Abstract
We have examined the effect of continuous perfusion with antifungals on Candida albicans biofilms under conditions of flow, closely mimicking physiological conditions encountered within patients. Biofilms displayed high levels of resistance to fluconazole, and this antifungal exerted minor effects on dispersion levels. Amphotericin B proved effective in reducing viability of cells within the biofilms and dispersion, but only at high concentrations. Under flow conditions, caspofungin exhibited potent activity against biofilms and drastically reduced biofilm dispersion.
TEXT
Candida albicans is known to frequently colonize and develop biofilms on medical implants. One of the most important characteristics of biofilm cells is their high level of resistance to antifungals (4, 7, 10, 17). Also, dispersion of cells from biofilms may be responsible for device-associated candidemia and subsequent disseminated invasive disease, the gravest forms of candidiasis (3, 22). In the laboratory, C. albicans biofilms have been formed both under static conditions and under flow conditions (1, 4, 6, 9, 14, 17, 19–21, 23). However, the actual drug susceptibility assays have invariably been carried out under static conditions, in that preformed biofilms are incubated in a limited amount of medium containing drugs, under nonshaking conditions (1, 4, 17, 20–21). This does not replicate certain scenarios in vivo, where the biofilms may continuously be in contact with body fluids/blood containing inhibitory levels of antifungal drugs. In addition, issues such as duration of action, instability, and saturation may render a drug ineffective upon prolonged static incubation periods (12). Here, to overcome these limitations, we have used a novel in vitro model that allows for the examination of the activity of antifungals against C. albicans biofilms under dynamic conditions of flow and for the assessment of the effect of antifungal drug treatment on biofilm dispersion.
Biofilms of C. albicans strain SC5314 were developed using a simple flow biofilm model system as previously described by us (21). Briefly, biofilms are developed on silicon elastomer strips for 16 h under a controlled flow of fresh yeast nitrogen base (YNB) medium (1 ml/min). For drug susceptibility assays under conditions of flow, biofilms were grown for 16 h in the absence of drug, after which time antifungal agents were simply added to the medium reservoir and biofilms were subjected to a continuous flow of medium containing a given concentration of a single antifungal agent over an additional 24-h period. The different concentrations for the antifungal agents studied were as follows: fluconazole (FLC), 1,024, 256, and 64 μg/ml; amphotericin B (AMB), 32, 16, 1, and 0.25 μg/ml; and caspofungin (CSP), 0.25, 0.125, 0.06, and 0.03 μg/ml. The biofilm inhibitory concentrations were determined using a semiquantitative colorimetric method to measure metabolic activity in cells within antifungal-treated biofilms compared to control (untreated) biofilms grown in parallel (16, 17). Under these conditions, biofilms were found to be completely resistant to all concentrations of FLC, including the highest, 1,024 μg/ml, despite being continuously exposed for 24 h to a constant flow of medium containing fresh FLC. AMB had a more pronounced effect on biofilm inhibition, and continuous exposure to 4 μg/ml of AMB resulted in >50% inhibition of cells within biofilms, while >80% inhibition was detected at 16 μg/ml of AMB. However, these AMB concentrations are generally considered toxic (18). CSP displayed high levels of activity under flow conditions against preformed biofilms, with sessile MIC50 and MIC80 (SMIC50 and SMIC80) values of 0.06 and 0.125 μg/ml, respectively, concentrations that are well within its therapeutic range. Thus, for these three drugs, representative of the three major classes of antifungal agents used in the clinics, results are similar to those observed under static incubation conditions. This is somewhat contrary to our initial expectations that continuous exposure of biofilms to fresh antifungal drugs may perhaps lead to improved antibiofilm activity. In any case, our observations indicate that biofilm resistance may not be a result of limited drug diffusion into the biofilm or the presence of quorum-sensing molecules in the biofilm (as these molecules are likely to be washed out by the continuous flow) or be related to limitations in the amount of the antifungal drug (1, 11, 13, 15).
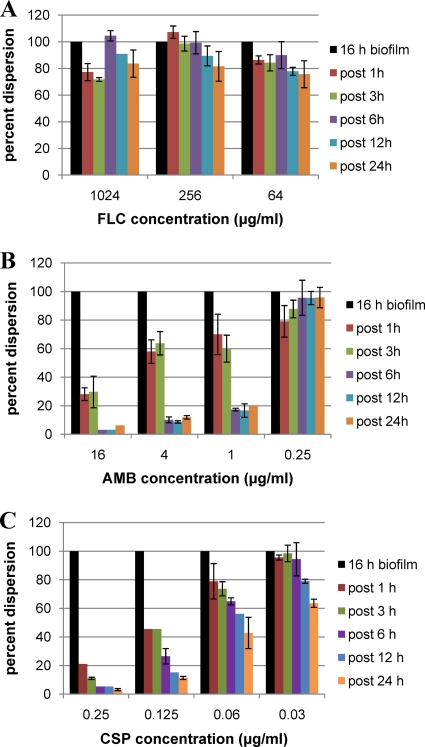
In addition, at various time points after the addition of antifungal agents, cells released from the biofilms in the flowthrough medium were collected, counted, and plated to assess the impact of antifungal drugs on biofilm dispersal and on the viability of dispersed cells. FLC treatment had only a minor effect on dispersion, even at 1,024 μg/ml, the highest concentration tested (Fig. 1). However, approximately 50% of the cells dispersed from biofilms after 12 h of continuous exposure to FLC (1,024 μg/ml) were nonviable, and by 24 h posttreatment, the proportion of the dead cells within the dispersed population increased to 66% (Table 1). At the highest concentration tested (16 μg/ml), AMB decreased the rate of biofilm dispersal by 70% within only 1 h of treatment, and by 6 h, an almost complete abrogation of biofilm dispersal was observed (Fig. 1B). Overall reductions in the numbers of cells released from the biofilms of greater than 80% were also observed after continuous perfusion for 6 to 24 h with AMB at concentrations of 1 and 4 μg/ml, whereas AMB at 0.25 μg/ml resulted in only minimal effects on biofilm dispersion. Treatment at all AMB concentrations tested resulted in decreased cell viability of dispersed cells (Table 1). Regarding CSP treatment, within only 1 h of flow over C. albicans biofilms, treatment with CSP at 0.25 μg/ml reduced biofilm dispersion by 80% and release of cells from the biofilms was virtually abolished by 6 h posttreatment (Fig. 1C). Notably, by 12 and 24 h after treatment with the two highest CSP concentrations, the majority (>80%) of dispersed cells were nonviable, reaching 98% killing after 24 h at 0.25 μg/ml (Table 1). Thus, in addition to their effect on preformed biofilms, we found that the antifungal drugs did significantly impact the process of biofilm dispersion, also with striking differences among the three agents tested.
Fig. 1.
Effect of FLC, AMB, and CSP treatment on the extent of biofilm dispersion. Biofilms developed under flow conditions were further treated under similar flow conditions with different concentrations of FLC, AMB, or CSP. Cells dispersed from untreated biofilms were enumerated at 16 h, and the dispersed cells from biofilms continuously perfused with FLC (A), AMB (B), and CSP (C) were also counted at various time points after addition of the antifungal agent to the top reservoir. Results are expressed as percentages, compared to levels of dispersion from untreated biofilms formed in parallel (in the absence of drug), which were considered 100%. Results are from a single experiment conducted in duplicate for each condition; the experiment was repeated with similar results.
Table 1.
Viability of cells dispersed from biofilms during continuous antifungal drug treatment under flow conditionsa
| Drug | Concn (μg/ml) | Nonviable dispersed cells (%) |
||||
|---|---|---|---|---|---|---|
| 1 h | 3 h | 6 h | 12 h | 24 h | ||
| FLC | 1,024 | 16.5 ± 4.8 | 31.3 ± 3.2 | 48.7 ± 3.8 | 47.0 ± 4.2 | 66.8 ± 2.3 |
| 256 | 9.5 ± 6.2 | 16.2 ± 2.6 | 26.5 ± 2.7 | 27.3 ± 4.9 | 37.5 ± 2.9 | |
| 64 | 5.5 ± 2.2 | 11.6 ± 1.4 | 15 ± 5.6 | 16.9 ± 4 | 14.5 ± 7.7 | |
| AMB | 16 | 13.8 ± 3.9 | 15.7 ± 0.0 | 34.5 ± 0.7 | 50 ± 14.1 | 64.2 ± 6 |
| 4 | 0.0 ± 0.0 | 11.8 ± 3.8 | 14.2 ± 8.0 | 46.6 ± 0.0 | 63.4 ± 10.3 | |
| 1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 3.8 ± 0.0 | 44.0 ± 0.0 | 38.0 ± 8.9 | |
| 0.25 | 10.9 ± 4.6 | 9.0 ± 1.4 | 6.1 ± 1.2 | 46.1 ± 8.8 | 40.8 ± 6.5 | |
| CSP | 0.25 | 23.6 ± 1.8 | 68.5 ± 4.0 | 74.5 ± 7.7 | 92.2 ± 0.3 | 98.2 ± 0.1 |
| 0.125 | 1.3 ± 1.8 | 51.7 ± 2.4 | 40.0 ± 4.0 | 81.2 ± 1.7 | 81.4 ± 1.9 | |
| 0.06 | 6.6 ± 0.0 | 2.6 ± 3.7 | 22.2 ± 4.7 | 34.3 ± 4.4 | 46.7 ± 2.3 | |
| 0.03 | 2.0 ± 1.0 | 1.6 ± 2.3 | 3.8 ± 5.3 | 11.4 ± 7.7 | 17.4 ± 2.2 | |
Results are percentages of nonviable cells and are expressed as averages and standard deviations for a single experiment conducted in duplicate. The experiments were repeated with similar results.
Biofilms also serve as a reservoir from which protected cells can detach and go on to establish infection at distal sites after hematogenous dissemination. We have recently characterized this “biofilm dispersal” phenomenon and described the dispersed cells to have several virulence traits, distinct from planktonic cells (22). Whether the dispersed cells inherit properties of antifungal drug resistance from their multidrug-resistant parent biofilms has not yet been investigated. Thus, we next used a slightly modified CLSI protocol (5) to investigate the in vitro activity of the three antifungal agents against cells dispersed from untreated biofilms in comparison to age-matched planktonic yeast cells grown in parallel under comparable conditions. We found the dispersed cells to be susceptible to therapeutic levels of AMB and CSP with identical MIC values compared to those of planktonic cells; however, dispersed cells were up to 8 times more resistant to FLC (MIC = 4 μg/ml) compared to their planktonic counterparts (MIC = 0.5 μg/ml).
Overall, our results suggest that cells within biofilms display high levels of resistance to most antifungal agents, even when these are continuously perfused over the biofilm for an expanded period of time. However, these antifungal treatments are able to ameliorate, to some extent, the dispersion of cells from biofilms. In particular, and supporting previous data acquired using static incubation models (2, 8, 18, 21), these results indicate a potent activity of CSP against C. albicans biofilms under dynamic conditions of flow, as well as against biofilm dispersion. As these conditions more closely mimic those encountered within the patient, CSP (and perhaps other echinocandin agents) should prove useful in the treatment of biofilm-associated candidiasis and in the prevention of biofilm dispersion, thereby decreasing dissemination to distal sites and subsequent establishment of invasive disease.
Acknowledgments
This work was supported by a grant from Merck & Co., Inc. Additional support was provided by grant number R21AI080930 from the National Institute of Allergy and Infectious Diseases. P.U. is supported by a postdoctoral fellowship, 10POST4280033, from the American Heart Association.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Merck, AHA, or the NIAID/NIH.
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Al-Fattani M. A., Douglas L. J. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55:999–1008 [DOI] [PubMed] [Google Scholar]
- 2. Bachmann S. P., et al. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blankenship J. R., Mitchell A. P. 2006. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9:588–594 [DOI] [PubMed] [Google Scholar]
- 4. Chandra J., et al. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard M27-A3, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Honraet K., Goetghebeur E., Nelis H. J. 2005. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J. Microbiol. Methods 63:287–295 [DOI] [PubMed] [Google Scholar]
- 7. Jabra-Rizk M. A., Falkler W. A., Meiller T. F. 2004. Fungal biofilms and drug resistance. Emerg. Infect. Dis. 10:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhn D. M., George T., Chandra J., Mukherjee P. K., Ghannoum M. A. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mukherjee P. K., Chand D. V., Chandra J., Anderson J. M., Ghannoum M. A. 2009. Shear stress modulates the thickness and architecture of Candida albicans biofilms in a phase-dependent manner. Mycoses 52:440–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mukherjee P. K., Chandra J. 2004. Candida biofilm resistance. Drug Resist. Updat. 7:301–309 [DOI] [PubMed] [Google Scholar]
- 11. Nett J., et al. 2007. Putative role of beta-1,3-glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 51:510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nett J. E., Cain M. T., Crawford K., Andes D. R. 2011. Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by XTT assay. J. Clin. Microbiol. 49:1426–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nett J. E., Crawford K., Marchillo K., Andes D. R. 2010. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob. Agents Chemother. 54:3505–3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nett J. E., Guite K. M., Ringeisen A., Holoyda K. A., Andes D. R. 2008. Reduced biocide susceptibility in Candida albicans biofilms. Antimicrob. Agents Chemother. 52:3411–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perumal P., Mekala S., Chaffin W. L. 2007. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob. Agents Chemother. 51:2454–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pierce C. G., et al. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3:1494–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramage G., Vande Walle K., Wickes B. L., Lopez-Ribot J. L. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramage G., VandeWalle K., Bachmann S. P., Wickes B. L., Lopez-Ribot J. L. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramage G., Vandewalle K., Wickes B. L., Lopez-Ribot J. L. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163–170 [PubMed] [Google Scholar]
- 20. Ramage G., Wickes B. L., Lopez-Ribot J. L. 2008. A seed and feed model for the formation of Candida albicans biofilms under flow conditions using an improved modified Robbins device. Rev. Iberoam. Micol. 25:37–40 [DOI] [PubMed] [Google Scholar]
- 21. Uppuluri P., Chaturvedi A. K., Lopez-Ribot J. L. 2009. Design of a simple model of Candida albicans biofilms formed under conditions of flow: development, architecture, and drug resistance. Mycopathologia 168:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uppuluri P., et al. 2010. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 6:e1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uppuluri P., Dinakaran H., Thomas D. P., Chaturvedi A. K., Lopez-Ribot J. L. 2009. Characteristics of Candida albicans biofilms grown in a synthetic urine medium. J. Clin. Microbiol. 47:4078–4083 [DOI] [PMC free article] [PubMed] [Google Scholar]