Abstract
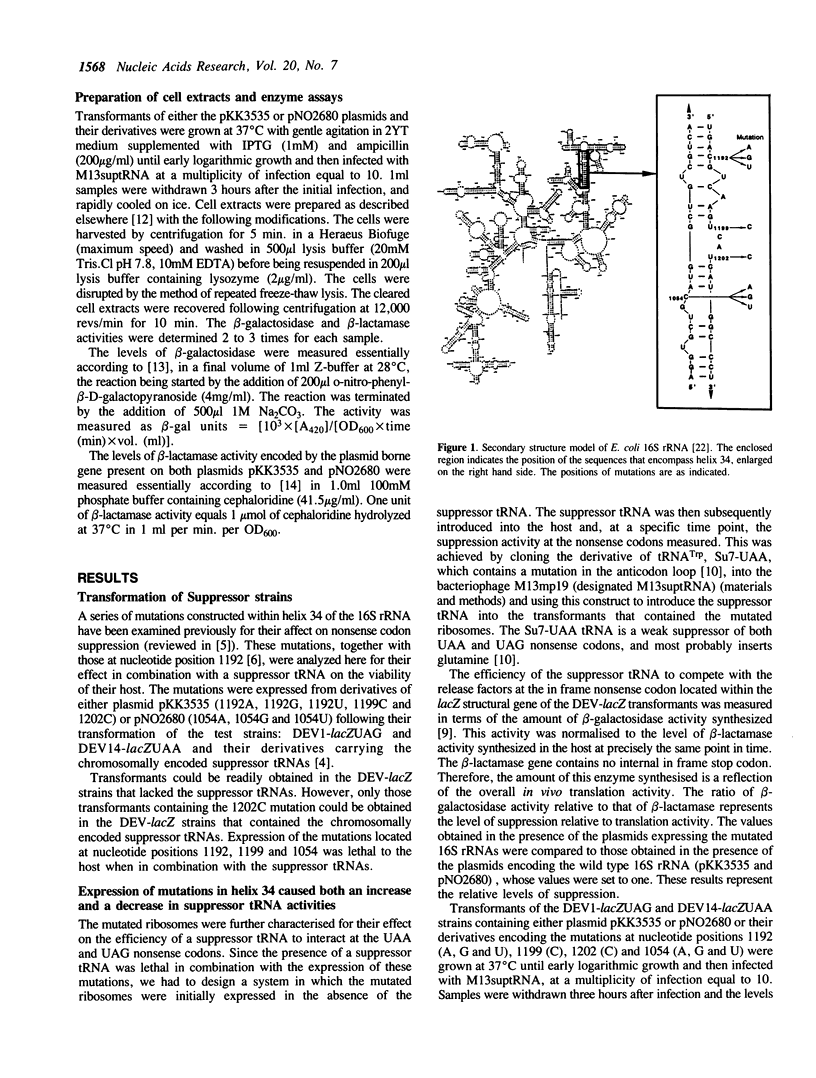
The in vivo expression of mutations constructed within helix 34 of 16S rRNA has been examined together with a nonsense tRNA suppressor for their action at stop codons. The data revealed two novel results: in contrast to previous findings, some of the rRNA mutations affected suppression at UAA and UAG nonsense codons. Secondly, both an increase and a decrease in the efficiency of the suppressor tRNA were induced by the mutations. This is the first report that rRNA mutations decreased the efficiency of a suppressor tRNA. The data are interpreted as there being competition between the two release factors (RF-1 and RF-2) for an overlapping domain and that helix 34 influences this interaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen B., Ross B. M., Squires C., Squires C. L. Transcriptional termination sequence at the end of the Escherichia coli ribosomal RNA G operon: complex terminators and antitermination. Nucleic Acids Res. 1991 Apr 25;19(8):1845–1852. doi: 10.1093/nar/19.8.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin F., Mougel M., Romby P., Eyermann F., Ebel J. P., Ehresmann B., Ehresmann C. Probing the phosphates of the Escherichia coli ribosomal 16S RNA in its naked form, in the 30S subunit, and in the 70S ribosome. Biochemistry. 1989 Jul 11;28(14):5847–5855. doi: 10.1021/bi00440a022. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Stockwell P. A., Trotman C. N., Tate W. P. The signal for the termination of protein synthesis in procaryotes. Nucleic Acids Res. 1990 Apr 25;18(8):2079–2086. doi: 10.1093/nar/18.8.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., Stark M. J., Dahlberg A. E. Regions of DNA involved in the stringent control of plasmid-encoded rRNA in vivo. Cell. 1983 Apr;32(4):1347–1354. doi: 10.1016/0092-8674(83)90315-x. [DOI] [PubMed] [Google Scholar]
- Göringer H. U., Hijazi K. A., Murgola E. J., Dahlberg A. E. Mutations in 16S rRNA that affect UGA (stop codon)-directed translation termination. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6603–6607. doi: 10.1073/pnas.88.15.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänfler A., Kleuvers B., Göringer H. U. The involvement of base 1054 in 16S rRNA for UGA stop codon dependent translational termination. Nucleic Acids Res. 1990 Oct 11;18(19):5625–5632. doi: 10.1093/nar/18.19.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makosky P. C., Dahlberg A. E. Spectinomycin resistance at site 1192 in 16S ribosomal RNA of E. coli: an analysis of three mutants. Biochimie. 1987 Aug;69(8):885–889. doi: 10.1016/0300-9084(87)90216-1. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Moffat J. G., Timms K. M., Trotman C. N., Tate W. P. Interaction of the release factors with the Escherichia coli ribosome: structurally and functionally-important domains. Biochimie. 1991 Jul-Aug;73(7-8):1113–1120. doi: 10.1016/0300-9084(91)90154-s. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Hijazi K. A., Göringer H. U., Dahlberg A. E. Mutant 16S ribosomal RNA: a codon-specific translational suppressor. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4162–4165. doi: 10.1073/pnas.85.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrullo L. A., Gallagher P. J., Elseviers D. The role of 2-methylthio-N6-isopentenyladenosine in readthrough and suppression of nonsense codons in Escherichia coli. Mol Gen Genet. 1983;190(2):289–294. doi: 10.1007/BF00330653. [DOI] [PubMed] [Google Scholar]
- Prescott C. D., Göringer H. U. A single mutation in 16S rRNA that affects mRNA binding and translation-termination. Nucleic Acids Res. 1990 Sep 25;18(18):5381–5386. doi: 10.1093/nar/18.18.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C. D., Kleuvers B., Göringer H. U. A rRNA-mRNA base pairing model for UGA-dependent termination. Biochimie. 1991 Jul-Aug;73(7-8):1121–1129. doi: 10.1016/0300-9084(91)90155-t. [DOI] [PubMed] [Google Scholar]
- Prescott C., Krabben L., Nierhaus K. Ribosomes containing the C1054-deletion mutation in E. coli 16S rRNA act as suppressors at all three nonsense codons. Nucleic Acids Res. 1991 Oct 11;19(19):5281–5283. doi: 10.1093/nar/19.19.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery L. A., Egan J. B., Cline S. W., Yarus M. Defined set of cloned termination suppressors: in vivo activity of isogenetic UAG, UAA, and UGA suppressor tRNAs. J Bacteriol. 1984 Jun;158(3):849–859. doi: 10.1128/jb.158.3.849-859.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. W., O'Callaghan C. H. Beta-lactamase assays. Methods Enzymol. 1975;43:69–85. doi: 10.1016/0076-6879(75)43081-6. [DOI] [PubMed] [Google Scholar]
- Shen Z. H., Fox T. D. Substitution of an invariant nucleotide at the base of the highly conserved '530-loop' of 15S rRNA causes suppression of yeast mitochondrial ochre mutations. Nucleic Acids Res. 1989 Jun 26;17(12):4535–4539. doi: 10.1093/nar/17.12.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate W. P., McCaughan K. K., Kastner B., Trotman C. N., Stoffler-Meilicke M., Stoffler G. Interaction of the release factor with the Escherichia coli ribosome: inhibition of the 30S subunit domain by specific antibodies. Biochem Int. 1988 Jul;17(1):179–186. [PubMed] [Google Scholar]



