Abstract
Previous in vivo studies have reported caspofungin dose escalation to be effective against Candida glabrata with reduced susceptibility. We hypothesized that higher doses of caspofungin would be effective against invasive candidiasis caused by the more virulent species Candida albicans, including isolates resistant to this echinocandin. Immunocompetent mice were inoculated with one of three C. albicans isolates, including one susceptible and two resistant isolates with different FKS1 hot spot 1 point mutations. Mice received daily caspofungin treatment for 7 days and were then followed off therapy for 2 weeks to assess survival. Kidney tissue and blood were collected, and fungal burden and serum (1→3)-β-d-glucan were measured. Significant differences in virulence were observed among the three C. albicans isolates, which translated into differences in responses to caspofungin. The most virulent of the resistant isolates studied (isolate 43001; Fks1p F641S) did not respond to caspofungin doses of up to 10 mg/kg of body weight, as there were no differences in survival (survival range, 0 to 12% with treatment), tissue burden, or (1→3)-β-d-glucan concentration compared to those for untreated controls. Higher doses of caspofungin did improve survival against the second resistant isolate (53264; Fks1p S645P) that demonstrated reduced virulence (5 and 10 mg/kg; 80% survival). In contrast, caspofungin doses as low as 1 mg/kg improved survival (85 to 95%) and reduced tissue burden and (1→3)-β-d-glucan concentration against the susceptible isolate (ATCC 90028). These data suggest that caspofungin dose escalation for invasive candidiasis may not be consistently effective against resistant C. albicans isolates, and this may be associated with the virulence of the strain.
INTRODUCTION
Echinocandins are safe and effective for the treatment of invasive candidiasis. In clinical trials at approved doses, these agents have been shown to have response rates of between 70 and 75% (15, 20, 25, 26). Overall, the toxicity profile of these agents is quite favorable, with reported adverse event rates similar to that of fluconazole and lower than those of amphotericin B formulations (15, 20, 29). In fact, a recent study demonstrated that daily doses of caspofungin of 150 mg, or three times the recommended daily dose of 50 mg, were safe and well tolerated (3). Similarly, high doses of micafungin have also been shown to be relatively well tolerated, with few toxicities in different patient populations, including patients with hematologic malignancies undergoing stem cell transplantation and children (12, 30, 31).
As is common with most antimicrobials, isolates of different fungal species have developed resistance to echinocandins. Case reports of reduced in vitro potency and associated clinical failures for each member of this class against different Candida species have been published previously (9–11, 14, 17, 19, 33). This reduced potency is associated with mutations within the FKS1 gene leading to amino acid changes within the glucan synthase enzyme Fks1p (27). Due to their excellent safety profile, some have considered dose escalation of echinocandins as a potential means to overcome resistance. Our group has previously reported that this strategy with caspofungin may overcome in vitro resistance in Candida glabrata (5, 39). However, it is unknown whether dosage escalation would be effective against the more virulent species Candida albicans. Our objective was to measure the in vivo efficacy of caspofungin dosage escalation in the treatment of invasive candidiasis caused by C. albicans clinical isolates resistant to caspofungin due to defined mutations in FKS1. We hypothesized that this strategy would be effective at improving survival and reducing tissue fungal burden.
(This work was presented in part at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 2010.)
MATERIALS AND METHODS
Isolates and antifungal agents.
Two caspofungin-resistant C. albicans clinical isolates, 53264 and 43001, were obtained from the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio. Candida albicans ATCC 90028 served as the susceptible, wild-type isolate for this study. The isolates were subcultured twice onto Sabouraud dextrose agar prior to in vitro and in vivo studies. For in vivo studies, the isolates were also grown in brain heart infusion broth overnight prior to animal inoculation.
In vitro growth, susceptibility testing, and determination of FKS1 point mutations.
The in vitro growth rates of isolates 53264 and 43001 were determined by measuring the change in optical density at 530 nm with the use of a microplate spectrophotometer over a 24-h period. The isolates were adjusted to an inoculum of 1 × 106 CFU/ml and added in duplicate to RPMI medium plus 0.165 M MOPS (morpholinepropanesulfonic acid) (pH 7.0) or half-strength RPMI medium diluted with sterile distilled water to a final inoculum of 0.5 × 103 to 3 × 103 CFU/ml in a 96-well cell culture plate. The plate was incubated with shaking at 37°C for 24 h, and optical density measurements were recorded every hour. The susceptibility of each isolate to caspofungin was determined using CLSI M27-A3 guidelines (6), and the caspofungin MIC was read as the concentration that resulted in a significant reduction in growth compared to the growth control after 24 h. The point mutations for the two isolates with reduced susceptibility have previously been reported (Table 1) (36).
Table 1.
In vitro susceptibilities and FKS1 point mutations against each of the three Candida albicans isolates studieda
| Isolate | MIC (μg/ml) | FKS1 gene mutation | Amino acid change |
|---|---|---|---|
| ATCC 90028 | 0.125 | None | Wild type |
| 53264 | 4 | T1933C | S645P |
| 43001 | 4 | T1922C | F641S |
MICs were measured after 24 h of incubation as the lowest concentrations of caspofungin that resulted in significant inhibition of growth. FKS1 mutations were determined by dideoxy sequencing as previously described (36).
Murine model of invasive candidiasis.
Immunocompetent outbred ICR mice (Harlan) weighing between 20 and 25 g were used and had access to food and water ad libitum. For all studies, mice were inoculated via the tail vein with a 0.2-ml volume of C. albicans cells. In the initial survival studies, mice were inoculated with each C. albicans isolate with different inocula ranging from 5.0 × 105 to 1.1 × 107 cells/animal. Mice were then followed until day 21 postinoculation to assess survival. Any animal that appeared moribund was humanely euthanized, and death was recorded as occurring the next day. The research staff who evaluated the mice was blinded to the virulence of the isolates, and morbidity was judged using objective criteria specified a priori. In caspofungin treatment studies, all mice were infected intravenously with a 0.2-ml volume with one of the three C. albicans isolates at an inoculum of ∼1 × 106 CFU/mouse on day 0. Treatment with intraperitoneal caspofungin (1, 5, or 10 mg/kg of body weight per day) began 24 h after inoculation and continued through day 7 postinoculation. In fungal burden studies, mice from each treatment group and untreated controls were humanely euthanized on days 1, 5, 6, and 7 postinoculation and blood and kidneys were collected. Kidneys from each animal were homogenized in sterile saline supplemented with antibiotics, and serial dilutions were prepared and plated in duplicate onto Sabouraud dextrose agar. After 24 h of incubation at 37°C, the colonies were counted and the numbers of CFU per gram of tissue were calculated. In survival studies, caspofungin was also continued through day 7, and mice were then followed off therapy until day 21 postinoculation to assess survival. In the initial survival studies that evaluated virulence, each inoculum level for each isolate consisted of ≥10 mice. All treatment studies were conducted in duplicate on separate occasions to ensure the reproducibility of the results. The total number of mice in the survival studies was ≥20 per treatment group, while fungal burden experiments consisted of 6 mice per group at each time point. This animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio, and all animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care (23).
Serum (1→3)-β-d-glucan assessment.
(1→3)-β-d-Glucan concentrations were measured within the serum samples collected from 6 mice per group on days 1, 5, 6, and 7 postinoculation using a commercially available kit (Fungitell; Associates of Cape Cod, East Falmouth, MA). Briefly, 5 μl of each sample was transferred in duplicate to a 96-well cell culture tray and processed according to the manufacturer's instructions. The mean rate of change in optical density at 405 nm for each sample was read over 40 min with a microplate spectrophotometer (Synergy HT; Biotek Instruments, Inc., Winooski, VT), and unknowns were interpolated from a standard curve.
Histopathology.
To assess the extent of fungal burden and damage within the tissues, kidneys separate from those used to assess fungal burden were collected from 2 mice per group on days 5, 6, and 7 postinoculation and placed into 10% buffered formaldehyde. The kidneys were embedded in paraffin wax, sectioned, and stained with Gomori methenamine silver (GMS). Kidney sections were viewed by light microscopy for the presence of infecting organisms.
Statistical analysis.
Survival was plotted by Kaplan-Meier analysis, and differences in median survival time and survival rates among groups were analyzed by the log rank test and Fisher's exact test, respectively. Differences in serum (1→3)-β-d-glucan concentrations and kidney fungal burden (CFU/g) were assessed for significance by analysis of variance with Tukey's posttest for multiple comparisons. A P value of ≤0.05 was considered statistically significant for all comparisons.
RESULTS
In vitro susceptibility and growth rates.
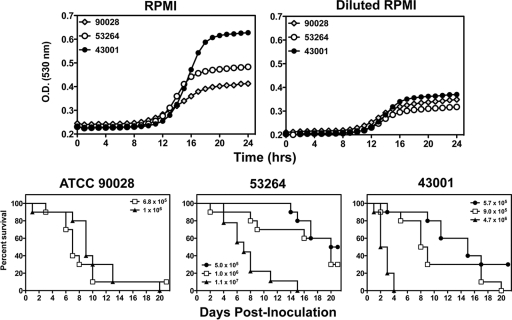
The objective of this study was to assess the in vivo effectiveness of higher doses of caspofungin against C. albicans isolates that are resistant to caspofungin. To achieve this objective, we used two resistant isolates and one susceptible strain, which served as a positive control in our murine model of invasive candidiasis. As shown in Table 1, the two resistant isolates had caspofungin MICs of 4 μg/ml. In addition, the resistant isolates had different point mutations within FKS1, resulting in different amino acid changes in hot spot 1 of Fks1p. We also assessed the in vitro fitness of the isolates by comparing their growth rates over time. As demonstrated in Fig. 1, the growth curves for ATCC 90028, 53264, and 43001 in RPMI medium were nearly superimposable over the first 14 h, after which the growth rate of 43001 was higher than those of 53264 and ATCC 90028. In the reduced-nutrient environment of half-strength RPMI medium, the growth rates were lower but similar among the three isolates. The differences in in vitro fitness between these isolates did suggest that differences in growth in vivo could be observed. However, similarities in the nutrient-limited environment suggested that they would be good choices for the evaluation of the in vivo effectiveness of caspofungin dosage escalation.
Fig. 1.
In vitro growth rates and initial inoculum studies used to assess the virulence of Candida albicans isolates with echinocandin resistance. Isolates ATCC 90028, 53264, and 43001 were inoculated into RPMI medium plus 0.165 M MOPS growth medium and RPMI medium diluted to half-strength with sterile distilled water, and the change in optical density (O.D.) at 530 nm was measured every hour over a 24-h period. In the initial studies to assess virulence, immunocompetent mice were inoculated with isolates ATCC 90028, 53264, and 43001 at different inocula ranging from 5.0 × 105 to 1.1 × 107 cells/animal. Animals were then followed until day 21 to assess survival. Any animal that appeared moribund was humanely euthanized, and death was recorded as occurring the next day. Each inoculum level for each isolate consisted of ≥10 mice.
In vivo virulence.
Significant differences in virulence were observed among the 3 C. albicans isolates. ATCC 90028 was the most virulent isolate at the lowest inoculum level (5.0 × 105 to 6.8 × 105 cells/animal) (Fig. 1). The median survival time of this wild-type isolate (7 days) was significantly shorter than that observed for both 43001 (15 days; P = 0.022) and 53264 (20.5 days; P = 0.001). Significant differences in virulence were also observed between the two caspofungin-resistant isolates. For these two isolates, 43001 was found to be more virulent. Neither resulted in 100% mortality at the lowest inoculum level. However, at the next inoculum level assessed (0.9 × 106 to 1.0 × 106 cells/animal), the median survival time in mice infected with 43001 (8.5 days) was significantly shorter than that observed for 53264 (20 days; P < 0.02). In addition, 30% of animals infected with 53264 survived to the study endpoint compared with none infected with 43001. No differences in survival were found between ATCC 90028 and 43001 at this inoculum level. However, the median survival time for ATCC 90028 (9 days) was significantly shorter than that observed for 53264 (P = 0.022). At the highest inoculum levels tested (4.7 × 106 and 1.1 × 107 cells/animal for 43001 and 53264, respectively), the median survival time in mice infected with 43001 was very short (2.5 days) and no animal survived past day 4 postinoculation. In contrast, the median survival time for isolate 53264 was longer (7 days; P < 0.0001), although all animals did succumb to infection by day 15.
In vivo survival in animals treated with escalating doses of caspofungin.
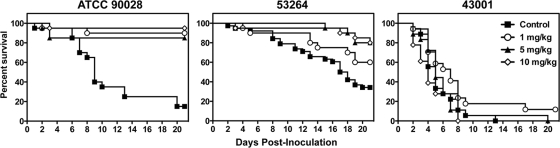
Differences in in vivo virulence between the two resistance isolates appeared to influence the effectiveness of caspofungin therapy. Against the susceptible isolate, ATCC 90028, caspofungin was effective at all doses tested. Each dose prolonged the median survival time (>21 days) and increased the percentage of animals surviving until the study endpoint (range, 85 to 95%) compared to those for untreated controls (9 days and 30%, respectively; P < 0.0001 for all comparisons) (Fig. 2). Against isolate 53264, caspofungin doses of 5 and 10 mg/kg improved the median survival time and overall survival percentage (>21 days and 80% for both doses) compared to those for untreated controls (17.5 days and 34%; P < 0.01) (Fig. 2). Although 60% of mice treated with 1 mg/kg caspofungin survived until day 21, this was not significantly different from the survival time of untreated controls due to the high survival rate observed in this group. The results for isolate 43001 were quite different. No dose of caspofungin resulted in a survival benefit in mice infected with this resistant strain. As shown in Fig. 2, the median survival time ranged between 5 and 7 days for untreated controls and all caspofungin groups. In addition, caspofungin did not significantly improve the percentage of animals surviving until day 21 postinoculation at any dose.
Fig. 2.
Survival in mice inoculated with Candida albicans isolates ATCC 90028, 53264, and 43001. Treatment groups consisted of mice that received intraperitoneal caspofungin (1, 5, or 10 mg/kg per day) or untreated controls, and therapy began 24 h after inoculation and continued until day 7 postinoculation. Mice were then followed off therapy until day 21. Any animal that appeared moribund was humanely euthanized, and death was recorded as occurring the next day. Each group consisted of ≥20 mice.
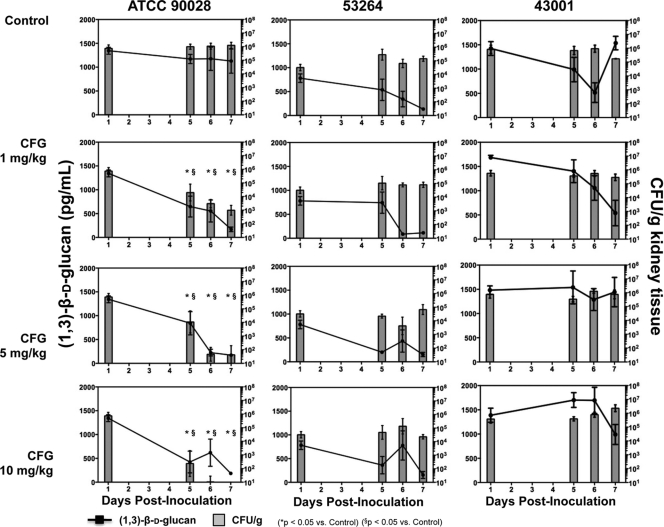
Tissue fungal burden and serum (1→3)-β-d-glucan.
In agreement with the survival data, each caspofungin dosage level significantly reduced both tissue fungal burden and (1→3)-β-d-glucan concentration compared to those for untreated controls infected with the susceptible isolate, ATCC 90028 (Fig. 3). These reductions in CFU counts within the kidneys and serum (1→3)-β-d-glucan concentrations were significant on day 5 postinoculation (means ranging from 2.4 to 4.3 log10 CFU/g and 424 to 841 pg/ml for the caspofungin treatment groups versus 6.0 log10 CFU/g and 1,170 pg/ml for untreated controls, respectively) and were further decreased on days 6 and 7. By day 6, the fungal burden in the 5- and 10-mg/kg dosage groups had fallen by >3 logs and was no longer detectable in mice that were administered 10 mg/kg caspofungin.
Fig. 3.
Changes in serum (1→3)-β-d-glucan concentration (pg/ml) and kidney tissue fungal burden (CFU/g) over time in mice infected intravenously with C. albicans isolates ATCC 90028, 53264, and 43001. Groups consisted of untreated controls and mice that received daily intraperitoneal doses of caspofungin (CFG) at 1, 5, or 10 mg/kg beginning at day 1 postinoculation. Animals were humanely euthanized on days 1, 5, 6, and 7 postinoculation, and sera and kidneys were harvested. Each group consisted of 6 mice per dose per time point. Mean values ± standard errors of the means are presented.
In contrast to the susceptible isolate, caspofungin was ineffective at reducing fungal burden within the kidneys of mice infected with resistant isolate 53264 (Fig. 3). The CFU counts at all dosage levels at all time points tested (means ranging from 3.6 to 5.1 log10 CFU/g) were not significantly different from those observed for untreated controls (4.5 to 5.2 log10 CFU/g). Interestingly, trends toward reductions in serum (1→3)-β-d-glucan concentrations compared to those measured on day 1 postinoculation were observed in all groups, including untreated controls. Against isolate 43001, caspofungin was ineffective at improving either marker of infection. In agreement with the lack of a survival benefit, high caspofungin doses did not lead to reductions in fungal burden with the kidneys or serum (1→3)-β-d-glucan concentrations compared to those for untreated controls.
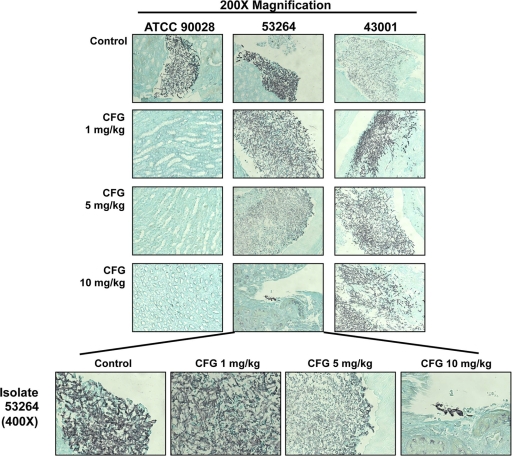
Histopathology.
Overall, the observations made from histopathology analysis supported the fungal burden results. As shown in Fig. 4, each caspofungin dose markedly reduced the amount of infecting organisms within the kidneys of mice inoculated with ATCC 90028. In mice infected with 53264, organisms could still be observed within the kidneys at all caspofungin dose levels. Interestingly, the morphology of the Candida cells appeared to be changed for the two doses that did improve survival (5 and 10 mg/kg). For histopathology analysis, the cells were blunted and somewhat distorted compared to the hyphae observed in untreated controls and mice treated with 1 mg/kg caspofungin. This was especially evident in mice that received 10 mg/kg caspofungin. In contrast, robust hyphae within the kidney tissue were observed in all groups of mice that were infected with 43001, the isolate that was also the most virulent in survival studies. These observations are consistent with the lack of a survival benefit and no reductions in tissue burden for any caspofungin dosage level against this resistant strain.
Fig. 4.
Representative histopathological sections of mouse kidneys. Mice were inoculated intraperitoneally with C. albicans isolates ATCC 90028, 53264, and 43001, and daily treatment with caspofungin (CFG) (1, 5, or 10 mg/kg administered by intraperitoneal injection) began at day 1 postinoculation. Two mice from the untreated control group and each caspofungin dose level were humanely euthanized on days 5, 6, and 7 postinoculation, and the kidneys were processed for histopathology. Sections were viewed by light microscopy at a magnification of ×200 or ×400.
DISCUSSION
Dose escalation of echinocandins has been proposed as a means to improve response rates in patients with invasive fungal infections. The rationale for this approach is based on the concentration-dependent pharmacodynamic activity for members of this class as well as the long half-lives and favorable adverse-effect profiles that are observed clinically. Previous studies with micafungin and caspofungin have demonstrated that doses of these agents greater than those currently approved for clinical use are relatively safe with no significant toxicities reported (3, 12, 30, 31).
Although there are no clinical data demonstrating that this strategy results in improved outcomes, in vivo studies have supported this approach. Taking into account the concentration-dependent activity and long half-lives of these agents, our group reported that high single doses of caspofungin and the investigational agent aminocandin (5 and 30 mg/kg) were effective at improving survival and reducing tissue fungal burden in mice infected with a susceptible C. albicans isolate when administered 7 days prior to inoculation (22). Two in vivo studies have also demonstrated that echinocandin dose escalation may be effective against resistant C. glabrata isolates. We have previously shown that daily caspofungin doses of 5 and 10 mg/kg were effective at reducing CFU counts in mice with invasive candidiasis caused by a C. glabrata isolate despite reduced potency, as demonstrated by elevated MIC values and limited activity as measured by in vitro pharmacodynamic assays (7, 39). Similarly, Brzankalski et al. reported that high daily doses of caspofungin (>1.4 mg/kg) were effective in reducing fungal burden in mice infected with this same C. glabrata isolate (MIC, 4 μg/ml) (5). However, this strategy was ineffective against a second C. glabrata isolate for which the caspofungin MIC was higher (>16 μg/ml).
The objective of the current study was to measure the in vivo efficacy of high caspofungin doses against caspofungin-resistant C. albicans isolates. Our results demonstrate that the effectiveness of this strategy may be isolate dependent. Although higher doses of this echinocandin did improve survival against one of the resistant isolates studied, this was probably influenced by the reduced in vivo fitness and virulence of this strain. Infection with 53264 did not result in 100% mortality unless mice were inoculated with a very high number of cells (>107 cells/animal), and serum (1→3)-β-d-glucan levels trended downward over time in all groups, including untreated controls. The decline in this surrogate marker of infection may reflect a reduced in vivo fitness for this isolate, which may be associated with the reduced virulence observed in the initial inoculum studies. The reasons for this reduced virulence and fitness are unknown; however, these results are consistent with those of other investigators that have reported reduced virulence in echinocandin-resistant C. albicans isolates. Ben-Ami et al. reported reduced in vitro fitness, compensatory increases in cell wall chitin, and an impaired capacity for yeast-to-hypha transformation in echinocandin-resistant C. albicans isolates with homozygous mutations in FKS1 (2). These in vitro findings were associated with reduced virulence in both Drosophila and murine models of invasive candidiasis. From our results, it is unclear whether an increase in cell wall chitin is associated with reduced virulence, as this was not evaluated. However, hyphae were present during histopathology analysis of the kidneys of mice infected with 53264 in both the uninfected control and low-dose caspofungin groups. In contrast, in mice that received higher doses of caspofungin, the Candida cells within the kidneys had a distorted appearance. This observation is consistent with previous reports of changes in cell morphology in Candida species exposed to echinocandins in vitro and may reflect changes in cell wall architecture due to deficiencies in (1→3)-β-d-glucan levels (4, 38), as well as increases in cell wall chitin, which has been reported to occur in Candida species in response to echinocandin exposure (8, 21, 28, 32).
In contrast, no dose of caspofungin resulted in improvements in survival or reductions in fungal burden in mice infected with 43001, the more virulent of the resistant isolates in our study. In addition, no significant reductions in serum (1→3)-β-d-glucan concentration were observed with caspofungin treatment, and concentrations of this surrogate marker remained elevated (>500 pg/ml) in all groups at all time points, which may be reflective of the enhanced in vivo fitness of this isolate. This observation may also have implications for the host response, as unmasking of β-glucans by echinocandins influences innate immunity due to recognition of this cell wall component by dectin-1 on macrophages, neutrophils, and dendritic cells (13, 16, 24, 34, 35). Although it is expected that the combination of caspofungin and the innate immune response would work in synergy against infections caused by Candida species, it is unknown if or how echinocandin resistance would influence β-glucan unmasking, the host immune response, and, potentially, the pathogenesis of invasive candidiasis. Against isolate 53264, it is possible that the immune response in our immunocompetent mice could have limited the in vivo fitness and virulence. However, this does not appear to have occurred against isolate 43001. Although one may speculate that the differences in FKS1 point mutations may have influenced virulence or in vivo fitness, no conclusions can be made from these results due to the limited number of isolates evaluated.
One limitation of the current study is that we did not assess the pharmacokinetics of high-dose caspofungin in our model. However, the doses that were used have been reported by others to result in clinically achievable concentrations (1 mg/kg), and the higher doses of 5 and 10 mg/kg have the potential to result in overall exposures at least two to three times higher than that achieved with the recommended clinical maintenance dose of 50 mg, as measured by area under the concentration-time curve (AUC) values (1, 18, 37). Additional studies are needed to determine whether even higher caspofungin exposures would be effective in overcoming the resistance and virulence observed with isolate 43001.
Overall, the results of this study suggest that caspofungin dose escalation is not consistently effective against C. albicans isolates that are resistant to this echinocandin. This lack of effect may be related to the in vivo fitness and virulence of individual isolates, which cannot be discerned from MIC values. Further work is warranted to understand how different FKS1 mutations may impact virulence and thus the response to antifungal therapy.
ACKNOWLEDGMENTS
This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck & Co., Inc.
The opinions expressed here are those of the authors and do not necessarily represent those of Merck & Co., Inc.
We thank Marcos Olivo, Randall Armstrong, Philip Graves, and Ashley Collazo for their assistance with the experiments.
N.P.W. has received research support from Pfizer, Schering-Plough, Merck, Basilea, and Astellas. T.F.P. has received research support from Basilea, Astellas, Merck, Pfizer, Schering-Plough, and Nektar Therapeutics and has served as a consultant for Basilea, Merck, Pfizer, and Toyama.
Footnotes
Published ahead of print on 18 April 2011.
REFERENCES
- 1. Andes D., et al. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 54:2497–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ben-Ami R., et al. 2009. Mutations in Candida albicans FKS1 conferring resistance are associated with attenuated virulence, abstr. M-446. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA [Google Scholar]
- 3. Betts R. F., et al. 2009. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin. Infect. Dis. 48:1676–1684 [DOI] [PubMed] [Google Scholar]
- 4. Bowman J. C., et al. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brzankalski G. E., et al. 2008. Evaluation of aminocandin and caspofungin against Candida glabrata including isolates with reduced caspofungin susceptibility. J. Antimicrob. Chemother. 62:1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed. Approved standard (M27-A3) Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Cota J., et al. 2006. In vitro pharmacodynamics of anidulafungin and caspofungin against Candida glabrata isolates, including strains with decreased caspofungin susceptibility. Antimicrob. Agents Chemother. 50:3926–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cota J. M., et al. 2008. Increases in SLT2 expression and chitin content are associated with incomplete killing of Candida glabrata by caspofungin. Antimicrob. Agents Chemother. 52:1144–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia-Effron G., Katiyar S. K., Park S., Edlind T. D., Perlin D. S. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 52:2305–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Effron G., Kontoyiannis D. P., Lewis R. E., Perlin D. S. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 52:4181–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hakki M., Staab J. F., Marr K. A. 2006. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 50:2522–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiemenz J., et al. 2005. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob. Agents Chemother. 49:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hohl T. M., Feldmesser M., Perlin D. S., Pamer E. G. 2008. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J. Infect. Dis. 198:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krogh-Madsen M., Arendrup M. C., Heslet L., Knudsen J. D. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938–944 [DOI] [PubMed] [Google Scholar]
- 15. Kuse E. R., et al. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519–1527 [DOI] [PubMed] [Google Scholar]
- 16. Lamaris G. A., et al. 2008. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J. Infect. Dis. 198:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laverdiere M., et al. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705–708 [DOI] [PubMed] [Google Scholar]
- 18. Louie A., et al. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 49:5058–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller C. D., Lomaestro B. W., Park S., Perlin D. S. 2006. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy 26:877–880 [DOI] [PubMed] [Google Scholar]
- 20. Mora-Duarte J., et al. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020–2029 [DOI] [PubMed] [Google Scholar]
- 21. Munro C. A., et al. 2007. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 63:1399–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Najvar L. K., et al. 2008. Therapeutic and prophylactic efficacy of aminocandin (IP960) against disseminated candidiasis in mice. Clin. Microbiol. Infect. 14:595–600 [DOI] [PubMed] [Google Scholar]
- 23. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 24. Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6:67–78 [DOI] [PubMed] [Google Scholar]
- 25. Pappas P. G., et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pappas P. G., et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883–893 [DOI] [PubMed] [Google Scholar]
- 27. Park S., et al. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfaller M., Riley J., Koerner T. 1989. Effects of cilofungin (LY121019) on carbohydrate and sterol composition of Candida albicans. Eur. J. Clin. Microbiol. Infect. Dis. 8:1067–1070 [DOI] [PubMed] [Google Scholar]
- 29. Reboli A. C., et al. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 356:2472–2482 [DOI] [PubMed] [Google Scholar]
- 30. Seibel N. L., et al. 2005. Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob. Agents Chemother. 49:3317–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sirohi B., et al. 2006. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 38:47–51 [DOI] [PubMed] [Google Scholar]
- 32. Stevens D. A., Ichinomiya M., Koshi Y., Horiuchi H. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:3160–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thompson G. R., III, et al. 2008. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob. Agents Chemother. 52:3783–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wheeler R. T., Fink G. R. 2006. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wheeler R. T., Kombe D., Agarwala S. D., Fink G. R. 2008. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 4:e1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiederhold N. P., Grabinski J. L., Garcia-Effron G., Perlin D. S., Lee S. A. 2008. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob. Agents Chemother. 52:4145–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wiederhold N. P., et al. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464–1471 [DOI] [PubMed] [Google Scholar]
- 38. Wiederhold N. P., Lewis R. E. 2003. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 12:1313–1333 [DOI] [PubMed] [Google Scholar]
- 39. Wiederhold N. P., et al. 2007. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 51:1616–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]