Abstract
A comparative genetic analysis of 42 clinical Klebsiella pneumoniae isolates, resistant to two or more antibiotics belonging to the broad-spectrum β-lactam group, sourced from Sydney, Australia, and three South American countries is presented. The study focuses on the genetic contexts of class 1 integrons, mobilizable genetic elements best known for their role in the rapid evolution of antibiotic resistance among Gram-negative pathogens. It was found that the class 1 integrons in this cohort were located in a number of different genetic contexts with clear regional differences. In Sydney, IS26-associated Tn21-like transposons on IncL/M plasmids contribute greatly to the dispersal of integron-associated multiple-drug-resistant (MDR) loci. In contrast, in the South American countries, Tn1696-like transposons on an IncA/C plasmid(s) appeared to be disseminating a characteristic MDR region. A range of mobile genetic elements is clearly being recruited by clinically important mobile class 1 integrons, and these elements appear to be becoming more common with time. This in turn is driving the evolution of complex and laterally mobile MDR units and may further complicate antibiotic therapy.
INTRODUCTION
The rapid emergence of multidrug-resistant phenotypes, including resistance to multiple β-lactam antibiotics conferred by broad-spectrum β-lactamases, within clinically important Gram-negative pathogens is a major global medical challenge (10, 21, 25, 32, 38, 47). The past 2 decades have seen different β-lactamase genes become closely linked to multiple-drug-resistant (MDR) regions. A good example of this is the association between various CTX-M-type genes within Gram-negative bacterial populations (7, 24, 42). MDR infections prolong hospital stays and can leave clinicians with limited or no therapeutic options. The evolution of MDR is relatively fast, as the main driving force is lateral gene transfer (LGT) (22), a process influenced by a wide range of mobile genetic elements. Notable among these elements are class 1 integrons, best known for their contribution to the emergence of antibiotic resistance worldwide (18, 23, 33).
Functionally, integrons include a site-specific recombination system capable of capturing, rearranging, and expressing mobile genes (17, 35). The units of capture are gene cassettes, which represent independent mobilizable elements and remain embedded in the core integron structure at a primary recombination site, attI, linked inevitably to the recombinase gene intI, together forming the core integron. When isolated from clinical microorganisms, gene cassettes predominantly contain antibiotic resistance genes. Integrons are a diverse family of mobile elements, but three classes in particular, classes 1, 2, and 3, are of clinical significance (5). Class 1 integrons are most frequently isolated from MDR pathogens, and the ongoing use of antibiotics has driven their numbers to swell in recent years (35).
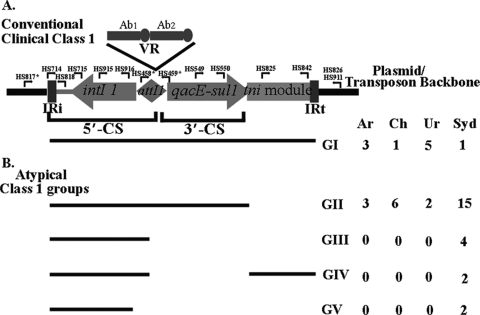
Structurally, clinical class 1 integrons (Fig. 1A) commonly have a physical link to the transposition module of Tn402-like transposons (6). However, in most cases, parts of the transposition genes are deleted. The characteristic Tn402-like inverted repeats (IRi and IRt) demarcate the boundaries of a mobilizable class 1 integron, within which are two conserved segments (CSs), the 5′-CS and the 3′-CS. A variable region (VR) made up of arrays of gene cassettes in tandem and captured by the particular class 1 integron as a result of site-specific recombination (Fig. 1A), is present between the two conserved regions. The events that created the 3′-CS also led to the loss of part of the Tn402 transposition module. Thus, class 1 integrons with defective Tn402-like transposition modules between the 3′-CS and IRt are very commonly reported. Although transposition defective, class 1 integrons can transpose when the essential functions are supplied in trans (6). Tn402-like transposition targets resolution (res) sites, commonly found in many transposons and conjugative plasmids (29).
Fig. 1.
Structural groups of class I integrons identified in this study. A conventional class I integron (A) versus the nonconventional groups (B) is shown. Lines representing the different “atypical” structural groups correspond to the genetic components present in each group and used to define them relative to the conventional structure. Primers used for PCR mapping are indicated in the figure. An asterisk indicates primers that produce different-sized amplicons (27). IR, inverted repeat; Ab1 and Ab2, antibiotic resistance genes in the variable region (VR) of a conventional class 1 integron; 5′-CS, 5′-conserved segment; 3′-CS, 3′-conserved segment. The table indicates the number of isolates representing each structural group from the four different countries. Ar, Argentina; Ch, Chile; Ur, Uruguay; Syd, Sydney, Australia.
Sequence-based analyses continue to recover complex structures of class 1 integrons from clinical and other environments (39, 40). These class 1 integron-associated sequences commonly include clustered non-cassette-associated resistance genes on other mobile elements. Thus, while most class 1 integrons from clinical environments possess both a defective transposition module and a 3′-CS between the IRi and IRt inverted repeats, additional rearrangements mediated by other types of elements have generated a plethora of Tn402-like class 1 integrons that show a high degree of structural diversity and are designated “atypical” clinical class 1 integrons for the purposes of this study. In relation to MDR microorganisms, these variants can serve as indicators of genealogy.
Klebsiella pneumoniae, a member of the Enterobacteriaceae, is a Gram-negative opportunistic pathogen that can survive equally well in the general environment. Clinically, K. pneumoniae is best known for nosocomial infections and is second in the list of pathogens mediating urinary tract infections (UTIs) worldwide (16, 19, 20). An increasing number of reports of K. pneumoniae strains expressing complex MDR phenotypes and associated therapeutic problems has resulted in the inclusion of K. pneumoniae in the ESKAPE list of pathogens by the Infectious Diseases Society of America (4).
We recently identified a complex class 1 integron-associated MDR locus, including a blaCTX-M-2 gene captured by an ISCR1 (insertion sequence common region 1) element, in a UTI-mediating Klebsiella pneumoniae strain, 12836, from Uruguay (27). The integron was located on a Tn5036-Tn21 hybrid transposon and represented a new class 1 integron lineage. Data from the same survey implied the prevalence of this novel transposon within the sampled population consisting of mixed genera belonging to the family Enterobacteriaceae, raising questions regarding its dominance and possible role in the dissemination of the blaCTX-M-2 gene in South American (SA) countries (1–3, 37, 43). Its presence elsewhere in the world, particularly in relation to the dispersal of CTX-M-type genes and other class 1 integron-linked MDR loci, is not known.
Extending on a previous study (27) here, ISCR1-associated class 1 integrons from MDR K. pneumoniae isolates were sourced from three neighboring SA countries, Argentina, Chile, and Uruguay, and compared to isolates from Sydney, Australia. Given the complex structure of the integron in strain 12386 and the fact that the associated transposon was novel, we tested whether similar complex MDR loci are common in K. pneumoniae. The genetic contexts of integron-associated MDR loci within Klebsiella pneumoniae were compared in an effort to identify regional and/or global trends in the dispersal routes. In Sydney, an ISCR1-linked qnrB2 gene within an integron-associated complex drug-resistant locus was commonly found to be disseminated by Tn21-like transposons on an IncL/M plasmid(s). A structurally similar integron, with ISCR1 associated with a CTX-M2 gene and located on Tn1696-like transposons, is being dispersed by IncA/C plasmids in South America.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and antimicrobial testing.
K. pneumoniae isolates positive for the class 1 integrase intI1 and their relevant MDR phenotypes identified by Vitek 2 (bioMérieux) screening are listed in Table 1. Bacteria were routinely grown at 37°C under aerobic conditions in Luria-Bertani (LB) broth. Antibiotic susceptibility was tested with 18 antibiotics, and multiple-drug resistance was defined as resistance to two or more unrelated drugs.
Table 1.
List of strains used in this studyc
| Country and isolate | Source | Plasmid incompatibility group(s) | PFGE profile | Integron structural groupa | Cassette array | Antibiotic profile |
|---|---|---|---|---|---|---|
| intI1- and ISCR1-positive isolates | ||||||
| Argentina | ||||||
| Kb201 | BC | A/C | XX | I | aacA4-blaOXA2-orfD | A, CZ, G, T, CF |
| Kb202 | BC | A/C | XXI | I | aacA4-blaOXA2-orfD | A, CZ, G, T |
| Kb205 | BC | A/C | XXII | I | aacA4-blaOXA2-orfD | A, CZ, G, T, CF |
| Kb300 | BC | A/C | XIII | II | aacA4-blaOXA2-orfD | A, CZ, G, T |
| Kb505 | BC | A/C | XXIV | II | aacA4-aadA1 | A, G, T, CF |
| Kb1001 | BC | A/C | XXV | II | aacA4-blaOXA2-orfD | A, CZ, G, T |
| Chile | ||||||
| Kp4 | PL | A/C | XXVI | II | aadA2 | A, CZ, G, T, ST, CF |
| Kp6 | UC | A/C | XXVI | II | aadA1 | A, CZ, G, T, ST, CF |
| Kp12 | UC | A/C | XXVI | II | aadA2 | A, CZ, G, T, ST, CF |
| V160 | Spt | A/C | XXVII | I | aadA2 | A, CZ, G, T, ST, CF |
| U722 | UC | A/C | XXVII | II | aadA2 | A, CZ, G, T, ST, CF |
| V89 | S | NP | XXVIII | II | aadA2 | A, CZ, G, T, ST, CF |
| Kp296 | Br Sc | A/C | XXIX | II | aacA4-blaOXA2-orfD | A, G, T |
| Uruguay | ||||||
| H-006 | BC | A/C | XV | I | aadA1 | A, CZ, G, T, ST, CF |
| H-007 | BC | A/C | XV | I | aadA1 | A, G, T, ST, CF |
| H-030 | BC | A/C | XV | I | aadA1 | A, CZ, G, ST, CF |
| U-002 | UC | A/C | XVI | I | aadA1 | A, CZ, G, ST, CF |
| U-056 | UC | A/C | XVII | I | dfrA17-aadA5 | A, CZ, G, T, CF |
| U-020 | UC | FII-A | XX | II | dfrA12-orfF-aadA2 | A, CZ, G, T, ST, CF |
| Sydney, Australia | ||||||
| C005968b | HE | L/M, FIIA | I | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T, ST |
| C15123b | HE | L/M, FIIA | I | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T, ST, CF |
| C15126b | HE | L/M, FIIA | I | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T, ST, CF |
| C15130b | HE | L/M, FIIA | I | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T, ST, CF |
| C189886b | Brn SC | L/M, FIIA | I | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T, ST, CF |
| C011712b | CVC-F | FIIA | I | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T, ST, CF |
| C15122b | HE | FIIA | I | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T, ST, CF |
| C15117 | HE | HI2 | II | II | aacA27-ereA2::IS1247-aacA3-ereA2 | A, CZ, G, T, ST, CF |
| C205965 | Br Sc | L/M | IV | II | ND | A, CZ, G, T |
| C106057b | CS | L/M | V | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T |
| C171302b | CVC-S | L/M | VII | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T |
| C169979b | UC | L/M | VII | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T |
| C68761 | UC | NP | XI | I | aadB* | A, ST |
| C131395 | UC | NP | XIV | II | ND | A, CZ, ST |
| intI1-positive but ISCR1-negative isolates from Sydney, Australia | ||||||
| C247823 | UC | FIIA | III | IV | dfrA14 | A, CZ, G, T |
| C188681 | UC | NP | VI | III | dfrA14 | A, CZ, T, ST, CP |
| C220427 | UC | NP | VIII | III | dfrA14 | A, CZ, G, T |
| C204742 | Spt | NP | IX | III | ND | A, G |
| C040830 | UC | L/M,FIIA | X | II | blaIMP4-qacG2-aacA4-catB3 | A, CZ, G, T |
| C71173 | UC | NP | XII | III | ND | A, CZ, G, T, ST, CF |
| TS31 | UC | FIIA | III | IV | ND | A, CZ, G, T, ST |
| TS298 | UC | NP | XIII | V | ND | A, CZ, ST |
| TS299 | UC | NP | XIII | V | ND | A, CZ, ST |
Represented in Fig. 1.
Sydney isolates that were also positive for the ISCR1-associated 3′-CS2.
NP, no products obtained in the assay; ND, not determined; BC, blood culture; UC, urine culture; PL, peritoneal liquid; Spt, sputum; S, surgery; Br Sc, bronchial secretion; HE, hospital environment; CVC-F, central venous catheter, femoral; CVC-S, central venous catheter, subclavian; Brn SC, burn skin culture; CS, chest swab; A, ampicillin; CZ, ceftazidime; G, gentamicin; T, tobramycin; CF, ciprofloxacin; ST, trimethoprim-sulfamethoxazole.
DNA manipulation and sequencing methods.
Genomic DNA was isolated from stationary-phase broth cultures, grown for 18 h, with a Puregene Qiagen yeast and bacterial DNA purification kit from Gentra (Qiagen Group) according to the manufacturer's instruction.
Standard PCRs were performed with MangoMix master mix (Bioline, Australia) according to previously reported methods (27). Primers were used at a final concentration of 0.5 μM per reaction and are listed in Table 2. PCR products were purified by using the Wizard SV gel and PCR cleanup system (Promega, Australia) prior to sequencing. Plasmid incompatibility groups and CTX-M types were identified according to previously reported methods (9, 44) and confirmed by sequencing. Plasmid DNA from large low-copy-number plasmids was purified by using a Qiagen plasmid maxikit according to the manufacturer's protocols (Qiagen).
Table 2.
List of primers used in this study
| Primer | Sequence (5′–3′) | Target genea | Reference |
|---|---|---|---|
| HS463A | CTGGATTTCGATCACGGCACG | intI1 (Fw) | 23 |
| HS464 | ACATGCGTGTAAATCATCGTCG | intI1 (Rv) | 23 |
| HS458 | GTTTGATGTTATGGAGCAGCAACG | 5′-CS attI1 end | 27 |
| HS459 | GCAAAAAGGCAGCAATTATGAGCC | 3′-CS | 27 |
| HS915 | CGTGCCGTGATCGAAATCCAG | intI1 | 27 |
| HS916 | TTCGTGCCTTCATCCGTTTCC | intI1 | 27 |
| HS549 | ACTAAGCTTGCCCCTTCCGC | sul1 | 27 |
| HS550 | CTAGGCATGATCTAACCCTCG | sul1 | 27 |
| HS817b | CCTCCAATTGCCGTTCC | Tn5036-like tnpR genes | 27 |
| HS818 | TCCTGGCGGATTCACTACC | 5′-CS-adjacent IRi | 27 |
| HS819 | GGGCCAGGTCTTGAGTATCG | ISCR1 (Fw) | 27 |
| HS820 | GCTTCGGCCATCACACC | ISCR1 (Rv) | 27 |
| HS825 | TGTTTTCGGAATCGTAGTCGC | Tn402-like tniA gene | 27 |
| HS826 | CTGACCGGCTTGTTCGTTC | Tn21merE gene | 27 |
| HS841 | GAAGGGTTACGCCAGTACCAG | IS26 | 27 |
| HS842 | ATGCTCAATACTCGTGTGCACC | Tn402-like tniA gene | 27 |
| HS856 | CATTCGCCGCTCAATGTTAAC | blaCTX-M-2 gene | 27 |
| HS857 | GAAGGTCTCATCACCCAACG | blaCTX-M-2 gene | 27 |
| HS911 | GCGCGGAATACGTCGAAC | Tn5036-like merE gene | 27 |
| FP | GGATGTGCTGCAAGGCGATTAAGTTGG | pCC1FOS sequencing primers (Fw) | 27 |
| RP | CTCGTATGTTGTGTGGAATTGTGAGC | pCC1FOS sequencing primers (Rv) | 27 |
| HS821 | GTTCGATCCATCACAGAGTCG | ISCR1-oriIS | This study |
| HS822 | AGCTGTCGATTGAAACACGG | Sul1-3′-CS2 | This study |
| HS1001 | GATCCCGCGATTCATCAACA | IS26-r | This study |
| HS1197 | GCTGCTCGCCAGTCGAAAG | qnrB2 (Fw) | This study |
| HS1198 | CGTGCGATGCTGAAAGATGC | qnrB2 (Rv) | This study |
| HS1199 | GGTTGAGGCTGGGTGAAGTA | blaCTX-M-15 (Fw) | This study |
| HS1200 | GGTTGAGGCTGGGTGAAGTA | blaCTX-M-15 (Rv) | This study |
Fw, forward; Rv, reverse.
HS817 in conjunction with HS818 produces a 360-bp fragment in the case of a Tn1696 backbone; a 376-bp product exemplifying the integron insertion point in a Tn5036-like backbone, as described previously (27); and a 700-bp fragment in the case of Tn21 and Tn21-Tn5051 backbones.
Fosmid library construction and screening.
Fosmid libraries were constructed from genomic DNAs of six isolates, U020, C005968, C188681, C220427, C247823, and C68761, by using the CopyControl fosmid library production kit (Epicentre, Madison, WI) according to standardized methods (23, 26). Fosmid DNA from intI1-positive clones was extracted by using a Wizard Plus SV miniprep kit (Promega, Australia), and the terminal ends of the insert DNA were sequenced by using the FP and RP vector primers (Table 2). A single fosmid clone per library was selected on the basis of end sequences and analyzed in detail by sequence walking. Sequences were assembled by using MacVector software and identified by NCBI BLAST analysis.
Conjugation assays.
Conjugation assays for isolates without the qnrB2 gene were set up by mixing mid-log-phase cells of the donor and nalidixic acid-resistant (25 μg/ml) Escherichia coli UB5201 (13) recipient cells at a ratio of 1:1 and incubating the mixed culture at 37°C for 2 h. Transconjugants were selected by plating 150 μl of the mating mixes onto LB plates supplemented with appropriate antibiotics.
Pulsed-field gel electrophoresis conditions.
Pulsed-field gel electrophoresis (PFGE) was performed on XbaI-digested samples, as reported previously (28), with a CHEFDRIII system (Bio-Rad Laboratories, Hercules, CA). Digital XbaI macrorestriction patterns were analyzed by using Fingerprinting II software (Bio-Rad Laboratories, Hercules, CA) and Salmonella enterica serotype Braenderup H9812 as a control. A phylogenetic tree was constructed by the use of the Dice similarity coefficient method (≥75%) and was interpreted according to guidelines reported previously (41). Each pulsotype was assigned a group designation (Table 1).
Nucleotide sequence accession numbers.
Regions of fosmids S1 (strain C005968), Syd12 (C188681), Syd15 (C220427), Syd8 (C247823), Syd23 (C68761), and U020 have been deposited in GenBank under accession numbers HQ419285, HQ419286, HQ419284, HQ419283, HQ419282, and HQ419281, respectively.
RESULTS
Class 1 integrons among MDR K. pneumoniae isolates.
In total, 199 MDR K. pneumoniae isolates were screened for class 1 integrons, made up of 25 isolates from Argentina, 100 from Chile, 15 from Uruguay, and 59 from Sydney. The abundance of class 1 integrons was high among MDR K. pneumoniae isolates in all three SA countries (22 in Argentina, 57 in Chile, and 11 in Uruguay). In comparison, class 1 integrons were less abundant but substantial in Sydney, with 23 intI1-positive isolates. All intI1-positive isolates were also screened for the presence of ISCR1 elements, and it was found that about half of the former also possessed the latter, with specific numbers by country and/or city being 17 in Argentina, 8 in Uruguay, 18 in Chile, and 14 in Sydney. Nineteen randomly selected isolates positive for both intI1 and ISCR1 from SA and 14 analogous isolates from Sydney were selected for a detailed analysis (Table 1). The SA cohort was comprised of seven isolates from Chile and six isolates each from Argentina and Uruguay.
Many studies of clinical class 1 integrons rely on PCR methods using primers that target the conserved regions, including the 3′-CS, flanking the inserted resistance genes, to recover a product. The experimental design selects for the recovery of class 1 cohorts with specific regions being present. In contrast, lineages that possess alterations of the basic structure (“atypical” integrons, as shown in Fig. 1) may provide insights into the evolution of class 1 integrons at global and regional levels. In this study we analyzed strains in a way that would provide detailed structural information for the sample set and not preclude integrons with unconventional structures. As a starting point, we used a PCR mapping method (Fig. 1A) to group integrons into structural types and to identify the prevalence of any particular type within the test MDR population. Structural group II integrons (Fig. 1B), defined here by the absence of a Tn402-like tniA-specific amplicon, were most frequently identified in isolates from all four countries, especially Chile (6 of 7) and Sydney, Australia (13 of 14). There was clonal diversity among isolates even where cassette arrays were identical. This was most notable for the 10 Sydney isolates that possessed the blaIMP4-qacG2-aacA4-catB3 array, where this array was distributed across three different PGFE pulsotypes (pulsotypes I, V, and VII) and harbored different combinations of IncL/M and FIIA plasmids (Table 1).
Genetic contexts of the class 1 integrons.
The Tn402 transposition system targets resolution (res) sites associated with plasmids and transposons (29). Taking advantage of this, the genetic contexts of the class 1 integrons in all strains were initially investigated by using primers that target these sites along with sets of primers (27) targeting IRi and IRt boundaries (Fig. 1A). The res-specific primers were designed to target the transposon backbones observed in the previous Uruguay study (Table 2) (27). These included the res regions for the Tn5036 and Tn21 transposon backbones. Transposon-integron boundaries within the SA cohort could be identified mostly at the IRi end (Table 3) with such primer pairs, but the majority of the Sydney isolates failed to produce an amplicon at either the IRi or IRt junction. At the IRt end this strategy did not work as effectively because group II integrons (Fig. 1 and Table 1) that lack a tniA gene and, hence, have no binding site for HS825 (Fig. 1) mostly dominated the Sydney cohort and were present in a significant number within the SA cohort.
Table 3.
Genetic contexts of the class 1 integrons identified within the MDR K. pneumoniae isolates
| Country and isolate | Transposon backboneb |
Presence of structure beyond the 3′-CS of the integronc |
Integron structural group | ||||
|---|---|---|---|---|---|---|---|
| IRi boundary | IRt boundary | ISCR1 | ISCR1 to 3′-CS2 | CTX-M2 | Tn1000/Tn2*-like | ||
| Argentina | |||||||
| Kb201 | Tn1696 | Tn21 | + | + | + | + | I |
| Kb202 | Tn1696 | Tn21 | + | + | + | + | I |
| Kb205 | Tn1696 | NP | + | + | + | + | I |
| Kb300 | Tn1696 | NP* | + | + | + | + | II |
| Kb505 | Tn21 | NP* | + | + | + | + | II |
| Kb1001 | Tn1696 | NP* | + | + | + | − | II |
| Chile | |||||||
| Kp12 | Tn21 | NP* | + | + | + | − | II |
| V160 | Tn1696 | Tn5036 | + | + | + | + | I |
| U722 | Tn21 | NP* | + | + | + | − | II |
| Kp4 | Tn21 | NP* | + | + | + | − | II |
| Kp296 | Tn1696 | NP* | + | + | + | + | II |
| Kp6 | Tn1696 | Tn21 | + | + | + | − | II |
| V89 | NP | NP* | + | − | − | − | II |
| Uruguay | |||||||
| H006 | Tn5036 | Tn5036 | + | + | + | + | I |
| H007 | Tn5036 | Tn5036 | + | + | + | + | I |
| H030 | Tn5036 | Tn5036 | + | + | + | + | I |
| U002 | Tn5036 | NP | + | + | + | − | I |
| U056 | Tn1696 | Tn21 | + | + | + | + | I |
| U020 | NP | NP* | + | − | − | − | II |
| Sydney, Australiaa | |||||||
| C205965 | Tn5036 | NP* | + | − | − | − | II |
| C131395 | Tn1696 | NP* | + | − | − | − | II |
| TS31 | Tn21 | NP* | − | − | − | − | III |
Only isolates that produced an amplicon with the IRi boundary PCR (27) are included in the list.
NP, no product; NP*, product not expected, as the isolate had a structural group II integron.
+, positive for the relevant genes; −, negative for the relevant genes.
The IRi boundaries could be identified in all but two isolates, V89 and U020, in the SA cohort with HS817-HS818 PCR (Table 3). Three of the six Uruguayan isolates have an integron in a Tn5036 transposon backbone at the same novel location described previously (27) from Uruguay across both the IRi and the IRt ends. Isolate U056 had a hybrid Tn1696-Tn21 backbone across the IRi and IRt ends, respectively. The Tn5036-class 1 integron structure observed in Uruguay and Tn1696 have identical transposition modules (i.e., Tn5036-like) but differ by 16 bases in relation to the insertion point of their respective integrons in the transposon backbone (27). While transposon backbones can be the same, different integron insertion points require independent integron capture events and therefore represent independent lineages (27). The Argentinean isolates and half of the Chilean isolates represented a Tn1696-like lineage at the IRi end, while the IRt ends in isolates that harbored a structural group I integron were commonly of the Tn21 type. The Tn5036 backbone with a class 1 integron insertion point, first observed in Uruguay, was therefore the major dispersal unit of class 1 integrons harboring an ISCR1-linked CTX-M-2 gene in that country, while the second Tn1696-like lineage was the dominant type dispersing CTX-M-2 genes in Argentina and Chile.
Twelve of the 14 Sydney isolates and all but 1 (V89) SA isolate generated plasmid-typing amplicons (Table 1). The IncA/C group of plasmids was dominant in SA generally, and IncL/M was dominant within the Sydney cohort (Table 1). Isolates from Sydney clustered into seven different PFGE pulsotypes and carried either one or both plasmids IncL/M and IncFIIA. Notably, 6 of the 14 Sydney isolates with a structural group II integron were from the general hospital environment (Table 1), with a subset of five isolates, C05968, C15123, C15126, C15130, and C15122, clustering into PFGE pulsotype I, indicating their clonal nature. Interestingly, isolate C15122 was different when plasmid profiles were compared (Table 1), indicating a possible lateral movement of an atypical group II integron within the local population.
The finding that the integrons were located on the plasmids described above was confirmed by conjugation assays using a representative isolate from each country containing the cassette array most prevalent in the respective country (Table 1). Transconjugants from each set were plasmid typed, and the presence of an integron was confirmed by PCR. Isolate C005968 from Sydney harbored a structural group II integron and appeared to have two plasmids belonging to the IncL/M and FIIA types. Pulsed-field gel electrophoresis was used to physically separate the two replicons, and the integron was located on the IncL/M replicon by plasmid-typing PCR (9). Purified C005968 plasmid DNA was also used as a template for sequencing the IRi boundary, which led to the identification of an IS26 element inserted 20 bases from IRi on a Tn21-like backbone. The IRi-IS26 boundary was previously reported for a clinical K. pneumoniae isolate, pJIBE401, from Sydney in 2008 (15) (GenBank accession no. AJ609296.2).
An IS26-based primer, HS1001 (Fig. 2 B), was designed and paired with HS818 to screen the Sydney cohort for this element. Ten isolates, all of which also harbored a structural group II integron and the blaIMP-4-containing cassette array, generated an amplicon (575 bp) consistent with the boundary in isolate C005968. Two isolates, C68761 and C15117, produced different-sized amplicons of 814 bp and 531 bp, respectively. Sequence analysis revealed a Tn21 transposon backbone (GenBank accession no. AF071413) with IS26 inserted at different points. Thus, 12 of 14 Sydney isolates had an IS26-coupled Tn21-like transposon boundary at the IRi end, suggesting a clear regional difference in the genetic contexts of the ISCR1-linked intI1-positive isolates sourced from the two continents.
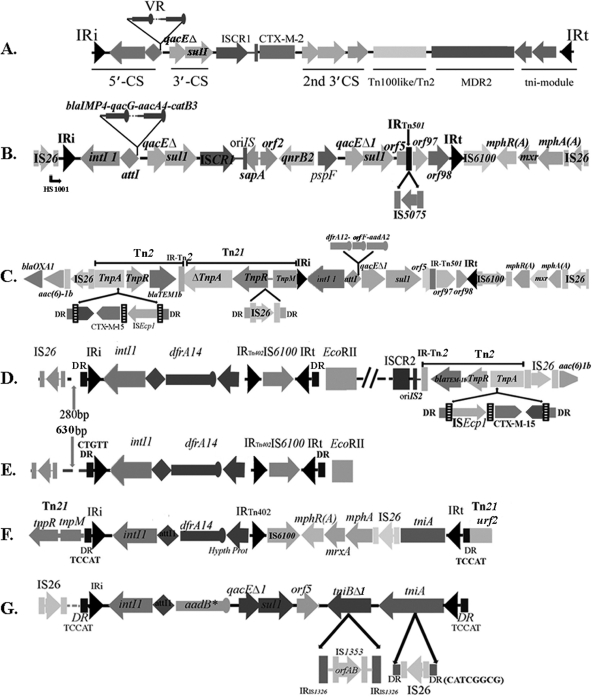
Fig. 2.
Details of the integron-associated MDR regions identified in the course of this study. The filled arrowheads identify inverted repeat sequences. (A) Diagrammatic representation of the complex MDR loci identified in K. pneumoniae strain 12386 (27). (B) Structure identified from fosmid S1 of strain C005968. (C to G) Structures of the integrons studied from isolate U020 (C), fosmid S12 from isolate C188681 (D), fosmid S15 from isolate C220427 (E), fosmid S8 from isolate C247823 (F), and fosmid S23 from isolate C68761 (G). DR, direct repeat.
MDR regions beyond the 3′-CS.
An unusually large and complex MDR locus, consisting of two 3′-CSs and multiple insertion sequence (IS) elements identified between the variable region (VR) and IRt (Fig. 2A), was identified in K. pneumoniae strain 12836 (27). The second 3′-CS was adjacent to an ISCR1 element, the latter of which included a blaCTX-M-2 gene. The presence of analogous regions in isolates recovered in this study was tested with targeted PCRs. Seventeen of the 19 SA isolates (except V89 and U020) tested positive for the second 3′-CS and the CTX-M-2 gene (Table 3), with the CTX-M-2 gene located in the same relative location (Fig. 2A) in all isolates. A Tn1000-Tn2* hybrid module (27) was common within the Uruguayan and Argentinean cohorts. The Sydney isolates tested negative for all of the above-described genetic components, including the CTX-M-2 gene, although a majority of them (10 of 14) (Table 1) possessed an ISCR1 element associated with a second 3′-CS. Sequencing of three representative amplicons generated with primers specific for ISCR1 and 3′-CS2 indicated the presence of a qnrB2 gene, which was subsequently confirmed for all 10 ISCR1/3′-CS2-positive isolates harboring the structural group II integron and an IS26-Tn21 IRi boundary. A fosmid library was made from isolate C005968, a representative of 10 isolates of the Sydney cohort that hosted a similar integron in an identical genetic context. Sequence analysis of an intI1-positive fosmid clone revealed the presence of a complex class 1 integron-associated MDR locus, previously identified in pJIBE401 (GenBank accession no. AJ609296) (15), with the exception of IS5075 inserted at the same location as that seen for IS4321 in pJIBE401 (Fig. 2B).
Structure and context of intI1-positive Sydney isolates without an ISCR1 element.
Nine intI1-positive MDR isolates that tested negative for ISCR1 elements, obtained from two different Sydney hospitals, were additionally analyzed in an effort to identify differences, if any, in the associated drug-resistant loci and genetic contexts of class 1 integrons. The nine isolates clustered into four unusual integron groups, groups II (1 isolate), III (4 isolates), IV (2 isolates), and V (2 isolates) (Fig. 1B), characterized by the absence of one or more PCR products specific for the 3′-CS and/or the tniA gene.
Tn21 transposon-specific genes at the IRi ends of the integron were identified in isolates C247823 and TS31 (Table 3), while isolates C205965 and C131395 tested positive for Tn5036 and Tn1696 boundaries, respectively (30). IS26 was detected at different locations beyond the IRi boundaries (i.e., outside the integron) in isolates C188681, C220427, C204742, and C71173, but they all were in an orientation opposite of that commonly found in the Sydney cohort of ISCR1-positive isolates. Analysis of three IS26-IRi boundary amplicons within the cohort of ISCR1-negative isolates revealed an identical sequence beyond IRi up to the respective IS26 insertion points. The longest amplicon, from isolate C204742, was 99% identical to a truncated resP site found in plasmid pK245 (GenBank accession no. DQ449578.1), reported from Taiwan in 2006 (12). The IS26 insertion points in isolates C188681 and C220427 were novel and have not been reported previously.
The blaCTX-M-15 gene was detected in four Sydney isolates (C188681, C71173, C247823, and TS31). None of the genetic features identified in the study so far could be detected in the ISCR1-negative isolates, including the qnrB2 gene, suggesting distinct identities. Isolates C188681 and C71173 harbored a structural group III integron each and did not generate a plasmid incompatibility group PCR amplicon. This could be explained by the presence of a plasmid of a type not detectable by the plasmid group primers used (9) or by an insertion of the MDR region into the chromosome. Isolates C247823 and TS31 (both CTX-M-15 positive) had a structural group IV integron, an FIIA plasmid(s), and a type III PFGE pattern, although they were isolated from patients in two different Sydney hospitals.
Identification of the integron-transposon boundaries in the additional Sydney isolates.
Fosmid libraries were constructed to characterize integron-associated structures and their context with representative isolates belonging to each atypical integron group within the ISCR1-negative subset of Sydney isolates. Additionally, a library was constructed with isolate U020 from Uruguay, since PCR mapping indicated that it harbors an integron with a structure not usually found in integrons analyzed from that country, including the presence of a blaCTX-M-15 gene, less commonly reported for SA.
Sequence analysis of an intI1-positive fosmid clone selected from the U020 library revealed the presence of a macrolide resistance operon flanked by a copy of IS6100 at one end and IS26 at the other. This structure was located immediately beyond the IRt of the resident structural group II integron (Fig. 2C). This same structure was also present in a similar genetic location in isolate C005968 (Fig. 2B) from Sydney and has several precedents in the literature (15, 36), suggesting that it is part of a Tn21-like transposon (40). In U020, a Tn21-specific transposition module, interrupted by an IS26 element and a Tn2 transposon, was present at the IRi boundary (Fig. 2C). The Tn2 transposon was associated with a blaTEM-1b gene and also hosted a blaCTX-M15 gene linked to an ISEcp1 element. This formed a complex drug-resistant locus beyond the 5′-CS of the integron, containing three β-lactamase genes (blaTEM-1b, blaCTX-M-15, and blaOXA-1) and an aminoglycoside acetyltransferase gene [aac(6)-1b (also known as aacA4)] (Fig. 2C).
Two fosmid libraries were constructed with isolates harboring structural group III integrons from Sydney, C220427 and C188681, with the latter isolate additionally containing a blaCTX-M-15 gene. Sequence analysis of intI1-positive clones from the respective libraries revealed the presence of an identical In4-like (34) class 1 integron (Fig. 2D and E) located at the same point in the respective backbones. Sequencing from both fosmid ends of clone S15 of isolate C220427 revealed sequences identical to an extended-spectrum β-lactamase (ESBL) plasmid, pK245, isolated from K. pneumoniae (12), implying that the whole MDR region of C220427 is inserted into a pK245-type backbone. In contrast, sequencing of the insert ends of fosmid S12 in isolate C188681 revealed sequence identity at each end matching to different plasmids. One end was found in a plasmid from Klebsiella oxytoca, and the other was from K. pneumoniae (8, 11). This finding suggests a homologous rearrangement in one of these plasmids that possibly involved sequences within the MDR region. The blaCTX-M-15 gene in isolate C188681 was located in a context identical to that of isolate U020, including the presence of blaTEM-1b and aac(6)-1b.
The integron in isolate C247823 had a single gene cassette (dfrA14) and was also an In4 derivative with a deleted 3′-CS. Beyond the inserted cassette, changes have collectively resulted in the acquisition of the macrolide resistance operon (Fig. 2F) flanked by IS6100 and IS26, as described above. In4 relatives are most commonly associated with Tn1696-like transposons (34); however, isolate C247823 has a Tn21 backbone. This implies that the integron-linked MDR locus was generated by an independent transposition event for the In4-like integron. The urf2 gene at the IRt end (Fig. 2F) was identical to Tn21. The best match of the assembled fosmid DNA sequence, including that from clone insert ends (data not shown), was to that of an IncF plasmid, pRSB107 (GenBank accession no. AJ851089), reported previously to belong to an unculturable bacterium from a sewage treatment plant in Europe in 2005 (40). pRSB107 harbors multiple drug resistance genes, iron acquisition operons, virulence-associated genes, and mercury resistance genes similar to those of Tn21-like transposons. Our isolate is probably a close relative and further implies that genetic elements common in bacteria in the broader environment are a source of genes via LGT for clinical isolates.
Isolate C68761 was the only MDR isolate recovered from Sydney with a conventional clinical class 1 integron but no ISCR1 element. Sequencing of the intI1-positive clone isolated from the fosmid library indicated the presence of an integron closely related to In2 in Tn21 (GenBank accession no. AF071413). Differences from the prototypical Tn21 were observed in the variable region (VR) and in the 3′-CS (Fig. 2G), where IS1326 had suffered an internal deletion. An additional 17 bases were present at the attI/attC junction of the sole aadB gene cassette of the integron compared to other examples of this cassette. A similar insertion was recently reported for a truncated class 1 integron on an IncN plasmid, pNL194, isolated from K. pneumoniae in Greece (30), although in this case the insertion was followed by a truncated dfrA1 cassette.
DISCUSSION
The most interesting observation from this study is the diversity and novelty of clinical class 1 integrons in the sample set when extensive context analysis is undertaken. Such unusual integrons are most frequently markers of clustered non-cassette-encoded drug resistance genes, forming complex MDR loci. When present between the boundaries of Tn402-like transposon-integron boundaries, such complex drug-resistant units are capable of comovement with this transposition system, as in the case of isolate C247823.
The blaIMP-4-qacG-aacA4-catB3 cassette array provided an example of cassette array analysis alone being a poor indicator of how resistance genes are being spread. This array was first identified in Sydney in a 2003 isolate (15), and we found the same array in our Sydney samples from 2006 and 2009 derived from both patients and the hospital environment. However, the isolates were not clonal by PFGE analysis, indicating a lateral movement of the drug-resistant loci, probably via conjugation, since many were linked to an IncL/M plasmid. This ongoing plasmid dissemination of a metallo-β-lactamase severely compromises infection treatment with carbapenems and extended-spectrum cephalosporins, reinforcing the need for rigorous infection control measures. Interestingly, this complex integron also harbored a qnrB2 gene, encoding low-level resistance to fluoroquinolones. It was recently demonstrated (14) that the qnrB2 promoter is under the control of a LexA-type SOS regulator, which can be activated by the antibiotic itself in an SOS response. Thus, the treatment of a microorganism with a drug to which it has low-level resistance could potentially become a problem in the management of infectious disease treatment. This is particularly true for qnrB2-containing pathogens or commensals because ciprofloxacin is generally the accepted empirical drug of choice for urinary tract infections and gastrointestinal infections in hospitals. Therefore, mobilized qnrB2 genes could lead to the rapid dissemination and selection of resistant pathogens, and it is likely to begin appearing in South America and other regions where it is absent.
The general architectures of the integron-linked MDR regions in the majority of K. pneumoniae isolates with an ISCR1 element were similar, irrespective of the country of isolation. The recruitment of genes by ISCR1 elements appears to be region specific. In these isolates CTX-M-2 predominated in South America, and qnrB2 predominated in Sydney. The linkage of the blaCTX-M-2 gene to ISCR1 was also reported previously in some parts of South America (27), which with data from this study can be extended to Chile. Context analysis of the isolates with ISCR1 indicated a trend toward the predominance of the Tn1696 family of transposons on IncA/C plasmids in SA as the principal disseminative units. Different examples of this backbone, however, have arisen through homologous recombination forming hybrid transposon backbones.
It is known that blaCTX-M gene flow occurs from the community to hospital settings (46). Previous studies in Colombia and Peru demonstrated the role of an IncA/C plasmid in the LGT of blaCTX-M-2 genes in commensal E. coli strains (31), and this same link between an IncA/C plasmid and the ISCR1-blaCTX-M-2 complex is also seen for K. pneumoniae 12836 from Uruguay. Given that this plasmid type is common in the region generally, it is likely that IncA/C plasmids serve as major conduits in the infiltration of blaCTX-M-2 genes from the community to hospitals in much of South America. Further analysis is required to determine the diversity of IncA/C plasmid backbones involved in this process.
While IncL/M plasmids were the major vehicles of movement of ISCR1-associated qnrB2 genes in Sydney, associations with IncFIIA plasmids were also observed. Notably, the integron-linked MDR locus in isolate U020, the only SA K. pneumoniae isolate without the second 3′-CS and a CTX-M-15 gene, was located within a Tn21 transposon on an IncFIIA plasmid. The association of qnrB2 with ISCR1 elements on IncL/M and IncFIIA plasmids found here further extends the observation reported in 2008 for a cohort of blaIMP-4-carrying strains where all isolates with the qnrB2 genes from Sydney were located exclusively on an IncL/M plasmid (15) and supports our conclusion of a lateral movement of integron-associated clustered MDR genes within isolates in Sydney.
IS26 appears to be very widespread in all types of MDR isolates in Sydney and is possibly driving the horizontal movement of clustered drug-resistant genes. This raises the possibility of a major role being played by IS26-based composite transposons in the dissemination of MDR regions. Recent reports have also indicated a lateral movement of resistance determinants on composite IS26 transposons in Salmonella isolates (45). Isolates C005968 and U020 indicate the possible role of such events within the Sydney isolates over many years.
Although class 1 integrons have been implicated in the dissemination of resistance genes since their discovery, it is now becoming evident that the genetic contexts in which these integrons are present actually also have an impact on the dispersal of clustered resistance genes. Region-specific transposon and plasmid types clearly influence the spread of antibiotic resistance genes. The complex interplay of many elements in a region-specific manner clearly emphasizes the need for extensive genome-scale sequencing of multiple isolates to have a better understanding of the drug resistance “mobilome” within defined populations. This will be valuable for epidemiological purposes and for managing MDR nosocomial infections more effectively.
ACKNOWLEDGMENTS
This work was supported by grants from the National Health and Medical Research Council of Australia, the Ministry of Education and Culture of Uruguay, the Development Programme of Basic Sciences of Uruguay, ANPCyT grant PICT 0690 to D.C., and grant DIUC 207036032-1.0 of the Universidad de Concepción, Concepción, Chile, to H.B.T.
We thank Nola Hitchick from the Sydney Adventist Hospital, Sydney, Australia, for providing some of the strains used in this study.
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Arduino S. M., Catalano M., Orman B. E., Roy P. H., Centron D. 2003. Molecular epidemiology of orf513-bearing class 1 integrons in multiresistant clinical isolates from Argentinean hospitals. Antimicrob. Agents Chemother. 47:3945–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arduino S. M., et al. 2002. blaCTX-M-2 is located in an unusual class 1 integron (In35) which includes Orf513. Antimicrob. Agents Chemother. 46:2303–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bado I., et al. 2010. Detection of class 1 and 2 integrons, extended-spectrum β-lactamases and qnr alleles in enterobacterial isolates from the digestive tract of intensive care unit inpatients. Int. J. Antimicrob. Agents 36:453–458 [DOI] [PubMed] [Google Scholar]
- 4. Boucher H. W., et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 5. Boucher Y., Labbate M., Koenig J. E., Stokes H. W. 2007. Integrons: mobilizable platforms that promote genetic diversity in bacteria. Trends Microbiol. 15:301–309 [DOI] [PubMed] [Google Scholar]
- 6. Brown H. J., Stokes H. W., Hall R. M. 1996. The integrons In0, In2, and In5 are defective transposon derivatives. J. Bacteriol. 178:4429–4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canton R., Coque T. M. 2006. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 9:466–475 [DOI] [PubMed] [Google Scholar]
- 8. Carattoli A., et al. 2010. Complete nucleotide sequence of the IncN plasmid pKOX105 encoding VIM-1, QnrS1 and SHV-12 proteins in Enterobacteriaceae from Bolzano, Italy compared with IncN plasmids encoding KPC enzymes in the USA. J. Antimicrob. Chemother. 65:2070–2075 [DOI] [PubMed] [Google Scholar]
- 9. Carattoli A., et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 10. Chanawong A., M'Zali F. H., Heritage J., Lulitanond A., Hawkey P. M. 2001. SHV-12, SHV-5, SHV-2a and VEB-1 extended-spectrum β-lactamases in Gram-negative bacteria isolated in a university hospital in Thailand. J. Antimicrob. Chemother. 48:839–852 [DOI] [PubMed] [Google Scholar]
- 11. Chen Y. T., et al. 2004. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene 337:189–198 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y. T., et al. 2006. Complete nucleotide sequence of pK245, a 98-kilobase plasmid conferring quinolone resistance and extended-spectrum-β-lactamase activity in a clinical Klebsiella pneumoniae isolate. Antimicrob. Agents Chemother. 50:3861–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collis C. M., Recchia G. D., Kim M. J., Stokes H. W., Hall R. M. 2001. Efficiency of recombination reactions catalyzed by class 1 integron integrase IntI1. J. Bacteriol. 183:2535–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Da Re S., et al. 2009. The SOS response promotes qnrB quinolone-resistance determinant expression. EMBO Rep. 10:929–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espedido B. A., Partridge S. R., Iredell J. R. 2008. bla(IMP-4) in different genetic contexts in Enterobacteriaceae isolates from Australia. Antimicrob. Agents Chemother. 52:2984–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gruteke P., et al. 2003. Patterns of resistance associated with integrons, the extended-spectrum β-lactamase SHV-5 gene, and a multidrug efflux pump of Klebsiella pneumoniae causing a nosocomial outbreak. J. Clin. Microbiol. 41:1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hall R. M., Collis C. M. 1998. Antibiotic resistance in Gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist. Updat. 1:109–119 [DOI] [PubMed] [Google Scholar]
- 18. Hall R. M., et al. 1999. Mobile gene cassettes and integrons in evolution. Ann. N. Y. Acad. Sci. 870:68–80 [DOI] [PubMed] [Google Scholar]
- 19. Inglis T. J., Kumarasinghe G., Chow C., Liew H. Y. 1994. Multiple antibiotic resistance in Klebsiella spp. and other Enterobacteriaceae isolated in Singapore. Singapore Med. J. 35:602–604 [PubMed] [Google Scholar]
- 20. Ko W. C., et al. 2002. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg. Infect. Dis. 8:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koh T. H., Sng L. H., Wang G. C., Hsu L. Y., Zhao Y. 2007. IMP-4 and OXA β-lactamases in Acinetobacter baumannii from Singapore. J. Antimicrob. Chemother. 59:627–632 [DOI] [PubMed] [Google Scholar]
- 22. Labbate M., Case R. J., Stokes H. W. 2009. The integron/gene cassette system: an active player in bacterial adaptation. Methods Mol. Biol. 532:103–125 [DOI] [PubMed] [Google Scholar]
- 23. Labbate M., Chowdhury P. R., Stokes H. W. 2008. A class 1 integron present in a human commensal has a hybrid transposition module compared to Tn402: evidence of interaction with mobile DNA from natural environments. J. Bacteriol. 190:5318–5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee S. G., et al. 2009. Spread of CTX-M-type extended-spectrum β-lactamases among bloodstream isolates of Escherichia coli and Klebsiella pneumoniae from a Korean hospital. Diagn. Microbiol. Infect. Dis. 63:76–80 [DOI] [PubMed] [Google Scholar]
- 25. Livermore D. M., Hawkey P. M. 2005. CTX-M: changing the face of ESBLs in the UK. J. Antimicrob. Chemother. 56:451–454 [DOI] [PubMed] [Google Scholar]
- 26. Maniatis T., Fritsch E. F., Sambrook J. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 27. Marquez C., et al. 2008. Urinary tract infections in a South American population: dynamic spread of class 1 integrons and multidrug resistance by homologous and site-specific recombination. J. Clin. Microbiol. 46:3417–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matushek M. G., Bonten M. J., Hayden M. K. 1996. Rapid preparation of bacterial DNA for pulsed-field gel electrophoresis. J. Clin. Microbiol. 34:2598–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minakhina S., Kholodii G., Mindlin S., Yurieva O., Nikiforov V. 1999. Tn5053 family transposons are res site hunters sensing plasmidal res sites occupied by cognate resolvases. Mol. Microbiol. 33:1059–1068 [DOI] [PubMed] [Google Scholar]
- 30. Miriagou V., et al. Sequence of pNL194, a 79.3-kilobase IncN plasmid carrying the blaVIM-1 metallo-β-lactamase gene in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:4497–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pallecchi L., et al. 2007. Rapid dissemination and diversity of CTX-M extended-spectrum β-lactamase genes in commensal Escherichia coli isolates from healthy children from low-resource settings in Latin America. Antimicrob. Agents Chemother. 51:2720–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Partridge S. R., Hall R. M. 2005. Evolution of transposons containing blaTEM genes. Antimicrob. Agents Chemother. 49:1267–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Partridge S. R., Hall R. M. 2003. In34, a complex In5 family class 1 integron containing orf513 and dfrA10. Antimicrob. Agents Chemother. 47:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Partridge S. R., Recchia G. D., Stokes H. W., Hall R. M. 2001. Family of class 1 integrons related to In4 from Tn1696. Antimicrob. Agents Chemother. 45:3014–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Partridge S. R., Tsafnat G., Coiera E., Iredell J. R. 2009. Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol. Rev. 33:757–784 [DOI] [PubMed] [Google Scholar]
- 36. Poole T. L., Callaway T. R., Bischoff K. M., Warnes C. E., Nisbet D. J. 2006. Macrolide inactivation gene cluster mphA-mrx-mphR adjacent to a class 1 integron in Aeromonas hydrophila isolated from a diarrhoeic pig in Oklahoma. J. Antimicrob. Chemother. 57:31–38 [DOI] [PubMed] [Google Scholar]
- 37. Power P., Galleni M., Di Conza J., Ayala J. A., Gutkind G. 2005. Description of In116, the first blaCTX-M-2-containing complex class 1 integron found in Morganella morganii isolates from Buenos Aires, Argentina. J. Antimicrob. Chemother. 55:461–465 [DOI] [PubMed] [Google Scholar]
- 38. Psichogiou M., et al. 2008. Ongoing epidemic of blaVIM-1-positive Klebsiella pneumoniae in Athens, Greece: a prospective survey. J. Antimicrob. Chemother. 61:59–63 [DOI] [PubMed] [Google Scholar]
- 39. Sarno R., McGillivary G., Sherratt D. J., Actis L. A., Tolmasky M. E. 2002. Complete nucleotide sequence of Klebsiella pneumoniae multiresistance plasmid pJHCMW1. Antimicrob. Agents Chemother. 46:3422–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szczepanowski R., et al. 2005. The 120 592 bp IncF plasmid pRSB107 isolated from a sewage-treatment plant encodes nine different antibiotic-resistance determinants, two iron-acquisition systems and other putative virulence-associated functions. Microbiology 151:1095–1111 [DOI] [PubMed] [Google Scholar]
- 41. Tenover F. C., et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tzouvelekis L. S., Tzelepi E., Tassios P. T., Legakis N. J. 2000. CTX-M-type β-lactamases: an emerging group of extended-spectrum enzymes. Int. J. Antimicrob. Agents 14:137–142 [DOI] [PubMed] [Google Scholar]
- 43. Vignoli R., et al. 2006. Entorno genetico de CTX-M2 en aislamientos de Klebsiella pneumoniae provenientes de pacientes hospitalizados en Uruguay. Rev. Argent. Microbiol. 38:84–88 [PubMed] [Google Scholar]
- 44. Woodford N., Fagan E. J., Ellington M. J. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases. J. Antimicrob. Chemother. 57:154–155 [DOI] [PubMed] [Google Scholar]
- 45. Yau S., Liu X., Djordjevic S. P., Hall R. M. 2010. RSF1010-like plasmids in Australian Salmonella enterica serovar Typhimurium and origin of their sul2-strA-strB antibiotic resistance gene cluster. Microb. Drug Resist. 16:249–252 [DOI] [PubMed] [Google Scholar]
- 46. Zahar J. R., et al. 2009. Addressing the challenge of extended-spectrum β-lactamases. Curr. Opin. Invest. Drugs 10:172–180 [PubMed] [Google Scholar]
- 47. Zong Z., Partridge S. R., Thomas L., Iredell J. R. 2008. Dominance of blaCTX-M within an Australian extended-spectrum β-lactamase gene pool. Antimicrob. Agents Chemother. 52:4198–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]