Abstract
Case reports indicate that psychiatrists administered ±3,4-methylenedioxymethamphetamine (MDMA) as a catalyst to psychotherapy before recreational use of MDMA as ‘Ecstasy’ resulted in its criminalization in 1985. Over two decades later, this study is the first completed clinical trial evaluating MDMA as a therapeutic adjunct. Twenty patients with chronic posttraumatic stress disorder, refractory to both psychotherapy and psychopharmacology, were randomly assigned to psychotherapy with concomitant active drug (n = 12) or inactive placebo (n = 8) administered during two 8-h experimental psychotherapy sessions. Both groups received preparatory and follow-up non-drug psychotherapy. The primary outcome measure was the Clinician-Administered PTSD Scale, administered at baseline, 4 days after each experimental session, and 2 months after the second session. Neurocognitive testing, blood pressure, and temperature monitoring were performed. After 2-month follow-up, placebo subjects were offered the option to re-enroll in the experimental procedure with open-label MDMA. Decrease in Clinician-Administered PTSD Scale scores from baseline was significantly greater for the group that received MDMA than for the placebo group at all three time points after baseline. The rate of clinical response was 10/12 (83%) in the active treatment group versus 2/8 (25%) in the placebo group. There were no drug-related serious adverse events, adverse neurocognitive effects or clinically significant blood pressure increases. MDMA-assisted psychotherapy can be administered to posttraumatic stress disorder patients without evidence of harm, and it may be useful in patients refractory to other treatments.
Keywords: combat disorders, MDMA, Posttraumatic stress disorder, psychedelics, PTSD
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating anxiety disorder characterized by re-experiencing, hyperarousal and avoidance symptoms, and is a major worldwide public health problem. The high incidence of PTSD and the limited effectiveness of existing treatments combine to create an urgent need for the development of new treatments. In the United States, the lifetime prevalence of PTSD in the general population is between 6% and 10% (Kessler et al., 2005), and is much higher in countries where there is endemic armed conflict (de Jong et al., 2003; Thabet and Vostanis, 1999; Weine et al., 1995). In US soldiers returning from service in Iraq and/or Afghanistan, the incidence of PTSD is as high as 18% (Hoge et al., 2004), and it is estimated that those with PTSD will number between 75,000 and 225,000 (Tanielian and Jaycox, 2008). PTSD is typically a chronic illness (Breslau and Davis, 1992; Kessler et al., 2005) associated with high rates of psychiatric and medical comorbidity, disability, suffering, drug abuse, and suicide (Breslau, 2001; Cohen et al., 2009; Frayne et al., 2004; Kessler et al., 2005; Perkonigg et al., 2000).
Existing treatments for PTSD include both pharmacotherapy and psychotherapies. Although a variety of drugs are used to treat symptoms of PTSD, they have limited efficacy. To date, the selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine are the only two drugs approved by the Food and Drug Administration (FDA) for this indication (Brady et al., 2000; Marshall et al., 2001). Nine clinical trials of SSRIs for PTSD published between 1994 and 2007 demonstrated a mean between-group effect size (Cohen’s d) of 0.5 for drug effect compared with placebo (Foa et al., 2009). After identifying 22 individual drugs in seven different drug classes, a 2008 review of PTSD treatment studies by the Institute of Medicine was inconclusive regarding evidence for use of any of the drugs studied (Committee on Treatment of Posttraumatic Stress Disorder, 2008). Other recent reviews of the literature have reached more favorable conclusions about pharmacotherapy. Several meta-analyses found that, while some studies did not show a significant drug effect, in general the response rate to pharmacotherapy was 20–22% greater than the response to placebo, and in SSRI trials 30% of subjects can achieve complete remission at 12 weeks. All these reviews have emphasized the need for further research into more effective agents for PTSD (Foa et al., 2009; Ipser et al., 2006; Stein et al., 2009; Ursano et al., 2004).
The most widely recognized methods of psychotherapy for PTSD are cognitive behavioral therapy (particularly Prolonged Exposure and Cognitive Processing Therapy), Eye Movement Desensitization and Reprocessing (EMDR), and psychodynamic psychotherapy (Foa et al., 2009; Ursano et al., 2004). A recent meta-analysis of direct comparison studies of ‘bona fide psychotherapies’ concluded that there is no statistically significant difference in between-group effect size (Benish et al., 2008). A review of cognitive behavioral therapy for PTSD listed 107 studies (Foa et al., 2009). Of the 36 studies using the Clinician-Administered PTSD Scale (CAPS) as an outcome measure and intent-to-treat analysis, the mean effect size was 0.88 when compared against wait-list controls (Foa et al., 2009). In clinical trials of psychotherapy for PTSD, the dropout rate is typically 20–30%, and the response rate is between 60% and 95% among subjects who receive active treatment and complete the trials (Cloitre, 2009; Hembree et al., 2003; Rothbaum et al., 2006). Data about combined psychotherapy and pharmacotherapy are limited to a few small studies with mixed results (Foa et al., 2009; Davis et al., 2006; Rothbaum et al., 2006). Overall, the evidence for pharmacotherapy and psychotherapies in the above studies indicates that existing therapies for PTSD are ineffective for between 25% and 50% of patients who enroll in clinical trials. An effective treatment that could reduce the substantial treatment failure rates associated with existing PTSD treatments is needed.
Methylenedioxymethamphetamine (MDMA) is a ring-substituted phenylisopropylamine derivative with a unique profile of psychopharmacological effects. MDMA was patented in 1914 by the German chemical and pharmaceutical company Merck KGaA as an intermediate compound in the synthesis of other drugs (Benzenhoefer and Passie, 2006; Freudenmann et al., 2006). Before MDMA was classified in the United States as a Schedule I controlled substance in 1985, a number of psychiatrists and other therapists in the United States and Europe used MDMA as an adjunct to psychotherapy. MDMA was reported to decrease feelings of fear while maintaining a clear-headed, alert state of consciousness (Greer and Tolbert, 1998). More recently, Phase I clinical trials have demonstrated that MDMA can be administered without evidence of harm to pre-screened subjects, and induces a 2–4-h experience typically characterized by euphoria, increased well being, sociability, self-confidence, and extroversion (Cami et al., 2000; Harris et al., 2002; Kolbrich et al., 2008; Liechti et al., 2001; Tancer and Johanson, 2001; Vollenweider et al., 1998). To test the potential efficacy of the clinical use of MDMA, we present the first rigorous data on its therapeutic application in this pilot Phase II clinical trial.
The decreased fear response induced by MDMA administration may be useful in the treatment of PTSD, a condition that involves exaggerated and uncontrolled fear responses. Many psychotherapies for PTSD involve the induction and extinction of these abnormal autonomic responses through revisiting traumatic experiences in psychotherapy with an appropriate level of emotional engagement (Foa et al., 2009). Frequently, however, treatment may be ineffective when patients are unable to tolerate feelings associated with revisiting the trauma, or when emotional numbing during exposure to traumatic memories precludes a level of engagement necessary for extinction (Jaycox and Foa, 1999). Therefore, if a drug could temporarily reduce fear and increase interpersonal trust, without clouding the sensorium or inhibiting access to emotions, it might prove an effective catalyst to psychotherapy for PTSD. The use of drugs to catalyze psychotherapy has been discussed in the psychiatric literature since the 1940s and has included the use of barbiturates, amphetamines, nitrous oxide, LSD, and others (Sargent et al., 1972). This report contains findings from the first completed pilot study designed to explore the possibility that MDMA could serve as such a catalyst. The hypothesis tested is that MDMA could be administered without harm to people with chronic, treatment-resistant PTSD and, in conjunction with psychotherapy, would lead to a significant decrease in PTSD symptoms compared with the same psychotherapy in conjunction with inactive placebo.
Possible mechanisms
Several possible mechanism of action for MDMA-assisted psychotherapy can be postulated. Learning theory, emotional processing theory and social-cognitive theories aimed at explaining the therapeutic effects of exposure therapy have been summarized by Foa et al. (Foa et al., 2009). To be effective, exposure must be accompanied by a degree of emotional engagement or ‘fear activation’ while avoiding dissociation or overwhelming emotion (Foa et al., 2007). This has been referred to as working within the ‘optimal arousal zone’ or ‘window of tolerance’ (Ogden and Pain, 2006; Siegel, 1999; Wilbarger and Wilbarger, 1997). Patients with PTSD are prone to extremes of emotional numbing or overwhelming anxiety, and often have a narrow window between thresholds of under and over-arousal (Ogden and Pain, 2006). MDMA may exert its therapeutic effect by widening this window. If MDMA allows patients to stay emotionally engaged without being overwhelmed by anxiety while revisiting traumatic experiences, it may thereby catalyze effective exposure therapy.
The pharmacological effects of MDMA include serotonin release, 5HT2 receptor stimulation, and increase in levels of the neurohormones oxytocin, prolactin and cortisol (Dumont et al., 2009; Grob et al., 1996; Harris et al., 2002; Mas et al., 1999; Thompson et al., 2007; Wolff et al., 2006). Serotonin release plays an important role in producing the subjective effects of MDMA (Farre et al., 2007; Liechti et al., 2000; Liechti and Vollenweider, 2000; Tancer and Johanson, 2007). Pretreatment with SSRIs reduces most acute subjective and physiological effects of MDMA, including effects on mood and perception. Serotonin release directly or indirectly leads to an elevation in oxytocin, possibly by stimulating 5HT1A receptors (Baggott et al., 2008; Dumont et al., 2009; Thompson et al., 2007). Recent findings suggest that oxytocin is involved in affiliation, trust and accurate perception of emotion (Domes et al., 2007; Kirsch et al., 2005; Zak et al., 2005), so elevated oxytocin might help participants form a therapeutic alliance and revisit traumatic experiences in an emotionally engaged state. In human volunteers, oxytocin reduces activation of the amygdala in response to fear-inducing stimuli (Kirsch et al., 2005) and increases trust (Baumgartner et al., 2008; Kosfeld et al., 2005). A recent study reported that elevation in oxytocin after MDMA was associated with greater sociability and gregariousness (Dumont et al., 2009). It has been postulated that prolactin release following MDMA administration may contribute to a post-orgasmic-like sense of relaxation and receptivity (Passie et al., 2005). The extent to which each of these pharmacological mechanisms may play a role in possible therapeutic effects of MDMA is speculative.
The ‘neurocircuitry model’ of PTSD postulates a deficit in extinction of fear conditioning mediated by the amygdala and the ventral/medial prefrontal cortex (vmPFC) (Rauch et al., 2006), a model supported by findings of reduced hippocampal activity and volume, increased activity in the amygdala and decreased activation of the medial prefrontal cortex in people with PTSD (Rauch et al., 2006). A human Positron Emission Tomography (PET) study 75 min following MDMA administration has shown increases in cerebral blood flow in the ventromedial frontal and occipital cortex, and decreases in the left amygdala (Gamma et al., 2000). MDMA may produce some of its effects through these acute changes in brain activity, possibly reversing abnormalities known to be associated with PTSD and thereby allowing for effective processing of traumatic material during the therapy sessions.
Methods and materials
Subjects
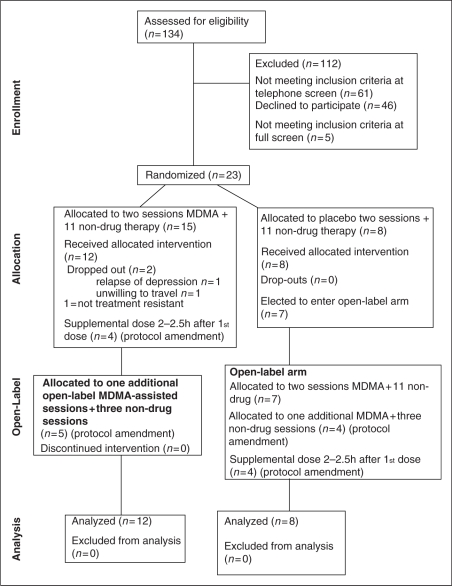
Subjects were recruited via letters to psychotherapists and internet advertisements. Potential subjects aged 21–70 years were screened using a scripted telephone interview to identify previously diagnosed medical or psychiatric exclusion criteria. Candidates who passed telephone screening and gave informed consent were evaluated in an outpatient office by an independent rater and a physician. Of these, 20 met all enrollment criteria and were recruited for the study, with replacement of two dropouts (Figure 1). For this initial pilot study, a minimum sample size of eight per group with oversampling for the experimental group was determined adequate to produce useful estimates of effect size. The study was approved by the Copernicus Group Independent Review Board (IRB), Research Triangle Park, NC, USA, and was conducted according to their regulatory guidance for protection of human subjects and relevant Federal regulations and international standards. Written informed consent was obtained from each subject by the investigators. Capacity to give informed consent was assessed clinically and with a written quiz testing understanding of the consent document. Enrollment began in March 2004 and ended in January 2008. Follow-up was completed in September 2008.
Figure 1.
Flow Diagram.
MDMA
The MDMA used was from a supply produced by David E Nichols, PhD, for the sponsor, which holds an FDA Drug Master File and Investigational New Drug permit (IND) for the study drug.
Design
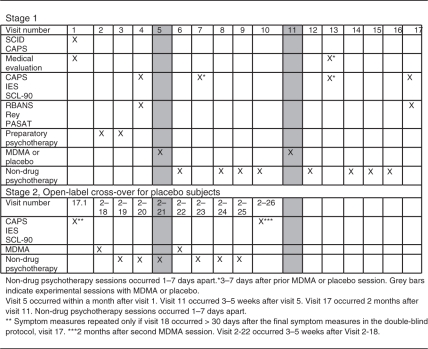
After enrollment, subjects were randomized, in double-blind fashion, to receive two experimental sessions of either psychotherapy with concomitant MDMA administration or the same psychotherapy accompanied by inactive placebo (lactose) administration (psychotherapy-only). The blind was broken for each subject after the follow-up visit 2 months after the second experimental session. All subjects who initially received placebo were offered participation in an open-label crossover segment (‘Stage 2’) (Figure 2). After the 2-month follow-up, nine subjects were given a third session of MDMA with psychotherapy, as allowed in a protocol amendment. However, because not all subjects received a third session and placebo subjects received only two sessions, data related to the third session were omitted from analysis, and for simplicity are omitted from Figure 2.
Figure 2.
Study Visits.
Subjects were required to taper and abstain from all psychotropic medication during study participation except sedative hypnotics or anxiolytics used as-needed between MDMA or placebo sessions (referred to as ‘rescue medications’). After preliminary evidence of safety and efficacy had been established, a protocol amendment was approved allowing the last nine subjects to receive a supplemental dose of MDMA or placebo in all experimental sessions. The purpose of this supplemental dose, half the initial dose administered 2 h afterwards, was to prolong the therapeutic window of MDMA effects and gather pilot data about dose for design of future clinical trials.
Assessments
Study entry screening consisted of a Structured Clinical Interview for Axis I Diagnosis (SCID) (First et al., 1994), the SCID II for personality disorder (First et al., 1997), CAPS, medical history, physical examination, serum chemistry profile, complete blood count, thyroid-stimulation hormone (TSH), free thyroxine, HIV serology, urinalysis, and electrocardiogram (ECG). Subjects were required to meet DSM-IV-R criteria for the diagnosis of crime or war-related chronic PTSD, and to have treatment-resistant symptoms, defined as a CAPS score of ≥50 (signifying moderate to severe symptoms) following at least 3 months of prior SSRI or serotonin–norepinephrine reuptake inhibitor (SNRI) treatment in addition to at least 6 months of psychotherapy. Exclusion criteria required freedom from any major medical conditions. In addition, psychiatric exclusion criteria included Borderline Personality Disorder or any current Axis I disorder with the following exceptions which were allowed: anxiety disorders, affective disorders other than bipolar disorder type 1, substance abuse or dependence in remission for ≥60 days, and eating disorder without active purging. Urine drug testing for cocaine, marijuana, amphetamines, MDMA, opiates and benzodiazepines was performed during initial screening and immediately before each experimental session. All screens were negative in all subjects.
Outcome measures
Primary
The CAPS is a widely used structured interview for quantifying PTSD symptoms that has excellent psychometric properties of reliability and validity (Weathers et al., 2001). This measure produces a global symptom severity score as well as a categorical ranking as to whether or not a subject meets DSM-IV-R criteria for PTSD.
Secondary
Impact of Events Scale-Revised (IES-R) is a widely used self-report measure that assesses psychological response to stress. It was revised to parallel the DSM-IV criteria for PTSD and found to have acceptable psychometric properties (Weiss, 1997). The Symptom Checklist 90-Revised (SCL-90-R) is a self-report of symptoms covering a wide range of psychiatric categories and yielding nine symptom scales as well as global indices (Derogatis, 1994).
Neurocognitive measures
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) tests attention and processing speed, expressive language, visual-spatial and constructional abilities, and memory (Randolph et al., 1998). The Paced Auditory Serial Addition Task (PASAT) assesses information processing speed and mental flexibility (Gronwall, 1977). The Rey-Osterrieth Complex Figure (RCFT) assesses visuospatial memory associated with a variety of neurological conditions that alter effective brain function (Mirushina et al., 1999).
Physiological measures
Blood pressure, pulse, and temperature were monitored during all experimental sessions.
Randomization and blind – Stage 1
Subjects were randomized to either MDMA or placebo, in conjunction with psychotherapy, in a ratio of 60% MDMA (n = 12) to 40% placebo (n = 8), replacing dropouts to preserve the group-assignment ratio and double-blinding. On the day of each subject’s first experimental session, an independent randomization monitor assigned a bottle containing either MDMA or placebo, determined by a computer-generated randomization list. The subjects, investigators and the independent rater who administered the outcome measures were all blinded. The independent rater did not have access to records of treatment sessions.
After screening and enrollment, participants completed baseline psychological and neurocognitive measures. Outcome measures were repeated approximately 4 days after each of two 8-h experimental sessions and 2 months after the second experimental session. Participants completed neurocognitive measures at baseline and again 2 months after the second experimental session (Table 1).
Table 1.
Study Visits
Stage 2
Placebo subjects who enrolled in the open-label arm completed outcome measures 2 months after their last experimental session. The schedule of visits in Stage 2 was nearly identical to the schedule in Stage 1 (Table 1).
Therapeutic intervention
The study was conducted in a comfortable, aesthetically pleasing outpatient office with facilities for the subject and a blinded consultant nurse to remain at the site overnight. The investigators maintained equipment and drugs for treatment of medical emergencies, and an emergency physician and nurse were on site for all experimental sessions. The experimental sessions lasted 8–10 h, followed by an overnight stay. One of the investigators maintained daily telephone contact with the subjects during the week after experimental sessions.
In addition to experimental sessions of MDMA or placebo during psychotherapy, the therapeutic intervention included non-drug psychotherapy sessions for preparation and integration. A male and female co-therapist team, one a psychiatrist, the other a psychiatric nurse, was present for all sessions. Each subject had two 90-min introductory sessions within 6 weeks before the first experimental session to prepare them for the structure of the sessions, the approach to therapy and possible effects of MDMA. In Stage 1, there were eight integration sessions focused on discussing the experimental sessions, additional emotional processing (Foa and Kozak, 1986) if necessary, and helping subjects incorporate any insights or new perspectives into their daily lives. One of these integration sessions occurred the morning after each of the two experimental sessions, and three more were scheduled during the month following each experimental session. Additional integration sessions were permitted if needed. A final integration session occurred 2 months after the second experimental session. In Stage 2, the schedule of integration sessions was the same as in Stage 1 except that there were three scheduled follow-up sessions after each experimental session instead of four.
The method of psychotherapy followed principles developed by Stanislav Grof, MD and others for LSD psychotherapy (Pahnke et al., 1971) and Holotropic Breathwork (Grof, 2000), and adapted for MDMA-assisted psychotherapy by Metzner and others (Greer and Tolbert, 1998; Metzner and Adamson, 2001). These methods were further modified by the investigators for application to PTSD treatment, and the psychotherapy technique was manualized to the extent possible prior to this initial pilot study. This draft treatment manual was written by the sponsor and investigators before the study began, and is being refined and operationalized with quantitative adherence measures based on this pilot study. It is available at http://www.maps.org/mdma/.
During experimental sessions, the subject sat or reclined on a futon bed and the co-therapists sat in chairs on either side. The first dose of MDMA (125 mg) or placebo was given in a capsule by mouth at 10 a.m. Subjects then rested in a comfortable position with eyes closed or wearing eyeshades, and listened to a program of music that was initially relaxing and later emotionally evocative. The programs of music used were identical for all subjects, though subjects or investigators could choose to skip over some selections or to replace them with periods of silence. Throughout the experimental sessions, periods of conversation alternated with periods during which subjects were encouraged to focus on introspection. The schedule for these alternating periods was flexible and determined by the desires of the subject and the judgment of the therapists. The therapists sought an approximately equal balance between quiet introspection and therapeutic discussion, but the actual ratio varied among individuals and between sessions.
The optional supplemental dose of 62.5 mg MDMA or placebo was administered 2–2.5 h after the initial dose if the investigators judged it to be safe and advisable and the subject agreed to it. The supplemental dose was administered in 22 of the 23 sessions in which it was an option. The therapists stayed with the subject until at least 5 p.m. or until the physical and psychological effects of the session had substantially subsided and the subject was judged to be in stable condition and at baseline mental status. If necessary, zolpidem or lorazepam were prescribed for insomnia. During psychotherapy on the following day, participants and investigators were asked to guess condition assignment and rate the certainty of their guess.
Statistical analysis
Per-protocol analysis with ANOVA with repeated measures was conducted to test CAPS, IES-R and SCL-90-R scores for differences between groups over time. The Holm’s sequential Bonferroni correction for multiplicity was employed after a significant ANOVA for the four post-hoc tests for differences between groups.
Results
Table 2 shows participant characteristics. Fifteen of the 20 subjects had previously undergone multiple medication trials (mean 4.2 different psychiatric drugs) and 15 had completed more than one course of psychotherapy. Average duration of PTSD was estimated at 19+ years. At baseline there were no significant differences between groups with the exception of duration of previous therapy. Analysis of co-variance with repeated measures was conducted for CAPS using number of months of previous therapy as covariate. The covariate proved non-significant (CAPS: F = 0.35, p = 0.56). Index traumas for participants are shown in Table 3. Two subjects, not included in Tables 2 or 3, dropped out before the second experimental session. One did so because she required resumption of medication for relapse of depression 42 days after her one MDMA-assisted session. The other withdrew because he found travel to the study site problematic due to limits on reimbursement of travel expenses set by the IRB, which were increased at the sponsor’s request after this subject dropped out.
Table 2.
Participant characteristics
| CHARACTERISTIC | All Subjects n = 20 | MDMA group n = 12 | Placebo Group n = 8 | p |
|---|---|---|---|---|
| Mean age (std) | 40.4 (7.2) | 40.2 (7.6) | 40.8 (7.0) | 0.79 |
| Gender | 0.66 | |||
| Male | 3 15% | 2 17% | 1 13% | |
| Female | 17 85% | 10 83% | 7 87% | |
| Caucasian | 20 100% | 12 100% | 8 100% | |
| Marital status | 0.27 | |||
| Never married | 7 35% | 6 50% | 1 12.5% | |
| Divorced/separated | 2 10% | 1 8% | 1 12.5% | |
| Widowed | 1 5% | 0 | 1 12.5% | |
| Married/living with partner | 10 50% | 5 42% | 5 62.5% | |
| On disability for PTSD | 3 15% | 2 17% | 1 13% | 0.85 |
| Hx alcohol abuse/dependency | 2 10% | 1 8% | 1 13% | 0.63 |
| Hx other substance abuse/dependency | 1 5% | 0 | 1 12% | 0.62 |
| Prior MDMA use | 9 43% | 6 46% | 3 38% | 0.53 |
| Lifetime MDMA use (number of times) for prior MDMA users | 9 (1–5) | 6 (1–5) | 3 (1–2) | 0.32 |
| PTSD mean number of months of duration (std) | 248 (173) | 232 (201) | 273 (126) | 0.57 |
| PTSD Crime-related | 19 95% | 11 85% | 8 100% | 0.60 |
| PTSD War-related | 1 5% | 1 8% | 0 | 0.60 |
| Comorbid major depression | 16 80% | 9* 75% | 7** 88% | 0.47 |
| Comorbid anxiety disorder | 3 15% | 2*** 17% | 1**** 13% | 0.65 |
| Mean # months of prior therapy (std) | 58.5 (49.5) | 40.6 (38.5) | 85.3 (54.2) | 0.04 |
| Mean Baseline CAPS score (std) | 79.4 (22.4) | 79.2 (23.6) | 79.6 (22.0) | 0.97 |
Bivariate statistical tests were conducted on all baseline measurements and subject characteristics to determine if significant differences existed initially between the placebo and MDMA treatment groups. All tests were non-significant except number of months subjects had of previous therapy.
two in remission
three in remission
one panic disorder in remission, one current generalized anxiety disorder and simple phobia
current panic disorder and obsessive compulsive disorder, in remission
Table 3.
Index traumas for study participants
| Index trauma | MDMA (n = 12) | Placebo (n = 8) | p |
|---|---|---|---|
| Sexual assault | 5 (42%) | 3 (38%) | p > 0.95 |
| Childhood abuse | |||
| Sexual | 4 (33%) | 4 (50%) | p > 0.95 |
| Physical, neglect | 2 (16%) | 0 | p = 0.35 |
| Emotional | 1 | 0 | p > 0.95 |
| Family violence, other | 1 | 0 | p > 0.95 |
| Violence (as stabbing) | 0 | 1 | p > 0.95 |
| Combat stress | 1 | 0 | p > 0.95 |
Physiological response and side effects
Onset of MDMA effects occurred 45–75 min after the initial dose. The effects reached a peak at 2–2.5 h and lasted 4–5 h in the 11 subjects who received a single dose, and 5–6 h in the nine who received a supplemental dose. Effects diminished gradually over several hours. Elevations of blood pressure, pulse, and body temperature were greater in the MDMA group, and spontaneously returned to baseline at session end in both groups (Table 4). There were no resulting medical complications or pharmacologic interventions.
Table 4.
Physiologic data: All experimental sessions
| MDMA | Placebo | |||
|---|---|---|---|---|
| Systolic BP | Mean (St. Dev.) | Range | Mean (St. Dev). | Range |
| Baseline | 117.9 (15.0) | 97/149 | 114.2 (11.6) | 92/140 |
| Maximum | 143.1 (16.3) | 117/179 | 136.4 (18.8) | 102/176 |
| Max Change | 25.2 (13.7) | 16/50 | 22.2 (15.3) | −3/57 |
| Diastolic BP | ||||
| Baseline | 74.6 (9.4) | 56/92 | 74.4 (8.1) | 58/89 |
| Maximum | 89.0 (9.2) | 74/113 | 88.2 (10.5) | 65/106 |
| Max Change | 14.4 (6.5) | 1/14 | 13.8 (7.5) | 2/28 |
| Pulse | ||||
| Baseline | 74.3 (11.7) | 54/98 | 69.9 (10.9) | 5/92 |
| Maximum | 103.1 (15.9) | 67/135 | 92.1 (17.8) | 68/141 |
| Max Change | 28.8 (11.5) | −7/52 | 22.2 (13.7) | 3/57 |
| Temperature | ||||
| Baseline | 36.6 (0.5) | 35.6/37.8 | 36.3 (0.6) | 35/37.2 |
| Maximum | 37.1 (0.3) | 36.7/37.8 | 37.1(0.4) | 36.7/37.8 |
| Max Change | 0.5 (0.4) | −0.6/1.5 | −0.7 (0.6) | −0.06/1.9 |
Group comparisons of vital signs were tested for change pre-session (15 min prior) to highest recorded and pre-session to post-session (6 h post) using t tests. There was a significantly greater increase in all physiologic measures from pre-session to highest recorded value during experimental sessions for the MDMA group than for the placebo group (p < 0.05). There were no significant differences when comparing change from pre-session to post-session (p > 0.05). All values returned to pre-session norms by 6 h after session completion.
Spontaneously reported side effects occurring within 7 days of MDMA or placebo administration are shown in Table 5. The most common side effects that occurred more frequently in the MDMA group on the day of experimental sessions were: jaw tightness, nausea, feeling cold, dizziness, loss of appetite, and impaired balance. Equally or more common in the placebo group on the day of experimental sessions were: anxiety, insomnia, headache and fatigue. In the week following experimental sessions, some of the most common side effects were reported at similar incidence by both groups: fatigue, anxiety, low mood, headache and nausea, with anxiety being slightly more frequent in the MDMA group and low mood slightly more frequent in the placebo group. During this week, irritability and loss of appetite were more frequently reported in the MDMA group and insomnia was reported more often in the placebo group. Side effects typically resolved over a period of hours or days, usually spontaneously; sometimes with short-term symptomatic treatment such as sedative hypnotics or non-steroidal anti-inflammatory drugs following experimental sessions. No medical treatment was required during any experimental sessions. No serious drug-related adverse effects occurred.
Table 5.
Side effects: Number of instances of spontaneously reported side effects in association with both experimental sessions in Stage 1, with instances a function of number of subjects and experimental sessions in which each side effect occurred during the session or within 7 days following
| Day of MDMA sessions (2) | Day of placebo sessions (2) | Within 7 days after MDMA sessions | Within 7 days after placebo session | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Subjects = 12 | Subjects = 8 | Subjects = 12 | Subjects = 8 | |
| Sessions N = 24 | Sessions N = 16 | Sessions N = 24 | Sessions N = 16 | |
| Anxiety | 14 (58%) | 13 (81%) | 13 (54%) | 7 (44%) |
| Decreased concentration | 3 (13%) | 1 (6%) | 6 (25%) | 0 |
| Derealization, detachment | 0 | 0 | 1 (4%) | 0 |
| Diarrhea | 0 | 0 | 3 (13%) | 0 |
| Dizziness | 9 (38%) | 2 (13%) | 3 (13%) | 1 (6%) |
| Drowsiness | 2 (8%) | 3 (19%) | 0 | 2 (13%) |
| Dry mouth | 4 (17%) | 1 (6%) | 1 (4%) | 0 |
| Fatigue | 11 (46%) | 8 (50%) | 18 (75%) | 12 (75%) |
| Feeling cold | 10 (42%) | 3 (19%) | 1 (4%) | 0 |
| General infection | 1 (4%) | 0 | 0 | 2 (13%) |
| Headache | 14 (58%) | 9 (56%) | 6 (25%) | 4 (25%) |
| Heavy legs | 2 (8%) | 0 | 1 (4%) | 0 |
| Impaired balance | 6 (25%) | 0 | 0 | 0 |
| Insomnia | 13 (54%) | 10 (63%) | 9 (38%) | 9 (56%) |
| Irritable | 2 (8%) | 3 (19%) | 8 (33%) | 3 (19%) |
| Loss of appetite | 8 (33%) | 1 (6%) | 9 (38%) | 0 |
| Low mood | 4 (17%) | 2 (13%) | 10 (42%) | 8 (50%) |
| Muscle tension | 4 (17%) | 2 (13%) | 2 (8%) | 1 (6%) |
| Nausea | 12 (50%) | 2 (13%) | 7 (29%) | 4 (25%) |
| Need more sleep | 0 | 1 (6%) | 5 (21%) | 2 (13%) |
| Nystagmus | 1 (4%) | 0 | 0 | 0 |
| Pain | 1(4%) | 4 (25%) | 1 (4%) | 1 (6%) |
| Panic, re-experiencing | 0 | 0 | 1 (4%) | 1 (6%) |
| Parasthesias | 2 (8%) | 0 | 0 | 0 |
| Perspiration | 4 (17%) | 1 (6%) | 0 | 1 (6%) |
| Restless | 5 (21%) | 2 (13%) | 1(4%) | 0 |
| Rumination | 1 (4%) | 1 (6%) | 3 (13%) | 2 (13%) |
| Somatic sensations | 1 (4%) | 1 (4%) | ||
| Thirst | 2 (8%) | 1 (6%) | 0 | 0 |
| Tight jaw | 19 (79%) | 3 (19%) | 6 (25%) | 2 (13%) |
| Upper GI burning | 1 (4%) | 0 | 0 | 0 |
| Upper respiratory infection | 0 | 0 | 2 (8%) | 2 (13%) |
| Visual disturbance | 1(4%) | 0 | 2 (8%) | 0 |
| Weakness | 1 (4%) | 1 (6%) | 6 (25%) | 0 |
Symptom levels Stage 1 – double blind
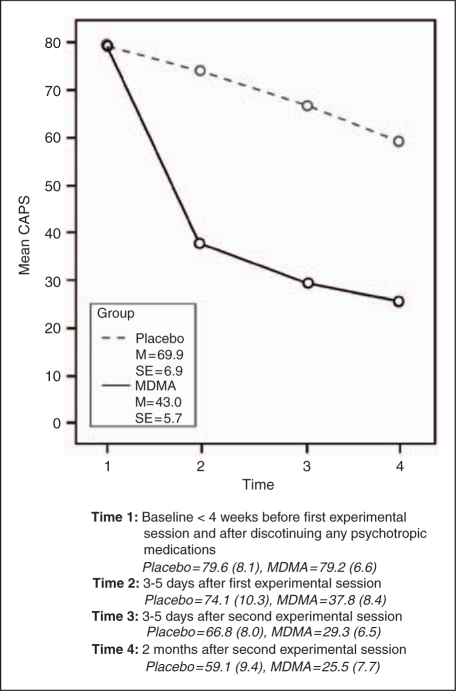
Figure 3 illustrates that PTSD symptoms, as measured by CAPS, improved over time in both groups (Time: F(3, 17) = 40.292, p < 0.0005), but the MDMA group showed significantly greater improvement (Time*Group interaction F(1, 17) = 7.173, p = 0.015). Mean differences between ‘group × time’ were examined using independent t-tests with Holm’s sequential Bonferroni correction (α) for multiplicity. Statistical significance (*) is indicated where p < α: Time 1 p = 0.966, α = 0.050, Time 2* p = 0.013, α = 0.017, Time 3* p = 0.002, α = 0.012, Time 4* p = 0.013, α = 0.025. Similar results were found for the IES-R, shown in Figure 4. PTSD symptoms, as measured by IES-R, improved over time in both groups (Time: F(3, 17) = 11.003, p < 0.0005), but the MDMA group showed significantly greater improvement (Time*Group interaction F(1, 17) = 3.290, p = 0.027). Mean differences between ‘group × time’ were examined using independent t-tests with Holm’s sequential Bonferroni correction (α) for multiplicity. Statistically significance (*) is indicated where p < α: Time 1 p = 0.976, α = 0.050, Time 2* p = 0.016, α = 0.017, Time 3* p = 0.006, α = 0.012, Time 4 p = 0.038, α = 0.025. The CAPS scores of the two subjects who dropped out, both of whom were randomized to receive MDMA, fell from 110 and 107, respectively, at baseline, to 17 and 27, respectively, 4 days after their only MDMA-assisted psychotherapy session. These data are not included in the analysis.
Figure 3.
CAPS Mean Scores by Group for Time 1-Time 4.
Figure 4.
IES-R Mean Scores by Group for Time 1-Time 4.
Symptom levels Stage 2 – open-label crossover
Seven of the eight placebo subjects chose to enroll in the crossover arm. One of the placebo responders, whose CAPS score had fallen from 67 to 15 after placebo, elected not to enroll in the crossover because she was satisfied with her improvement. The other placebo responder had a transient decline in CAPS from 54 to 15 after placebo, but her CAPS increased to 64 in the 3 months prior to enrollment in Stage 2. Paired t-tests were used to analyze change in outcome measures from Time 1c to Time 4c in the crossover arm. For the seven placebo subjects who completed the open-label crossover, there were significant decreases in CAPS (Table 6) and IES-R scores (mean IES-R decrease = 15.9, SD = 12.1, p = 0.013) from end of the control trial to 4–6 weeks after two MDMA sessions were completed. These decreases are similar in magnitude to the CAPS and IES-R decreases in the subjects initially randomized to full-dose MDMA.
Table 6.
Crossover Post-hoc Group Comparisons of CAPS at Time 1c–Time 4c
| Crossover Arm | CAPS | Time 1c (Baseline)* | Time 4c** | Change Time 1c - Time 4c |
|---|---|---|---|---|
| MDMA | Mean | 65.6 | 33.9 | −31.7*** |
| n | 7 | 7 | 7 | |
| Std. Dev. | 24.2 | 12.8 | 15.0 |
Crossover Time 1c is pre-MDMA and at least 2 months post-placebo. Crossover trial Time 4 = Time 1c, unless >30 days until starting crossover, in which case baseline measures repeated <30 days before crossover.
4–6 weeks after second MDMA session
p < 0.05 Post-hoc paired t-test determined statistical significance of mean difference between times
Neurocognitive measures
At baseline, there were no significant group differences on any of the cognitive measures including the RBANS total score (t = 1.78, p = 0.09), PASAT Trial 1 (t = 0.95, p = 0.35), PASAT Trial 2 (t = −0.16, p = 0.88) and the Rey-Osterrieth Figure 30-minute delay (t = 0.06, p = 0.95). To test whether the experimental condition had an adverse impact on cognition, between-group comparisons were performed at the study follow-up 2 months after the second experimental session. There were no significant group differences on any of the major index scores. This is best captured by the RBANS total score (t = 1.05, p = 0.31), PASAT Trial 1 (t = 0.35, p = 0.19), PASAT Trial 2 (t = 0.41, p = 0.69) and the Rey-Osterrieth 30-minute delay score (t = 0.98, p = 0.35).
Clinical response
Clinical response was defined as >30% reduction from baseline in CAPS total severity score. In Stage 1, the clinical response was 83.3% (10/12) in the MDMA group versus 25% (2/8) in the placebo group. Likewise, 10 of the MDMA group no longer met DSM-IV criteria for PTSD compared with two of the placebo group. In Stage 2, the clinical response rate was 100% in the seven subjects, six of whom had failed to respond to placebo and one of whom had relapsed after an initial placebo response. An unplanned observation was that all three subjects who reported being unable to work due to PTSD were able to return to work.
Additional psychotherapy sessions
As allowed in the protocol, additional psychotherapy sessions occurred when the investigators judged them to be necessary to support integration in subjects who experienced anxiety or other difficulties following experimental sessions. Only one additional session was conducted following placebo sessions, whereas 20 such sessions were provided to seven of 13 subjects following MDMA-assisted sessions. The data does not allow meaningful statistical characterization of the relationship between extra visits and changes in symptom measures; for one to three extra sessions there was a trend toward correlation with improved CAPS scores, but any increase beyond three extra sessions was inversely related to improvement in CAPS scores.
Supplemental doses
Comparing CAPS scores over the four time periods between the four subjects who received a supplemental dose of MDMA (per protocol amendment) and the eight who did not, there were no significant differences in Group by time (ANOVA with repeated measures F(1, 9) = 0.413, p = 0.637).
Evaluation of blind
Nineteen of 20 participants (95%) correctly guessed their condition assignment, though three were uncertain about their guess. The therapists guessed correctly in all cases, but were uncertain in three instances.
Rescue medication
Zolpidem was administered following 31 of 51 MDMA-assisted sessions (60.7%) and after 11 of 16 psychotherapy-only sessions (68.8%) (p = 0.77). Benzodiazepines were administered following (though usually not the same day as) 24 of 51 MDMA-assisted sessions (47.0%) and after six of 16 psychotherapy-only sessions (37.5%) (p = 0.57 – both with Fisher’s Exact Test). Seventeen of 20 subjects, the majority of whom had pre-existing sleep disturbance related to PTSD, received zolpidem for insomnia during study participation. In five cases this was limited to one or two nights following MDMA or placebo sessions. Fourteen subjects received benzodiazepines during study participation. Eleven of 14 reported taking benzodiazepines before enrollment. Two of the three who had not taken benzodiazepines before study enrollment did so for 1 and 7 days, respectively. The mean decrease in CAPS scores from time 1 to time 4 was nearly equal for the 14 who received benzodiazepines and the six who did not: 40.3 (SD = 39) and 40.7 (SD = 27.2), respectively (p = 0.98).
Discussion
This pilot study demonstrates that MDMA-assisted psychotherapy with close follow-up monitoring and support can be used with acceptable and short-lived side effects in a carefully screened group of subjects with chronic, treatment-resistant PTSD. In this group, MDMA-assisted psychotherapy compared with the same psychotherapy with inactive placebo produced clinically and statistically significant improvements in PTSD symptoms as measured by standard symptom scales. This difference was immediate and was maintained throughout the time period. There were no drug-related serious adverse events and no evidence of impaired cognitive function as measured by neuropsychological testing. The between-group effect size (1.24) of the study drug compares favorably with other treatment modalities for PTSD (Foa et al., 2009), particularly given the treatment-refractory nature of the current sample. The clinical significance of the symptom reductions is indicated by the high percentage of subjects attaining a >30% reduction in CAPS scores and no longer meeting criteria for PTSD 2 months after MDMA-assisted psychotherapy, and by the report that all three subjects who had been unable to work because of PTSD were able to return to work.
The strengths of this study are its prospective, double-blind, crossover design, the use of a standardized primary outcome measure (CAPS) that is widely used for PTSD research (Weathers et al., 2001), enrollment of chronic, treatment-resistant subjects who had moderate to severe PTSD, and the use of a blinded, independent rater. Subjects met well-defined inclusion and exclusion criteria. Groups were well matched; at baseline, subjects in both groups had nearly identical CAPS scores.
This study has several limitations and should be considered only a preliminary step toward exploring MDMA as a possible therapeutic adjunct. Sample size is small, as is appropriate in a Phase II pilot study. The majority of participants were female and all were Caucasian. Gender and/or ethnic differences in response to MDMA-assisted psychotherapy could exist. At baseline, the placebo group had a history of more prior psychotherapy than the MDMA group, which could mean that the placebo group was more treatment-resistant; however, this covariate proved non-significant. Furthermore, in the open-label phase, the placebo group had a response comparable to the MDMA group, so in fact, the placebo group proved not to be more resistant to MDMA-assisted psychotherapy.
Another important weakness of this study is the transparency of the blinding. We chose to use an inactive placebo in this initial trial in order to compare side effects of MDMA with those of placebo in this patient population. Although the independent rater remained effectively blinded because he was not present during experimental sessions, the novel subjective experience was a strong clue for the subjects, as was the subjects’ increase in pulse and blood pressure for the investigators. We have recently obtained a ‘may proceed’ letter from the FDA for a protocol for a three-arm, dose–response study that we expect will result in successful blinding. An argument against a placebo response having accounted for between-group outcome differences is the maintenance of treatment effect at 2-month follow-up, and the subjects’ having failed to respond to prior treatments during a course of PTSD lasting a mean of nearly 20 years. Nevertheless, a placebo effect cannot be ruled out and future studies must address this limitation.
Additional psychotherapy sessions were conducted more often after MDMA-assisted sessions than after psychotherapy-only sessions. This is a potentially confounding factor, but it is not a likely explanation for the difference in final outcome. Additional psychotherapy cannot explain the significant improvements in CAPS scores recorded 4 days after the first MDMA-assisted session, before any additional psychotherapy sessions occurred. In future studies, attempts should be made to limit additional psychotherapy sessions while retaining some flexibility to ensure subject safety while maintaining therapeutic effect. Another limitation is that measurement of the durability of symptom improvement was limited to 2 months after the second experimental session. We chose this interval because it is well beyond the acute effects of the drug and the immediate expectancy effects, but short enough to minimize effects from intervening events. A long-term outcome study is currently being conducted evaluating subjects from 1–5 years post-treatment. The absence of therapist adherence measures was an unavoidable weakness of this first pilot study. A treatment manual and adherence measures based on audio and video recordings from this study have now been developed for use in future clinical trials.
The use of zolpidem for sleep or benzodiazepines for anxiety during the study did not differ between the MDMA and placebo conditions, but deserves some discussion to clarify the intent. As is common with PTSD, most of the study subjects had pre-existing sleep disturbance. Zolpidem was offered frequently, often the night after an experimental session when subjects spent the night in the relatively unfamiliar environment of the clinic after a long day of emotional processing. Benzodiazepines were offered more sparingly so as to avoid suppressing ongoing emotional processing, which is considered an important element of the integration phase of therapy, while providing some symptomatic relief to help subjects effectively balance emotional processing with rest, work and other daily activities. The vast majority of benzodiazepine doses went to people who had used them before, and all subjects had previously taken psychiatric medications with significant anxiolytic properties that were not allowed during the study. It is important to note that a temporary increase in anxiety was sometimes a side effect of this treatment, but the fact that neither zolpidem nor benzodiazepines were administered significantly more often after MDMA sessions than after placebo sessions suggests that this side effect was caused by the psychotherapy in the setting of chronic PTSD rather than by MDMA administration.
An obvious feature of this treatment model is that it involves an initial period of concentrated patient-therapist contact (31 h over 2 months) including all-day therapy sessions and an overnight stay in the clinic. These are not usual features of psychotherapy practice in the outpatient setting. If MDMA-assisted psychotherapy is ultimately approved for use in clinical practice, it would likely occur in clinics specifically equipped for longer treatment sessions and overnight stays. This method also involves patient preparation and close follow-up to support further processing of emotions and integration of cognitive shifts that may occur. Both the preparation sessions and the integration sessions appear to be important for safety and therapeutic effect. In future studies we recommend that the number of 90-min preparation sessions be increased from two to three. This approach is initially more expensive than other outpatient treatments; however, given the chronic nature of treatment-resistant PTSD requiring ongoing psychiatric treatment and the associated high rates of medical comorbidity and disability, it has the potential to be more cost effective over time for a significant segment of the patient population. In this study the second session appeared to add depth to the overall therapeutic process and it provided the reassurance that subjects would have more than one opportunity to work through their issues. Nevertheless, the fact that most of the symptom improvement occurred after the first MDMA-assisted psychotherapy session suggests that it would be worthwhile for future studies to investigate the impact of number of sessions on strength and duration of PTSD symptom reduction.
The promising results of this initial pilot study suggest that further research is warranted to confirm our findings, distinguish and refine the essential elements of this approach, enhance the methodology, and elucidate the mechanisms involved.
Acknowledgements
We wish to thank the subjects who participated in this study, the Copernicus IRB and our Data Safety Monitoring Board for their careful oversight, the emergency physicians and nurses who provided stand-by medical backup, the psychiatric nurses who attended our subjects during their overnight stays, Yvonne Michel, PhD, for statistical analysis and guidance, Nicole Vilardo for formatting and proofing, Heather Mithoefer for graphics and John H Halpern, MD, Harvard Medical School, Harrison G Pope, MD, MPH, Harvard Medical School. Andrew Sewell, MD, VA Connecticut Healthcare/Yale University School of Medicine and Timothy D Brewerton, MD, Medical University of South Carolina, who provided helpful comments on an previous version of the manuscript.
Funding/Support
All funding was provided by The Multidisciplinary Association for Psychedelic Studies, a non-profit organization. The sponsor played a role in study design, data analysis and writing of the report. The investigators performed all data collection. The corresponding author wrote the first draft of the report, had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest statement
Two of the authors, Lisa Jerome and Rick Doblin, are employed by the sponsor. Michael Mithoefer is medical monitor for other studies of MDMA-assisted psychotherapy currently being conducted by the sponsor. He and Ann Mithoefer both receive payment from the sponsor for conducting this research, working on development of a treatment manual, investigator training program, and protocols for additional studies planned by the sponsor. Mark Wagner received payment from the sponsor for acting as the independent rater for this study.
Trial Registration
ClinicalTrials.gov Identifier: NCT00090064
References
- Baggott MJ, Galloway G, Jang M, Didier R, Pournajafi-Nazarloo H, Carter CS, et al. (2008) 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) and prazosin interactions in humans. 70th Annual Meeting of the College on Problems of Drug Dependence. San Juan, Puerto Rico.
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. (2008) Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58: 639–650 [DOI] [PubMed] [Google Scholar]
- Benish SG, Imel ZE, Wampold BE. (2008) The relative efficacy of bona fide psychotherapies for treating post-traumatic stress disorder: a meta-analysis of direct comparisons. Clin Psychol Rev 28: 746–758 [DOI] [PubMed] [Google Scholar]
- Benzenhoefer UP, Passie T. (2006) The early history of ecstasy. Nervenartz 77: 95–96 (98–99 [DOI] [PubMed] [Google Scholar]
- Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, et al. (2000) Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA 283: 1837–1844 [DOI] [PubMed] [Google Scholar]
- Breslau N. (2001) The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry 62: 16–22 [PubMed] [Google Scholar]
- Breslau N, Davis GC. (1992) Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry 149: 671–675 [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M, Mas M, Roset PN, Poudevida S, Mas A. (2000) Human pharmacology of 3,4-methylenedioxymethamphetamine (“ecstasy”): psychomotor performance and subjective effects. J Clin Psychopharmacol 20: 455–466 [DOI] [PubMed] [Google Scholar]
- Cloitre M. (2009) Effective psychotherapies for posttraumatic stress disorder: a review and critique. CNS Spectr 14: 32–43 [PubMed] [Google Scholar]
- Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. (2009) Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA 302: 489–492 [DOI] [PubMed] [Google Scholar]
- Committee on Treatment of Posttraumatic Stress Disorder, Institute of Medicine (2008) Posttraumatic stress disorder: an assessment of the evidence. Washington, DC: The National Academies Press [Google Scholar]
- Davis M, Barad M, Otto M, Southwick S. (2006) Combining pharmacotherapy with cognitive behavioral therapy: traditional and new approaches. J Trauma Stress 19: 571–581 [DOI] [PubMed] [Google Scholar]
- de Jong JT, Komproe IH, Van Ommeren M. (2003) Common mental disorders in post-conflict settings. Lancet 36: 2128–2130 [DOI] [PubMed] [Google Scholar]
- Derogatis LR. (1994) SCL-90-R: Administration, Scoring and Procedures Manual. Minneapolis: National Computer Systems, Inc [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. (2007) Oxytocin improves “mind-reading” in humans. Biol Psychiatry 61: 731–733 [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Sweep FC, van der, Steen R, Hermsen R, Donders AR, Touw DJ, et al. (2009) Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci 4: 359–366 [DOI] [PubMed] [Google Scholar]
- Farre M, Abanades S, Roset PN, Peiro AM, Torrens M, O’Mathuna B, et al. (2007) Pharmacological interaction between 3,4-methylenedioxymethamphetamine (ecstasy) and paroxetine: pharmacological effects and pharmacokinetics. J Pharmacol Exp Ther 323: 954–962 [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams J, Benjamin L. (1997) Structured clinical interview for Axis II DSM-IV disorders: Patient Edition (SCID II). Washington, DC: American Psychiatric Press, Inc [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. (1994) Structured clinical interview for Axis I DSM -IV disorders: Patient Edition (SCID-I/P). New York: NYSPI [Google Scholar]
- Foa EB, Hembree EA, Rothbaum BO. (2007) Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences: therapist guide. New York, NY: Oxford University Press [Google Scholar]
- Foa EB, Keane TM, Friedman MJ, Cohen JA. (2009) Effective treatments for PTSD, practice guidelines from the international society for traumatic stress studies, 2nd edn New York, NY: Guilford Press [Google Scholar]
- Foa EB, Kozak MJ. (1986) Emotional Processing of Fear: Exposure and corrective information. Psychol Bull 99: 20–35 [PubMed] [Google Scholar]
- Frayne SM, Seaver MR, Loveland S, Christiansen CL, Spiro A, Parker VA. (2004) Burden of medical illness in women with depression and posttraumatic stress disorder. Arch Intern Med 164: 1306–1312 [DOI] [PubMed] [Google Scholar]
- Freudenmann RW, Oxler F, Bernschneider-Reif S. (2006) The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents. Addiction 101: 1241–1245 [DOI] [PubMed] [Google Scholar]
- Gamma A, Buck A, Berthold T, Liechti ME, Vollenweider FX. (2000) 3,4-Methylenedioxymethamphetamine (MDMA) modulates cortical and limbic brain activity as measured by [H(2)(15)O]-PET in healthy humans. Neuropsychopharmacology 23: 388–395 [DOI] [PubMed] [Google Scholar]
- Greer GR, Tolbert R. (1998) A method of conducting therapeutic sessions with MDMA. J Psychoactive Drugs 30: 371–379 [DOI] [PubMed] [Google Scholar]
- Grob CS, Poland RE, Chang L, Ernst T. (1996) Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: methodological considerations and preliminary observations. Behav Brain Res 73: 103–107 [DOI] [PubMed] [Google Scholar]
- Grof S. (2000) Psychology of the Future. Albany, NY: State University of New York Press [Google Scholar]
- Gronwall DM. (1977) Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills 44: 367–373 [DOI] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. (2002) Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 162: 396–405 [DOI] [PubMed] [Google Scholar]
- Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X. (2003) Do patients drop out prematurely from exposure therapy for PTSD?. J Trauma Stress 16: 555–562 [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. (2004) Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 351: 13–22 [DOI] [PubMed] [Google Scholar]
- Ipser J, Seedat S, Stein DJ. (2006) Pharmacotherapy for post-traumatic stress disorder – a systematic review and meta-analysis. S Afr Med J 96: 1088–1096 [PubMed] [Google Scholar]
- Jaycox LF, Foa EB. (1999) Obstacles in implementing exposure therapy for PTSD: Case discussions and practical solutions. Clin Psychol Psychother 3: 176–184 [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. (2005) Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25: 11489–11493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. (2008) Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit 30: 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. (2005) Oxytocin increases trust in humans. Nature 435: 673–676 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Baumann C, Gamma A, Vollenweider FX. (2000) Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology 22: 513–521 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. (2001) Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 154: 161–168 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. (2000) The serotonin uptake inhibitor citalopram reduces acute cardiovascular and vegetative effects of 3,4-methylenedioxymethamphetamine (‘Ecstasy’) in healthy volunteers. J Psychopharmacol 14: 269–274 [DOI] [PubMed] [Google Scholar]
- Marshall RD, Beebe KL, Oldham M, Zaninelli R. (2001) Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry 145: 1982–1988 [DOI] [PubMed] [Google Scholar]
- Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, et al. (1999) Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 290: 136–145 [PubMed] [Google Scholar]
- Metzner R, Adamson S. (2001) Using MDMA in healing, psychotherapy and spiritual practice. Ecstasy: the complete guide. Rochester, VT: Inner Traditions; 182–207 [Google Scholar]
- Mirushina MN, Boone KB, D’Elia LF. (1999) Handbook of normative data for neuropsychological assessment. New York, NY: Oxford University Press [Google Scholar]
- Ogden PKM, Pain C. (2006) Trauma and the body. New York, NY: W.W. Norton & Company [Google Scholar]
- Pahnke WN, Kurland AA, Unger S, Savage C, Grof S. (1971) The experimental use of psychedelic (LSD) psychotherapy. Int J Clin Pharmacol 4: 446–454 [PubMed] [Google Scholar]
- Passie T, Hartmann U, Schneider U, Emrich HM, Kruger TH. (2005) Ecstasy (MDMA) mimics the post-orgasmic state: impairment of sexual drive and function during acute MDMA-effects may be due to increased prolactin secretion. Med Hypotheses 64: 899–903 [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Kessler RC, Storz S, Wittchen HU. (2000) Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta Psychiatr Scand 101: 46–59 [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. (1998) The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20: 310–319 [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. (2006) Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research – past, present, and future. Biol Psychiatry 60: 376–382 [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Cahill SP, Foa EB, Davidson J, Compton J, Connor KM, et al. (2006) Augmentation of sertraline with prolonged exposure in the treatment of posttraumatic stress disorder. J Trauma Stress 19: 625–638 [DOI] [PubMed] [Google Scholar]
- Sargent W, Slater E, Kelly D. (1972) An Introduction to Physical Methods of Treatment in Psychiatry. Edinburgh and London: Churchill Livingstone [Google Scholar]
- Siegel D. (1999) The developing mind. New York, NY: Guilford Press [Google Scholar]
- Stein DJ, Ipser J, McAnda N. (2009) Pharmacotherapy of posttraumatic stress disorder: a review of meta-analyses and treatment guidelines. CNS Spectr 14: 25–31 [PubMed] [Google Scholar]
- Tancer ME, Johanson CE. (2001) The subjective effects of MDMA and mCPP in moderate MDMA users. Drug Alcohol Depend 65: 97–101 [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. (2007) The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 189: 565–573 [DOI] [PubMed] [Google Scholar]
- Tanielian TL, Jaycox L. the Rand Corporation (2008) Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND [Google Scholar]
- Thabet AA, Vostanis P. (1999) Post-traumatic stress reactions in children of war. J Child Psychol Psychiatry 40: 385–391 [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. (2007) A role for oxytocin and 5-HT (1A) receptors in the prosocial effects of 3,4-methylenedioxymethamphetamine (“ecstasy”). Neuroscience 146: 509–514 [DOI] [PubMed] [Google Scholar]
- Ursano RJ, Bell C, Eth S, Friedman M, Norwood A, Pfefferbaum B, et al. (2004) Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry 161: 3–31 [PubMed] [Google Scholar]
- Vollenweider FX, Gamma A, Liechti M, Huber T. (1998) Psychological and cardiovascular effects and short-term sequelae of MDMA (“ecstasy”) in MDMA-naive healthy volunteers. Neuropsychopharmacology 19: 241–251 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. (2001) Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 13: 132–156 [DOI] [PubMed] [Google Scholar]
- Weine SM, Becker DF, McGlashan TH, Laub D, Lazrove S, Vojvoda D, et al. (1995) Psychiatric consequences of “ethnic cleansing”: clinical assessments and trauma testimonies of newly resettled Bosnian refugees. Am J Psychiatry 152: 536–542 [DOI] [PubMed] [Google Scholar]
- Weiss DM. (1997) The Impact of Event Scale-Revised. In: Wilson JP, Keanne TM. (eds) Assessing Psychological Trauma and PTSD. New York: Guilford Press [Google Scholar]
- Wilbarger P, Wilbarger J. (1997) Sensory defensiveness and related social/emotional and neurological problems. Van Nuys, CA: Wilbarger [Google Scholar]
- Wolff K, Tsapakis EM, Winstock AR, Hartley D, Holt D, Forsling ML, et al. (2006) Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. J Psychopharmacol 20: 400–410 [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. (2005) Oxytocin is associated with human trustworthiness. Horm Behav 48: 522–527 [DOI] [PubMed] [Google Scholar]