Abstract
Multiple sclerosis is a chronic inflammatory disease of the central nervous system, associated with demyelination and neurodegeneration. The mechanisms of tissue injury are currently poorly understood, but recent data suggest that mitochondrial injury may play an important role in this process. Since mitochondrial injury can be triggered by reactive oxygen and nitric oxide species, we analysed by immunocytochemistry the presence and cellular location of oxidized lipids and oxidized DNA in lesions and in normal-appearing white matter of 30 patients with multiple sclerosis and 24 control patients without neurological disease or brain lesions. As reported before in biochemical studies, oxidized lipids and DNA were highly enriched in active multiple sclerosis plaques, predominantly in areas that are defined as initial or ‘prephagocytic’ lesions. Oxidized DNA was mainly seen in oligodendrocyte nuclei, which in part showed signs of apoptosis. In addition, a small number of reactive astrocytes revealed nuclear expression of 8-hydroxy-d-guanosine. Similarly, lipid peroxidation-derived structures (malondialdehyde and oxidized phospholipid epitopes) were seen in the cytoplasm of oligodendrocytes and some astrocytes. In addition, oxidized phospholipids were massively accumulated in a fraction of axonal spheroids with disturbed fast axonal transport as well as in neurons within grey matter lesions. Neurons stained for oxidized phospholipids frequently revealed signs of degeneration with fragmentation of their dendritic processes. The extent of lipid and DNA oxidation correlated significantly with inflammation, determined by the number of CD3 positive T cells and human leucocyte antigen-D expressing macrophages and microglia in the lesions. Our data suggest profound oxidative injury of oligodendrocytes and neurons to be associated with active demyelination and axonal or neuronal injury in multiple sclerosis.
Keywords: multiple sclerosis, demyelination, neurodegeneration, oxidative damage
Introduction
Multiple sclerosis is a chronic inflammatory disease of the CNS, leading to focal plaques of primary demyelination with a variable degree of axonal and neuronal degeneration (Lassmann et al., 2007). Although different mechanisms may contribute to demyelination and neurodegeneration in multiple sclerosis, it recently became clear that mitochondrial injury and subsequent energy failure is a major factor driving tissue injury (Lu et al., 2000; Dutta et al., 2006; Mahad et al., 2008; Trapp and Stys 2009; Witte et al., 2010). Active multiple sclerosis lesions show profound alterations of proteins of the mitochondrial respiratory chain (Mahad et al., 2008, 2009) and mitochondrial DNA deletions are present in neurons, in particular in the progressive stage of the disease (Campell et al., 2011). Such mitochondrial changes may explain characteristic pathological features of multiple sclerosis lesions, including demyelination and oligodendrocyte apoptosis (Veto et al., 2010), preferential destruction of small-calibre axons (Mahad et al., 2008, 2009), differentiation arrest of oligodendrocyte progenitor cells and remyelination failure (Ziabreva et al., 2010) and astrocyte dysfunction (Sharma et al., 2010; Campbell et al., unpublished data). Mitochondrial proteins and DNA are highly vulnerable to oxidative damage (Higgins et al., 2010), and it is thus expected that free radical-mediated mechanisms may drive mitochondrial injury in multiple sclerosis (Lu et al., 2000; Kalman and Leist, 2003; Mao and Reddy, 2010; van Horssen et al., 2011). Oxidized lipids and oxidized DNA have been detected biochemically in brain tissue from patients with multiple sclerosis (Vladimirova et al., 1998; Smith et al., 1999; Bizzozero et al., 2005; Quin et al., 2007) and some studies have analysed their cellular localization in multiple sclerosis lesions by immunocytochemistry (Newcombe and Cuzner, 1994; Lu et al., 2000; Van Horssen et al., 2008). The results of the latter studies, however, are disappointing, since immunoreactivity was seen in macrophages and astrocytes, but not in those cells or components that actually are destroyed in the lesions, such as oligodendrocytes, neurons and axons. The aim of this study was a systematic analysis of the presence and location of oxidized DNA and lipids at a cellular level in different stages of lesion formation in multiple sclerosis. We show that oxidized DNA and lipids are concentrated within active portions of the lesions. We further found that oxidized lipids—both oxidized phospholipids and malondialdehyde (MDA)—are excellent markers for acute cell injury and degeneration of neurons and glia. Furthermore, different oxidized lipids preferentially accumulate in different cell types or cellular compartments.
Materials and methods
This study was performed on paraffin-embedded archival autopsy material from 30 patients with multiple sclerosis, one patient with neuromyelitis optica and 24 controls without neurological disease or brain lesions (Table 1). Controls included 17 normal controls of different age and seven patients, who died under septic conditions. The presence of concomitant vascular (ischaemic) lesions, which could by itself lead to oxidative damage in human brain tissue, was excluded by detailed neuropathological studies, performed on multiple tissue blocks from each patient. The clinical course was defined by retrospective chart review according to established criteria before and blinded to the pathological analysis (Lublin and Reingold, 1996). The multiple sclerosis cohort included seven cases of acute multiple sclerosis (Marburg, 1906), who died within 1 year after disease onset (Table 1). Further, two cases of relapsing/remitting multiple sclerosis, 12 cases of secondary progressive multiple sclerosis and eight cases of primary progressive multiple sclerosis were included. Due to insufficient clinical data one additional multiple sclerosis case could only be classified as progressive multiple sclerosis. The study was approved by the Ethik Kommission of the Medical University of Vienna (EK Nr 535/2004).
Table 1.
Clinical data of controls and patients with multiple sclerosis included in this study
| Clinical multiple sclerosis type | Age | Gender | Disease course | Total disease duration (months) | Lesion types |
|---|---|---|---|---|---|
| AMS 1 | 45 | Male | Monophasic | 0.2 | Active (Pattern III); inactive |
| AMS 2 | 45 | Male | Monophasic | 0.6 | Active (Pattern III), inactive |
| AMS 3 | 52 | Male | Monophasic | 1.5 | Active (Pattern II), inactive |
| AMS 4 | 35 | Male | Monophasic | 2 | Active (Pattern II; inactive |
| AMS 5 | 78 | Male | Monophasic | 2 | Active (Pattern III); inactive |
| AMS 6 | 34 | Female | Monophasic | 4 | Active (Pattern III); inactive |
| AMS 7 | 51 | Female | Monophasic | 5 | Active (Pattern II), inactive |
| RRMS 1 | 40 | Female | RRMS | 120 | Active (Pattern III); inactive |
| RRMS 2 | 57 | Female | RRMS | 156 | Active (Pattern II); inactive |
| SPMS 1 | 66 | Female | SPMS with attacks | 96 | Inactive |
| SPMS 2 | 34 | Male | SPMS with attacks | >120 | Inactive |
| SPMS 3 | 62 | Female | SPMS without attacks | 144 | Active (Pattern I) |
| SPMS 4 | 84 | Female | SPMS without attacks | 264 | Inactive |
| SPMS 5 | 61 | Female | SPMS with attacks | 288 | Slowly expanding active; inactive |
| SPMS 6 | 64 | Female | SPMS without attacks | 336 | Inactive |
| SPMS 7 | 56 | Male | SPMS without attacks | 372 | Active (Pattern I); inactive |
| SPMS 8 | 76 | Male | SPMS without attacks | 372 | Inactive |
| SPMS 9 | 78 | Female | SPMS without attacks | 372 | Inactive |
| SPMS 10 | 69 | Female | SPMS without attacks | 384 | Inactive |
| SPMS 11 | 81 | Female | SPMS without attacks | 432 | Inactive |
| SPMS 12 | 46 | Female | SPMS with attacks | 444 | Slowly expanding active; inactive |
| PPMS 1 | 55 | Female | PPMS | 60 | Slowly expanding active; inactive |
| PPMS 2 | 67 | Male | PPMS | 87 | Active (Pattern I), inactive |
| PPMS 3 | 53 | Male | PPMS | 168 | Slowly expanding active; inactive |
| PPMS 4 | 77 | Female | PPMS | 168 | Slowly expanding active; inactive |
| PPMS 5 | 34 | Female | PPMS | 204 | Slowly expanding active; inactive |
| PPMS 6 | 71 | Female | PPMS | 264 | Slowly expanding active; inactive |
| PPMS 7 | 83 | Female | PPMS | 360 | Inactive |
| PPMS 8 | 75 | Female | PPMS | 372 | Inactive |
| ProgMS | 68 | Female | Progressive MS | 120 | Inactive |
| NMO | 20 | Female | Relapsing NMO | 48 | Neuromyelitis optica |
| WMS 1 | 85 | Female | Acute stroke | 2 days | Initial white matter stroke lesion |
| WMS 2 | 65 | Female | Recurrent stroke | 48 | Initial and advanced stroke lesions |
| Control 1 | 30 | Female | Normal control | – | – |
| Control 2 | 36 | Female | Normal control | – | – |
| Control 3 | 37 | Male | Normal control | – | – |
| Control 4 | 39 | Female | Normal control | – | – |
| Control 5 | 42 | Female | Normal control | – | – |
| Control 6 | 45 | Female | Normal control | – | – |
| Control 7 | 46 | Male | Normal control | – | – |
| Control 8 | 47 | Female | Normal control | – | – |
| Control 9 | 48 | Male | Normal control | – | – |
| Control 10 | 65 | Male | Normal control | – | – |
| Control 11 | 70 | Male | Normal control | – | – |
| Control 12 | 71 | Female | Normal control | – | – |
| Control 13 | 71 | Female | Normal control | – | – |
| Control 14 | 72 | Male | Normal control | -– | – |
| Control 15 | 83 | Male | Normal control | – | – |
| Control 16 | 84 | Female | Normal control | – | – |
| Control 17 | 97 | Female | Normal control | – | – |
| S-Control | 42 | Female | Septic control | – | – |
| S-Control | 45 | Male | Septic control | – | – |
| S-Control | 51 | Female | Septic control | – | – |
| S-Control | 74 | Male | Septic control | – | – |
| S-Control | 88 | Male | Septic control | – | – |
| S-Control | 89 | Female | Septic control | – | – |
| S-Control | 95 | Female | Septic control | – | – |
AMS = acute multiple sclerosis; MS = multiple sclerosis; NMO = neuromyelitis optica; PPMS = primary progressive multiple sclerosis; RRMS = relapsing/remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis; WMS = white matter stroke.
Neuropathological techniques and immunocytochemistry
All cases underwent detailed neuropathological examination on multiple tissue blocks from various brain regions and lesion activity was evaluated as described in detail previously (Frischer et al., 2009). Immunocytochemistry was performed on paraffin sections according to established techniques (King et al., 1997; Bauer et al., 2007). A detailed list of primary antibodies, dilutions and corresponding pretreatment of sections is shown in Table 2. Since immunocytochemical analysis of oxidized molecules in tissue sections may potentially lead to false-positive results in sections stained with a peroxidase detection system, an additional detection system, based on alkaline phosphatase was used, which showed identical results.
Table 2.
Antibodies used for immunocytochemistry
| Antibody | Antibody type | Target | Source | Staining |
|---|---|---|---|---|
| E06 | Mouse (mAB) | Oxidized phospholipids | Palinski et al., 1996 | 10 µg/ml |
| E014 | Mouse (mAB) | Malondialdehyde–lysine | Palinski et al., 1996 | 0.4 µg/ml |
| MDA2 | Mouse (mAB) | Malondialdehyde–lysine | Palinski et al., 1990 | 1 : 1000 |
| 8-OHdG | Goat (polyAB) | 8-Hydroxy 2-deoxy Guanosine | Abcam, ab10802 | 1 : 1000; E |
| CD3 | Rabbit (polyAB) | T-cell antigen | Dako, A0452 Lot128 | 1 : 2000; E |
| IBA-1 | Rabbit (polyAB) | Ionized calcium binding adaptor molecule 1 | Courtesy of Dr Sonja Forss-Petter/Japan | 1 : 3000; E |
| CD68 | Mouse (mAB) | CD68 110-kD transmembrane glycoprotein | Dako, M0814 | 1 : 100; E |
| HLA-D | Mouse (mAB) | Human leucocyte antigen-DP, DQ, DR | Dako, M0775 | 1 : 100; C |
| TPPP/p25 | Rat (polyAB) | Tubulin polymerization promoting protein | Produced by Gergö Botond | 1 : 3000; E |
| CNPase | Mouse (mAB) | 2',3'-cyclic nucleotide 3' phosphodiesterase | Sternberger Monoclonals, SMI91 | 1 : 2000; E |
| MOG Y10 and Z12 | Mouse (mABs) | Myelin oligodendrocyte glycoprotein | Piddlesden et al., 1991 | 1 : 100; C |
| PLP | Mouse (mAB) | Proteolipid protein | Serotec, MCA 839 G | 1 : 1000; E |
| APP | Mouse (mAB) | Alzheimer amyloid precursor protein | Chemicon, MAB 348 | 1 : 1000; C |
| SY | Rabbit (polyAB) | Synaptophysin | Epitomics YE269, Burlingame, CA, USA | 1 : 100; E |
| SMI 32 | Mouse (mAB) | Non-phosphorylated 200 kDa neurofilament | Sternberger Monoclonals SMI32 | 1 : 1000; E |
| SMI 311 and 312 | Mouse (mAB) | Neurofilament | Sternberger Monoclonals SMI 311 and 312 | 1 : 18000; E |
| GFAP | Rabbit (polyAB) | Glial fibrillary acidic protein (intermediate filament of mature astrocytes) | Dako, Z0334 Lot 096 | 1 : 3000; E |
AB = antibody; C = citrate buffer; E =ethylenedinitrilotetraacetic acid buffer.
Double labelling was performed using primary antibodies from different species. Oxidative markers were first stained using primary antibodies from mouse or goat, followed by respective species-specific secondary antibodies, labelled with alkaline phosphatase and development in fast blue BB reagent (blue reaction product). Then cell-specific rabbit or rat antibodies were applied (TPPP-p25 for oligodendrocytes, glial fibrillary acidic protein for astrocytes, synaptophysin for axons with disturbed axonal transport, Iba-1 for macrophages and microglia and CD3 for T cells). This was followed by species-specific biotinylated anti-rabbit or anti-rat immunoglobulin, avidin–peroxidase and development in amino ethyl carbazole reagent (red reaction product; for details see Marik et al., 2007).
Confocal laser fluorescence microscopy
For identifying E06 (oxidized phospholipid)-labelled axonal spheroids, fluorescence immunohistochemistry was performed on paraffin sections as described above with few modifications. The primary antibodies, monoclonal anti-E06 and polyclonal anti-synaptophysin, were applied simultaneously at 4°C in a humid chamber overnight. After washing with phosphate-buffered saline, secondary antibodies consisting of goat-anti-rabbit Cy3 (Jackson ImmunoResearch, 1 : 200) and biotinylated anti-mouse (Jackson; 1 : 500) were applied simultaneously for 1 h at room temperature. The staining was finished by incubation with streptavidin-Cy2 (Jackson ImmunoResearch; 1 : 75) for 1 h at room temperature. Fluorescent preparations were examined using a Leica SP2 confocal laser scan microscope.
Quantitative analysis
For quantification of immunocytochemistry for oxidative markers, two different approaches were applied. In the first approach, we performed densitometry of sections stained with the antibody against oxidized phospholipids. In a second approach, we manually counted different cell types or dystrophic axons stained selectively with the markers 8-hydroxy-d-guanosine (oxidized DNA), E06 (oxidized phospholipids), MDA2 (malondialdehyde–lysine), CD3 (T cells), Human leucocyte antigen-DP, DQ, DR (HLA-D; mainly macrophages and microglia) and amyloid precursor protein (dystrophic axons with disturbed axonal transport).
Densitometric analysis
By means of Luxol fast blue Kluver’s staining as well as haematoxylin eosin staining and proteolipid protein immunocytochemistry, regions of interest (n = 761, each 0.61 mm × 0.46 mm) were defined and numbered, giving each individual region an ID. The locations of these regions were chosen on the basis of multiple sclerosis pathology differing between types of multiple sclerosis lesions or normal-appearing white matter. Photomicrographs were taken from the corresponding regions in the E06-stained slides under standardized conditions (identical settings, controlled by white balance values) with a Reichert Polyvar 2 microscope using Nikon NIS-Elements D3.10. Pictures were captured with Gain 1.2 at an exposure time of 6 ms. All images were saved as JPEG files with lowest compression. They were processed with Adobe® Photoshop CS2, image size was reduced to a width of 10 cm and resolution set on 300 pixel/inch and cold filter with 25% was applied. A threshold level (output level = 128) was set and pixels above this value were deleted. The images were saved as TIF files and opened with ImageJ, which measures the per cent area covered by the signal. The data were structured in order to allow following of each database value back to the original microscopic image. Averages for individual densitometric values were calculated per lesion area per case and these averages were compared between multiple sclerosis samples and white matter of controls as well as between different lesion areas within the multiple sclerosis sample.
Manual counting of immunostained cells or dystrophic axons
Manual counting was performed in the same section areas used for densitometric analysis. Counting was performed at the microscope in areas defined by an ocular grid as described previously (Frischer et al., 2009) for CD3, HLA-D and amyloid precursor protein-stained sections. For the oxidative markers, counting was performed in 10 microscopic fields of 0.25 mm2 per lesion area. Average counts per square millimetre were calculated for each region of interest (normal-appearing white matter, active lesions, slowly expanding lesions and inactive lesions) per case and compared by statistical analysis.
Statistical analysis
Due to the uneven distribution of our data, statistical analysis was performed with non-parametric tests. Descriptive analysis included median value and range. Differences between two groups were assessed with Wilcoxon–Mann–Whitney U-test. Differences between more than two groups were assessed with Kruskal–Wallis test, followed by pair-wise Wilcoxon–Mann–Whitney U-tests. In case of multiple testing (comparison of more than two groups) significant values were corrected with Bonferroni procedure. Interdependence of variables was evaluated by Spearman non-parametric correlation test. The reported P-values are results of two-sided tests. A P-value ≤ 0.05 was considered statistically significant. For all statistical analysis, mean values per patient for each lesion type and normal-appearing white matter were used.
Results
Oxidative damage in multiple sclerosis tissue is related to lesion activity and inflammation
In a first approach, we analysed the accumulation of oxidized DNA and oxidized lipids within multiple sclerosis lesions of different activity from patients with acute, relapsing, remitting and progressive disease by immunocytochemistry. As controls, we used brain tissue from patients without neurological disease, who died under conditions of systemic inflammation (septic controls) or non-inflammatory conditions (normal controls). Sections were stained under identical conditions, scanned and evaluated by densitometry or by manual counting of immunoreactive cells within the tissue. In comparison with normal and septic controls, increased immunocytochemical signals were detected in the global multiple sclerosis population (Figs 1A and B, 2A–C and Table 3). This was seen in a similar way for markers for oxidized DNA or lipids. Within multiple sclerosis sections, the highest signal for oxidative damage was found in active lesion areas, while inactive lesions showed a low signal. Within active lesions, more intense staining was present in classical active lesions of acute and relapsing multiple sclerosis compared with slowly expanding lesions, which dominate in the progressive stage of the disease. We also subtyped lesions according to patterns of demyelination (Lucchinetti et al., 2000). We found higher expression (on average 8.2-fold) of oxidized lipids and oxidized DNA in lesions following Pattern III (hypoxia-like tissue injury) compared with Pattern II (complement-associated demyelination). This was seen for global staining intensity for oxidized phospholipids, determined by densitometry, as well as by counting the number of cells with nuclear 8-hydroxy-d-guanosine reactivity or with cytoplasmic expression of MDA2. However, due to the low number of cases analysed, statistical significance was reached only for MDA2 (P = 0.01) and a trend (P = 0.072) for 8-hydroxy-d-guanosine. The values in multiple sclerosis Pattern II lesions were similar to those seen in a case of neuromyelitis optica, which was included as a control for antibody-mediated tissue injury. Interestingly, astrocytes, which are the primary target for destruction in neuromyelitis optica, did not show 8-hydroxy-d-guanosine-reactive nuclei. This may be due to their rapid and efficient antibody- and complement-mediated destruction. Quantification of inflammation regarding T cells (CD3) and macrophages/microglia (HLA-D) revealed a relative distribution of values, similar to that seen for markers of oxidative damage. In addition, we found a weak but significant correlation between T cells or macrophages/microglia and the presence of oxidized DNA or lipids within the sections (data not shown).
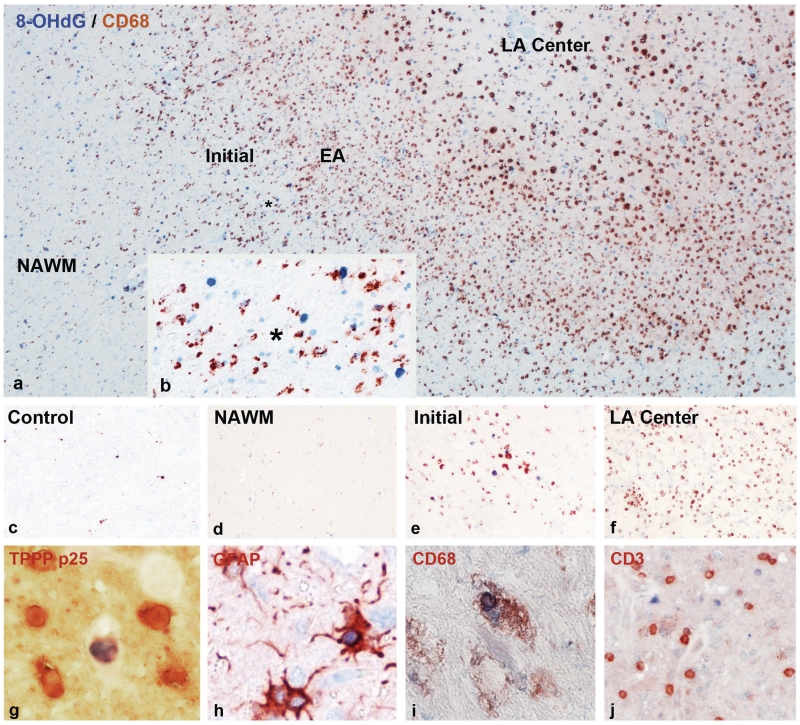
Figure 1.
Oxidized DNA in multiple sclerosis lesions, visualized by immunocytochemistry for 8-hydroxy-d-guanosine (8-OHdG). (a) Actively demyelinating lesion in a patient with primary progressive multiple sclerosis; the figure shows areas of the normal-appearing white matter (NAWM) (left), the zone of ongoing demyelinating activity with profound microglia activation and macrophage infiltration (brown immunoreactivity for the macrophage marker CD68) and the inactive portion of the lesion with low macrophage infiltration. Oxidized DNA (8-OHdG) reactivity is shown as blue nuclei, which are mainly present in the active lesion edge (×60). EA = early active demyelination with macrophages containing myelin-reactive degradation products; initial = initial stage of demyelination with profound microglia activation and oligodendrocyte apoptosis; LA centre = late active stage of demyelination in lesions centre; macrophages with neutral lipid degradation products. (b) Higher magnification of the ‘initial’ stage of the active plaque in a, shows profound microglia activation (brown cells; CD68 positive) and many blue nuclei, representing cells with DNA oxidation; the asterisks in a and b indicate the position of b within the lesion shown in a (×200). (c) Normal white matter of a control patient without neurological disease and brain lesions; only very few microglia cells show cytoplasmic CD68 reactivity (brown); there are no blue nuclei, suggesting DNA oxidation (×100). (d) Normal-appearing white matter (NAWM) of a patient with acute multiple sclerosis (Marburg’s type); similar pattern as in the control white matter, shown in c (×100). (e) Very early active lesion area in a patient with acute multiple sclerosis (Marburg’s type); profound microglia activation (brown cells; CD68) adjacent to cell nuclei, positive for 8-hydroxy-d-guanosine (blue) (×100). (f) Late active lesion centre in a patient with Marburg’s type of acute multiple sclerosis; numerous CD68-positive macrophages (brown), but no nuclei with blue 8-hydroxy-d-guanosine reactivity (×100). (g) Double staining for oxidized DNA (8-hydroxy-d-guanosine; blue) and the oligodendrocyte marker TPPP-p25 (red) at the lesion edge of an active lesion of acute multiple sclerosis. One of the six oligodendrocytes shows blue 8-hydroxy-d-guanosine reactivity in a nucleus with nuclear condensation and fragmentation (apoptotic cell) (×900). (h) Double staining for oxidized DNA (8-hydroxy-D-guanosine, blue) and glial fibrillary acidic protein (GFAP, red) at the edge of an active lesion of acute multiple sclerosis. One astrocyte contains a blue nucleus, indicating oxidized DNA (×600). (i) Double staining for oxidized DNA (8-hydroxy-d-guanosine, blue) and the macrophage marker CD68 (red) in an active lesion of acute multiple sclerosis. The macrophage contains a nucleus with oxidized DNA, possibly representing phagocytosis of an apoptotic cell (×600). (j) Double staining for oxidized DNA (8-hydroxy-d-guanosine, blue) and the T-cell marker CD3 (red) in an active lesion of acute multiple sclerosis. No DNA oxidation is seen in the CD3-positive T-cell population (×200).
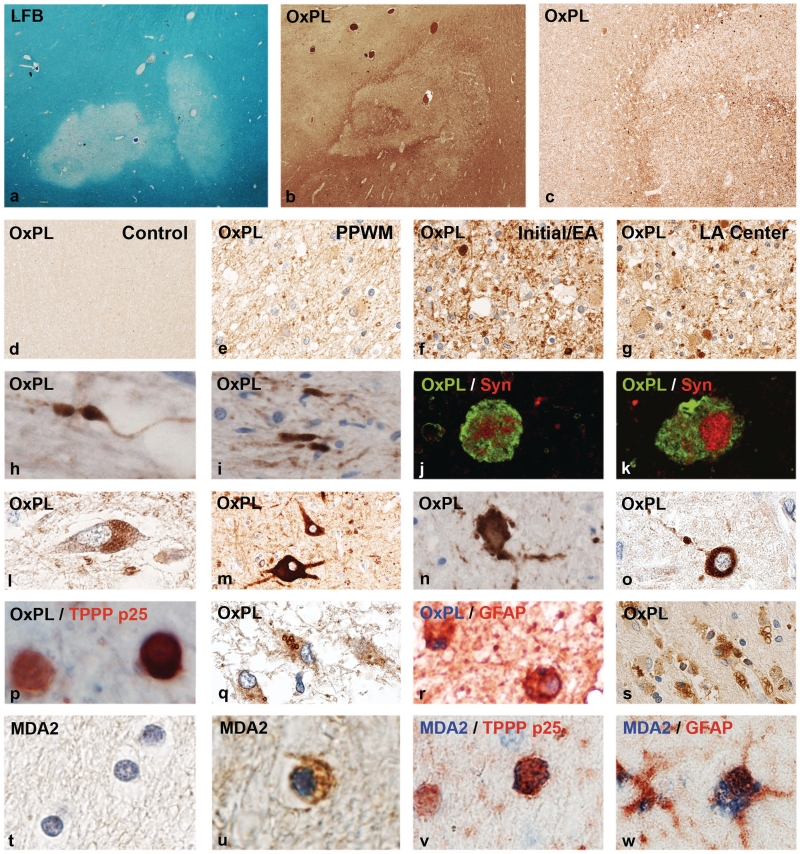
Figure 2.
Oxidized lipids in multiple sclerosis lesions. (a) Myelin loss in an active white matter lesion in acute multiple sclerosis, Marburg’s type (×8). (b) Adjacent serial section from a, stained with the marker E06 for oxidized phospholipids (OxPL) with profound immunoreactivity at the lesion edge and the adjacent peri-plaque white matter (×8). (c) Higher magnification of the sections shown in b, showing increased immunoreactivity for oxidized phospholipids at the lesion edge (×45). (d) Normal white matter of a control patient without neurological disease or CNS lesions, without immunoreactivity for oxidized phospholipids (×45). (e) Peri-plaque white matter (PPWM) from the area shown in b, with increased immunoreactivity, in comparison with the control white matter, but much lower reactivity as at the active lesion edge. Immunoreactivity is mostly associated with myelin (×150). (f) High immunoreactivity for oxidized phospholipids in myelin sheaths and some globular structures, representing axonal spheroids, are present at the edge of the active lesions shown in b (×150). (g) Inactive lesion centre of the same lesion shown in b, with low immunoreactivity for oxidized phospholipids; intensive immunoreactivity, however, is seen in some axonal spheroids (×150). (h–k) Oxidized phospholipids are selectively accumulated within dystrophic axons and axonal spheroids (h, i); the nature of dystrophic axons is documented by double staining confocal microscopy for oxidized phospholipids and synaptophysin (Syn: j and k) (h, i: ×900; j, k: ×2500). (l–o) Accumulation of oxidized phospholipids in neurons in patients with multiple sclerosis with active lesions: (l) lipofuscin reactivity in cortical neuron; (m) ballooned cortical neuron adjacent to destructive subcortical lesion with intense immunoreactivity for oxidized phospholipids; (n, o) neurons with intense cytoplasmic reactivity for oxidized phospholipids with fragmented cell processes (dendrites) in active lesions in the basal ganglia (h) and cortex (o) (×600). (p) Active multiple sclerosis lesions; double staining for the oligodendrocyte marker TPPP-p25 (red) and (black/brown), showing one of the two oligodendrocytes with intense cytoplasmic E06 immunoreactivity (×900). (q, r) In astrocytes, oxidized phospholipids are sequestered in the cytoplasm in the form of larger granules, possibly representing autophagic vacuoles (q: ×450; r: ×750). (s) Macrophages with granular cytoplasmic reactivity for oxidized phospholipids in the centre of an active multiple sclerosis plaque (×150). (t) Normal white matter of a control patient without neurological disease or brain lesions; no MDA2 immunoreactivity (×900). (u, v) MDA2 immunoreactivity in oligodendrocytes and myelin in active multiple sclerosis lesions; the nature of the cells as oligodendrocytes is shown by double staining in v, using TPPP-p25 as a marker (×900). (w) Granular cytoplasmic MDA2 immunoreactivity (blue) in the cytoplasm of a glial fibrillary acidic protein (GFAP)-positive astrocyte (red) in an active multiple sclerosis lesion (×800). LFB = Luxol fast blue.
Table 3.
Quantification of oxidized lipids and oxidized DNA in different types of multiple sclerosis lesions in comparison with controls
| Active lesion |
Slowly expanding lesion |
Inactive lesion |
NAWM multiple sclerosis |
White matter controls | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median (range) | P-value* | Median (range) | P-value* | Median (range) | P-value* | Median (range) | P-value* | Median (range) | |
| Percentage of E06 positive area | 3.1 (26.5) | 0.004 | 1.6 (16.7) | 0.245 | 0.6 (9.2) | 0.02 | 0.8 (17.1) | 0.029 | 0.06 (2.9) |
| E06 axon spheroids | 1.3 (18.2) | 0.000 | 1.2 (121.2) | 0.000 | 0.4 (2.3) | 0.000 | 0.0 (1.2) | 0.061 | 0.00 (0.0) |
| MDA-2 OG | 1.6 (25.8) | 0.005 | 0.9 (5.4) | 0.048 | 0.9 (1.6) | 0.286 | 0.4 (9.2) | 0.147 | 0.00 (1.6) |
| 8-OHdG nuclei | 5.6 (78.1) | 0.023 | 9.0 (19.0) | 0.136 | 1.6 (6.8) | 0.544 | 1.4 (12.0) | 0.486 | 0.40 (10.4) |
| APP | 44.3 (197.6) | 0.000 | 16.1 (40.0) | 0.000 | 0.3 (1.5) | 0.022 | 0.0 (1.0) | 1.002 | 0.00 (0.8) |
| CD3 | 73.6 (298.8) | 0.000 | 51.2 (105.6) | 0.008 | 10.0 (69.1) | 2.835 | 4.8 (38.4) | 0.35 | 9.60 (49.6) |
| HLA-D | 324.8 (585.6) | 0.000 | 148.3 (398.0) | 0.020 | 29.0 (246.5) | 3.718 | 99.3 (190.0) | 0.289 | 43.10 (114.4) |
Values represent medians and range (95th percentile range values); P-values are corrected by Bonferroni for multiple testing; all values represent counted cells/mm2. Active lesions = classical actively demyelinating lesions; slowly expanding lesions = lesions with inactive lesion centre, surrounded by a small rim of activated microglia with recent myelin-degradation products; inactive lesions = lesions without any recent demyelinating activity; NAWM multiple sclerosis = normal-appearing white matter from patients with multiple sclerosis; white matter controls = normal white matter of all controls; APP = amyloid precursor protein reactive axonal spheroids or end bulbs; CD3 = T cells; percentage of E06 positive area = densitometric analysis of area covered by E06 immunoreactivity; E06 axon spheroids = axonal spheroids or end bulbs stained by E06 antibody; HLA-D = class II MHC-positive macrophages/microglia; MDA-2 OG = oligodendrocyte-like cells, immunoreactive for MDA-2; 8-OHdG nuclei = number of cell nuclei containing 8-hydroxy-D-guanosine immunoreactivity; Our data show a highly significant accumulation of oxidized DNA and oxidized lipids in active multiple sclerosis lesions in comparison with controls. Oxidized DNA and lipids are predominantly seen in lesions with high T-cell and macrophage infiltrates and with profound microglia activation. *P-value: Bold values represent significant differences (P < 0.05) in comparison to respective control values.
Within the global multiple sclerosis population, patients dying in the course of acute multiple sclerosis showed more accumulation of oxidized lipids and DNA in the lesions as compared with those dying in the progressive stage of the disease. The difference, however, did not reach statistical significance. Similarly, we did not see a significant global age- or gender-dependent effect on the presence of oxidized lipids and DNA in multiple sclerosis or control cases.
Cellular localization of oxidized DNA and lipids and its relation to lesion activity
Fine mapping of actively demyelinating lesions in multiple sclerosis revealed several different zones, representing the evolution of the plaques (Figs 1A–F, 2A–G). The centre of an active plaque is densely packed with foamy macrophages. Towards the edge of the lesions, macrophage density is even higher, most of them containing myelin-degradation products, which still have the staining properties of intact myelin (including even low-abundance myelin proteins such as myelin oligodendrocyte glycoprotein; early active lesion according to Brück et al., 1995). In between this area and the normal peri-plaque white matter, there is another zone that contains activated microglia, dispersed between seemingly intact myelin sheaths, but containing oligodendrocytes with apoptotic-like nuclear changes. This zone is regarded as the initial demyelinating lesion (Marik et al., 2007) or the ‘prephagocytic’ stage of active multiple sclerosis lesions, as defined by Barnett and Prineas (2004). Immunoreactivity for oxidized DNA and oxidized lipids was most prominent in this zone of initial lesion formation, followed by the adjacent peri-plaque white matter, but being much lower in the zone of early phagocytic myelin digestion and nearly absent in the centre of the active lesion (Figs 1A–F, 2A–G).
Oxidized DNA
Immunocytochemistry for 8-hydroxy-d-guanosine revealed selective staining of cell nuclei, reflecting oxidized DNA. Immunoreactive cell nuclei were most numerous in classical active lesions (Fig. 1A, B and Table 3), followed by slowly expanding lesions. Low numbers of immunoreactive nuclei were present in inactive lesions and in the normal-appearing white matter. Only exceptional nuclei were positive in sections of normal and septic controls (Fig. 1C). Immunoreactivity for oxidized DNA was mainly found in areas with profound microglia activation at the lesion edge (‘initial’ lesion area; Fig. 1A–F). The vast majority of cells with oxidized DNA were small round cells, morphologically resembling oligodendrocytes (Fig. 1G). Double staining with specific cellular markers showed oxidized DNA in TPPP-p25-positive oligodendrocytes, which in part revealed nuclear alterations resembling apoptosis (Fig. 1G). Only 3–4% of cells with 8-hydroxy-d-guanosine-positive nuclei were astrocytes, identified by double staining with glial fibrillary acidic protein (Fig. 1H), and nuclear reactivity was occasionally seen in neurons in cortical lesions. In addition, oxidized DNA was seen in some Iba-1- or CD68-positive microglia and macrophages (Fig. 1I) but not in CD3-positive T cells (Fig. 1J).
Oxidized phospholipids
In both multiple sclerosis and control tissue, E06 immunoreactivity was seen in lipofuscin granules of macrophages and neurons (Fig. 2L and S). In addition, oxidized phospholipids were present in multiple sclerosis tissue in various different cell types, predominantly in active white matter and cortical lesions. The most prominent expression was found in acutely injured axons, mainly located at the edges of actively expanding lesions (Fig. 2H–K). Reactivity was most intense at the sites of axonal transection and less in the proximal or distal axon (Fig. 2H and I). Double staining with synaptophysin revealed a co-localization of oxidized phospholipids with sites of disturbance of fast axonal transport (Fig. 2J and K); however, on average only 10% of amyloid precursor protein-positive axons also contained E06 immunoreactivity (Table 3). Quantitative analysis showed the highest density of axonal spheroids containing oxidized phospholipids in active lesions, followed by slowly expanding lesions, inactive lesions and normal-appearing white matter (Table 3). Only exceptional axonal spheroids reactive for oxidized phospholipids were encountered in brain sections from normal and septic controls (Fig. 2D). Dense cytoplasmic staining for oxidized phospholipids was also present in the perinuclear cell bodies and dendrites of some neurons in active lesions in the cortex and the basal ganglia. Many of the neurons with cytoplasmic staining for oxidized phospholipids revealed irregularities or fragmentation of their dendritic processes (Fig. 2M–O).
In addition to neurons and axons, oxidized phospholipids were also located in myelin (Fig. 2F), in TPPP-p25-positive oligodendrocytes (Fig. 2P) and in glial fibrillary acidic protein-positive astrocytes (Fig. 2Q and R). While in oligodendrocytes the entire cytoplasm was stained, in astrocytes the immunoreactivity was restricted to cytoplasmic inclusions, possibly reflecting autophagic vacuoles. A granular reactivity for oxidized phospholipids was also seen in the cytoplasm of macrophages, consistent with their content of lipofuscin (Fig. 2S).
Malondialdehyde
Similar to oxidized phospholipids, MDA immunoreactivity was seen in lipofuscin granules in neurons and macrophages in multiple sclerosis and control tissue. In multiple sclerosis tissue, additional staining was predominantly present in myelin sheaths in active lesions, which in many instances were fragmented. In addition, reactivity was present in small round cells with morphological features of oligodendrocytes (Fig. 2U). These cells could be in part identified as oligodendrocytes by double staining with TPPP-p25 (Fig. 2V). Similar to other markers for oxidative damage, the number and density of MDA-positive oligodendroglial-like cells was highest in classical active lesions, followed by slowly expanding lesions, inactive lesions and the normal-appearing white matter (Table 3). MDA2 reactivity was also seen in the cytoplasm of astrocytes and macrophages. In these cells, reactivity was present in granular inclusions, similar to the staining pattern for oxidized phospholipids (Fig. 2W). No MDA2 immunoreactivity was seen in CD3-positive T cells.
White matter stroke lesions
We have shown before (Aboul Enein et al., 2003) that the patterns of tissue injury in Pattern III multiple sclerosis lesions closely resemble those seen in the initial stages of white matter stroke lesions, consistent of microglia activation, distal oligodendrogliopathy, oligodendrocyte apoptosis and acute axonal injury, followed by demyelination and reactive gliosis. However, in contrast to multiple sclerosis, these lesions occur in the absence of major lymphocytic infiltrates. Using the same panel of oxidative markers, as described above, we found very similar distribution of oxidized DNA and oxidized lipids within the lesions. As in multiple sclerosis, 8-hydroxy-d-guanosine reactive nuclei were mainly seen in oligodendrocytes and in neurons within cortical lesions. MDA2 and E06 immunoreactivity was seen in myelin and diffusely in the oligodendrocyte cytoplasm, while in astrocytes reactivity was sequestered in lipofuscin-like granules. In addition, numerous axonal spheroids and end bulbs were reactive for oxidized phospholipids.
Discussion
To date, the mechanisms of tissue injury and neurodegeneration in multiple sclerosis are poorly understood. Demyelination and axonal injury occurs on a background of chronic inflammation in the relapsing as well as in the progressive stage of the disease (Frischer et al., 2009) and close contacts between activated microglia or macrophages and degenerating axons, myelin sheaths and oligodendrocytes have been described (Ferguson et al., 1997; Trapp et al., 1998; Kornek et al., 2000). Reactive oxygen species and nitric oxide intermediates are produced by activated macrophages and microglia and are, thus, likely candidates to be involved in tissue injury in multiple sclerosis (Van Hoorsen et al., 2010). Indeed, biochemical studies on multiple sclerosis tissue provided clear evidence for oxidized lipids and DNA within active multiple sclerosis lesions (Vladimirova et al., 1998; Smith et al., 1999; Lu et al., 2000) and upregulation of antioxidative proteins has been reported mainly in astrocytes (Van Hoorssen et al., 2008, 2010). However, attempts to directly localize oxidized molecules to degenerating oligodendrocytes, axons and neurons have so far been unsatisfactory. In the current study, immunocytochemistry for oxidized lipids revealed their presence within macrophages, located in structures that morphologically resemble lipofuscin. This is not surprising, since lipofuscin appears to contain non-digestible remnants of oxidized cellular components, accumulating in macrophages or resident cells of the CNS, for instance neurons (Keller et al., 2004; Wang et al., 2008). Similarly, nitrosylated epitopes, recognized by antibodies against nitrotyrosine, were mainly seen in macrophages (Cross et al., 1998; Liu et al., 2001). The only exception is immunoreactivity in oligodendrocytes for nitrotyrosine, previously described in some active multiple sclerosis lesions, which suggests a role of peroxynitrite in their destruction (Jack et al., 2007; Zeis et al., 2009). In contrast to these previous studies, we show here that DNA and lipid oxidation is associated with ongoing demyelination and neurodegeneration in active multiple sclerosis lesions. Furthermore, we show for the first time that acute cell injury and cell death of oligodendrocytes, axons and neurons in multiple sclerosis is linked to profound cytoplasmic and nuclear oxidative damage. The reason for the different results between our current and previous studies is not entirely clear. The most likely explanation comes from our observation that oxidized DNA and lipids were mainly present in a small zone of active multiple sclerosis lesions, which represents that previously described as the area of initial demyelination (Marik et al., 2007) or the ‘prephagocytic’ lesion (Barnett and Prineas, 2004; Henderson et al., 2009). Such lesions or lesion areas may not have been included in earlier studies. It has been shown previously that oxidized phospholipids and MDA epitopes are present in apoptotic cells as well as in apoptotic bodies ingested by macrophages (Chang et al., 1999). Apoptotic oligodendrocytes are predominantly seen in multiple sclerosis lesions in areas of initial (prephagocytic) demyelination (Barnett and Prineas, 2004; Marik et al., 2007). Furthermore, apoptotic cell death through oxidative mechanisms may exert pro-inflammatory and immunogenic actions (Chang et al., 2004), which in part may explain the progressive increase in inflammation with lesion maturation in multiple sclerosis (Marik et al., 2007; Henderson et al., 2009).
Analysing DNA oxidation in active multiple sclerosis, we found that the cells most severely affected are those morphologically resembling oligodendrocytes and that these cells in part show condensed and fragmented nuclei, morphologically resembling apoptosis. These cells could in part be identified by their expression of the oligodendrocyte marker TPPP-p25 (Höftberger et al., 2010). Identification of dying oligodendrocytes by double staining is difficult, since many cellular proteins, used as specific cell markers, are degraded in the course of apoptosis or necrosis. Nevertheless, the selective demyelination and oligodendrocyte apoptosis related to the expression of apoptosis-inducing factor (Veto et al., 2010) in active multiple sclerosis lesions is in line with the profound DNA oxidation seen in this study. The prominent labelling of myelin and oligodendrocyte-like cells by immunocytochemistry for MDA and oxidized phospholipids, as shown in this study, further supports the view that oxidative damage plays a major role in demyelination and oligodendrocyte destruction in active multiple sclerosis lesions. It must, however, be emphasized that DNA oxidation in active multiple sclerosis lesions is not restricted to oligodendrocytes, but also affects astrocytes in low incidence.
Immunocytochemistry for oxidized phospholipids provided further insights into the mechanisms of tissue injury in multiple sclerosis lesions. Besides the presence of oxidized phospholipids in oligodendrocytes and myelin, we also found a highly selective and profound expression of these neo-epitopes within degenerating axons and neurons. Accumulation of oxidized phospholipids in dystrophic axons may accumulate at sites of disturbed axonal transport. Alternatively, they may indicate radical-mediated damage as an initial change in axonal demise. We think that the second possibility is more likely. The half-life of oxidized lipids in a cell is estimated by minutes or few hours only (Keller and Matson, 1998). Furthermore, only a small fraction of amyloid precursor protein reactive dystrophic axons were also stained for oxidized phospholipids and they were concentrated at sites of initial lesions. In contrast, abundant amyloid precursor protein reactive axonal spheroids, which are present in the centre of the lesions, were devoid of immunoreactivity for oxidized phospholipids. This suggests that for the first time it is possible to directly visualize acute injury of axons, neuronal cell bodies or the fragmentation of their dendrites as a direct consequence of radical-mediated injury.
Oxidized phospholipids were also present in astrocytes in active multiple sclerosis lesions. In contrast to oligodendrocytes and neurons, these cells, however, do not degenerate but appear to be capable of sequestering damaged cytoplasmic components into (autophagic) vacuoles. However, it has been shown before that astrocytes, too, show signs of injury, mainly reflected by retraction of their cell processes and altered expression of molecules, related to the formation of the glia limitans (Parrat and Prineas, 2010; Sharma et al., 2010).
It was interesting to note that different cell types within the multiple sclerosis lesions differentially accumulated MDA and oxidized phospholipids. While both types of oxidized lipid epitopes were seen in myelin and oligodendrocytes, axons and neurons exclusively accumulated oxidized phospholipids. One possible explanation could be that the cellular content of phosphatidylcholine, rich in polyunsaturated fatty acids, is higher in the grey matter compared with the white matter (Svennerholm, 1968), suggesting that such fatty acids are preferentially present in neurons and axons.
In summary, our study provides evidence for an important role of oxidative damage in the pathogenesis of demyelination and neurodegeneration in multiple sclerosis lesions, which may act in addition to, or in cooperation with nitric oxide radicals, as described previously (Bagasra et al., 1995; Zeis et al., 2009). It further shows—for the first time—that the analysis of oxidized lipid epitopes in multiple sclerosis lesions allows identification of acute damage of oligodendrocytes, axons and neurons at different stages of lesion formation. Our data also suggest that oxidative damage is in part related to inflammation, that it affects different cellular components of the CNS, but that myelin, oligodendrocytes, neurons and axons may be more sensitive to oxidative damage than astrocytes.
Funding
This study was funded by the Austrian Science Fund (FWF Project P 19854-B02 and the PhD Doktoratskolleg CCHD; APW 1205-B09).
Acknowledgements
We thank Marianne Leiszer, Ulrike Köck and Angela Kury for expert technical assistance and Prof. Dr Johannes Berger for advise in lipid metabolism.
Glossary
Abbreviations
- MDA
malondialdehyde
References
- Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Brück W, et al. Preferential loss of myelin associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropath Exp Neurol. 2003;62:25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Michaels FH, Zheng YM, Bobroski LE, Spitsin SV, Fu ZF, et al. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:12041–5. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–68. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Bauer J, Elger CE, Hans VH, Schramm J, Urbach H, Lassmann H, et al. Astrocytes are a specific immunological target in Rasmussen's encephalitis. Ann Neurol. 2007;62:67–80. doi: 10.1002/ana.21148. [DOI] [PubMed] [Google Scholar]
- Bizzozero OA, Dejesus G, Callaha K, Pastuszyn A. Elevated protein carbonylation in the brain white matter and grey matter of patients with multiple sclerosis. J Neurosci Res. 2005;81:687–95. doi: 10.1002/jnr.20587. [DOI] [PubMed] [Google Scholar]
- Brück W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–96. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Ziabreva I, Reeve AK, Krishnan KJ, Reynolds R, Howell O, et al. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann Neurol. 2011;69:481–92. doi: 10.1002/ana.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, et al. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–70. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353–8. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AH, Manning PT, Keeling RM, Schmidt RE, Misko TP. Peroxynitrite formation within the central nervous system in active multiple sclerosis. J Neuroimmunol. 1998;88:45–56. doi: 10.1016/s0165-5728(98)00078-2. [DOI] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–89. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–9. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Frischer JM, Bramow S, Dal Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–89. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson APD, Barnett MH, Parratt JDE, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–53. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P. Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimers Dis. 2010;20:S453–73. doi: 10.3233/JAD-2010-100321. [DOI] [PubMed] [Google Scholar]
- Höftberger R, Fink S, Aboul-Enein F, Botond G, Olah J, Berki T, et al. Tubulin polymerization promoting protein (TPPP/p25) as a marker for oligodendroglial changes in multiple sclerosis. Glia. 2010;58:1847–57. doi: 10.1002/glia.21054. [DOI] [PubMed] [Google Scholar]
- Jack C, Antel J, Brück W, Kuhlmann T. Contrasting potential of nitric oxide and peroxynitrite to mediate oligodendrocyte injury in multiple sclerosis. Glia. 2007;55:926–34. doi: 10.1002/glia.20514. [DOI] [PubMed] [Google Scholar]
- Kalman B, Leist TP. A mitochondrial component of neurodegeneration in multiple sclerosis. Neuromolecular Med. 2003;3:147–58. doi: 10.1385/NMM:3:3:147. [DOI] [PubMed] [Google Scholar]
- Keller JN, Mattson MP. Roles of lipid peroxidation in modulation of cellular signalling pathways, cell dysfunction, and death in the nervous system. Rev Neurosci. 1998;9:105–16. doi: 10.1515/revneuro.1998.9.2.105. [DOI] [PubMed] [Google Scholar]
- Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, Ding Q. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol. 2004;36:2376–91. doi: 10.1016/j.biocel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- King G, Payne S, Walker F, Murray GI. A highly sensitive detection method for immunohistochemistry using biotinylated tyramine. J Pathol. 1997;183:237–41. doi: 10.1002/(SICI)1096-9896(199710)183:2<237::AID-PATH893>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–76. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Brück W, Lucchinetti C. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–8. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JSH, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Amer J Pathol. 2001;158:2057–66. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Selak M, O’Connor J, Croul S, Lorenzana C, Butunoi C, et al. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci. 2000;177:95–103. doi: 10.1016/s0022-510x(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008;131:1722–35. doi: 10.1093/brain/awn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D, Ziabreva I, Campbell G, Lax N, Hanson PS, Lassmann H, Turnbull DH. Mitochondrial changes within axons in multiple sclerosis. Brain. 2009;132:1161–74. doi: 10.1093/brain/awp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao P, Reddy H. Is multiple sclerosis a mitochondrial disease? Biochim Biophys Acta. 2010;1802:66–79. doi: 10.1016/j.bbadis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marburg O. Die sogenannte akute multiple sklerose. Jahrbuch Psychiatrie. 1906;27:211–312. [Google Scholar]
- Marik C, Felts P, Bauer J, Lassmann H, Smith KJ. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain. 2007;130:2800–15. doi: 10.1093/brain/awm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe J, Cuzner ML. Low density lipoprotein uptake by macrophages in multiple sclerosis plaques: implications for pathogenesis. Neuropath Appl Neurobiol. 1994;20:152–62. doi: 10.1111/j.1365-2990.1994.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Parratt JD, Prineas JW. Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult Scler. 2010;16:1156–72. doi: 10.1177/1352458510382324. [DOI] [PubMed] [Google Scholar]
- Palinski W, Hörkkö S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–14. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W, Ylä-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, et al. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–35. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Amer J Pathol. 1993;143:555–64. [PMC free article] [PubMed] [Google Scholar]
- Quin J, Goswami R, Balabanov R, Dawson G. Oxidised phosphatidylcholine is a marker for neuroinflammation in multiple sclerosis brain. J Neurosci Res. 2007;85:977–84. doi: 10.1002/jnr.21206. [DOI] [PubMed] [Google Scholar]
- Sharma R, Fischer MT, Bauer J, Felts PA, Smith KJ, Misu T, et al. Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol. 2010;120:223–36. doi: 10.1007/s00401-010-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Kapoor PA, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L. Distribution of fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968;9:570–9. [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Trapp B, Stys P. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurology. 2009;8:80–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Witte ME, Schreibelt G, de Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochem Biophys Acta. 2011;1812:141–50. doi: 10.1016/j.bbadis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, et al. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radical Biol Med. 2008;45:1729–37. doi: 10.1016/j.freeradbiomed.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Veto S, Acs P, Bauer J, Lassmann H, Berente Z, Setalo G, Jr, et al. Inhibiting poly(ADP-ribose) polymerase: a potential therapy against oligodendrocyte death. Brain. 2010;133:822–34. doi: 10.1093/brain/awp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirova O, O’Connor J, Cahill A, Alder H, Butunoi C, Kalman B. Oxidative damage to DNA in plaques of MS brains. Mult Scler. 1998;4:413–8. doi: 10.1177/135245859800400503. [DOI] [PubMed] [Google Scholar]
- Wang X, Liao Y, Li G, Yin D, Sheng S. A comparative study of artificial ceroid/lipofuscin from different tissue materials of rats. Exp Aging Res. 2008;34:282–95. doi: 10.1080/03610730802070282. [DOI] [PubMed] [Google Scholar]
- Witte ME, Geurts JJ, deVires HE, van der Valk P, van Horssen J. Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration. Mitochondrion. 2010;10:411–8. doi: 10.1016/j.mito.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Zeis T, Propst A, Steck AJ, Stadelmann C, Brück W, Schaeren-Wiemers N. Molecular changes in white matter adjacent to an active demyelinating lesion in early multiple sclerosis. Brain Pathol. 2009;19:459–66. doi: 10.1111/j.1750-3639.2008.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziabreva I, Campbell G, Rist J, Zambonin J, Rorbach J, Wydro MM, et al. Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes. Glia. 2010;58:1827–37. doi: 10.1002/glia.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]