Abstract
The olfactocentric paralimbic cortex plays a critical role in the regulation of emotional and neurovegetative functions that are disrupted in core features of bipolar disorder. Adolescence is thought to be a critical period in both the maturation of the olfactocentric paralimbic cortex and in the emergence of bipolar disorder pathology. Together, these factors implicate a central role for the olfactocentric paralimbic cortex in the development of bipolar disorder and suggest that abnormalities in this cortex may be expressed by adolescence in the disorder. We tested the hypothesis that differences in olfactocentric paralimbic cortex structure are a morphological feature in adolescents with bipolar disorder. Subjects included 118 adolescents (41 with bipolar disorder and 77 healthy controls). Cortical grey matter volume differences between adolescents with and without bipolar disorder were assessed with voxel-based morphometry analyses of high-resolution structural magnetic resonance imaging scans. Compared with healthy comparison adolescents, adolescents with bipolar disorder demonstrated significant volume decreases in olfactocentric paralimbic regions, including orbitofrontal, insular and temporopolar cortices. Findings in these regions survived small volume correction (P < 0.05, corrected). Volume decreases in adolescents with bipolar disorder were also noted in inferior prefrontal and superior temporal gyri and cerebellum. The findings suggest that abnormalities in the morphology of the olfactocentric paralimbic cortex may contribute to the bipolar disorder phenotype that emerges in adolescence. The morphological development of the olfactocentric paralimbic cortex has received little study. The importance of these cortices in emotional and social development, and support for a central role for these cortices in the development of bipolar disorder, suggest that study of the development of these cortices in health and in bipolar disorder is critically needed.
Keywords: bipolar disorder, magnetic resonance imaging, brain cortex, prefrontal, anterior temporal lobe
Introduction
The hallmark of bipolar disorder is affective dysregulation characterized by the emotional elevations of manic episodes and the emotional decreases of depressive episodes. Although the affective changes are prominent features of bipolar disorder, the acute mood episodes of bipolar disorder invariably also include symptoms ranging from disruptions in neurovegetative functions to deficits in higher order cognition. The neural basis for components of this constellation of symptoms in bipolar disorder is suggested by reports of morphological differences in varying combinations of amygdala, paralimbic and heteromodal prefrontal cortices in adults with bipolar disorder (Drevets et al., 1997; Pearlson et al., 1997; Strakowski et al., 1999; Altshuler et al., 2000; Blumberg et al., 2002, 2003a, 2006; Lopez-Larson et al., 2002; Kasai et al., 2003; Lochhead et al., 2004; McIntosh et al., 2004; Lyoo et al., 2006; Nugent et al., 2006; Rosso et al., 2007; Arnone et al., 2009; Ha et al., 2009; Stanfield et al., 2009; Womer et al., 2009a; Zuliani et al., 2009; Ellison-Wright et al., 2010). However, a unifying model that can help to explain the development of the range of behaviours central to bipolar disorder is needed. We previously suggested that as adolescence is a critical period in the emergence of bipolar disorder, brain regions that undergo maturational changes by this epoch, and that subserve the functions characteristically involved in the disorder, are likely to be important in the developmental pathophysiology of bipolar disorder (Blumberg et al., 2002, 2003b, 2004, 2006; Fredericks et al., 2006; Kalmar et al., 2009a; Womer et al., 2009a). These factors implicate the olfactocentric paralimbic cortex (OPC) as central in the development of bipolar disorder.
The OPC, including orbitofrontal, insular and temporopolar cortices, is a group of highly interconnected structures that develop in concert to comprise critical elements in the neural system subserving emotional regulation (Mesulam et al., 1985; Mesulam, 1998, 2000). As a transition zone from agranular to granular cortex, the OPC sits between autonomic, limbic and heteromodal structures with which it shares major reciprocal connections. The principal limbic afferents to the OPC are derived from the amygdala (Amaral et al., 1984; Mesulam, 2000). The OPC also contains cortical regions with the highest density of connections with the hypothalamus (Nauta, 1971; Mesulam, 2000). Thus, the OPC is in position to process information regarding internal states to provide feedback to adaptively regulate affective processing, as well as circadian rhythms, sleep, appetite and sexual functions that are often the first functions to show disruptions at the onset of bipolar disorder episodes (Bunney et al., 1972). OPC connections from sensory, motor and higher order heteromodal cortices provide a continual update of conditions in the extra-personal world (Morecraft et al., 1992; Carmichael et al., 1995; Rempel-Clower et al., 1998; Mesulam, 2000; Olson et al., 2007). The OPC is thus in position to regulate emotional responses to be optimally adaptive by integrating information from both the internal milieu and extra-personal world and returning feedback to help to regulate the responses of the connection sites (Mesulam, 1998, 2000). This suggests that OPC abnormalities could lead to emotional responses that are maladaptive given internal and external conditions and could potentially result in the range of affective, vegetative and cognitive symptoms of bipolar disorder.
A role for the OPC in bipolar disorder is supported by longstanding reports of OPC lesions producing symptoms similar to those of bipolar disorder, including manic- and depressive-type symptoms in association with orbitofrontal and temporal lesions, as well as cycling between mania and depression in association with temporal pathology (Flor-Henry, 1976; Starkstein et al., 1990; Migliorelli et al., 1993; Damasio et al., 1994; Rolls et al., 1994; Bechara et al., 1999; Murai et al., 2003). In addition to structural neuroimaging studies, functional neuroimaging studies of adults with bipolar disorder report varying combinations of abnormalities in orbitofrontal, insular and temporopolar cortices, as well as their amygdala, prefrontal and cerebellum connection sites (Altshuler et al., 2005; Blumberg et al., 2005; Strakowski et al., 2005; Fredericks et al., 2006; Keener et al., 2007; Malhi et al., 2007, 2008; Wessa et al., 2007; McIntosh et al., 2008; Kalmar et al., 2009a; Kim et al., 2009; Womer et al., 2009a). Dysregulated ventral prefrontal cortex and insula responses have been reported in functional neuroimaging studies of adolescents with bipolar disorder during emotional processing, implicating pathology in the OPC in adolescents with the disorder (Chang et al., 2004; Rich et al., 2006; Dickstein et al., 2007; Pavuluri et al., 2007, 2008, 2009). Structural neuroimaging studies of small samples of adolescents with bipolar disorder have reported abnormalities in one or more OPC structures, including orbitofrontal volume decreases in 10 adolescents with bipolar disorder (Wilke et al., 2004) and in 11 adolescent males with bipolar disorder (Najt et al., 2007), and insular volume decreases in eight adolescents with bipolar disorder (Farrow et al., 2005). These suggest the presence of OPC morphological differences in adolescents with bipolar disorder.
In the present study, we used high-resolution structural MRI and voxel-based morphometry to study cortical morphology in adolescents with bipolar disorder, compared with adolescent healthy controls. We hypothesized that volume decreases would be present in the OPC, and might also be present in cortical regions highly connected to the OPC, in adolescents with bipolar disorder.
Materials and methods
Subjects
The adolescent healthy control group was composed of 77 adolescents [ages 11–21 years, mean age = 16.55 ± 3.08 years, 33 (43%) female]. The bipolar disorder group was composed of 41 adolescents with bipolar disorder [ages 10–21 years, mean age = 16.93 ± 2.62 years, 21 (51%) female]. Participants were without a history of other neurological disorders or loss of consciousness >5 min or significant medical disorders, with the exception of one subject with bipolar disorder with treated hypothyroidism. The presence or absence of Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; American Psychiatric Association 1994) Axis I disorders and mood state were confirmed by administration of the revised Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version to participants and parents/guardians of participants aged ≤18, or the Structural Clinical Interview for DSM-IV Axis I Disorders for participants over the age of 18 years. At the time of scanning, 14 (34.1%) participants with bipolar disorder met criteria for an elevated mood episode (manic, mixed or hypomanic), eight (19.5%) for a depressive episode and 19 (46.3%) were euthymic. Seventeen (41.5%) participants with bipolar disorder met criteria for rapid cycling, and eight (19.5%) reported chronic daily cycling; cycling rate could not be determined for one participant. Fifteen (36.6%) participants with bipolar disorder had a history of psychotic symptoms and 10 (24.4%) had a history of substance-related disorders. Psychotropic medications prescribed to the adolescents with bipolar disorder at the time of scanning included lithium carbonate (n = 7, 17.1%), anticonvulsants (n = 16, 39.0%), atypical antipsychotics (n = 18, 43.9%), antidepressants (n = 12, 29.3%), stimulants (n = 8, 19.5%) and benzodiazepines (n = 3, 7.3%). Ten (24.4%) of the bipolar disorder participants were not medicated at the time of scanning.
Following a complete description of the research, written informed consent was obtained from parents/guardians and participants 18 years and older. Written informed assent was obtained from minors. This research was approved by the human investigation committees of the Yale School of Medicine and the Department of Veterans Affairs.
Structural imaging data acquisition
High-resolution structural MRI scanning was performed on a 3-Tesla Siemens Trio Magnetic Resonance scanner (Siemens). A 3D magnetization prepared rapid acquisition gradient echo T1-weighted sequence was used to acquire sagittal images with parameters: repetition time = 1500 ms, echo time = 2.83 ms, field of view = 256 × 256 mm2, matrix = 256 × 256, slice thickness = 1.0 mm without gap, 160 slices and two averages.
Structural MRI data processing and analysis
Images were processed and analysed with Statistical Parametric Mapping 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm), as detailed in our previous publications (Tang et al., 2007; Kalmar et al., 2009b). Briefly, the SPM5 segmentation function was implemented for bias correction, spatial normalization and segmentation of the original structural images in the same model. Bias correction produced images with more uniform intensities. SPM5 tissue probability maps (voxel size 2 × 2 × 2 mm3) were used to guide normalization and segmentation. During spatial normalization, a ‘modulation’ step was used to ensure that the overall amount of tissue in a class was not altered. The segmented, normalized and modulated grey matter images were smoothed with an 8 mm, full width at half maximum, isotropic Gaussian kernel. Following the preprocessing, voxel-based analysis of covariance (ANCOVA) was used to compare grey matter volume between the bipolar disorder and healthy control groups with SPM5, including age as a covariate. Findings were considered significant at a height threshold of P < 0.001 uncorrected for multiple comparisons and an extent threshold of 100 voxels. Analyses were also performed with small volume correction for multiple comparisons (P < 0.05, corrected) to further confirm the findings for the hypothesized OPC regions. Regression analyses were performed in the bipolar disorder group with duration of illness as a continuous, independent variable to explore the relationship between duration of illness and the volumes of the regions showing significant differences between the adolescent healthy control and bipolar disorder groups. An exploratory analysis (univariate analysis of variance) assessed potential diagnosis by age interactions in the regions of significant volume differences. Additional exploratory analyses (ANCOVA) were performed for effects of mood state, rapid cycling, history of psychotic symptoms, history of substance-related disorders and medications (overall presence or absence of medication, as well as the presence or absence of lithium carbonate, anticonvulsants, atypical antipsychotics and stimulants) on the regions that showed significant differences between the adolescent healthy control and bipolar disorder groups.
Results
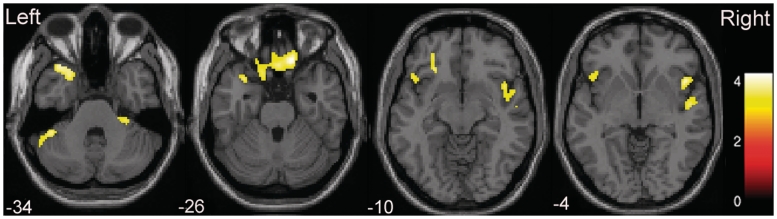
The adolescent healthy control and bipolar disorder groups did not differ significantly in age (P = 0.5) or sex distribution (P = 0.36). Volumes were significantly reduced in adolescents with bipolar disorder, compared with adolescent healthy controls, in OPC regions, including orbitofrontal cortex [Brodmann areas (BA) 11/47], insula and temporopolar cortex (BA 38). Additional areas of decreased volume in the adolescent bipolar disorder group, compared with the adolescent healthy control group, included the inferior frontal gyrus (BA 44/45), superior temporal gyrus (BA 22) and cerebellum (Fig. 1, Table 1). The OPC findings remained significant with small volume correction (P < 0.05, corrected). There was no region showing significantly increased grey matter volumes in the bipolar disorder group, compared with the adolescent healthy control group.
Figure 1.
Regions of reduced cortical volume in adolescents with bipolar disorder. The axial images (z = −34 mm, −26 mm, −10 mm and −4 mm Montreal Neurological Institute coordinate planes) show the regions of significantly decreased volumes in olfactocentric paralimbic cortices in adolescents with bipolar disorder, relative to adolescent healthy controls. Statistically significant differences in grey matter volume were defined as P < 0.001, with a cluster size of 100 adjacent voxels. The colour bar represents the range of T-values.
Table 1.
Areas of reduced cortical volume in adolescents with bipolar disorder compared with healthy control adolescents
| Cortical regions (Brodmann area) | Cluster size | MNI coordinates |
T-values | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Bilateral orbitofrontal (11/47) | 416 | 14 | 32 | −26 | 4.18 |
| 2 | 26 | −26 | 3.75 | ||
| −14 | 20 | −26 | 3.58 | ||
| Left orbitofrontal (47), left temporal pole (38) | 357 | −26 | 28 | −12 | 4.12 |
| −26 | 20 | −36 | 4.07 | ||
| −34 | 22 | −34 | 3.93 | ||
| Left orbitofrontal (47), left insula | 150 | −52 | 20 | 0 | 3.90 |
| −46 | 22 | −10 | 3.50 | ||
| −36 | 28 | 0 | 3.42 | ||
| Right inferior frontal (44/45), right insula | 643 | 54 | 20 | 30 | 4.24 |
| Right superior temporal (22) | 46 | 14 | −4 | 3.95 | |
| 52 | −8 | −6 | 3.88 | ||
| Left cerebellum | 162 | −42 | −42 | −36 | 3.89 |
| −52 | −48 | −38 | 3.78 | ||
| Right cerebellum | 130 | 32 | −40 | −46 | 3.74 |
| 26 | −40 | −56 | 3.65 | ||
| 32 | −30 | −34 | 3.56 | ||
There was no significant effect of illness duration or interaction between diagnosis and age in the regions of significant volume difference between the adolescent healthy control and bipolar disorder groups. Exploratory analyses did not reveal significant effects of clinical factors, including mood state (P > 0.1), rapid cycling (P > 0.1), history of psychotic symptoms (P > 0.2) or of substance-related disorders (P > 0.3), within the bipolar disorder group in the regions of group differences.
There were no significant effects of medication on volumes in the regions that differed between the adolescent healthy control and bipolar disorder groups. However, trends were noted at the low threshold of P < 0.05 (uncorrected) for higher frontotemporal volumes, including in orbital and inferior frontal, temporal polar and association cortices, in adolescents with bipolar disorder who were taking medications at the time of study than in those who were not; there were no frontotemporal areas in which medication was associated with lower volume. Lithium carbonate appeared to contribute to the increases in association with medication in orbitofrontal cortex as there was a region of increased volume in orbitofrontal cortex (BA 11) in adolescents prescribed lithium carbonate, compared with adolescents not prescribed lithium carbonate. Anticonvulsants also appeared to contribute to the effects in orbitofrontal cortex, showing increased volume in orbitofrontal cortex (BA 47) in adolescents prescribed anticonvulsants, as compared with adolescents who were not. In addition, the adolescents taking anticonvulsants or stimulants had higher volumes in temporal association cortex (BA 22).
Discussion
This study supports the presence of hypothesized decreases in OPC volume in adolescents with bipolar disorder, compared with healthy adolescents, including in orbitofrontal cortex, insula and temporopolar cortices. Additional regions of decreased volume in adolescents with bipolar disorder also included the inferior frontal cortex, superior temporal gyrus and cerebellum.
The presence of structural abnormalities in the OPC in adolescents with bipolar disorder implicates the OPC in the development of the disorder. Childhood and adolescence are periods of significant cortical development. Among prefrontal cortex regions, the dorsolateral prefrontal cortex has often been the focus of studies of development, demonstrating synaptic progression prior to adolescence followed by regression during adolescence thought to subserve the development of more adaptive behavioural control (Goldman-Rakic, 1987; Bourgeois et al., 1994; Lewis, 1997). The OPC structures have received less direct study in animal and human models of child and adolescent development. The similarities between OPC and dorsal prefrontal cortex development, and the extent to which they occur in sequence or in parallel, have been debated and remain unknown (Goldman-Rakic, 1987; Machado et al., 2003). Changes demonstrated in OPC neural systems in models of adolescence include development of limbic connections to paralimbic homologues in rat models of adolescence (Cunningham et al., 2002). Structural and functional development of orbitofrontal cortex have also been theorized to subserve the maturation of emotional and social behaviours in adolescence (Machado et al., 2003; Galvan et al., 2006). Less is known about the development of the insular and temporopolar cortices, though these structures subserve affective processes important in bipolar disorder. For example, temporopolar cortex is associated with the integration of sensory with affective processing (Starkstein et al., 1990) and the insula is implicated in self-reflection and monitoring of internal affective states (Damasio et al., 2000; Shafritz et al., 2006; Modinos et al., 2009).
It cannot be determined from this study when in the course of bipolar disorder the OPC abnormalities developed. We speculate that the OPC abnormalities in bipolar disorder may reflect earlier developmental differences. In embryonic development, OPC structures develop in concert, sharing cytoarchitectonic features and connectivity (Mesulam, 2000; Schmahmann et al., 2006). They develop around the allocortical olfactory cortex, shifting from agranular to granular cortex with increased distance from the allocortical core (Mesulam, 1985). Several post-mortem studies have noted cellular differences in the OPC in bipolar disorder. For example, differences in neuronal size and density, and glial number and density in caudal orbitofrontal and subgenual prefrontal cortex regions have been reported in bipolar disorder (Ongur et al., 1998; Rajkowska, 2002; Cotter et al., 2005; Toro et al., 2006; Bielau et al., 2007). It is not known how these cellular findings may relate to development of the transition from agranular to granular cortex in the OPC. We suggest that architectonic study of the transitional cortex of these structures may hold important clues for understanding the developmental neuropathophysiology of bipolar disorder.
We previously reported a progressive divergence in prefrontal cortex volume in a longitudinal within-subject study of a small sample of adolescents and young adults with bipolar disorder over an approximately 2-year period (Kalmar et al., 2009c). The prefrontal cortex regions found in the longitudinal study included ventral prefrontal cortex regions and also extended to regions more rostral to those reported herein. This suggests that differences in more rostral prefrontal cortex regions may be more prominent in the later adolescent and early adult years. We did not detect a significant effect of illness duration or interaction between diagnosis and age in this sample. We suggest that differences in the more caudal, paralimbic prefrontal cortex may emerge earlier than the more rostral ones. However, whether there are progressive changes in OPC over adolescence cannot be determined from the current cross-sectional study. Larger, prospective longitudinal imaging studies of adolescents with bipolar disorder are needed.
Decreases in volume in orbitofrontal and temporopolar cortices have been relatively consistent findings in adults with bipolar disorder (Kasai et al., 2003; Blumberg et al., 2006; Nugent et al., 2006; Stanfield et al., 2009), supporting continuous differences that persist from adolescence into adulthood in the disorder. However, for the insula, although there have been findings of volume decreases, including in recent meta-analyses (Ha et al., 2009; Ellison-Wright et al., 2010), there have been reports of no differences or increases (Kasai et al., 2003; Lochhead et al., 2004; Ha et al., 2009; Ellison-Wright et al., 2010). This raises questions about potential relationships with age in insula in bipolar disorder. Longitudinal examination of orbitofrontal, insular and temporopolar cortices, as well as more rostral cortices, and the relative timing that differences in these subregions emerge, may help to point towards underlying neurodevelopmental mechanisms in bipolar disorder and understanding of age-dependent features of regional differences.
Additional regions in which volume decreases were found in the adolescents with bipolar disorder included the inferior frontal gyrus, superior temporal gyrus, and the cerebellum. The inferior frontal gyrus and superior temporal gyrus are proximal to OPC regions and share substantial connections with them (Morecraft et al., 1992). Functions of the inferior frontal gyrus include adaptive behavioural inhibition (Jonides et al., 1998). Differences in inferior frontal gyrus volume have been reported previously in studies of bipolar disorder (Lopez-Larson et al., 2002) and have been associated with duration of illness, suggesting that changes in inferior frontal gyrus may be the result of episodes. Rich et al. (2008) reported abnormalities in connectivity to temporal association cortex in paediatric bipolar disorder, suggested to contribute to early abnormalities in misperception of social face cues in the disorder. The cerebellum also has substantial interconnections with the OPC (Anand et al., 1959; Snider et al., 1976; Ramnani, 2006), has been implicated in affective functions, and cerebellar lesions have been associated with manic-like symptoms (Yadalam et al., 1985; Supple et al., 1987, 1988; Lauterbach, 1996; Schmahmann et al., 1998; Tavano et al., 2007). Neuroimaging studies of bipolar disorder have reported both structural and functional abnormalities in the cerebellum (DelBello et al., 1999; Ketter et al., 2001; Strakowski et al., 2005; Malhi et al., 2007; Womer et al., 2009b). The relationship between the development of these structures in bipolar disorder and whether they develop simultaneously with, contribute to, or result from early OPC abnormalities is not known.
Among the participants with bipolar disorder, no significant effects were detected for the presence or absence of psychotropic medication on the regions that showed significant differences between the adolescent healthy control and bipolar disorder groups. However, when explored with a less strict threshold, a trend was noted towards higher OPC volumes in adolescents with bipolar disorder prescribed medications than those not taking medications. Lithium carbonate and anticonvulsants appeared to contribute to orbitofrontal increases. This suggests that the volume decreases in the adolescents with bipolar disorder were not due to medication. It also suggests that medications may have the potential to attenuate or reverse volumes deficits in adolescents with bipolar disorder. These findings are consistent with preclinical studies of neurotrophic and neuroprotective effects of these medications, previous studies showing higher volumes as well as normalization of functioning in ventral prefrontal regions in bipolar disorder in association with lithium (Moore et al., 2000; Blumberg et al., 2006), and higher mesial temporal volume and functional normalization in adolescents and adults with bipolar disorder in association with anticonvulsants (Blumberg et al., 2005; Chang et al., 2005). While these data raise hopeful possibilities regarding the potential of medications, as well as the interesting possibility that different medication subtypes may have their most prominent effects in different OPC components, these data should be considered with caution. Our naturalistic study design with regard to medication limits causal inferences that can be made about the association of medication use with brain morphology in bipolar disorder. Future systematic studies with randomized medication assignment of specific medications are needed for more definitive study.
While the distribution of the findings in OPC regions implicates them highly in the social and emotional behaviours and neurovegetative functions disrupted in bipolar disorder, direct associations were not tested in this study. Future studies that directly assess associations to regional brain dysfunction and behaviours are warranted.
In conclusion, these findings suggest that abnormalities in OPC in adolescence may contribute to the emergence of key components of the bipolar disorder phenotype at this time in development. The neurodevelopment of OPC has received little study. The importance of the OPC in the development of emotional and social behaviour in adolescence, and the support for a central role for the OPC in the development of bipolar disorder, suggest that study of OPC development in health and in bipolar disorder is critically needed.
Funding
National Institute of Health (NIH) (R01MH69747 to H.P.B.; RC1MH088366 to H.P.B.; R01MH070902 to H.P.B.; RL1DA024856 to H.P.B., F.W. and J.H.K.; K01MH086621 to F.W.; T32MH14276 to J.H.K. and L.G.C.); NIH National Centre for Research Resources (NCRR) (UL1 RR0249139); Department of Veterans Affairs Research Enhancement Award Program (REAP) (to H.P.B. and L.G.C.); National Alliance for Research on Schizophrenia and Depression (Great Neck, NY) (to F.W., J.H.K. and H.P.B.); The Attias Family Foundation (to H.P.B.); Marcia Simon Kaplan (to J.H.K.); Women's Health Research at Yale – The Ethel F. Donaghue Women's Health Investigator Program at Yale (New Haven, CT) (to H.P.B.); Klingenstein Foundation (to F.W. and J.H.K.).
Acknowledgements
The authors thank Susan Quatrano, B.A. and Philip Markovich, B.A. for their assistance with the study, and the research subjects for their participation.
Glossary
Abbreviations
- OPC
olfactocentric paralimbic cortex
References
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–62. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–3. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–96. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Anand BK, Malhotra CL, Singh B, Dua S. Cerebellar projections to limbic system. J Neurophysiol. 1959;22:451–7. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielau H, Steiner J, Mawrin C, Trubner K, Brisch R, Meyer-Lotz G, et al. Dysregulation of GABAergic neurotransmission in mood disorders: a postmortem study. Ann N Y Acad Sci. 2007;1096:157–69. doi: 10.1196/annals.1397.081. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Charney DS, Krystal JH. Frontotemporal neural systems in bipolar disorder. Semin Clin Neuropsychiatry. 2002;7:243–54. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005;183:308–13. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann NY Acad Sci. 2004;1021:376–83. doi: 10.1196/annals.1308.048. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003a;60:1201–8. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, Durkin K, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59:611–8. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003b;160:1345–7. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Jr, Murphy DL, Goodwin FK, Borge GF. The ‘switch process’ in manic-depressive illness. I. A systematic study of sequential behavioral changes. Arch Gen Psychiatry. 1972;27:295–302. doi: 10.1001/archpsyc.1972.01750270005001. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–41. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–92. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–73. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005;7:358–69. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–30. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–5. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacology. 1999;21:63–8. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, et al. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord. 2007;9:679–92. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58:713–23. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P. Lateralized temporal-limbic dysfunction and psychopathology. Ann NY Acad Sci. 1976;280:777–97. doi: 10.1111/j.1749-6632.1976.tb25541.x. [DOI] [PubMed] [Google Scholar]
- Fredericks CA, Kalmar JH, Blumberg HP. The ventral prefrontal cortex and mood disorders. Oxford: Oxford University Press; 2006. [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–22. [PubMed] [Google Scholar]
- Ha TH, Ha K, Kim JH, Choi JE. Regional brain gray matter abnormalities in patients with bipolar II disorder: a comparison study with bipolar I patients and healthy controls. Neurosci Lett. 2009;456:44–8. doi: 10.1016/j.neulet.2009.03.077. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci USA. 1998;95:8410–3. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar JH, Shah MP, Blumberg HP. Cortico-limbic brain circuitry development in bipolar disorder: a neuroimaging view. New York: Cambridge University Press; 2009a. [Google Scholar]
- Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009b;48:636–42. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Spencer L, Edmiston E, Lacadie CM, Martin A, et al. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J Int Neuropsychol Soc. 2009c;15:476–81. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Onitsuka T, Toner SK, Yurgelun-Todd D, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003;60:1069–77. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- Keener MT, Phillips ML. Neuroimaging in bipolar disorder: a critical review of current findings. Curr Psychiatry Rep. 2007;9:512–20. doi: 10.1007/s11920-007-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Kim E, Jung YC, Ku J, Kim JJ, Lee H, Kim SY, et al. Reduced activation in the mirror neuron system during a virtual social cognition task in euthymic bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1409–16. doi: 10.1016/j.pnpbp.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC. Bipolar disorders, dystonia, and compulsion after dysfunction of the cerebellum, dentatorubrothalamic tract, and substantia nigra. Biol Psychiatry. 1996;40:726–30. doi: 10.1016/0006-3223(96)82516-9. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–98. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55:1154–62. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Das P, Moss K, Berk M, Coulston CM. A functional MRI study of Theory of Mind in euthymic bipolar disorder patients. Bipolar Disord. 2008;10:943–56. doi: 10.1111/j.1399-5618.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, Ketter T. Is a lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disord. 2007;9:345–57. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56:544–52. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Whalley HC, McKirdy J, Hall J, Sussmann JE, Shankar P, et al. Prefrontal function and activation in bipolar disorder and schizophrenia. Am J Psychiatry. 2008;165:378–84. doi: 10.1176/appi.ajp.2007.07020365. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Principles of behavioral and cognitive neurology. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Mesulam MM, Mufson EJ. The insula of Reil in man and monkey. In: Peters A, Jones EG, editors. Cerebral cortex. Vol. 4. New York, NY: Plenum Press; 1985. [Google Scholar]
- Migliorelli R, Starkstein SE, Teson A, de Quiros G, Vazquez S, Leiguarda R, et al. SPECT findings in patients with primary mania. J Neuropsychiatry Clin Neurosci. 1993;5:379–83. doi: 10.1176/jnp.5.4.379. [DOI] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Activation of anterior insula during self-reflection. PLoS One. 2009;4:e4618. doi: 10.1371/journal.pone.0004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–2. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–58. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Murai T, Fujimoto S. Rapid cycling bipolar disorder after left temporal polar damage. Brain Inj. 2003;17:355–8. doi: 10.1080/0269905031000070170. [DOI] [PubMed] [Google Scholar]
- Najt P, Nicoletti M, Chen HH, Hatch JP, Caetano SC, Sassi RB, et al. Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neurosci Lett. 2007;413:183–6. doi: 10.1016/j.neulet.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJ. The problem of the frontal lobe: a reinterpretation. J Psychiatr Res. 1971;8:167–87. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30:485–97. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–5. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–67. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–55. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:308–19. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, et al. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–92. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–22. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, et al. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. J Child Psychol Psychiatry. 2008;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA. 2006;103:8900–5. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–24. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Killgore WD, Cintron CM, Gruber SA, Tohen M, Yurgelun-Todd DA. Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter. Biol Psychiatry. 2007;61:743–9. doi: 10.1016/j.biopsych.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31:468–75. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. J Neurosci Res. 1976;2:133–46. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- Stanfield AC, Moorhead TW, Job DE, McKirdy J, Sussmann JE, Hall J, et al. Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disord. 2009;11:135–44. doi: 10.1111/j.1399-5618.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Mayberg HS, Berthier ML, Fedoroff P, Price TR, Dannals RF, et al. Mania after brain injury: neuroradiological and metabolic findings. Ann Neurol. 1990;27:652–9. doi: 10.1002/ana.410270612. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162:1697–705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–60. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Supple WF, Jr, Cranney J, Leaton RN. Effects of lesions of the cerebellar vermis on VMH lesion-induced hyperdefensiveness, spontaneous mouse killing, and freezing in rats. Physiol Behav. 1988;42:145–53. doi: 10.1016/0031-9384(88)90290-9. [DOI] [PubMed] [Google Scholar]
- Supple WF, Jr, Leaton RN, Fanselow MS. Effects of cerebellar vermal lesions on species-specific fear responses, neophobia, and taste-aversion learning in rats. Physiol Behav. 1987;39:579–86. doi: 10.1016/0031-9384(87)90156-9. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wang F, Xie G, Liu J, Li L, Su L, et al. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: a voxel-based morphometric magnetic resonance imaging study. Psychiatry Res. 2007;156:83–6. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–60. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- Toro CT, Hallak JE, Dunham JS, Deakin JF. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci Lett. 2006;404:276–81. doi: 10.1016/j.neulet.2006.05.067. [DOI] [PubMed] [Google Scholar]
- Wessa M, Houenou J, Paillere-Martinot ML, Berthoz S, Artiges E, Leboyer M, et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry. 2007;164:638–46. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Womer FY, Kalmar JH, Wang F, Blumberg HP. A ventral prefrontal-amygdala neural system in bipolar disorder: a view from neuroimaging research. Acta Neuropsychiatrica. 2009a;21:228–38. doi: 10.1111/j.1601-5215.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- Womer FY, Wang F, Chepenik LG, Kalmar JH, Spencer L, Edmiston E, et al. Sexually dimorphic features of vermis morphology in bipolar disorder. Bipolar Disord. 2009b;11:753–8. doi: 10.1111/j.1399-5618.2009.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadalam KG, Jain AK, Simpson GM. Mania in two sisters with similar cerebellar disturbance. Am J Psychiatry. 1985;142:1067–9. doi: 10.1176/ajp.142.9.1067. [DOI] [PubMed] [Google Scholar]
- Zuliani R, Moorhead TW, Job D, McKirdy J, Sussmann JE, Johnstone EC, et al. Genetic variation in the G72 (DAOA) gene affects temporal lobe and amygdala structure in subjects affected by bipolar disorder. Bipolar Disord. 2009;11:621–7. doi: 10.1111/j.1399-5618.2009.00731.x. [DOI] [PubMed] [Google Scholar]