Abstract
The objective of this study was to investigate the potential role of ceftaroline, a new broad-spectrum cephalosporin, as a therapeutic option for the treatment of daptomycin-nonsusceptible (DNS) methicillin-resistant Staphylococcus aureus (MRSA) infections. Four clinical DNS MRSA strains, R5717, R5563, R5996 (heteroresistant vancomycin-intermediate S. aureus) and R5995 (vancomycin-intermediate S. aureus) were evaluated in a two-compartment hollow-fiber in vitro pharmacokinetic/pharmacodynamic model at a starting inoculum of 107 CFU/ml for 96 h. Simulated regimens were ceftaroline at 600 mg every 12 h (q12h) (maximum free-drug concentration [fCmax], 15.2 μg/ml; serum half-life [t1/2], 2.3 h), daptomycin at 6 mg/kg q24h (fCmax, 7.9 μg/ml; t1/2, 8 h), and daptomycin at 10 mg/kg q24h (fCmax, 15.2 μg/ml; t1/2, 8 h). Differences in CFU/ml between 24 and 96 h were evaluated by analysis of variance with Tukey's post-hoc test. Bactericidal activity was defined as a ≥3-log10 CFU/ml decrease in the colony count from the initial inoculum. The ceftaroline MIC values were 0.25, 0.5, 0.5, and 0.5 μg/ml, and the daptomycin MIC values were 2, 2, 4, and 4 μg/ml for R5717, R5563, R5996, and R5995, respectively. Ceftaroline displayed sustained bactericidal activity against 3 of the 4 strains at 96 h (R5717, −3.1 log10 CFU/ml; R5563, −2.5 log10 CFU/ml; R5996, −5.77 log10 CFU/ml; R5995, −6.38 log10 CFU/ml). Regrowth occurred during the daptomycin at 6-mg/kg q24h regimen (4 strains) and the daptomycin at 10-mg/kg q24h regimen (3 strains). At 96 h, ceftaroline was significantly more active, resulting in CFU/ml counts lower than those obtained with daptomycin at 6 mg/kg q24h (4 strains, P ≤ 0.008) and daptomycin at 10 mg/kg q24 h (3 strains, P ≤ 0.001). Isolates with increased MIC values for daptomycin (all 4 strains) but not for ceftaroline were recovered. Ceftaroline was effective against the 4 isolates tested and may provide a clinical option for the treatment of DNS MRSA infections.
INTRODUCTION
Daptomycin (DAP)-nonsusceptible (DNS) Staphylococcus aureus is defined as a strain having an MIC of >1 μg/ml (5). In North America, the rate of DNS S. aureus is 0.01 to 0.1%, with no trend of increasing MIC values observed over the past several years (4, 27, 29, 33). DNS S. aureus infections occur mainly in patients with high-inoculum infections requiring long-term treatment such as osteomyelitis, medical devices, septic arthritis, or endocarditis (3, 35). Bactericidal therapies with a favorable safety profile are preferred to treat these types of infections. The recent guidelines from the Infectious Diseases Society of America for the treatment of methicillin-resistant S. aureus (MRSA) list telavancin, linezolid, trimethoprim-sulfamethoxazole, and quinupristin-dalfopristin as potential therapeutic options for the treatment of DNS S. aureus infections (19). Unfortunately, some of these agents and most other available alternative antistaphylococcal antibiotics, including tigecycline, clindamycin, and the tetracyclines, are typically bacteriostatic or may be limited by safety concerns. The optimal therapy for the treatment of infections caused by DNS S. aureus infections is therefore currently unknown (19, 35). Since the area under the concentration-time curve (AUC)/MIC or peak/MIC ratio is the pharmacodynamic parameter that best predicts efficacy, a “high dose” of DAP of 8 to 12 mg/kg/day has been evaluated clinically, showing both efficacy and a favorable safety profile (11, 22, 24). For this reason, high-dose DAP (10 mg/kg/day) is included in the recent MRSA guidelines to treat a variety of infections (19).
Ceftaroline (CPT), the active form of the prodrug CPT fosamil, is a novel broad-spectrum cephalosporin approved by the United States Food and Drug Administration in 2010 for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections. The current FDA-approved breakpoint for CPT against S. aureus (skin infections only) is ≤1 μg/ml (12). Similar to the mechanism of action of other cephalosporins, CPT binds to penicillin-binding proteins (PBPs), leading to inhibition of cell wall synthesis (17, 23, 40). CPT displays activity against many Gram-positive pathogens, including Staphylococcus spp. and Streptococcus spp. (10, 14, 15, 20, 25, 26, 30–32, 34). CPT has a strong affinity for PBP1, -2, and -3 and also maintains affinity for PBP2a, which is present in MRSA and confers resistance to methicillin and most other β-lactams (23, 40). In addition, CPT appears to maintain its susceptibility profile against strains of S. aureus displaying reduced susceptibility to vancomycin and DAP, including vancomycin-intermediate S. aureus (VISA), heteroresistant VISA (hVISA), vancomycin-resistant S. aureus, and DNS S. aureus, as evidenced by MIC50 values of 0.5 μg/ml for DNS S. aureus (31, 34). CPT displays a favorable safety profile similar to those of other cephalosporins, with most adverse effects being mild (37, 41).
The objective of the present study was to investigate the potential of CPT as a therapeutic option for the treatment of DNS MRSA infections by using an in vitro pharmacokinetic/pharmacodynamic (PK/PD) model.
(This study was presented as an oral presentation at the 21st European Congress of Clinical Microbiology and Infectious Diseases/27th International Congress of Chemotherapy, Milan, Italy, 2011.)
MATERIALS AND METHODS
Bacterial strains.
A total of four clinical isolates of DNS MRSA obtained from patients with bacteremia at the Detroit Medical Center were evaluated, i.e., R5717, R5563, R5996 (hVISA), and R5995 (VISA). All isolates were tested by the previously described modified population analysis profile method using Mu3 as a control strain to determine hVISA or VISA status (42).
Antimicrobials and media.
CPT (lot no. FMD-CEF-019 and FMD-CEF-028) was provided by its manufacturer (Forest Laboratories, Inc., New York, NY). CPT solutions were prepared daily in 50% dimethyl sulfoxide and 50% sterile water. DAP was commercially purchased. Mueller-Hinton broth II (MHBII; Difco, Detroit, MI) with 25 mg/liter calcium and 12.5 mg/liter magnesium was used for MIC testing and in vitro PK/PD models with CPT. MHBII supplemented to 50 mg/liter of calcium was used for MIC testing, and in vitro PK/PD models were used to evaluate DAP simulations due to the dependency of DAP on calcium for antimicrobial activity (18). Colony counts were determined using Tryptic Soy Agar (TSA; Difco, Detroit, MI) plates. Development of resistance was evaluated using either Mueller-Hinton agar (MHA; Difco, Detroit, MI) supplemented with 50 mg/liter of calcium or brain heart infusion agar (Difco, Detroit, MI) plates containing DAP and CPT, respectively.
Susceptibility testing.
MIC values were determined by broth microdilution at 106 CFU/ml according to CLSI guidelines (5). All samples were incubated at 35°C for 24 h.
In vitro PK/PD model.
A previously described in vitro PK/PD model consisting of a two-compartment hollow-fiber model chamber (Fiber Cell Systems, Inc., Frederick, MD) with multiple ports for the addition and removal of growth medium, delivery of antibiotics, and collection of samples was used (39). Due to differences in the DAP penetration of various fiber model cartridges, a cellulosic fiber cartridge (C3008; Fiber Cell Systems, Inc., Frederick, MD) was utilized for all experiments with DAP and CPT. The apparatus was prefilled with medium, and antibiotics were administered as boluses over a 96-h time period. Prior to each experiment, several colonies from an overnight growth on TSA were suspended and added to each model to obtain a starting inoculum of ∼107 CFU/ml. Fresh medium was continuously supplied to and removed from the central compartment along with the drug via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL) set to simulate the human half-lives (t1/2) of the antibiotics. The antibiotic regimens evaluated included CPT simulations of 600 mg every 12 h (q12h; maximum free-drug concentration [fCmax], 15.2 μg/ml; t1/2, 2.3 h; protein binding, 20%) and DAP simulations of 6 mg/kg q24h (DAP6; fCmax, 7.9 μg/ml; t1/2, 8 h; protein binding, 93%) and 10 mg/kg q24h (DAP10; fCmax, 13.17 μg/ml; t1/2, 8 h; protein binding, 93%) (2, 13). All models were done in duplicate to ensure reproducibility.
Pharmacodynamic analysis.
Samples from each model were collected at 0, 2, 4, 8, 24, 32, 48, 56, 72, and 96 h and diluted in cold 0.9% saline. Colony counts were determined by spiral plating appropriate dilutions using an automatic spiral plater (WASP; DW Scientific, West Yorkshire, England) to enumerate the CFU/ml. Colonies were counted using a laser colony counter (ProtoCOL; Synoptics Limited, Frederick, MD). If the drug concentration at the anticipated dilution was within 1 tube dilution of the MIC or higher, then vacuum filtration was used to avoid antibiotic carryover. When vacuum filtration was used, samples were washed through a 0.45-μm filter with normal saline to remove the antimicrobial agent and recover the bacteria on the filter, which was then placed on TSA plates. For both methods, plates were incubated at 37°C for 24 h, after which colony counts were performed. These methods have a lower limit of reliable detection of 1 log10 CFU/ml. The total reduction in log10 CFU/ml over 96 h was determined by plotting time-kill curves based on the number of remaining organisms. Bactericidal activity (99.9% killing) was defined as a ≥3-log10 CFU/ml decrease in the colony count from the initial inoculum. Bacteriostatic activity was defined as a <3-log10 CFU/ml reduction in the colony count from the initial inoculum, and inactivity was defined as no observed reduction of the initial inoculum. The time required to achieve a 99.9% bacterial load reduction was determined by linear regression or by visual inspection (if r2 was ≤0.95). Antibiotic activity was assessed at every time point.
Pharmacokinetic analysis.
Pharmacokinetic samples were obtained through the injection port of each model at 0.5, 1, 2, 4, 8, 24, 32, 48, 56, 72, and 96 h in duplicate for the verification of target antibiotic concentrations. All samples were stored at −70°C until ready for analysis. CPT and DAP concentrations were determined by bioassay. For CPT, blank 0.25-in. disks were placed on preswabbed (Bacillus subtilis ATCC 6633) agar plates (antibiotic medium number 11) and spotted with 10 μl of the standards (2.5, 10, and 40 μg/ml) or samples. For DAP, 0.25-in. holes were punched in preswabbed (Micrococcus luteus ATCC 9341) agar plates (antibiotic medium number 5) and filled with 50 μl of the standards (2.5, 7.5, and 15 μg/ml) or samples. Each standard and sample was tested in duplicate. Plates were incubated for 18 to 24 h at 37°C, at which time the zone sizes were measured using a protocol reader (Protocol; Microbiology International, Frederick, MD). The t1/2, fCmax, and fCmin of CPT and DAP were determined from the concentration-versus-time plots. The time above the MIC was calculated using first-order elimination concepts. The area under the concentration-time curve (AUC) and the subsequent AUC/MIC ratio were determined by the trapezoidal method utilizing PK Analyst Software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
Resistance.
Development of resistance was evaluated at multiple time points throughout the simulation at 24, 48, 72, and 96 h. A sample (100 μl) from each time point was plated on MHA or brain heart infusion plates supplemented with 3 × MIC DAP or CPT to assess the development of resistance. Plates were then examined for growth after 24 and 48 h of incubation at 35°C.
Statistical analysis.
Changes in the number of CFU/ml at 24, 48, 56, 72, and 96 h were compared by two-way analysis of variance with Tukey's post-hoc test. A P value of ≤0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistical Software (release 19.0; SPSS, Inc., Chicago, IL).
RESULTS
The MIC results for the isolates tested are summarized in Table 1. One isolate, R5717, displayed a CPT MIC value of 0.25 μg/ml, while the other three isolates displayed a MIC value of 0.5 μg/ml. The first two isolates, R5717 and R5563, were confirmed to be non-hVISA and non-VISA by population analysis and vancomycin susceptibility testing and displayed a DAP MIC value of 2 μg/ml. Isolate R5995, a VISA strain (ratio with Mu3, 1.23), and R5996, an hVISA strain (ratio with Mu3, 1.15), both displayed a DAP MIC value of 4 μg/ml.
Table 1.
MICs of tested isolates and recovered mutantsa
| Isolate and populationa | MIC (μg/ml) |
||
|---|---|---|---|
| CPTb | DAP6 | DAP10 | |
| R5717 | 0.25 | 2 | 2 |
| T96 | 0.25 | 8 | 4–8 |
| T96 M | NAc | 8 | 8 |
| R5563 | 0.5 | 2 | 2 |
| T96 | 0.5 | 8 | 4 |
| T96 M | NA | 8 | NA |
| R5995 (VISA) | 0.5 | 4 | 4 |
| T96 | 0.5 | 4 | 4 |
| T96 M | NA | 16 | NA |
| R5996 (hVISA) | 0.5 | 4 | 4 |
| T96 | 0.5 | 8–16 | 4–8 |
| T96 M | NA | 8–16 | 16 |
T96, overall bacterial population at 96 h; T96 M, mutant bacterial population at 96 h.
CPT was used at 600 mg q12h.
NA, no resistant mutants recovered.
The observed PK parameter values were within 12% of the target values for both drugs. For CPT, the observed fCmax values were 13.77 to 15 μg/ml (goal, 15.2 μg/ml) and the observed t1/2 values were 2.25 to 2.47 h (goal, 2.3 h). The free time above the MIC (fT>MIC) was maintained for 100% of the 12-h dosing interval for R5717 and 95% (11.4 h) for the other three isolates. The observed fCmax and t1/2 values were 8.65 to 8.84 μg/ml and 7.2 to 8.8 h for DAP6 (goal fCmax, 7.9 μg/ml; t1/2, 8 h). The observed fAUC for DAP6 was approximately 108.7 μg-h/ml, corresponding to fAUC/MIC ratios of 54 and 27 for isolates with DAP MIC values of 2 and 4 μg/ml, respectively. DAP10 (goal fCmax, 13.17 μg/ml; t1/2, 8 h) produced values of 13.59 μg/ml and 7.62 h for fCmax and t1/2, respectively. The observed fAUC for DAP10 was approximately 149.4 μg-h/ml, corresponding to fAUC/MIC ratios of 75 and 37 for isolates with DAP MIC values of 2 and 4 μg/ml, respectively.
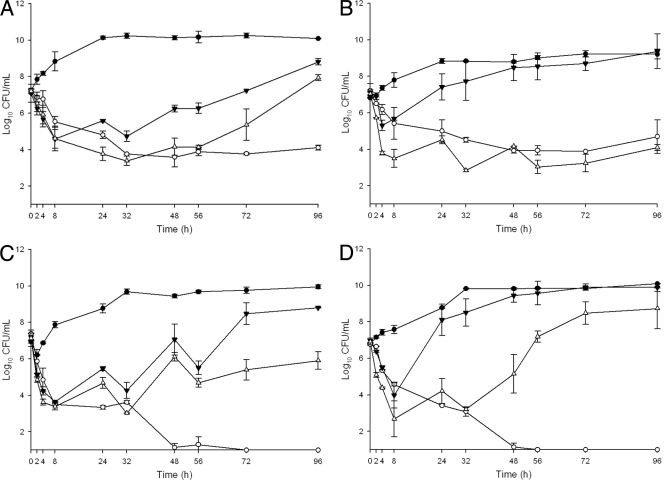
The quantitative changes in log10 CFU/ml are displayed in Fig. 1 A to D. CPT displayed sustained bactericidal activity against 3 of the 4 strains, with overall decreases of 3.1 log10 CFU/ml (R5717), 2.5 log10 CFU/ml (R5563), 6.38 log10 CFU/ml (R5995), and 5.77 log10 CFU/ml (R5996) at 96 h. No mutants of any of the strains with elevated CPT MIC values were recovered, and the overall population MIC value did not change at 96 h (Table 1). The DAP6 regimen produced initial bactericidal activity against R5995 and R5996 and reductions in cell titers of 1.8 and 2.5 log10 for R5563 and R5717, respectively; however, this activity was not sustained and substantial regrowth of all four strains occurred. Under exposure to the DAP6 simulated regimen, the MIC values of the overall population increased from 2 to 8 μg/ml for R5717 and R5563 and from 4 to 8 to 16 μg/ml for R5996. Mutants of R5717/R5563 and R5995/R5996 displaying increased DAP MIC values of 8 and 16 μg/ml, respectively, were recovered. At 96 h, reductions in viable counts were significantly greater with CPT than DAP6 for all four strains (P ≤ 0.008).
Fig. 1.
Activities of CPT, DAP6, and DAP10 against R5717 (A), R5563 (B), R5995 (VISA) (C), and R5996 (hVISA) (D). Closed circles, growth control; open circles, CPT at 600 mg q12h; closed triangles, DAP6; open triangles, DAP10. Error bars are standard deviations of 2 independent experiments.
The simulated regimen of DAP10 displayed a dose-dependent increase in activity compared to DAP6. DAP10 was initially bactericidal against all four isolates. Regrowth of R5996 and R5717 occurred, with changes at 96 h of +1.8 and +0.7 log10 CFU/ml, respectively, versus the baseline. Mutants of both of these strains with DAP MIC values of 16 and 8 μg/ml, respectively, were recovered. At 96 h, decreases in bacterial counts for the DAP10 regimen were 1.2 log10 CFU/ml for R5995 and 2.9 log10 CFU/ml for R5563. Mutants of neither of these strains were recovered during exposure to the DAP10 regimen. At 96 h, CPT resulted in reductions in viable counts of R5717 (P = 0.001), R5995 (P < 0.001), and R5996 (P = 0.001) significantly greater than those obtained with DAP10.
DISCUSSION
Although DNS S. aureus remains rare, when encountered as the cause of a clinical infection, it presents a treatment challenge, as optimal therapy is undefined (35). Recommended treatment options for infections caused by DNS S. aureus that also displays reduced susceptibility to vancomycin are limited to a small number of agents (if the strain is still susceptible) such as linezolid, quinupristin-dalfopristin, trimethoprim-sulfamethoxazole, and telavancin (19). Since DNS infections occur most commonly in patients with complicated deep-seated infections such as osteomyelitis, septic arthritis, and endocarditis, the optimal therapy to treat these infections would be an agent that is both bactericidal and relatively safe for longer treatment durations (3, 35).
In the present study, we examined the activity of CPT against four DNS MRSA strains, including an hVISA and a VISA strain. CPT displayed sustained bactericidal activity against three strains and achieved a sustained decrease in the bacterial counts of the fourth of 2.5 log10 CFU/ml, corresponding to a fT>MIC of 95 to 100%. During this experiment, no CPT-resistant mutants were recovered. In addition, CPT displayed enhanced activity with a greater than 5.5 log10 CFU/ml decrease in the colony counts of the two strains also displaying decreased susceptibility to vancomycin. This enhanced activity may be clinically relevant, because decreased DAP susceptibility has been noted in some VISA strains (9). In addition to its bactericidal activity, CPT appears to have a safety profile similar to that of other cephalosporins based on data from its clinical trials (6–8, 41). The combination of bactericidal activity and a favorable safety profile could make CPT a valuable potential option to treat MRSA infections, including those caused by DNS MRSA. We also observed variable activity of DAP at 6 and 10 mg/kg/day against the 4 DNS MRSA strains tested. DAP displayed a dose-dependent effect against all 4 strains tested. A DAP ƒAUC/MIC ratio of 56 to 222 is required for bactericidal activity (38). The ƒAUC/MICs achieved were in this range for DAP at 10 mg/kg versus R57175 and R5563 at 75, and sustained bactericidal activity was observed for R5563. In addition, exposure to DAP at 6 and 10 mg/kg/day produced mutants against 4 and 2 of the strains tested, respectively. The variable activity of high-dose DAP in vitro against DNS MRSA is consistent with previous reports from our laboratory (28).
Although the exact mechanisms of DNS in S. aureus have yet to be clearly elucidated, several changes in the cell membrane and surface have been associated with these strains. S. aureus strains with elevated DAP MIC values often display increased cell wall thickness, an increased cell membrane surface charge (causing repulsion of the DAP-Ca2+ complex), and changes in membrane fluidity (16, 21, 36). As CPT acts via the PBPs, including PBP2a in S. aureus, these phenotypic changes would not be expected to have a large effect, if any, on the activity of CPT (23, 40). The bactericidal activity and magnitude of the colony count decrease in this study are similar to those of a previously published study with a similar hollow-fiber model examining the activity of CPT against six S. aureus strains that were DAP susceptible, suggesting that the addition of DNS has little effect on CPT activity (39). Of these six strains, two also had decreased susceptibility to vancomycin with an hVISA phenotype. CPT displayed enhanced activity against one hVISA strain, R1629, killing to the limit of detection. For the other hVISA strain, regrowth occurred during exposure to the CPT regimen and this was explained by the higher CPT MIC value of 2 μg/ml and the heterogeneous population analysis profile.
Possible limitations of this study include its short duration, inoculum amounts, and the use of only one DNS strain each displaying the hVISA or VISA phenotype. As stated previously, most infections with DNS S. aureus require antibiotic treatment for long durations. It is therefore possible that the study period of 96 h (4 days) is not sufficient to elicit what the full antibiotic and DNS S. aureus interaction would be under longer exposures. The bacterial inoculum utilized for this study of 7 log10 CFU/ml is moderate compared to some infections typically associated with DNS S. aureus infections such as infective endocarditis. While the CPT MIC value range of strains utilized was 0.25 to 0.5 μg/ml, the CPT fT>MIC would be approximately 80% of the dosing interval for strains with a CPT MIC value of 1 μg/ml, easily exceeding the CPT fT>MIC target of ∼45% for Gram-positive organisms (1). In this study, CPT displayed enhanced activity against the two DNS S. aureus strains also displaying an hVISA or VISA phenotype. Because there was only one strain of each type, the ability to extrapolate these results to other DNS S. aureus strains displaying decreased vancomycin susceptibility is limited.
In conclusion, CPT has the potential to provide a new therapeutic option for treating DNS MRSA infections, as it is a relatively well-tolerated antibiotic which could be more suitable for longer treatment durations while providing bactericidal activity. This is the first study evaluating the activity of CPT against DNS MRSA in an in vitro PK/PD model. CPT activity appeared to be enhanced for DNS S. aureus strains also displaying the hVISA or VISA phenotype. Further study of this enhanced activity and the efficacy of CPT for treating DNS S. aureus infections is warranted.
ACKNOWLEDGMENTS
This study was funded by a research grant from Forest Laboratories. Scientific Therapeutics Information, Inc. (Springfield, NJ), provided editorial assistance with the manuscript. Funding for editorial assistance was provided by Forest Laboratories, Inc.
M.J.R. has received grant support, has served as a consultant, or has participated as a speaker for Astellas, Cerexa, Cubist, Forest, Pfizer, and Theravance. C.V. and M.E.S. have no conflicts of interest to declare.
Footnotes
Published ahead of print on 16 May 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Andes D., Craig W. A. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benvenuto M., Benziger D. P., Yankelev S., Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boucher H. W., Sakoulas G. 2007. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:601–608 [DOI] [PubMed] [Google Scholar]
- 4. Castanheira M., Jones R. N., Sader H. S. 2008. Update of the in vitro activity of daptomycin tested against 6710 Gram-positive cocci isolated in North America (2006). Diagn. Microbiol. Infect. Dis. 61:235–239 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—eighth edition: approved standard M7–A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Corey G. R., et al. 2010. Integrated analysis of CANVAS 1 and 2: phase 3, multicenter, randomized, double-blind studies to evaluate the safety and efficacy of ceftaroline versus vancomycin plus aztreonam in complicated skin and skin-structure infection. Clin. Infect. Dis. 51:641–650 [DOI] [PubMed] [Google Scholar]
- 7. Corey G. R., et al. 2010. CANVAS 1: the first phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl. 4):iv41–iv51 [DOI] [PubMed] [Google Scholar]
- 8. Corrado M. L. 2010. Integrated safety summary of CANVAS 1 and 2 trials: phase III, randomized, double-blind studies evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl. 4):iv67–iv71 [DOI] [PubMed] [Google Scholar]
- 9. Cui L., Tominaga E., Neoh H. M., Hiramatsu K. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fenoll A., et al. 2008. In vitro activity of ceftaroline against Streptococcus pneumoniae isolates exhibiting resistance to penicillin, amoxicillin, and cefotaxime. Antimicrob. Agents Chemother. 52:4209–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Figueroa D. A., et al. 2009. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin. Infect. Dis. 49:177–180 [DOI] [PubMed] [Google Scholar]
- 12. Forest Pharmaceuticals Inc 2010. Teflaro prescribing information. Forest Pharmaceuticals Inc., St. Louis, MO [Google Scholar]
- 13. Ge Y., Redman R., Floren L., Liao S., Wikler M. 2006. The pharmacokinetics (PK) and safety of ceftaroline (PPI-0903) in healthy subjects receiving multiple-dose intravenous (IV) infusions, poster A-1937. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA [Google Scholar]
- 14. Ge Y., Biek D., Talbot G. H., Sahm D. F. 2008. In vitro profiling of ceftaroline against a collection of recent bacterial clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:3398–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones R. N., Fritsche T. R., Sader H. S. 2008. Ceftaroline activity against organisms causing skin and skin-structure infections (SSSI) isolated in US and European medical centers in 2008, poster C1-160. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting, Washington, DC [Google Scholar]
- 16. Jones T., et al. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kosowska-Shick K., McGhee P. L., Appelbaum P. C. 2010. Affinity of ceftaroline and other beta-lactams for penicillin-binding proteins from Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 54:1670–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamp K. C., Rybak M. J., Bailey E. M., Kaatz G. W. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu C., et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 20. McGee L., et al. 2009. In vitro evaluation of the antimicrobial activity of ceftaroline against cephalosporin-resistant isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 53:552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mishra N. N., et al. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohr J. F., Friedrich L. V., Yankelev S., Lamp K. C. 2009. Daptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE). Int. J. Antimicrob. Agents 33:543–548 [DOI] [PubMed] [Google Scholar]
- 23. Moisan H. P. M., Malouin F. 2008. Binding of ceftaroline (CPT) to penicillin-binding proteins (PBPs) of Streptococcus pneumoniae (SPN) and methicillin-resistant Staphylococcu aureus (MRSA), poster C1-183. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting, Washington, DC [Google Scholar]
- 24. Moise P. A., Hershberger E., Amodio-Groton M. I., Lamp K. C. 2009. Safety and clinical outcomes when utilizing high-dose (> or =8 mg/kg) daptomycin therapy. Ann. Pharmacother. 43:1211–1219 [DOI] [PubMed] [Google Scholar]
- 25. Morrissey I., Ge Y., Janes R. 2009. Activity of the new cephalosporin ceftaroline against bacteraemia isolates from patients with community-acquired pneumonia. Int. J. Antimicrob. Agents 33:515–519 [DOI] [PubMed] [Google Scholar]
- 26. Patel S. N., McGeer A., Green K., Pong-Porter S., Low D. E. 2008. Activities of ceftaroline, ceftibiprole, and cethromycin against multi-drug resistant (MDR) Streptococcus pneumoniae isolates from Canadian bacterial surveillance network (CBSN), poster C1-3843. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting, Washington, DC [Google Scholar]
- 27. Pfaller M. A., Sader H. S., Jones R. N. 2007. Evaluation of the in vitro activity of daptomycin against 19615 clinical isolates of Gram-positive cocci collected in North American hospitals (2002-2005). Diagn. Microbiol. Infect. Dis. 57:459–465 [DOI] [PubMed] [Google Scholar]
- 28. Rose W. E., Leonard S. N., Rybak M. J. 2008. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:3061–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sader H. S., et al. 2009. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob. Agents Chemother. 53:4127–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sader H. S., Fritsche T. R., Jones R. N. 2008. Antimicrobial activities of ceftaroline and ME1036 tested against clinical strains of community-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 52:1153–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sader H. S., Fritsche T. R., Kaniga K., Ge Y., Jones R. N. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sader H. S., Jones R. N. 2009. Antimicrobial activity of ceftaroline tested against contemporary (2008) bacteria isolated from community-acquired respiratory tract infections (CARTI), including oxacillin (methicillin)-resistant Staphylococcus aureus. Staphylococcus Symposium, Honolulu, HI [Google Scholar]
- 33. Sader H. S., Jones R. N. 2009. Antimicrobial susceptibility of Gram-positive bacteria isolated from US medical centers: results of the Daptomycin Surveillance Program (2007–2008). Diagn. Microbiol. Infect. Dis. 65:158–162 [DOI] [PubMed] [Google Scholar]
- 34. Saravolatz L., Pawlak J., Johnson L. 2010. In vitro activity of ceftaroline against community-associated methicillin-resistant, vancomycin-intermediate, vancomycin-resistant, and daptomycin-nonsusceptible Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 54:3027–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma M., Riederer K., Chase P., Khatib R. 2008. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 27:433–437 [DOI] [PubMed] [Google Scholar]
- 36. Silverman J. A., Oliver N., Andrew T., Li T. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Talbot G. H., Thye D., Das A., Ge Y. 2007. Phase 2 study of ceftaroline versus standard therapy in treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 51:3612–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torrico M., et al. 2010. Bactericidal activity of daptomycin versus vancomycin in the presence of human albumin against vancomycin-susceptible but tolerant methicillin-resistant Staphylococcus aureus (MRSA) with daptomycin minimum inhibitory concentrations of 1-2microg/mL. Int. J. Antimicrob. Agents 35:131–137 [DOI] [PubMed] [Google Scholar]
- 39. Vidaillac C., Leonard S. N., Rybak M. J. 2009. In vitro activity of ceftaroline against methicillin-resistant Staphylococcus aureus and heterogeneous vancomycin-intermediate S. aureus in a hollow fiber model. Antimicrob. Agents Chemother. 53:4712–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Villegas-Estrada A., Lee M., Hesek D., Vakulenko S. B., Mobashery S. 2008. Co-opting the cell wall in fighting methicillin-resistant Staphylococcus aureus: potent inhibition of PBP 2a by two anti-MRSA beta-lactam antibiotics. J. Am. Chem. Soc. 130:9212–9213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilcox M. H., et al. 2010. CANVAS 2: the second phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl. 4):iv53–iv65 [DOI] [PubMed] [Google Scholar]
- 42. Wootton M., MacGowan A. P., Walsh T. R., Howe R. A. 2007. A multicenter study evaluating the current strategies for isolating Staphylococcus aureus strains with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 45:329–332 [DOI] [PMC free article] [PubMed] [Google Scholar]