers J, Holstege G. In cat four times as many lamina I neurons project to the parabrachial nuclei and twice as many to the periaqueductal grey as to the thalamus. Neuroscience. 2005;134:189–97. doi: 10.1016/j.neuroscience.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Knecht S, Kunesch E, Schnitzler A. Parallel and serial processing of haptic information in man: effects of parietal lesions on sensorimotor hand function. Neuropsychologia. 1996;34:669–87. doi: 10.1016/0028-3932(95)00148-4. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Koski L, Paus T, Hofle N, Petrides M. Increased blood flow in the basal ganglia when using cues to direct attention. Exp Brain Res. 1999;129:241–6. doi: 10.1007/s002210050894. [DOI] [PubMed] [Google Scholar]
- Koyama T, Kato K, Mikami A. During pain-avoidance neurons activated in the macaque anterior cingulate and caudate. Neurosci Lett. 2000;283:17–20. doi: 10.1016/s0304-3940(00)00894-6. [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The single-epoch functional MRI design: validation of a simplified paradigm for the collection of subjective ratings. Neuroimage. 2003;19:976–87. doi: 10.1016/s1053-8119(03)00119-8. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analysed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83:155–71. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–6. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengual E, de las Heras S, Erro E, Lanciego JL, Gimenez-Amaya JM. Thalamic interaction between the input and the output systems of the basal ganglia. J Chem Neuroanat. 1999;16:187–200. doi: 10.1016/s0891-0618(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–46. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Morgan S, Huston JP, Pritzel M. Effects of reducing sensory-motor feedback on the appearance of crossed nigro-thalamic projections and recovery from turning induced by unilateral substantia nigra lesions. Brain Res Bull. 1983;11:721–7. doi: 10.1016/0361-9230(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol. 1984;227:109–20. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Severe tactual memory deficits in monkeys after combined removal of the amygdala and hippocampus. Brain Res. 1983;270:340–4. doi: 10.1016/0006-8993(83)90610-8. [DOI] [PubMed] [Google Scholar]
- Nobre AC. The attentive homunculus: now you see it, now you don’t. Neurosci Biobehav Rev. 2001;25:477–96. doi: 10.1016/s0149-7634(01)00028-8. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting spatial discrimination of pain. J Neurosci. 2007;27:3388–94. doi: 10.1523/JNEUROSCI.5128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, De Bellefeuille L. Organization of efferent projections from the internal segment of globus pallidus in primate as revealed by fluorescence retrograde labeling method. Brain Res. 1982;245:201–13. doi: 10.1016/0006-8993(82)90802-2. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Parent A, Mackey A, Smith Y, Boucher R. The output organization of the substantia nigra in primate as revealed by a retrograde double labeling method. Brain Res Bull. 1983;10:529–37. doi: 10.1016/0361-9230(83)90151-x. [DOI] [PubMed] [Google Scholar]
- Pedersen JL, Kehlet H. Hyperalgesia in a human model of acute inflammatory pain: a methodological study. Pain. 1998a;74:139–51. doi: 10.1016/s0304-3959(97)00160-7. [DOI] [PubMed] [Google Scholar]
- Pedersen JL, Kehlet H. Secondary hyperalgesia to heat stimuli after burn injury in man. Pain. 1998b;76:377–84. doi: 10.1016/S0304-3959(98)00070-0. [DOI] [PubMed] [Google Scholar]
- Pertovaara A, Martikainen IK, Hagelberg N, Mansikka H, Nagren K, Hietala J, et al. Striatal dopamine D2/D3 receptor availability correlates with individual response characteristics to pain. Eur J Neurosci. 2004;20:1587–92. doi: 10.1111/j.1460-9568.2004.03622.x. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, et al. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122(Pt 9):1765–80. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Piche M, Arsenault M, Rainville P. Dissection of perceptual, motor and autonomic components of brain activity evoked by noxious stimulation. Pain. 2010;149:453–62. doi: 10.1016/j.pain.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–74. [PubMed] [Google Scholar]
- Price DD. Psychological mechanisms of pain and analgesia. Vol. 15. Seattle, WA: IASP Press; 1999. [Google Scholar]
- Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–26. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol. 1989;62:1270–9. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–23. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, et al. Affective components and intensity of pain correlate with structural differences in grey matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, et al. Striatal grey matter increase in patients suffering from fibromyalgia–a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–16. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Schneider JS. Interactions between the basal ganglia, the pontine parabrachial region, and the trigeminal system in cat. Neuroscience. 1986;19:411–25. doi: 10.1016/0306-4522(86)90271-x. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased grey matter density in young women with chronic vulvar pain. Pain. 2008;140:411–9. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–95. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Henson RN, Dolan RJ, Rugg MD. Functional MRI correlates of the episodic retrieval of emotional contexts. Neuroimage. 2004;22:868–78. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Sprenger T, Berthele A, Platzer S, Boecker H, Tolle TR. What to learn from in vivo opioidergic brain imaging? Eur J Pain. 2005;9:117–21. doi: 10.1016/j.ejpain.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Tailarach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–40. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vankova M, Arluison M, Leviel V, Tramu G. Afferent connections of the rat substantia nigra pars lateralis with special reference to peptide-containing neurons of the amygdalo-nigral pathway. J Chem Neuroanat. 1992;5:39–50. doi: 10.1016/0891-0618(92)90032-l. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey. I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–70. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Willer JC. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain. 1977;3:69–80. doi: 10.1016/0304-3959(77)90036-7. [DOI] [PubMed] [Google Scholar]
- Willis WD. Nociceptive pathways: anatomy and physiology of nociceptive ascending pathways. Philos Trans R Soc Lond B Biol Sci. 1985;308:253–70. doi: 10.1098/rstb.1985.0025. [DOI] [PubMed] [Google Scholar]
- Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–82. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.