Abstract
A growing body of preclinical evidence indicates that addiction to cocaine is associated with neuroadaptive changes in frontostriatal brain systems. Human studies in cocaine-dependent individuals have shown alterations in brain structure, but it is less clear how these changes may be related to the clinical phenotype of cocaine dependence characterized by impulsive behaviours and compulsive drug-taking. Here we compared self-report, behavioural and structural magnetic resonance imaging data on a relatively large sample of cocaine-dependent individuals (n = 60) with data on healthy volunteers (n = 60); and we investigated the relationships between grey matter volume variation, duration of cocaine use, and measures of impulsivity and compulsivity in the cocaine-dependent group. Cocaine dependence was associated with an extensive system of abnormally decreased grey matter volume in orbitofrontal, cingulate, insular, temporoparietal and cerebellar cortex, and with a more localized increase in grey matter volume in the basal ganglia. Greater duration of cocaine dependence was correlated with greater grey matter volume reduction in orbitofrontal, cingulate and insular cortex. Greater impairment of attentional control was associated with reduced volume in insular cortex and increased volume of caudate nucleus. Greater compulsivity of drug use was associated with reduced volume in orbitofrontal cortex. Cocaine-dependent individuals had abnormal structure of corticostriatal systems, and variability in the extent of anatomical changes in orbitofrontal, insular and striatal structures was related to individual differences in duration of dependence, inattention and compulsivity of cocaine consumption.
Keywords: orbitofrontal, insula, caudate, grey matter, cocaine dependence, sustained attention, stop signal, impulsivity, compulsivity
Introduction
According to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), cocaine is the second most widely used illicit drug in Europe (after cannabis; EMCDDA, 2010). Approximately 14 million Europeans are believed to have used cocaine at least once in their lifetime (EMCDDA, 2010), but not everybody who uses cocaine becomes addicted to it. It has been estimated that ∼20% of cocaine users develop dependence (Wagner and Anthony, 2002). Impulsive individuals, i.e. people who tend to show behaviour that is premature, poorly planned and often inappropriate for the context (Moeller et al., 2001a), seem to be particularly vulnerable to making the transition from recreational to compulsive cocaine use (Verdejo-Garcia et al., 2008; Potenza and Taylor, 2009). Impulsivity, as assessed by self-report, has been shown to further increase with chronic cocaine exposure (Ersche et al., 2010). This is of concern because in drug-dependent individuals, impulsivity also increases the risk of adverse life events (Hayaki et al., 2005) and the likelihood of early treatment drop-out (Moeller et al., 2001b). Impulsivity can also be assessed by behavioural tasks; but, in healthy individuals, self-report and behavioural measures of impulsivity are only weakly correlated (Reynolds et al., 2006; Meda et al., 2009).
Here we investigate impulsivity in individuals who have become dependent on cocaine using both self-report and behavioural measures. There is convincing preclinical evidence indicating that addiction is associated with neuroadaptive changes in the frontostriatal networks, which may influence both impulsivity and drug-related compulsivity (Jentsch and Taylor 1999; Porrino et al., 2002, 2007; Everitt and Robbins, 2005; Schoenbaum and Shaham, 2008). Compulsivity of drug use is defined as a maladaptive tendency to repeat or perseverate in a previously rewarded behaviour (e.g. cocaine-seeking or consumption) even in the face of significant aversive or disadvantageous consequences (e.g. failure of relationships, loss of employment, imprisonment, etc). Previous studies in humans with cocaine dependence have found significant changes in grey matter in prefrontal and striatal brain regions (Jacobsen et al., 2001; Fein et al., 2002; Franklin et al., 2002; Matochik et al., 2003; Sim et al., 2007). More recently, neuroimaging studies have used computational techniques in order to relate aspects of impulsivity to the structural MRI scans of psychiatric patients (Matsuo et al., 2009; Schiffer et al., 2010; Schwartz et al., 2010). The present study aims to build on this work by investigating the relationship between individual differences in impulsivity and cocaine-related compulsivity and grey matter volume variation in large-scale brain systems, in a sizeable sample of cocaine-dependent individuals and healthy volunteers. We hypothesized that the increased levels of impulsivity and compulsivity in cocaine users would be associated with anatomical changes in frontostriatal brain systems.
Materials and methods
Participants
Sixty individuals with a history of chronic cocaine abuse, satisfying the DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Revised, American Psychiatric Association, 2000) criteria for cocaine dependence, and 60 healthy control volunteers without a history of drug abuse took part in the study. All participants were aged 18–50 years and in good physical health. Participants were psychiatrically evaluated using the structured clinical interview for DSM-IV (First et al., 2002). Exclusion criteria included a major medical or neurological illness, a lifetime history of a psychotic disorder, a history of a traumatic head injury or any contra-indications to MRI scanning. The cocaine users were non-treatment seeking and recruited from the local community by advertisements and word-of-mouth. All cocaine users were actively using cocaine, as verified by positive urine screens for cocaine on the day of scanning. On average, cocaine users had been using cocaine for 10 years ± 7.1 standard deviation (SD), starting at the age of 21 years ± 5.7 SD. On the Obsessive–Compulsive Drug Use Scale (OCDUS; Franken et al., 2002; Ersche et al., 2010), the cocaine users typically reported moderate levels of cocaine-related compulsivity (OCDUS mean = 21.3 ± 8.5 SD; range 5–40). One cocaine user was prescribed mirtazapine, two were prescribed benzodiazepines and one regularly used over-the-counter paracetamol. Fifty cocaine users also met DSM-IV criteria for nicotine dependence, 16 for alcohol dependence, 11 for cannabis dependence and four for heroin dependence. The majority of the cocaine users were smoking cannabis regularly (68%) and many also consumed other drugs (ecstasy 28%, amphetamines 18%, hallucinogens 15%, benzodiazepines 11% and opiates 7%).
The healthy volunteers were partly recruited from the GlaxoSmithKline healthy volunteer panel, and partly by advertisement in the local community. They did not satisfy criteria for alcohol abuse or dependence, and were not taking prescribed or illicit drugs on a regular basis. Urine samples provided on the testing day were negative for all illicit substances tested. Seventeen per cent of this sample reported recreational cannabis use in the past, 7% were occasional tobacco smokers and 36% had smoked tobacco in the past. All participants completed the National Adult Reading Test (NART; Nelson, 1982), as an estimate of verbal IQ and the Beck Depression Inventory (BDI-II, Beck et al., 1996) to assess depressive mood.
The study protocol received ethical approval from the Cambridge Research Ethics Committee and written informed consent was obtained from all volunteers prior to study enrolment.
Impulsivity assessment
Impulsivity was assessed using both self-report questionnaire measures and behavioural tasks. The two self-report measures comprised: (i) the Barratt Impulsiveness Scale (Patton et al., 1995), a 30-item questionnaire, which assesses impulsive personality traits in three dimensions: attention (inattention and cognitive instability), motor behaviour (spontaneous actions) and non-planning (lack of forethought); and (ii) the Behavioural Inhibition/Activation System scale (Carver and White, 1994), a 20-item questionnaire that measures both inhibitory and excitatory tendencies in behaviour. The behavioural inhibition system subscale assesses the individual's behaviour in the anticipation of punishment; the behavioural activation system subscale assesses behaviour in the anticipation of rewarding outcomes, i.e. the tendency to respond with heightened energy and positive affect in the context of rewarding events (reward responsiveness), the pursuit of rewarding goals (drive) and the impulsive approach towards potential rewards (fun-seeking).
For the behavioural assessment of impulsivity, we focused on those aspects of impulsivity that have been classified as impulsive actions (Schachar et al., 2007), i.e. behaviours involving either the cancellation of an ongoing response or the inhibition of inappropriate actions. Impulsive actions are believed to be particularly relevant for the development of compulsive patterns of cocaine abuse (Belin et al., 2008; Winstanley et al., 2010), and in the present study were measured by two computerized tests: (i) The Stop-Signal task (Logan et al., 1997), which measures the time that an individual needs to withhold an ongoing response (stop-signal reaction time). The calculation of stop-signal reaction time is based on the assumption that go and stop processes are independent; reaction time on successful go-trials and on unsuccessful stop-trials was recorded in addition to the outcome of each stop trial; additionally, we also computed relative slowing on the go trials after a stop trial; and (ii) the Rapid Visual Information Processing Task (RVIP) (www.camcog.com) is a test of sustained attention equivalent to the Continuous Performance Test; it measures a person's capacity to discriminate between targets and non-targets (target sensitivity, A′) and evaluates their tendency to respond irrespective of the presence of a target (response bias, B′′). Errors are calculated either by the number of targets missed (omission errors) or by responses to non-targets (commission errors). Impulsivity is reflected by an increased number of commission errors paired with short response latencies.
Behavioural data on four individuals were lost due to technical problems (Stop-Signal task: one control, two cocaine users; and RVIP task: one control, one cocaine user); and the Behavioural Inhibition/Activation System scale scores for one cocaine user were incomplete, and not included in the analysis.
Statistical analysis of demographic and impulsivity measures
Non-imaging data were analysed using the Statistical Packages for the Social Sciences version 13 (SPSS Inc.). All tests were two-tailed and an effect was deemed significant at P < 0.05. Independent-sample t-tests were used to explore group differences in demographic variables, including measures of mood. Chi-square or Fisher's exact tests were used, as appropriate, for the analyses of categorical data. An exploratory factor analysis with principal components extraction was performed to identify a few major components of variation/covariation underlying all (self-report and behavioural) impulsivity measures in all participants. As healthy volunteers had no cocaine-taking experiences, the OCDUS scores were not available in this group, and therefore the OCDUS score was not included in the principal component analysis. The participants’ scores on each impulsivity component were then subject to group comparisons using analysis of covariance with years of education and depressive mood (Beck Depression Inventory, Version 2; BDI-II) scores included as covariates. Pearson correlations were estimated between each of the impulsivity component scores and the duration of cocaine abuse.
Magnetic resonance imaging data acquisition and preprocessing
The MRI data were acquired at the Wolfson Brain Imaging Centre, University of Cambridge, UK, using a Siemens Magentom Trio Tim scanner operating at 3 Tesla (www.medical.siemens.com). For the T1-weighted MRI scans, a magnetically prepared rapid acquisition gradient echo sequence (MPRAGE) was used (176 slices of 1 mm thickness, repetition time = 2300 ms, echo time = 2.98 ms, inversion time = 900 ms, flip angle = 9°, field of view = 240 × 256). All magnetic resonance images were screened for normal radiological appearance by a specialist in neuroradiology.
The grey matter volume maps were constructed from each participant's image using FSLVBM 1.1 (http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html). First, structural images were skull-stripped using the brain extraction tool (Smith, 2002) and tissue-type segmentation was conducted using FAST (Zhang et al., 2001). The resulting grey matter partial volume images were aligned to MNI standard space using the affine registration tool FLIRT (Jenkinson and Smith 2001; Jenkinson et al., 2002), followed by a non-linear registration using FNIRT (Andersson et al., 2007a, b) implementing a b-spline representation of the registration warp field (Rueckert et al., 1999). The images were averaged to create a study-specific template, to which the native grey matter images were then non-linearly re-registered. The registered partial volume images were modulated (to correct for local expansion or contraction) by dividing by the Jacobian of the warp field, and smoothed with an isotropic Gaussian kernel with full-width half-maximum = 2.3 mm to minimize slight misregistration errors.
Magnetic resonance imaging statistical analysis
The smoothed grey matter maps were statistically analysed using CamBA software, version 2.3.0 (http://www-bmu.psychiatry.cam.ac.uk/software/). For statistical inference, we used permutation methods and spatially extended statistics with nominal type I error control and greater sensitivity than voxel-based metrics (Suckling and Bullmore 2004). First, we performed a whole-brain analysis of group differences in grey matter volume using the general linear model with a single-factor two independent groups ANOVA design. This resulted in a map of brain areas that demonstrated significant differences in grey matter volume in cocaine users compared with healthy volunteers. Secondly, we explored variations in abnormal brain anatomy that were associated with individual differences in impulsivity, compulsivity and duration of cocaine use. For these analyses, we focused on the data from cocaine users only and tested associations with behavioural, clinical and cognitive variables in those brain regions that were significantly abnormal in the cocaine user group compared with healthy volunteers. In other words, the map of between-group differences in brain anatomy obtained by the first analysis was used as an inclusive mask to define a restricted search volume for the secondary analyses, which entailed regressing grey matter volume at each voxel within the mask on the following variables: principal component scores for inattention and impulsive reward-seeking dimensions of impulsivity; duration of cocaine use; and compulsivity of cocaine use (OCDUS scores). Regional mean grey matter volumes for those regions that showed significant association with any of the behavioural or clinical variables were graphically examined to evaluate the possible effects of outlier observations.
For both whole-brain analysis of between-group differences, and the masked analysis of associations between grey matter and clinical or behavioural variables, statistical inference was by permutation testing at the level of spatially contiguous voxel clusters (Suckling and Bullmore, 2004). The P-value for significance was adjusted to control for multiple comparisons so that the expected number of false positive clusters in each analysis was less than one. Thus the cluster-wise probability threshold for significance in the whole-brain analysis was P = 0.001 and the corresponding threshold for each of the masked analyses was P ∼ 0.002. The slightly more lenient threshold for significance in the masked analysis reflects the smaller search volume (number of voxel clusters) tested.
Results
Demographics and group differences in impulsivity
The two groups were reasonably well matched in terms of age, gender and verbal intelligence (Table 1). Eighty-three per cent of the cocaine users had a high school education; although this level is comparable with other studies in cocaine dependence (e.g. Buchanan et al., 2006), it is falling behind education levels in the control group, in which 98% of volunteers had a high school degree (Fisher's exact P = 0.008). The cocaine users also reported more dysphoric mood compared with the healthy volunteers, which is not unusual for substance-dependent individuals (Buckley et al., 2001). In keeping with previous research, the cocaine users reported increased trait-impulsivity and appetitive motivation (Moeller et al., 2004; 2005; Franken and Muris 2006; Ersche et al., 2010). However, these high levels of self-reported impulsivity were not reflected in their behavioural performance. On the Stop-Signal task, cocaine users showed an overall slowing of responses that was not limited to the stop-signal reaction time; latencies on both stop- and go-trials were prolonged. Their poor target detection accuracy on a test of sustained attention was due to the fact that cocaine users missed significantly more targets than controls. We found evidence for generally impaired attentional control rather than the more specific pattern of an increased rate of false alarms and speeded-up responding, which has traditionally been considered to be a marker of impulsive behaviour. Statistical details of all self-report and behavioural measures are shown in Table 1.
Table 1.
Demographic and impulsivity measures for healthy volunteers and cocaine-dependent individuals
| Group characteristics | Healthy volunteers | Cocaine dependent | t-value or χ2 | P-value |
|---|---|---|---|---|
| Demographics | n = 60 | n = 60 | ||
| Age (years) | 32.3 ± 8.3 | 32.5 ± 8.5 | −0.12 | 0.905 |
| Gender (M : F) | 46 : 14 | 53 : 7 | 2.83 | 0.148 |
| IQ (NART) | 110.0 ± 7.0 | 109.5 ± 6.9 | 0.36 | 0.716 |
| Depressive mood (BDI-II total score) | 2.1 ± 3.2 | 13.2 ± 11.6 | −7.20 | <0.001 |
| Education (years of formal education) | 12.3 ± 1.6 | 11.5 ± 1.7 | 2.78 | 0.006 |
| Trait-impulsivity (BIS-11 scale, total score) | 60.8 ± 7.5 | 76.4 ± 9.6 | −9.89 | <0.001 |
| BIS-11 attention | 14.3 ± 2.8 | 18.6 ± 3.9 | −6.91 | <0.001 |
| BIS-11 motor | 22.8 ± 3.3 | 27.5 ± 5.4 | −5.77 | <0.001 |
| BIS-11 non-planning | 23.8 ± 4.0 | 31.3 ± 4.3 | −8.63 | <0.001 |
| Anxiety-avoidance (BIS/BAS scale) | ||||
| BIS score | 18.6 ± 3.4 | 19.4 ± 3.7 | −1.14 | 0.258 |
| Reward-approach (BIS/BAS scale) | ||||
| BAS drive | 11.0 ± 1.9 | 12.1 ± 2.6 | −2.50 | 0.014 |
| BAS fun-seeking | 12.0 ± 1.8 | 13.6 ± 1.8 | −4.91 | <0.001 |
| BAS reward responsiveness | 16.4 ± 1.9 | 16.9 ± 2.2 | −1.16 | 0.248 |
| Sustained attention (CANTAB-RVIP) | ||||
| Signal detection (A’) | 0.92 ± 0.05 | 0.89 ± 0.04 | 3.22 | 0.002 |
| Response bias (B′′) | 0.9 ± 0.3 | 1.0 ± 0.0 | −1.71 | 0.093 |
| Commission errors (number) | 1.5 ± 2.1 | 1.0 ± 1.2 | 1.42 | 0.158 |
| Omission errors (number) | 8.5 ± 4.8 | 11.5 ± 4.7 | −3.43 | 0.001 |
| Correct responses/hits (number) | 18.5 ± 4.8 | 15.5 ± 4.7 | 3.42 | 0.001 |
| Mean reaction time (ms) | 407.8 ± 96.3 | 439.4 ± 85.2 | −1.89 | 0.061 |
| Response inhibition (stop-signal task) | ||||
| Percentage of successful stops | 54.4 ± 3.1 | 53.4 ± 4.8 | 1.32 | 0.189 |
| Mean reaction time on successful Go-trials (ms) | 481.3 ± 61.6 | 532.9 ± 87.7 | −3.68 | <0.001 |
| Mean reaction time on unsuccessful Stop-trials (ms) | 447.1 ± 51.2 | 476.7 ± 56.0 | −2.97 | 0.003 |
| Stop-signal reaction time (ms) | 234.9 ± 46.2 | 263.2 ± 55.2 | −3.01 | 0.003 |
| Post-stop slowing (ms) | 485.7 ± 99.2 | 560.1 ± 253.1 | −2.10 | 0.038 |
| Principal components | F(1,111) | P-value | ||
| Inattention | − 0.51 ± 0.81 | 0.54 ± 0.90 | 19.43 | <0.001 |
| Impulsive reward-seeking | − 0.40 ± 0.89 | 0.42 ± 0.94 | 17.16 | <0.001 |
| Response slowing | 0.00 ± 0.92 | 0.00 ± 1.09 | 0.32 | 0.572 |
| Impulsive responding | 0.27 ± 1.00 | −0.28 ± 0.92 | 3.30 | 0.072 |
| Anxious responding | −0.03 ± 1.09 | 0.03 ± 0.91 | 0.60 | 0.439 |
BDI-II = Beck Depression Inventory, Version 2; BIS-11 = Barratt Impulsiveness Scale; BIS/BAS = Behavioural Inhibition/Activation System scale; CANTAB = Cambridge Neuropsychological Test Automated Battery; NART = National Adult Reading Test.
We used principal component analysis to examine how the different task measures were related to each other in all participants. A five-component solution, comprising all components with standardized eigenvalues >1, accounted for 71% of the total variance/covariance. As shown in Table 2, the first component, labelled tentatively ‘inattention’, loaded on the behavioural measures of target detection during sustained attention (RVIP) and response latencies during the response inhibition task. The second component, ‘impulsive reward-seeking’, loaded strongly on self-report measures reflecting reward-driven behaviours (behavioural activation system items) as well as motor and cognitive impulsivity [Barratt Impulsiveness Scale-11 (BIS-11)]. The third component, ‘response slowing’, loaded strongly on prolonged response times on the stop-signal task. The fourth component, ‘impulsive responding’, summarized self-reported behaviours in anticipation of reward [Behavioural Activation System (BAS)] and lack of forward thinking (BIS-11). The fifth component, ‘anxious responding’, loaded highly on self-reported avoidance behaviour (Behavioural Inhibition/Activation System scale) and also on impulsive errors on the RVIP.
Table 2.
The eigenvector matrices of the principal component analysis including 16 impulsivity variables in all participants
| Component |
|||||
|---|---|---|---|---|---|
| Inattention | Impulsive reward-seeking | Response slowing | Impulsive responding | Anxious responding | |
| Per cent variance (cumulative variance), % | 27 (27) | 14 (41) | 12 (52) | 10 (62) | 8 (71) |
| BIS-11 attention | 0.41 | 0.54 | 0.05 | −0.47 | 0.21 |
| BIS-11 motor | 0.43 | 0.57 | 0.02 | −0.39 | 0.03 |
| BIS-11 non-planning | 0.49 | 0.29 | −0.19 | −0.56 | 0.24 |
| BIS score (BIS/BAS) | 0.20 | 0.13 | 0.05 | 0.03 | 0.65 |
| BAS drive | 0.16 | 0.63 | 0.03 | 0.48 | −0.29 |
| BAS fun-seeking | 0.19 | 0.80 | −0.10 | 0.11 | −0.16 |
| BAS reward responsiveness | 0.09 | 0.63 | 0.02 | 0.58 | −0.03 |
| RVIP A′ (response accuracy) | −0.82 | 0.23 | 0.49 | −0.14 | −0.01 |
| RVIP B′′ (response bias) | 0.32 | −0.14 | −0.01 | −0.33 | −0.42 |
| RVIP mean RT correct responses | 0.45 | −0.10 | −0.13 | 0.26 | −0.18 |
| RVIP commission errors | −0.02 | −0.07 | −0.11 | 0.45 | 0.69 |
| RVIP omission errors | 0.82 | −0.22 | −0.48 | 0.10 | −0.05 |
| RVIP correct responses | −0.82 | 0.22 | 0.48 | −0.11 | 0.05 |
| Stop-Signal mean successful go-RT | 0.65 | −0.19 | 0.62 | 0.00 | −0.01 |
| Stop-Signal mean unsuccessful stop-RT | 0.67 | −0.19 | 0.60 | 0.07 | 0.04 |
| Stop-Signal reaction time | 0.63 | −0.06 | 0.45 | 0.13 | 0.17 |
| Stop-Signal post-stop-RT | 0.47 | −0.06 | 0.49 | 0.12 | −0.20 |
Component loadings of ≥0.5 were considered significant and are given in bold.
BIS-11 = Barratt Impulsiveness Scale; BIS/BAS = Behavioural Inhibition/Activation System scale; RT = reaction time.
Group comparisons on the five components were controlled for the between-group differences in years of education and dysphoric mood ratings. The analyses revealed significant group differences on the components reflecting inattention [F(1,111) = 20.46, P < 0.001] and self-reported impulsive reward-seeking [F(1,111) = 16.48, P < 0.001]; statistical details of the group comparisons are shown in Table 1.
Group differences in grey matter volume
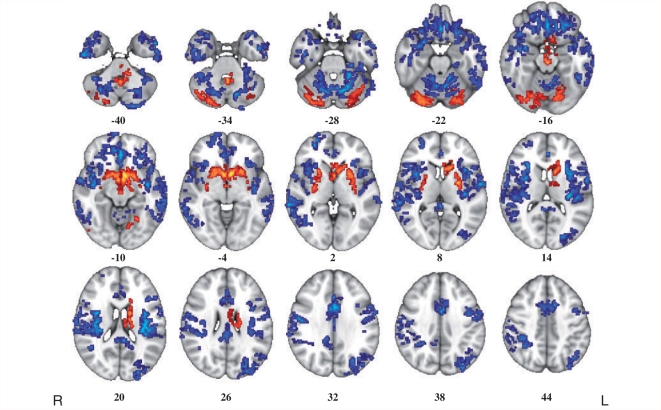
There were significant differences in grey matter volume between the two groups (Fig. 1). There was widespread significant loss of grey matter in orbitofrontal cortex bilaterally in the cocaine user group. Grey matter volume was also abnormally reduced in the insula, the medial frontal and anterior cingulate cortex, temporoparietal cortex and the cerebellum. In contrast to this extensive system of decreased cortical grey matter volume, cocaine users also showed a significant increase of grey matter volume mainly localized to basal ganglia structures (including putamen, caudate nucleus and pallidum), and cerebellum.
Figure 1.
Whole-brain maps of significant differences in grey matter volume between healthy volunteers and cocaine users. Voxels coloured blue indicate brain areas in which cocaine users have reduced grey matter volume compared with healthy volunteers, and voxels coloured red indicate brain areas in which cocaine users have abnormally increased grey matter volume. These results were generated by permutation testing of voxel cluster statistics with cluster-wise P < 0.001, at which level we expect less than one false positive cluster per map. The statistical results are overlaid on the FSL MNI152 standard T1 image and the numbers beneath each section of the image refer to its position (mm) relative to the intercommissural plane in standard stereotactic space. L = left; R = right.
Individual differences in impulsivity, compulsivity and grey matter volume
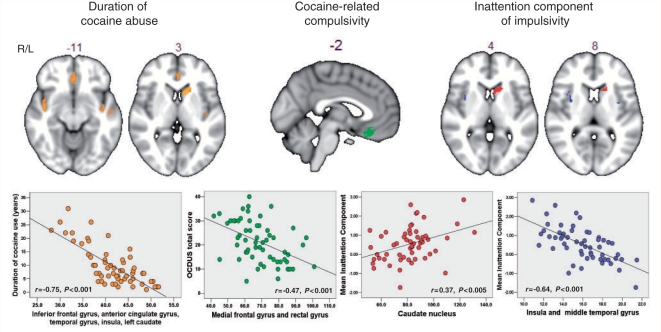
To investigate how the significant components of impulsivity were associated with the abnormal grey matter systems in the cocaine-dependent group, we separately regressed cocaine users’ individual scores on each of the two abnormal components (inattention and impulsive reward-seeking) on grey matter volume in each voxel of the corticostriatal system that was abnormal in the cocaine-dependent individuals group compared with healthy participants. This procedure identified a set of voxels where grey matter volume was significantly positively correlated with the first impulsivity component (inattention) in the cocaine users (coloured in red in Fig. 2) in the left caudate nucleus [Montreal Neurological Institute coordinates (x, y, z; mm): −18, 18, 8], and negatively correlated with grey matter volume in the insula bilaterally [coloured in blue (38, −8, 18) and (−36, 0, 8)], and in the right middle temporal gyrus (56, 0, −18). The second component (impulsive reward-seeking) was not significantly correlated with grey matter volume variation in the cocaine-dependent group.
Figure 2.
Maps of brain regions demonstrating significant association between grey matter volume and measures of duration of cocaine use, compulsivity and impulsivity in the group of cocaine users. Regions where grey matter volume correlated significantly with the duration of cocaine use in drug users are indicated in orange. Regions that correlated significantly with compulsive cocaine-taking (as assessed by the OCDUS) are coloured in green. Regions where grey matter volume correlated significantly with the inattention component of impulsivity in cocaine users are indicated in red (if the correlation was positive) and blue (if the correlation was negative). The scatter plots beneath each section of the brain image show the correlation between these measures and the total grey matter volume for each drug user in those regions found to be significantly correlated by permutation testing of cluster-level statistics in the restricted search volume or mask defined by the areas of significant between-group difference in grey matter anatomy (Fig. 1). The probability threshold for significance was P ∼ 0.002 for each analysis, at which level we expect less than one false positive cluster per map. The statistical results are overlaid on the FSL MNI152 standard T1 image and the numbers above each section of the image refer to its plane position (mm) relative to the origin in MNI stereotactic space. L = left; R = right.
We also regressed the OCDUS score of cocaine-related compulsivity on those grey matter systems in the cocaine group that differed from control volunteers. As shown in Fig. 2, drug-related compulsivity was significantly associated with grey matter loss in the orbitofrontal cortex (−2, 32, −18). The OCDUS score was correlated with the inattention score (r = 0.31, P < 0.05) (Table 3), and the OCDUS-related decline in grey matter in the orbitofrontal cortex was correlated with the inattention-related decline in grey matter in the insula and middle temporal gyrus (r = 0.36, P < 0.01).
Table 3.
Correlation matrix of impulsivity, compulsivity and duration of cocaine use in cocaine-dependent individuals
| Inattention | Impulsive reward-seeking | Response slowing | Impulsive responding | Anxious responding | OCDUS | Duration of abuse | |
|---|---|---|---|---|---|---|---|
| Inattention | |||||||
| Pearson Correlation | 1.00 | −0.15 | 0.12 | 0.25 | 0.01 | 0.31 | 0.04 |
| P-value (two-tailed) | 0.269 | 0.363 | 0.066 | 0.941 | 0.020 | 0.756 | |
| Impulsive reward-seeking | |||||||
| Pearson Correlation | −0.15 | 1.00 | 0.01 | 0.20 | −0.10 | 0.16 | −0.24 |
| P-value (two-tailed) | 0.269 | 0.947 | 0.139 | 0.461 | 0.238 | 0.077 | |
| Response slowing | |||||||
| Pearson Correlation | 0.12 | 0.01 | 1.00 | 0.16 | −0.21 | −0.10 | 0.04 |
| P-value (two-tailed) | 0.363 | 0.947 | 0.247 | 0.118 | 0.457 | 0.751 | |
| Impulsive responding | |||||||
| Pearson Correlation | 0.25 | 0.20 | 0.16 | 1.00 | −0.52 | −0.25 | −0.02 |
| P-value (two-tailed) | 0.066 | 0.139 | 0.247 | 0.000 | 0.067 | 0.866 | |
| Anxious responding | |||||||
| Pearson Correlation | 0.01 | −0.10 | −0.21 | −0.52 | 1.00 | 0.24 | 0.13 |
| P-value (two-tailed) | 0.941 | 0.461 | 0.118 | 0.000 | 0.076 | 0.357 | |
| OCDUS | |||||||
| Pearson Correlation | 0.31 | 0.16 | −0.10 | −0.25 | 0.24 | 1.00 | 0.12 |
| P-value (two-tailed) | 0.020 | 0.238 | 0.457 | 0.067 | 0.076 | 0.375 | |
| Duration of cocaine abuse | |||||||
| Pearson Correlation | 0.04 | −0.24 | 0.04 | −0.02 | 0.13 | 0.12 | 1.00 |
| P-value (two-tailed) | 0.756 | 0.077 | 0.751 | 0.866 | 0.357 | 0.375 |
Relationship between duration of cocaine dependence and grey matter volume
To investigate whether or not altered grey matter volume was related to the duration of cocaine abuse, we regressed the number of years of cocaine abuse of each cocaine user on the map of grey matter volume differences. We found that the individuals who had been using cocaine for longer periods of time, had greater extent of grey matter volume reduction in the anterior and middle cingulate gyrus, middle frontal cortex (orbital part), rectus gyrus, supplementary motor area, superior temporal gyrus, insula, cerebellum and in the left caudate (r = −0.75, P > 0.001; Fig. 2).
Discussion
By comparing grey matter volume between chronic cocaine users and healthy volunteers, we confirmed findings from previous studies of significant grey matter loss in large parts of frontal and parietal cortices and the enlargement of striatal structures in cocaine dependence (Jacobsen et al., 2001; Fein et al., 2002; Franklin et al., 2002; Matochik et al., 2003; Sim et al., 2007). We further found that the caudate enlargement in cocaine users was associated with significant attentional impairments, whereas the reduction in grey matter in the orbitofrontal cortex was associated with cocaine-related compulsivity. The abnormal changes in grey matter in the striatum and in the orbitofrontal cortex were both related to the duration of cocaine abuse, i.e. the longer cocaine users have been using cocaine, the greater the loss of grey matter. Our observations are in keeping with the findings from preclinical studies indicating that neuroadaptive changes in frontostriatal networks are associated with cocaine dependence (Jentsch and Taylor 1999; Everitt and Robbins, 2005; Koob and Le Moal, 2005). More specifically they show that individual differences in the duration of cocaine dependence, attentional impairment and compulsivity of drug use are correlated with each other and with the extent of grey matter volume abnormality in orbitofrontal cortex, insula and the caudate nucleus.
Relationships between behaviour and related brain structure
As hypothesized, and consistent with previous studies, the cocaine users perceived themselves as highly impulsive, scoring significantly higher on the impulsivity questionnaires compared with healthy volunteers (Moeller et al., 2004; 2005; Franken and Muris, 2006; Ersche et al., 2010). However, their behavioural performance was not impulsive in the sense of being premature, which might reflect a ceiling effect of task performance, but did confirm the significant attentional problems that have been previously reported in chronic cocaine users (Horner, 1999; Aharonovich et al., 2003, 2006; Jovanovski et al., 2005; Goldstein et al., 2007; Tomasi et al., 2007; Gooding et al., 2008). The fact that the cocaine users had prolonged (not speeded) response latencies in both tasks may reflect a failure in attentional processing (Sarter et al., 2001). Successful performance on both tasks requires sustained attention and performance monitoring, which involves both prefrontal and subcortical structures including the insula and the caudate nucleus (Coull et al., 1996; Lawrence et al., 2003; Ray Li et al., 2008). Specifically, increased functional activation of the caudate has been associated with improved performance on the RVIP task (Lawrence et al., 2002) and the Stop-Signal task (i.e. shortened stop-signal reaction times) (Ray Li et al., 2008). Indeed, the inattentive performance profile in our cocaine users was significantly correlated with grey matter volume changes in the insula and the caudate. Both brain areas have been associated with the acute effects of cocaine in humans (Breiter et al., 1997) and chronic cocaine use in experimental monkeys (Porrino et al., 2007). It is thus conceivable that cocaine-induced structural changes in cortical organization cause abnormalities of sustained attention and attentional control in cocaine-dependent individuals. A similar inattentive performance profile was observed on an analogous test of sustained attention and response inhibition in rats with lesions to the dorsomedial striatum (which corresponds to the caudate nucleus in humans) (Rogers et al., 2001; Eagle and Robbins 2003), and following direct local infusion of dopamine D2 receptor antagonists into this structure (Eagle et al., 2011), supporting the notion of caudate neuropathology as well as reduced dopamine D2 receptor functioning in our cocaine-dependent group.
Relationships between brain structure and duration of cocaine use
We found that overall, compared with healthy volunteers, cocaine users had significantly increased grey matter volume in subcortical structures including the caudate nucleus. However, we also found a strong, negative correlation between duration of cocaine use and grey matter volume in frontal and cingulate cortex, insula and caudate. In other words, greater duration of cocaine use was associated with relatively reduced grey matter volume in these structures.
Like most drugs of abuse, cocaine exerts its pharmacological effects in the ventral striatum (Di Chiara and Imperato, 1988). Enlarged striatal structures have been reported previously in chronic cocaine users (Jacobsen et al., 2001) and methamphetamine users (Chang et al., 2005; Jernigan et al., 2005), but also in individuals with autism and fragile X syndrome (Voelbel et al., 2006; Langen et al., 2007; Hallahan et al., 2011). However, the neuropathology underlying this enlargement is not fully understood. Blockade of dopamine D2 receptors by antipsychotic drugs has been shown to increase the volume of basal ganglia structures in both animals and humans (Benes et al., 1985; Keshavan et al., 1994; Chakos et al., 1998; Corson et al., 1999; Scherk and Falkai 2006), possibly indicating that striatal enlargement is associated with an under-active dopamine system. It has recently been shown in humans that variation in grey matter volume correlates both positively and negatively with individual differences in the expression of D2-like receptors in various brain regions, including the caudate (Woodward et al., 2009). Cocaine dependence has also been associated with significant reduction in striatal dopamine D2 receptor density (Volkow et al., 1997; Martinez et al., 2004), along with significant reduction in dopamine transmission [i.e. reduced endogenous dopamine release and presynaptic re-uptake (Wu et al., 1997; Martinez et al., 2009)].
Thus there is reasonable prior evidence, consistent with the between-group difference observed in these data, to suggest that striatal enlargement is an imaging marker of cocaine dependence, which may reflect reduced dopamine neurotransmission and could indeed be a predisposing factor rather than a consequence of cocaine use. In this context, the relatively reduced striatal and cortical volumes associated with greater duration of cocaine use could conceivably represent a ‘normalization’ of striatal volume due to repeated exposure to the dopamine-enhancing effects of cocaine. In support of this hypothesis, we note a similar inverse association between striatal volume and cumulative methamphetamine use has previously been reported by Chang et al. (2005). It is also notable that caudate volume is smaller in children with attention deficit hyperactivity disorder who have been treated with methylphenidate compared with unmedicated children with attention deficit hyperactivity disorder (Bussing et al., 2002). As methylphenidate is pharmacologically very similar to cocaine (Volkow et al., 1995), it has been speculated that the reduced caudate volume might be related to a methylphenidate-induced increase in dopamine neurotransmission, reflecting an opposite effect to the volume change observed in patients with schizophrenia following treatment with dopamine antagonists (Bussing et al., 2002). However, the literature regarding caudate volume in childhood attention deficit hyperactivity disorder is inconsistent (Castellanos et al., 2002) and the exact mechanisms underlying the striatal volume changes over time in both treated attention deficit hyperactivity disorder and cocaine dependence require further investigation to test causal explanatory models.
Brain structure—behaviour relationships
Impulsivity is thought to be a vulnerability factor for substance abuse and dependence (Dalley et al., 2007; de Wit, 2009). Yet, the high levels of self-reported impulsivity were only weakly related to the behavioural measures of impulsivity in the present study and also in previous studies (Reynolds et al., 2006; Meda et al., 2009). However, one has to bear in mind that impulsivity is a multifaceted construct (Evenden, 1999). As can be seen in Table 2, the self-report and the behavioural measures loaded on different components, suggesting that they are not measuring the same aspects of impulsivity. While performance in the two behavioural tasks was associated with altered grey matter volume, the questionnaire measures were unrelated to brain structure. Several lines of research have shown significant associations between Barratt Impulsiveness Scale-11 impulsivity and responses to dopaminergic drugs (Cools et al., 2007; Clatworthy et al., 2009; Lee et al., 2009; Buckholtz et al., 2010). It is thus conceivable that self-reported impulsivity, as measured by the Barratt Impulsiveness Scale, indexes the functional integrity of the brain dopamine system rather than the structure of the corticostriatal networks it modulates.
Relationships between brain structure and compulsivity
Frontostriatal dysfunction in cocaine dependence is thought to underlie the compulsive pattern of drug consumption and behavioural rigidity in the face of negative consequences (Jentsch and Taylor, 1999; Robbins and Everitt, 1999; Volkow and Fowler, 2000; Schoenbaum and Shaham, 2008). In the present study, cocaine-related compulsivity was associated with a significant loss of grey matter in the orbitofrontal cortex, which may reflect the shift in the control of behaviour from the prefrontal cortex to the striatum that has been demonstrated by preclinical research (Everitt and Robbins 2005; Porrino et al., 2007). Neuroimaging studies using PET have also shown that hypometabolic activity in the orbitofrontal cortex of cocaine users is associated with reduced dopamine receptor density in the striatum (Volkow et al., 1993). Presumably, reduction in grey matter in the orbitofrontal cortex may reflect the lack of top-down control that reduces drug users’ ability to optimally guide their behaviour. This lack of orbitofrontal control may result in drug-craving and disinhibition when faced with drug-related cues (Volkow and Fowler, 2000). The OCDUS scale measures the subjective interference and distress caused by drug-related thoughts and compulsive behaviour patterns. Indeed, those cocaine users reporting high levels of cocaine-related compulsivity, as indexed by high scores on the OCDUS scale, showed the greatest reduction in grey matter volume in the orbitofrontal cortex.
Methodological limitations and summary
The study sample was sizeable in comparison with previous neuroimaging studies of cocaine dependence. However, the cocaine users were somewhat heterogeneous in terms of their exposure to alcohol, nicotine and other illicit drugs; and the study was not designed powerfully to investigate possible differences in brain anatomy between subgroups of the cocaine user group defined by their concurrent use of alcohol and other drugs. Larger studies will be required in future to address this issue although it will likely always prove challenging to identify cocaine-dependent individuals who are not also dependent on one or more other substances. To conduct statistical testing we used permutation-based methods that have been previously described and validated in terms of nominal type 1 error control (Bullmore et al., 1999; Suckling and Bullmore, 2004). This non-parametric approach to voxel cluster-level analysis offers considerable advantages in terms of sensitivity compared with mass univariate analysis of individual voxels, or parametric testing of voxel clusters (Bullmore et al., 1999). However, it does entail some assumptions, including the assumption that the spatial covariance or smoothness of the voxel statistic maps is homogeneous. This assumption is unlikely to be entirely justified in analysis of ‘raw’ MRI data, which typically demonstrate local inhomogeneities of spatial covariance, e.g. in subcortical structures and at the boundaries between grey and white matter (Flitney and Jenkinson, 2000). To address this possible concern, we applied a Gaussian filter to the statistic maps before significance testing (which will have rendered the spatial covariance more homogeneous than in the raw data). We have also corroborated the results of cluster-level mapping by graphical and statistical examination of grey matter volume at a regional level for key structures such as the striatum.
We acknowledge that impulsivity is not a unitary construct (Evenden, 1999), and we have not investigated all aspects of this construct. In particular, we did not examine impulsive choice, which may possibly involve more cognitive aspects of impulsivity than the ‘impulsive actions’ investigated in this study (Winstanley et al., 2010). Not only impulsivity but also compulsivity may be a multifaceted construct. We only used the OCDUS scale as a measure of cocaine-related compulsivity but novel experimental tasks are now needed to quantify compulsive behaviour. Further studies will be required to investigate the neural substrates of compulsivity in addiction. It will also be necessary to expand the investigation to other drugs of abuse, as well as to analysing the effects of stimulants, such as those of cocaine we have reported here.
In summary, we identified extensive significantly decreased grey matter volume in orbitofrontal and other cortical regions, and a significant increase in grey matter volume of the basal ganglia, in cocaine-dependent individuals. We also showed that the changes in grey matter volume within this frontostriatal circuitry were associated with cocaine-related compulsivity and attentional impairments, suggesting that they may reflect the shift in the control of behaviour from the frontal cortex to the striatum that has previously been predicted by preclinical research. Finally, behavioural and brain imaging markers were significantly correlated with the duration of cocaine abuse, suggesting (but not proving) that changes in brain systems controlling attention and compulsive behaviour may be a consequence of prolonged cocaine consumption.
Funding
This work was funded and sponsored by GlaxoSmithKline (RG45422) and conducted within the GlaxoSmithKline (GSK) Clinical Unit Cambridge, UK. The Behavioural and Clinical Neuroscience Institute (BCNI) at the University of Cambridge is jointly funded by the Medical Research Council and The Wellcome Trust. K.D.E. and P.S.J. are supported by a grant from the Medical Research Council (MRC). A.B. is a recipient of the NARSAD Young Investigator Award. S.M.-Z. is funded by a Wellcome Trust Program Grant to T.W.R. T.W.R. consults for Cambridge Cognition and various pharmaceutical companies, including GlaxoSmithKline. E.T.B. is employed part-time by GlaxoSmithKline and part-time by the University of Cambridge. Funding to pay the Open Access publication charges for this article was provided by GlaxoSmithKline.
Acknowledgements
The authors would like to thank all the volunteers for their participation in this study, and staff at the GlaxoSmithKline Clinical Unit Cambridge and the Wolfson Brain Imaging Centre for their dedicated support. We are also grateful to Dr Sanja Abbot for her assistance with data extraction and to Dr John Suckling for advice on statistical analysis.
Glossary
Abbreviations
- OCDUS
Obsessive–Compulsive Drug Use Scale
- RVIP
Rapid Visual Information Processing Task
References
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–11. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–22. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn., text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1. 2007a. www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2. 2007b. www.fmrib.ox.ac.uk/analysis/techrep.
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Paskevich PA, Davidson J, Domesick VB. The effects of haloperidol on synaptic patterns in the rat striatum. Brain Research. 1985;329:265–73. doi: 10.1016/0006-8993(85)90532-3. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Buchanan D, Tooze JA, Shaw S, Kinzly M, Heimer R, Singer M. Demographic, HIV risk behavior, and health status characteristics of “crack” cocaine injectors compared to other injection drug users in three New England cities. Drug Alcohol Depend. 2006;81:221–9. doi: 10.1016/j.drugalcdep.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, Parker JD, Heggie J. A psychometric evaluation of the BDI-II in treatment-seeking substance abusers. J Subst Abuse Treat. 2001;20:197–204. doi: 10.1016/s0740-5472(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Tans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Bussing R, Grudnik J, Mason D, Wasiak M, Leonard C. ADHD and conduct disorder: an MRI study in a community sample. World J Biol Psychiatry. 2002;3:216–220. doi: 10.3109/15622970209150624. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral-inhibition, behavioral activation, and affective responses to impending reward and punishment - the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–33. [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental Trajectories of Brain Volume Abnormalities in Children and Adolescents With Attention-Deficit/Hyperactivity Disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA. Striatal enlargement in rats chronically treated with neuroleptic. Biol Psychiatry. 1998;44:675–684. doi: 10.1016/s0006-3223(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: A possible compensatory response. Biol Psychiat. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJG, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–6. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–14. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry. 1999;156:1200–4. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RSJ, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–95. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: Effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–17. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Wong JCK, Allan ME, Mar AC, Theobald DEH, Robbins TW. Contrasting roles for dopamine D1- and D2-receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioural inhibition in the stop-signal task in rats. J Neurosci. 2011;31:7349–56. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, et al. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Arch Gen Psychiatry. 2010;67:632–644. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–3. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Annual report 2010: the state of the drugs problem in Europe. Lisbon, Portugal: Office for Official Publications of the European Communities; 2010. [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Flitney DE, Jenkinson M. Technical report TR00DF1 - cluster analysis revisited. Oxford, UK: Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, University of Oxford; 2000. [Google Scholar]
- Franken IHA, Hendriks VM, Van den Brink W. Initial validation of two opiate craving questionnaires: The Obsessive Compulsive Drug Use Scale and the Desires for Drug Questionnaire. Addict Behav. 2002;27:675–85. doi: 10.1016/s0306-4603(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Muris P. BIS/BAS personality characteristics and college students’ substance use. Pers Indiv Differ. 2006;40:1497–1503. [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiat. 2002;51:134–42. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, Volkow ND. The effect of practice on a sustained attention task in cocaine abusers. NeuroImage. 2007;35:194–206. doi: 10.1016/j.neuroimage.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Burroughs S, Boutros NN. Attentional deficits in cocaine-dependent patients: converging behavioral and electrophysiological evidence. Psychiatry Res. 2008;160:145–54. doi: 10.1016/j.psychres.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, et al. In vivo brain anatomy of adult males with Fragile X syndrome: an MRI study. NeuroImage. 2011;54:16–24. doi: 10.1016/j.neuroimage.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Hayaki J, Stein MD, Lassor JA, Herman DS, Anderson BJ. Adversity among drug users: relationship to impulsivity. Drug Alcohol Depend. 2005;78:65–71. doi: 10.1016/j.drugalcdep.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Horner MD. Attentional functioning in abstinent cocaine abusers. Drug Alcohol Depend. 1999;54:19–33. doi: 10.1016/s0376-8716(98)00141-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry. 2001;158:486–9. doi: 10.1176/appi.ajp.158.3.486. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward- related stimuli. Psychopharmacology. 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–72. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exper Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Bagwell W, Haas G, Sweeney J, Schooler N, Pettegrew J. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–4. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Langen M, Durston S, Staal WG, Palmen SJMC, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007;62:262–6. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–38. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–48. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci. 1997;8:60–4. [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang YY, et al. Cocaine dependence and D-2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, et al. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D2/D3 receptors following acute dopamine depletion. Am J Psychiatry. 2009;166:1170–7. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti MA, Peluso MAM, Hatch JP, Nemoto K, Watanabe Y, et al. Anterior cingulate volumes associated with trait impulsivity in individuals with bipolar disorder. Bipolar Disord. 2009;11:628–36. doi: 10.1111/j.1399-5618.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, et al. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behav Pharmacol. 2009;20:390–9. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001a;158:1783–93. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Fischer CJ, Dougherty DM, Reilly EL, Mathias CW, et al. P300 event-related potential amplitude and impulsivity in cocaine-dependent subjects. Neuropsychobiology. 2004;50:167–73. doi: 10.1159/000079110. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001b;21:193–8. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–7. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National adult reading test manual. Windsor, UK: NFER-Nelson; 1982. [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, et al. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–94. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJR. The effects of cocaine: a shifting target over the course of addiction. Prog Neuro-Psychopharmacol Biol Psych. 2007;31:1593–600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Taylor JR. Found in translation: understanding impulsivity and related constructs through integrative preclinical and clinical research. Biol Psychiatry. 2009;66:714–6. doi: 10.1016/j.biopsych.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Li CS, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a stop signal task. NeuroImage. 2008;41:1352–63. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Indiv Differ. 2006;40:305–15. [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–70. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav Neurosci. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Tans Med Imaging. 1999;18:712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Rev. 2001;35:146–60. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2007;35:229–38. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Scherk H, Falkai P. Effects of antipsychotics on brain structure. Curr Opin Psychiatry. 2006;19:145–50. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Muller BW, Scherbaum N, Forsting M, Wiltfang J, Leygraf N, et al. Impulsivity-related brain volume deficits in schizophrenia-addiction comorbidity. Brain. 2010 doi: 10.1093/brain/awq153. doi: 10.1093/brain/awq153. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–62. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, et al. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. NeuroImage. 2010;50:1392–401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, et al. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–37. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling J, Bullmore E. Permutation tests for factorially designed neuroimaging experiments. Hum Brain Mapp. 2004;22:193–205. doi: 10.1002/hbm.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, et al. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res Neuroimaging. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehavioral Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Voelbel GT, Bates ME, Buckman JF, Pandina G, Hendren RL. Caudate nucleus volume and cognitive performance: are they related in childhood psychopathology? Biol Psychiatry. 2006;60:942–50. doi: 10.1016/j.biopsych.2006.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, et al. Is methylphenidate like cocaine - studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–63. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine-D(2) receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–77. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–3. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–88. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–18. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Zald DH, Ding Z, Riccardi P, Ansari MS, Baldwin RM, et al. Cerebral morphology and dopamine D2/D3 receptor distribution in humans: a combined [18F]fallypride and voxel-based morphometry study. NeuroImage. 2009;46:31–8. doi: 10.1016/j.neuroimage.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Bell K, Najafi A, Widmark C, Keator D, Tang C, et al. Decreasing striatal 6-FDOPA uptake with increasing duration of cocaine withdrawal. Neuropsychopharmacology. 1997;17:402–9. doi: 10.1016/S0893-133X(97)00089-4. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Tans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]