Abstract
Gait freezing and postural instability are disabling features of Parkinsonian disorders, treatable with pedunculopontine nucleus stimulation. Both features are considered deficits of proximal and axial musculature, innervated predominantly by reticulospinal pathways and tend to manifest when gait and posture require adjustment. Adjustments to gait and posture are amenable to pre-preparation and rapid triggered release. Experimentally, such accelerated release can be elicited by loud auditory stimuli—a phenomenon known as ‘StartReact’. We observed StartReact in healthy and Parkinsonian controls. However, StartReact was absent in Parkinsonian patients with severe gait freezing and postural instability. Pedunculopontine nucleus stimulation restored StartReact proximally and proximal reaction times to loud stimuli correlated with gait and postural disturbance. These findings suggest a relative block to triggered, pre-prepared movement in gait freezing and postural instability, relieved by pedunculopontine nucleus stimulation.
Keywords: deep brain stimulation, gait freezing, Parkinson’s disease, pedunculopontine nucleus, StartReact
Introduction
Freezing of gait and postural instability are the major causes of falls in Parkinsonian disorders, including Parkinson’s disease (Factor, 2008; Kerr et al., 2010) and are often poorly responsive to dopaminergic medication (Bloem et al., 2004). Informed by experimental studies in animal models (Nandi et al., 2002; Jenkinson et al., 2004, 2006), deep brain stimulation of the pedunculopontine nucleus has emerged as a novel therapy for freezing of gait/postural instability (Mazzone et al., 2005; Plaha and Gill, 2005; Ferraye et al., 2009; Moro et al., 2010).
The pathophysiology of freezing of gait and postural instability is poorly understood—but their frequent coexistence raises the possibility of shared mechanisms (Giladi et al., 2001; Karachi et al., 2010) Both conditions are considered deficits of axial and proximal musculature (Jankovic, 2008). In postural instability, postural reflexes that adjust for body sway and environmental perturbations are diminished (Bloem, 1992). Similarly, freezing of gait typically occurs when adjustments are required to the locomotor rhythm—for example, with gait initiation, turning, overcoming reduced stride length and negotiating tight spaces and obstacles (Okuma, 2006; Chee et al., 2009; Almeida and Lebold, 2010).
Adjustments to posture and gait can be considerably accelerated (or even triggered involuntarily) by loud auditory stimuli of the type that may also elicit a startle reflex (MacKinnon et al., 2007; Reynolds and Day, 2007; Queralt et al., 2008). The speeding of responses when such a stimulus is delivered with the imperative cue is known as the ‘StartReact’ phenomenon (Valls-Sole et al., 1995, 1999). StartReact occurs when the relevant motor response can be prepared in advance, as seen experimentally in simple reaction time tasks (Valls-Sole et al., 1999; Carlsen et al., 2008). The assumption is that some motor programmes can be stored in a pre-prepared state and are subject to triggered reflex-like release, such as by loud auditory stimuli. The short latencies of StartReact responses have been interpreted to reflect direct subcortical release (Carlsen et al., 2004a). The triggering by loud auditory stimuli has further prompted speculation that StartReact responses may utilize the same efferent pathway as the startle reflex—the reticulospinal tract (Valls-Sole et al., 1995, 2008). The reticulospinal tract appears to predominantly innervate proximal and axial musculature, as supported by early lesioning studies in primates (Lawrence and Kuypers, 1968a, b). Accordingly, StartReact has been shown to be preferentially expressed in proximal compared to distal muscles (Carlsen et al., 2009).
Here, we explore the hypothesis that in freezing of gait/postural instability there is impairment of the system supporting the reflexic release of pre-prepared motor programmes. We therefore predicted that Parkinsonian patients with freezing of gait/postural instability will have a deficit in StartReact in proximal muscles, and, importantly, that this deficit would be reversed by pedunculopontine nucleus stimulation.
Subjects and methods
Subjects and clinical assessments
Three subject groups were assessed: (i) eight patients with Parkinson’s disease complicated by severe freezing of gait/postural instability, chronically implanted with bilateral pedunculopontine nucleus stimulators (Parkinson’s disease freezing of gait/postural instability group); (ii) eight patients with Parkinson’s disease of akinetic/rigid subtype without significant freezing of gait/postural instability (Parkinson’s disease no freezing of gait/postural instability group); and (iii) 10 age-matched healthy controls. Patients with Parkinson’s disease were matched for age, disease duration, motor severity and cognitive status. Subjects were recruited from centres in Oxford, UK and Brisbane, Australia. Subjects with bilateral deafness were excluded. Local ethics committee approval was obtained from both centres and participants gave written informed consent.
Parkinsonian patients were clinically assessed with the motor subsection (Part III) of the Unified Parkinson’s Disease Rating Scale (UPDRS, score/108). OFF medication assessments occurred after overnight withdrawal (>12 h) of dopaminergic therapy. Patients with pedunculopontine nucleus stimulators were also assessed on and off stimulation with a minimum 1 h washout period. These clinical assessments at both centres (in two countries) were performed unblinded by the same neurologist specialized in movement disorders (W.T.). UPDRS was segmented into items 27–30 (score/16) assessing posture, gait and balance and residual items 1–26 (R-UPDRS, score/92) assessing bradykinesia, rigidity and tremor. Patients also prospectively completed the Gait and Falls Questionnaire (GFQ, score/64) which assesses Parkinsonian gait disturbance including freezing of gait, festination and falls (Giladi et al., 2000). The Freezing of Gait Questionnaire (FOGQ, score/24) and Falls Question (FallsQ, score/4) are components of the Gait and Falls Questionnaire (Giladi et al., 2000, 2009). For all motor scales, higher scores indicate worse function. Additionally, cognition was assessed with the Mini-Mental State Examination (MMSE, score/30), with lower scores indicating worse function.
The dominant symptomatic issue in the patients with Parkinson’s disease with freezing of gait/postural instability was severe freezing of gait/postural instability persisting even ‘ON medication’, causing frequent falls. In Parkinson’s disease, freezing of gait/postural instability becomes more common and tends to be less medication-responsive as the disease progresses (Giladi et al., 2001; Bloem et al., 2004). The overall prevalence of freezing of gait/postural instability in Parkinson’s disease is ∼50% (Macht et al., 2007). However, severe ‘ON medication’ freezing of gait/postural instability as the predominant issue is unusual in Parkinson’s disease and raises the question of atypical pathologies (Factor, 2008; Jankovic, 2008). In the absence of a definitive test in life, we stress that the diagnosis of Parkinson’s disease is presumptive.
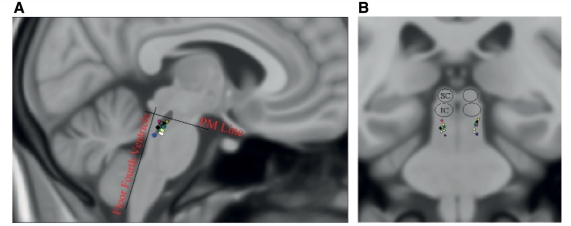
Patients with Parkinson’s disease with freezing of gait/postural instability were receiving chronic bilateral pedunculopontine nucleus stimulation. One patient was also receiving subthalamic nucleus stimulation (switched off an hour prior to and during experiments). No other patient had received surgery to any other brain target. Pedunculopontine nucleus electrodes were model 3387 (Medtronic), configured with four active contacts, each 1.5 mm in diameter with 1.5 mm spacing between adjacent contacts. Surgical implantation of the pedunculopontine nucleus has been described previously (Pereira et al., 2008). The lower pedunculopontine nucleus region was targeted, below the level of the inferior colliculus. Localization of stimulation sites (midpoint between active contacts for bipolar stimulation and cathodes for monopolar stimulation) is represented in Fig. 1. Contacts were identified on postoperative computerized tomography fused with preoperative magnetic resonance images and transformed onto Montreal Neurological Institute (MNI) space using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (Smith et al., 2004). Coordinates were calculated in millimetres from midline (laterality), ventrodorsal distance (d) from floor of the fourth ventricle and rostrocaudal distance (h) from a pontomesencephalic line connecting the pontomesencephalic junction to the caudal end of the inferior colliculi, as described previously (Ferraye et al., 2009). The relative location/extent of the pedunculopontine nucleus has been outlined, based on choline-acetyltransferase immunohistochemical (ChAT5) staining in the human (Mesulam et al., 1989). Parameters employed for chronic therapeutic stimulation were as follows: frequency range 30 or 35 Hz, voltage range 2.5–4.3V and pulse width 60 µs.
Figure 1.
Localization of the sites of stimulation—represented in Montreal Neurological Institute (MNI) space on sagittal (A) and coronal (B) views. In (A), the relative location/extent of the pedunculopontine nucleus has been outlined in dark grey, based on choline-acetyltransferase immunohistochemical (ChAT5) staining in the human (see text). IC = inferior colliculus; PM = pontomesencephalic line connecting the pontomesencephalic junction to the caudal end of the inferior colliculi; SC = superior colliculus.
Clinical details of the study participants are shown in Tables 1 and 2. Healthy controls, patients with Parkinson’s disease with no freezing of gait/postural instability and patients with Parkinson’s disease with freezing of gait/postural instability did not significantly differ with respect to age [F(2,23) = 0.365, P = 0.698]. Patients with Parkinson’s disease with no freezing of gait/postural instability and patients with Parkinson’s disease with freezing of gait/postural instability did not differ with respect to disease duration [t(14) = 0.1, P = 0.92], R-UPDRS subscore [t(14) = 0.311, P = 0.761] or Mini-Mental State Examination [t(12) = −0.408, P = 0.690]. Patients with Parkinson’s disease with freezing of gait/postural instability had significantly higher scores in items 27/30 [t(14) = −4.743, P < 0.001], Gait and Falls Questionnaire [t(14) = −5.281, P < 0.001], Freezing of Gait Questionnaire [t(14) = −5.967, P = 0.001] and Falls Questionnaire [t(14) = −6.325, P < 0.001].
Table 1.
Baseline characteristics
| Age (years) | Sex | PD Duration (years) | MMSE | R-UPDRS Off meds/ stim | IT27-30 Off meds/ stim | GFQ | FOGQ | FallsQ | |
|---|---|---|---|---|---|---|---|---|---|
| Healthy controls | 68.3 (7.6) | 6M, 4F | \ | \ | \ | \ | \ | \ | \ |
| PD NoFOG/PI | 65.1 (7.6) | 4M, 4F | 11.5 (3.7) | 29.2 (1.0) | 28.1 (9.5) | 3.3 (1.8) | 4.0 (3.9) | 2.0 (2.0) | 0.4 (0.7) |
| PD FOG/PI | 67.5 (8.8) | 6M, 2F | 11.3 (6.0) | 29.4 (0.9) | 26.8 (8.1) | 8.4 (2.4)a | 26.9 (11.6)a | 11.8 (4.2)a | 2.9 (0.8)a |
Data are mean (SD).
aDifferent from Parkinson’s disease with no freezing of gait/postural instability P ≤ 0.001.
Falls Q = Falls Questionnaire (score/4); FOG = freezing of gait; FOGQ = Freezing of Gait Questionnaire (score/24); GFQ = Gait and Falls Questionnaire (score/64); IT27-30 = items 27-30 Unified Parkinson’s disease rating scale part III, assessing gait, posture and balance (score/16); MMSE = Mini-Mental State Examination (score/30); PD = Parkinson’s disease; PI = postural instability; R-UPDRS = items 1-26 Unified Parkinson’s disease rating scale part III (score/92).
For Parkinson’s disease with freezing of gait/postural instability, scores are post-operative.
Table 2.
Patients with Parkinson’s disease with freezing of gait/postural instability
| Patient | Age/Sex | PD duration (years) | Post-operative duration (years, months) | L-dopa dose equivalent (mg/day) | UPDRS III OFF/ON meds (off stim) | IT27-30 off/on stim (OFF meds) | GFQ pre-/ post- operative | FOGQ pre-/post- operative | FallsQ pre-/post- operative | Supportive for UK brain bank criteriaa |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72F | 10 | 2 | 950 | 38/22 | 11/8 | 48/26 | 22/13 | 4/2 | D, A, T, P |
| 2 | 72M | 18 | 2,5 | 2500 | 25/17 | 6/6 | 30/16 | 14/11 | 4/2 | D, A, T, P |
| 3 | 76M | 6 | 2 | 600 | 26/14 | 6/4 | 51/18 | 22/7 | 3/3 | A, P |
| 4 | 61F | 10 | 2 | 800 | 40/23 | 10/9 | 61/36 | 24/16 | 4/3 | D, A, P |
| 5 | 77M | 6 | 0,6 | 1400 | 31/17 | 10/10 | 31/14 | ^/6 | ^/2 | A, P |
| 6 | 71M | 4 | 0,6 | 1550 | 27/18 | 5/5 | ^/21 | ^/9 | ^/3 | P |
| 7 | 55M | 20 | 1 | 850 | 51/19 | 8/6 | 38/40 | 14/15 | 4/4 | D, A, T, P |
| 8 | 56M | 16 | 2,10 | 1400 | 43/16 | 11/8 | 61/44 | 23/17 | 4/4 | D, A, T, P |
aAdditional to disease duration and levodopa response as documented elsewhere in the table.
Post-operative clinical assessments were performed on the same day as reaction time assessment. Patients 7 and 8 were recruited from Oxford, UK, other patients from Brisbane, Australia. Patient 7 also had subthalamic nucleus stimulators that were turned off 1 h prior to and during experiments.
^ = not known; A = asymmetry persistent; D = dyskinesias; Falls Q = Falls Questionnaire (score/4); FOGQ = Freezing of Gait Questionnaire (score/24); GFQ = Gait and Falls Questionnaire (score/64); IT27-30 = items 27-30 Unified Parkinson’s disease rating scale, assessing gait, posture and balance (score/16); MMSE = Mini-Mental State Examination (score/30); P = progressive disease course; PD = Parkinson’s disease; T = tremor at rest; UPDRS III = part III (motor) Unified Parkinson’s disease rating scale (score/108).
Experiments
Three tasks were administered:
Auditory blink and startle reflex task: patients were presented with 10 trials of loud auditory stimuli (LAS: 122 dB, 40 ms duration, 1000 Hz). Intertrial intervals were variable (10–15 s). Patients were advised that they would hear a series of sounds, some louder than others, and were requested to sit comfortably with eyes open, with no need to respond.
Proximal simple reaction time task: a warned simple reaction time task. All stimuli were auditory, to eliminate the possibility of intersensory facilitation (Hershenson, 1962). Serial presentation of 35 trials, each consisting of a warning cue (92 dB, 40 ms duration, 300 Hz) followed by the imperative ‘go’ cue (40 ms duration, 1000 Hz). The imperative stimulus was either normal intensity (89 dB—normal trials) or loud (122 dB—LAS trials). The first five trials were ‘practice’ normal trials, followed by 20 normal and 10 LAS trials randomly intermixed. Warning periods (1–3.5 s) and intertrial (6–10 s) intervals were variable. Patients were instructed to react as quickly as possible with ballistic elbow flexion.
Distal simple reaction time task: the same task as the proximal simple reaction time except patients responded with ballistic abduction of the forefinger. In this task, patients were seated with hand and forearm resting on a bench-top, flexed 90° at the elbow. The hand was positioned prone with digits adducted. Patients were instructed to react as quickly as possible with forefinger abduction then return to the resting hand position.
Stimuli were controlled through a digital to analogue converter (1401, Cambridge Electronic Design). Auditory tones were delivered binaurally through headphones (Audio Technica ATH-ES7). Sound pressure levels were assessed in a sound-proofed room with a modular precision sound analyser (Observer 2260, Bruel and Kjaer) via an artificial ear and headphone adaptor.
Bipolar surface EMG activity was recorded using 9 mm diameter silver–silver chloride electrodes. EMG electrodes were taped to skin overlying orbicularis oculi and sternocleidomastoid contralateral to the limb to be moved in reaction time assessments. Reaction times were assessed with both EMG and a triaxial accelerometer. For the proximal simple reaction time task, EMG was applied to biceps and accelerometer taped to the radial styloid. For the distal simple reaction time task, EMG was applied over the first dorsal interosseous and accelerometer taped to the tip of index finger. In one healthy subject, distal EMG reaction time was not assessable due to misplaced EMG electrodes. Data were sampled (or downsampled to) 256 Hz (Porti amplifier, TMSI). EMG recordings were amplified and low pass filtered at 500 Hz. Accelerometer (TMSI) was band pass filtered between 2 and 60 Hz.
Tasks were administered with subjects seated comfortably in a quiet, dimly lit room. The auditory blink and startle reflex task was always administered first, to minimize habituation effects on this task. The order of proximal and distal simple reaction time tasks was counterbalanced.
In patients with Parkinson’s disease, experiments were conducted after overnight withdrawal of dopaminergic medication to limit variance from fluctuating dopaminergic state. For patients with Parkinson’s disease with freezing of gait/postural instability, there were two conditions – on and off therapeutic bilateral pedunculopontine nucleus stimulation. Ordering of conditions was counterbalanced, with a minimum 1 h washout period between conditions. Subjects were blinded to condition and to experimental hypotheses.
Parameters and data analysis
Two reaction time parameters were assessed; accelerometer reaction time and EMG reaction time. Accelerometer analysis was automated by a script developed for Matlab (The Mathworks Inc). Priority is therefore given to this dataset. Accelerometer reaction times were computed for every trial before averaging to yield the task accelerometer reaction time. EMG reaction times for individual trials could not be reliably determined due to a poorer signal to noise ratio, partly due to resting EMG activity due to rigidity. For each task, EMG reaction time was therefore assessed from averages of the trials for a given condition in a given patient in Spike 2 (Cambridge Electronic Design). Note, however, that the onset of EMG activity in such averages tends to be dominated by trials with the shortest response times.
The first five trials in each simple reaction time task (always normal trials) were discarded as practice. Anticipatory responses (EMG response prior to the imperative cue) were discarded. The automated accelerometer analysis involved initial DC removal (time constant individualized for each trial from the average DC level 0.45 s prior to the imperative) before rectification. Accelerometer response onset was defined as an amplitude rise exceeding the mean of the prestimulus (0.5 s) baseline by 3 standard deviations (SDs). The EMG reaction time signal was first subject to DC removal using a fixed averaging interval of 0.002 s (the latter is defined as the time constant of the procedure in Spike 2). Thereafter the EMG signal was rectified and trials averaged across a given condition in each patient. EMG reaction time onset was defined in the latter as the first data point exceeding the mean plus 3 SD of the prestimulus (1 s) baseline that had a steep (>2 uV/ms) rise in amplitude sustained for >20 ms.
Auditory startle reflexes were assessed during reflex and simple reaction time tasks. An auditory startle reflex was considered present if there was a short latency (<130 ms in healthy subjects and <150 ms in subjects with Parkinson’s disease) sternocleidomastoid response following a loud stimulus, sustained above the background, which in simple reaction time tasks was required to precede the limb EMG response (determined from the native unrectified signals). Orbicularis and sternocleidomastoid EMGs were then subject to DC removal (individualized for each recording) then rectification. Amplitude and latency of the first occurring auditory startle reflex was assessed. For auditory blink reflexes, amplitude and latency of the averaged orbicularis response were assessed in the auditory blink and startle reflex task.
Statistics
The Kolmogorov–Smirnov Test demonstrated that the distribution of all measures was not different from the normal. Within each group, reaction times for each joint (proximal and distal) and stimulus (normal and LAS) were compared using repeated measures ANOVA with factors ‘Joint’ and ‘Stimulus’. Patients with Parkinson’s disease with freezing of gait/postural instability had an additional factor ‘deep brain stimulation’ (on and off). We report Joint effects only where they interact significantly with other factors (e.g. Joint × Stimulus). Post hoc tests were performed with paired t-tests. StartReact benefit (normal reaction time – LAS reaction time) was compared between groups with ANOVA and post hoc independent samples t-tests.
In patients with Parkinson’s disease with freezing of gait/postural instability, off and on stimulation results were considered together and correlations (Pearson’s) sought between LAS reaction times and two independent clinical measures of Parkinsonian gait and balance disturbance—the Gait and Falls Questionnaire (contains both the Freezing of Gait Questionnaire and Falls Questionnaire) and items 27/30.
Reflex latencies and amplitudes were compared between groups using single-factor ANOVA and post hoc independent samples t-tests. The frequency of individuals recording a recognizable auditory startle reflex was compared between groups using Pearson’s chi-square.
Post hoc tests were corrected for multiple comparisons (Bejamini and Hochberg, 1995). Level of significance was P < 0.05.
Results
Accelerometer reaction time
In healthy subjects, there was a significant main effect of Stimulus [F(1,9) = 44.3, P < 0.001]. Post hoc tests revealed the presence of StartReact with LAS trials significantly faster than normal trials [mean normal reaction time, averaged across proximal and distal muscles 135.3 ms versus LAS reaction time, averaged across proximal and distal muscles 113.4 ms, t(19) = 6.057, P < 0.001]. The Joint × Stimulus interaction was not significant.
In Parkinson’s disease with no freezing of gait/postural instability, there was again a significant effect of Stimulus [ F(1,7) = 9.2, P = 0.019]. Post hoc tests revealed the presence of StartReact with LAS trials significantly faster than normal trials [mean normal reaction time 164.8 ms versus LAS reaction time 130.2 ms, t(15) = 3.755, P = 0.002]. The Joint × Stimulus interaction was not significant.
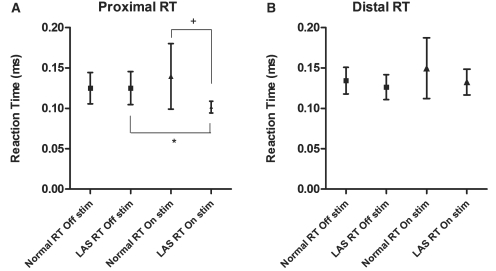
In contrast, in patients with Parkinson’s disease with freezing of gait/postural instability, the effect of Stimulus was absent [F(1,7) = 3.7, P = 0.097]. There was no main effect of deep brain stimulation nor a Deep brain stimulation × Joint interaction. There was a trend towards a Deep brain stimulation×Stimulus interaction [F(1,7) = 5.0, P = 0.060]. However, there was a significant Deep brain stimulation × Joint × Stimulus interaction [F(1,7) = 7.2, P = 0.031]. Post hoc tests revealed that this was due to a selective improvement in proximal LAS reaction time with pedunculopontine nucleus stimulation [mean LAS reaction time 125.0 ms off stimulation versus 101.5 ms on stimulation, t(7) = 3.6, P = 0.036] (Fig. 2). Pedunculopontine nucleus stimulation also meant that proximal LAS reaction times became faster than proximal normal reaction times, so that pedunculopontine nucleus stimulation restored proximal StartReact [mean normal reaction time 139.4 ms versus LAS reaction time 101.5 ms, t(7) = 3.0, P = 0.040].
Figure 2.
Proximal (A) and Distal (B) Accelerometer reaction times (means and SD) for patients with Parkinson’s disease with freezing of gait/postural instability, on and off bilateral pedunculopontine nucleus stimulation. *P = 0.036;+P = 0.040. Increased variance of normal reaction time (reflected in widened SD) is noted in the ‘on stimulation’ condition. This is mostly attributable to two patients who recorded more reaction time outliers ‘on stimulation’, suggestive of attentional lapses/fatigue. This figure without the results of these two patients can be viewed in Supplementary material, but the two significant differences remain. RT = reaction time.
In line with the above, an ANOVA of proximal StartReact benefit showed a significant difference between subject groups (patients with Parkinson’s disease with freezing of gait/postural instability off stimulation) [F(2,23) = 4.729, P = 0.019]. Post hoc tests revealed proximal StartReact benefit to be significantly less in patients with Parkinson’s disease with freezing of gait/postural instability (off stimulation) compared with patients with Parkinson’s disease with no freezing of gait/postural instability [−0.08 ms versus 41.3 ms, t(14) = −2.639, P = 0.040] and healthy controls [−0.08 ms versus 26.0 ms, t(16) = −2.600, P = 0.019]. However, with patients with Parkinson’s disease with freezing of gait/postural instability on stimulation, proximal StartReact did not differ between groups [F(2,23) = 0.615, P = 0.549].
Electromyography reaction time
EMG reaction time results followed a similar pattern as accelerometer reaction time. In healthy subjects, there was a significant effect of Stimulus [F(1,8) = 16.7, P = 0.003]. Post hoc tests revealed the presence of StartReact, with LAS trials significantly faster than normal trials [mean normal reaction time, averaged across proximal and distal muscles 86.4 ms versus LAS reaction time, averaged across proximal and distal muscles 77.2 ms, t(18) = 3.860, P = 0.001]. The Joint × Stimulus interaction was not significant.
In patients with Parkinson’s disease with no freezing of gait/postural instability, there remained a significant effect of Stimulus [F(1,7) = 8.7, P = 0.021]. Post hoc tests revealed the presence of StartReact, with loud trials significantly faster than normal trials [mean normal reaction time 114.3 ms versus LAS reaction time 92.1 ms, t(15) = 2.884, P = 0.011]. The Joint × Stimulus interaction was not significant.
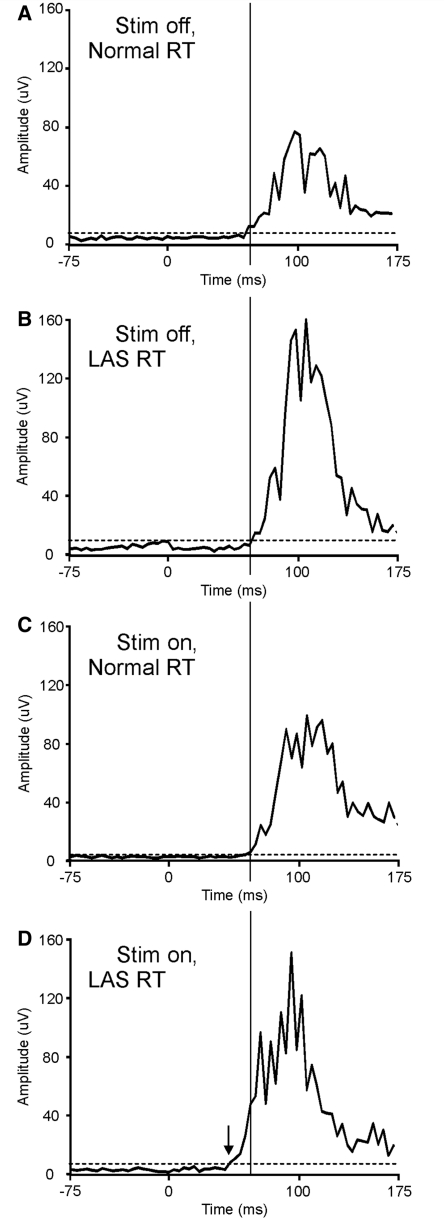
In patients with Parkinson’s disease with freezing of gait/postural instability, the effect of Stimulus was absent [F(1,7) = 0.00, P = 0.987]. There was no effect of deep brain stimulation. There were significant interactions between Deep brain stimulation × Joint [F(1,7) = 8.119, P = 0.025], Deep brain stimulation × Stimulus [F(1,7) = 8.312, P = 0.024] and Deep brain stimulation × Joint × Stimulus [F(1,7) = 5.669, P = 0.049]. Post hoc tests revealed that this was due to a selective improvement in proximal LAS reaction time with pedunculopontine nucleus stimulation [mean LAS reaction time 84.8 ms off stimulation versus 65.4 ms on stimulation, t(7) = 6.167, P < 0.001] (Fig. 3). Pedunculopontine nucleus stimulation significantly increased proximal StartReact benefit [proximal StartReact off stimulation −10.4 ms versus on stimulation 15.5 ms, t(7) = −3.363, P = 0.012].
Figure 3.
Proximal EMG reaction times for a patient with Parkinson’s disease with freezing of gait/postural instability. Speeded responses (ballistic elbow flexion) were recorded to normal (89 dB) auditory stimuli (normal reaction time) and loud (122 dB) auditory stimuli (LAS reaction time), off and on pedunculopontine nucleus stimulation. Traces are the averaged biceps EMG waveforms from the proximal simple reaction time task in Patient 3, with Parkinson’s disease with freezing of gait/postural instability. The onsets of such averaged waveforms tend to reflect the fastest occurring responses during the task. The dotted horizontal line represents the mean plus 3 SD of the pre-stimulus (1 s) baseline. The solid vertical line transects all traces at the same time-point (and at the onset defined for the stimulation on, normal reaction time). When off stimulation, the averaged LAS reaction time (B) is not faster than the normal reaction time (A); StartReact is absent. Pedunculopontine nucleus stimulation speeds the LAS reaction time (D, onset indicated by the arrow) but not the normal reaction time (C). Thus pedunculopontine nucleus stimulation restored proximal StartReact. RT = reaction time.
Accordingly, an ANOVA of proximal StartReact benefit showed a significant difference between subject groups (with patients with Parkinson’s disease with freezing of gait/postural instability off stimulation) [F(2,23) = 9.810, P = 0.001]. Post hoc tests revealed proximal StartReact to be significantly less in patients with Parkinson’s disease with freezing of gait/postural instability (off stimulation) compared with patients with Parkinson’s disease with no freezing of gait/postural instability [−10.4 ms versus 36.3 ms, t(14) = 3.580, P = 0.010] and healthy controls [−10.4 ms versus 10.9 ms, t(16) = 3.469, P = 0.006]. However, with patients with Parkinson’s disease with freezing of gait/postural instability on stimulation, proximal StartReact did not differ between groups [F(2,23) = 2.397, P = 0.113].
Correlations of reaction time with clinical measures
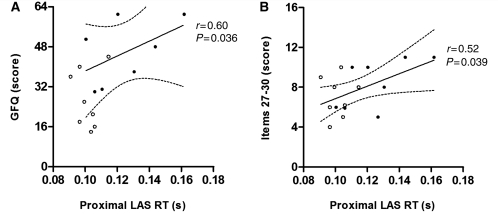
In patients with Parkinson’s disease with freezing of gait/postural instability (with off and on stimulation results considered together), proximal LAS accelerometer reaction time correlated with Gait and Falls Questionnaire (r = 0.60, P = 0.036) and items 27–30 (r = 0.52, P = 0.039) (Fig. 4). Distal LAS accelerometer reaction time and proximal and distal EMG LAS reaction times did not correlate with these measures.
Figure 4.
Correlation between proximal LAS accelerometer reaction time (RT) and clinical measures in patients with Parkinson’s disease with freezing of gait/postural instability. Linear regression (solid line) and 95% confidence intervals (dotted lines) are shown. Data from on stimulation (open circles) and off stimulation (filled circles) are both included, affording 16 potential data points [two results from each patient, except for one patient (A) in whom preoperative off stimulation Gait and Falls Questionnaire (GFQ) data were absent, as indicated in Table 2]. (A) Correlation between proximal LAS accelerometer reaction time and Gait and Falls Questionnaire. Off and on stimulation Gait and Falls Questionnaire scores were prospectively obtained preoperatively (off stimulation) and postoperatively (on stimulation). (B) Correlation between proximal LAS accelerometer reaction time and items 27–30 of the UPDRS. Items 27–30 scores were rated by the same examiner, off and on stimulation at the same postoperative visit with a minimum 1 h washout period.
Acoustic startle and blink reflexes
Auditory startle reflexes were identified in 7/10 healthy subjects, 5/8 subjects with Parkinson’s disease with no freezing of gait/postural instability and 1/8 subjects with Parkinson’s disease with freezing of gait/postural instability. The frequency of individuals with auditory startle reflex differed significantly between subject groups [χ2 (2,26) = 6.60, P = 0.037] (Table 3). There were significantly fewer auditory startle reflex in patients with Parkinson’s disease with freezing of gait/postural instability compared with patients with Parkinson’s disease with no freezing of gait/postural instability [χ2 (1,16) = 4.267, P = 0.030]. In all subjects, auditory startle reflexes were infrequent, usually occurring with the first LAS trial then rapidly habituating. In patients with Parkinson’s disease with freezing of gait/postural instability, pedunculopontine nucleus deep brain stimulation did not restore auditory startle reflex in any patient. Comparing healthy subjects and patients with Parkinson’s disease with no freezing of gait/postural instability, there were no significant differences in auditory startle reflex amplitudes [321.3 μV versus 238.5 μV, t(10) = 0.456, P = 0.658] or latencies [98.2 ms versus 101.9 ms, t(10) = -0.19, P = 0.854]. Insufficient patients with Parkinson’s disease with freezing of gait/postural instability had an auditory startle reflex to make comparisons with this group.
Table 3.
Summary data for acoustic blink and startle reflexes
| Acoustic blink reflex |
Acoustic startle reflex |
|||||
|---|---|---|---|---|---|---|
| Occurrence | Latency (ms) | Amplitude (μV) | Occurrence | Latency (ms) | Amplitude (μV) | |
| Healthy controls | 10/10 | 52.1 (7.8) | 89.3 (127.6) | 7/10 | 98.2 (29.7) | 321.36 (354.3) |
| PD NoFOG/PI | 8/8 | 42.4 (12.5) | 84.2 (80.3) | 5/8 | 101.9 (37.4) | 238.5 (229.8) |
| PD FOG/PI off stim | 7/7 | 48.5 (17.0) | 44.0 (55.5) | 1/8 | _ | _ |
| PD FOG/PI on stim | 7/7 | 47.3 (14.9) | 43.5 (53.6) | 0/8 | _ | _ |
Occurrence indicates proportion of patients demonstrating the response. Otherwise data are mean (SD). FOG = freezing of gait; PD = Parkinson’s disease; PI = postural instability.
An averaged auditory blink reflex in the auditory blink and startle reflex task was identifiable in all healthy subjects and patients with Parkinson’s disease with no freezing of gait/postural instability subjects and 7/8 subjects with Parkinson’s disease with freezing of gait/postural instability (in one patient with Parkinson’s disease with freezing of gait/postural instability, any auditory blink reflex was obscured by excessive blinking during recordings). Between subject groups (with Parkinson’s disease freezing of gait/postural instability off stimulation), no differences were found in auditory blink reflex amplitudes [F(2,22) = 0.495, P = 0.616] or latencies [F(2,22) = 1.387, P = 0.271]. In patients with Parkinson’s disease with freezing of gait/postural instability, pedunculopontine nucleus stimulation did not alter auditory blink reflex amplitudes [t(6) = 0.141, P = 0.892] or latencies [t(6) = 0.385, P = 0.714].
Discussion
We found that Parkinsonian patients with freezing of gait/postural instability can be distinguished from those without freezing of gait/postural instability by attenuation of the StartReact phenomenon in a proximal muscle, the biceps, and through the scarcity of auditory startle responses. The deficit of StartReact, but not that of the auditory startle response, was reversed by pedunculopontine nucleus stimulation.
The scarcity of auditory startle responses in Parkinsonian patients with freezing of gait/postural instability recalls the reduced frequency of startle in progressive supranuclear palsy (Vidailhet et al., 1992; Kofler et al., 2001; Gironell et al., 2003). Severe ‘ON medication’ freezing of gait/postural instability as a dominating complaint in Parkinson’s disease is unusual and itself flags the possibility of progressive supranuclear palsy (Jankovic, 2008). In the absence of a definitive test in life, the diagnosis of Parkinson’s disease in our patients with Parkinson’s disease with freezing of gait/postural instability should be considered presumptive, although the persistence of normal auditory blink reflexes differs from the absent or abnormal auditory blink reflexes reported in progressive supranuclear palsy (Vidailhet et al., 1992; Valldeoriola et al., 1998; Kofler et al., 2001; Gironell et al., 2003; Williams et al., 2008).
Regardless of pathological type, attenuation of startle in Parkinsonian patients with freezing of gait/postural instability implicates the pons as a site of significant functional disturbance in this phenotype, a conclusion further strengthened by the deficit in StartReact and its’ reversal by pedunculopontine nucleus stimulation (Brown et al., 1991; Vidailhet et al., 1992; Valls-Sole et al., 2008). Conversely, preservation of the auditory blink reflex suggests that the midbrain can be relatively spared in freezing of gait/postural instability (Hori et al., 1986).
The pedunculopontine nucleus region and release of preprogrammed movement
StartReact is described to occur when the relevant motor response can be fully anticipated, ‘preprogrammed’ and stored for release, as in simple reaction time tasks (Valls-Sole et al., 1999; Carlsen et al., 2008). In line with this task specificity, we previously found that pedunculopontine nucleus stimulation in patients with Parkinson’s disease with freezing of gait/postural instability selectively improved simple reaction time, but not choice or Go-NoGo reaction times (Thevathasan et al., 2010). Taken together with the current findings, it appears that pedunculopontine nucleus stimulation corrects a deficit in freezing of gait/postural instability in the release of pre-prepared responses, both in response to simple cues, or more strikingly, when cues are accompanied or replaced by loud auditory stimuli. This suggests that tonic low frequency activity in the pedunculopontine nucleus or a pathway in the region of the pedunculopontine nucleus supports the release of pre-prepared motor programmes in Parkinson’s disease. However, given that we only studied the effects of pedunculopontine nucleus stimulation in patients with Parkinson’s disease, one can only speculate about the relevance of this to the normal functioning of the pedunculopontine nucleus.
Some of our findings differ from those previously reported. We did not replicate our previous result that pedunculopontine nucleus stimulation improved simple reaction time in trials without LAS (‘normal reaction time’ in the present study) (Thevathasan et al., 2010). However, the tasks of the two studies are different. In the present study, we aimed to optimize StartReact by, for example, using warning cues and long intertrial intervals. This contrasts with the rapidly occurring unwarned visual cues of unchanging intensity employed previously, which could have promoted more reflexic simple reaction time responses. Furthermore, a small change in simple reaction time may have been undetected in this study as the long intertrial intervals meant that normal reaction times were averaged over only 20 trials per task, compared with 50 previously.
A previous study in young healthy subjects demonstrated StartReact to be greater in proximal compared with distal movements (Carlsen et al., 2009). Our study was not powered to demonstrate these differential effects, particularly in an elderly cohort. The same previous study in young healthy subjects suggested that an accompanying startle reflex might identify those responses with the greatest shortening of reaction time (Carlsen et al., 2009). However, we found that in elderly healthy subjects, a startle reflex seldom accompanied the intended motor response, despite the use of loud auditory stimuli. Aside from the age difference of subjects, the differing results could be explained by the different criteria used to define the presence of a startle reflex. We defined startle during reaction time tasks not only by virtue of short latency sternocleidomastoid EMG activity (e.g. <130 ms) but also, unlike the aforementioned study, by appearance of such activity before the limb response. This latter criterion was necessary to exclude sternocleidomastoid activity due to accessory muscle activation. Otherwise, responses sped by loud sounds (which had limb EMG latencies around 70–90 ms) might appear to be accompanied by startle. Other studies have similarly reported the absence of startle from trials where loud stimuli triggered rapid (sometimes even involuntary) responses – including where the released motor programmes were postural reflexes and stepping (MacKinnon et al., 2007; Reynolds and Day, 2007). Further evidence for the separable nature of StartReact and the startle reflex is that in LAS trials, an accompanying startle reflex does not alter the triphasic EMG pattern of the intended motor response (Carlsen et al., 2004b). Furthermore, unlike acoustic blink and startle reflexes, StartReact is not modified by prepulse inhibition (Valls-Sole et al., 2005). Such results are consistent with our finding that pedunculopontine nucleus stimulation restored StartReact without restoring startle in patients with Parkinson’s disease with freezing of gait/postural instability. As has been argued previously, such findings support that the StartReact phenomenon and startle reflex may be dissociated (Valls-Sole et al., 2005).
Relevance to the pathophysiology of freezing of gait/postural instability and the therapeutic mechanism of pedunculopontine nucleus stimulation
StartReact was absent in patients with Parkinson’s disease with freezing of gait/postural instability but conserved in disease matched patients with Parkinson’s disease with no freezing of gait/postural instability controls and healthy subjects. Unless the pedunculopontine nucleus electrodes themselves caused the reversible deficit in StartReact, then this deficit appears associated with gait freezing and postural instability.
Pedunculopontine nucleus stimulation sped proximal LAS reaction times significantly more than distal LAS reaction times—and proximal (and not distal) LAS accelerometer reaction times correlated with clinical measures of gait and postural disturbance. Proximal and axial musculature is predominantly innervated by reticulospinal pathways—the likely conduit of modifications to spinal pattern generated locomotion as well as postural reflexes (Lawrence and Kuypers, 1968b; Drew et al., 2004; Davidson et al., 2007; Stapley and Drew, 2009). Distal musculatures, particularly intrinsic hand muscles, receive predominantly corticospinal input (Lawrence and Kuypers, 1968a; Riddle et al., 2009). A lesser benefit of pedunculopontine nucleus stimulation on distal StartReact is therefore consistent with an effect mediated through reticulospinal pathways and also with the therapeutic role of pedunculopontine nucleus stimulation on the axial and proximal deficits of freezing of gait/postural instability.
In this study, we did not directly demonstrate that freezing of gait/postural instability involves a deficit in StartReact for gait and postural responses. However, adjustments to gait and posture are known to be amenable to StartReact—suggesting that some aspects of gait and posture are preprogrammed and potentially subject to the same triggered release we have shown is deficient in freezing of gait/postural instability and restored by pedunculopontine nucleus stimulation (Reynolds and Day, 2007; Queralt et al., 2008). This interpretation was supported by the presence of correlations between proximal LAS accelerometer reaction times and two independent measures of Parkinsonian gait and balance disturbance—the Gait and Falls Questionnaire and items 27–30. These correlations were found when proximal LAS reaction times were assessed with the accelerometer but not EMG. One explanation is that actual movement is more clinically relevant than the onset of motor recruitment. However, an additional consideration is that the methods of assessing EMG and accelerometer reaction times differed. In this study, EMG reaction times tended to reflect the fastest occurring responses whereas accelerometer reaction times were more representative of performance across all trials.
Optimization of motor systems releasing preprogrammed movement may not be the only mechanism by which pedunculopontine nucleus deep brain stimulation may improve gait as the strength of correlations suggested it accounts for only around a third of the variance in gait scores. In this regard, it is important to stress that the pedunculopontine nucleus has multiple functions. For example, local field potential studies and clinical observations have raised the possibility that attentional changes may also contribute to the effects of stimulation in this area (Androulidakis et al., 2008; Arnulf et al., 2010).
Our observation that StartReact is deficient in patients with Parkinson’s disease with freezing of gait/postural instability and restored by pedunculopontine nucleus stimulation offers insights into the pathophysiology of freezing of gait/postural instability and the mechanisms of pedunculopontine nucleus stimulation. However, we have not, in this study, addressed whether these findings might help predict response to pedunculopontine nucleus deep brain stimulation. The clinical utility of our findings remains to be investigated, although the variability in StartReact benefit may preclude any inferences at the single subject level.
In conclusion, our findings suggest that Parkinsonian patients with freezing of gait/postural instability have a deficit in the release of preprogrammed movement, when the latter should have been promoted by loud auditory stimuli. Delays in the release of preprogrammed movement correlated with the severity of the freezing of gait/postural instability phenotype. Pedunculopontine nucleus stimulation, while improving the phenotype, also restored the deficit in preprogrammed movement release. Accordingly, freezing of gait/postural instability may, in part, involve impairment of a pontine system supporting the reflexic release of pre-prepared motor programmes.
Funding
Medical Research Council (UK) and the NIHR Oxford Biomedical Research Centre.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- LAS
loud auditory stimuli
- UPDRS
Unified Parkinson’s Disease Rating Scale
References
- Almeida QJ, Lebold CA. Freezing of gait in Parkinson's disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry. 2010;81:513–8. doi: 10.1136/jnnp.2008.160580. [DOI] [PubMed] [Google Scholar]
- Androulidakis AG, Mazzone P, Litvak V, Penny W, Dileone M, Gaynor LM, et al. Oscillatory activity in the pedunculopontine area of patients with Parkinson’s disease. Exp Neurol. 2008;211:59–66. doi: 10.1016/j.expneurol.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Arnulf I, Ferraye M, Fraix V, Benabid AL, Chabardes S, Goetz L, et al. Sleep induced by stimulation in the human pedunculopontine nucleus area. Ann Neurol. 2010;67:546–9. doi: 10.1002/ana.21912. [DOI] [PubMed] [Google Scholar]
- Bejamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bloem BR. Postural instability in Parkinson’s disease. Clin Neurol Neurosurg. 1992;94(Suppl):S41–5. doi: 10.1016/0303-8467(92)90018-x. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–84. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain. 1991;114(Pt 4):1891–902. doi: 10.1093/brain/114.4.1891. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Dakin CJ, Sanderson DJ, Inglis JT, Franks IM. Startle reveals an absence of advance motor programming in a Go/No-go task. Neurosci Lett. 2008;434:61–5. doi: 10.1016/j.neulet.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res. 2004a;159:301–9. doi: 10.1007/s00221-004-1924-z. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Mot Behav. 2004b;36:253–64. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Differential effects of startle on reaction time for finger and arm movements. J Neurophysiol. 2009;101:306–14. doi: 10.1152/jn.00878.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing in Parkinson's disease and the stride length sequence effect interaction. Brain. 2009;132(Pt 8):2151–60. doi: 10.1093/brain/awp053. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci. 2007;27:8053–8. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–61. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- Factor SA. The clinical spectrum of freezing of gait in atypical parkinsonism. Mov Disord. 2008;23(Suppl 2):S431–8. doi: 10.1002/mds.21849. [DOI] [PubMed] [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. 2009;133(Pt 1):205–14. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56:1712–21. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000;6:165–70. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord. 2009;24:655–61. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- Gironell A, Kulisevsky J, Roig C, Pascual-Sedano B, Rodriguez-Fornells A, Otermin P. Diagnostic potential of acoustic startle reflex, acoustic blink reflex, and electro-oculography in progressive supranuclear palsy: a prospective study. Mov Disord. 2003;18:1273–9. doi: 10.1002/mds.10529. [DOI] [PubMed] [Google Scholar]
- Hershenson M. Reaction time as a measure of intersensory facilitation. J Exp Psychol. 1962;63:289–93. doi: 10.1037/h0039516. [DOI] [PubMed] [Google Scholar]
- Hori A, Yasuhara A, Naito H, Yasuhara M. Blink reflex elicited by auditory stimulation in the rabbit. J Neurol Sci. 1986;76:49–59. doi: 10.1016/0022-510x(86)90141-3. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ. Pedunculopontine nucleus stimulation improves akinesia in a Parkinsonian monkey. Neuroreport. 2004;15:2621–4. doi: 10.1097/00001756-200412030-00012. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Nandi D, Oram R, Stein JF, Aziz TZ. Pedunculopontine nucleus electric stimulation alleviates akinesia independently of dopaminergic mechanisms. Neuroreport. 2006;17:639–41. doi: 10.1097/00001756-200604240-00016. [DOI] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–54. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75:116–24. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- Kofler M, Muller J, Wenning GK, Reggiani L, Hollosi P, Bosch S, et al. The auditory startle reaction in parkinsonian disorders. Mov Disord. 2001;16:62–71. doi: 10.1002/1531-8257(200101)16:1<62::aid-mds1002>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968a;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968b;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Macht M, Kaussner Y, Moller JC, Stiasny-Kolster K, Eggert KM, Kruger HP, et al. Predictors of freezing in Parkinson's disease: a survey of 6,620 patients. Mov Disord. 2007;22:953–6. doi: 10.1002/mds.21458. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, et al. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol. 2007;97:4368–79. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport. 2005;16:1877–81. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol. 1989;283:611–33. doi: 10.1002/cne.902830414. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. 2010;133(Pt 1):215–24. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain. 2002;125(Pt 11):2418–30. doi: 10.1093/brain/awf259. [DOI] [PubMed] [Google Scholar]
- Okuma Y. Freezing of gait in Parkinson’s disease. J Neurol. 2006;253(Suppl. 7):VII27–32. doi: 10.1007/s00415-006-7007-2. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Muthusamy KA, De Pennington N, Joint CA, Aziz TZ. Deep brain stimulation of the pedunculopontine nucleus in Parkinson's disease. Preliminary experience at Oxford. Br J Neurosurg. 2008;22(Suppl 1):S41–4. doi: 10.1080/02688690802448335. [DOI] [PubMed] [Google Scholar]
- Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport. 2005;16:1883–7. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- Queralt A, Weerdesteyn V, van Duijnhoven HJ, Castellote JM, Valls-Sole J, Duysens J. The effects of an auditory startle on obstacle avoidance during walking. J Physiol. 2008;586(Pt 18):4453–63. doi: 10.1113/jphysiol.2008.156042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RF, Day BL. Fast visuomotor processing made faster by sound. J Physiol. 2007;583(Pt 3):1107–15. doi: 10.1113/jphysiol.2007.136192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–9. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol. 2009;101:1334–50. doi: 10.1152/jn.91013.2008. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Silburn PA, Brooker H, Coyne TJ, Khan S, Gill SS, et al. The impact of low-frequency stimulation of the pedunculopontine nucleus region on reaction time in parkinsonism. J Neurol Neurosurg Psychiatry. 2010;81:1099–104. doi: 10.1136/jnnp.2009.189324. [DOI] [PubMed] [Google Scholar]
- Valldeoriola F, Valls-Sole J, Tolosa E, Ventura PJ, Nobbe FA, Marti MJ. Effects of a startling acoustic stimulus on reaction time in different parkinsonian syndromes. Neurology. 1998;51:1315–20. doi: 10.1212/wnl.51.5.1315. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp Brain Res. 2008;187:497–507. doi: 10.1007/s00221-008-1402-0. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Kofler M, Kumru H, Castellote JM, Sanegre MT. Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp Brain Res. 2005;165:541–8. doi: 10.1007/s00221-005-2332-8. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516(Pt 3):931–8. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Sole A, Valldeoriola F, Munoz E, Gonzalez LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci Lett. 1995;195:97–100. doi: 10.1016/0304-3940(94)11790-p. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Rothwell JC, Thompson PD, Lees AJ, Marsden CD. The auditory startle response in the Steele-Richardson-Olszewski syndrome and Parkinson’s disease. Brain. 1992;115(Pt 4):1181–92. doi: 10.1093/brain/115.4.1181. [DOI] [PubMed] [Google Scholar]
- Williams DR, Doyle LM, Lees AJ, Brown P. The auditory startle response in parkinsonism may reveal the extent but not type of pathology. J Neurol. 2008;255:628–32. doi: 10.1007/s00415-008-0758-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.