LETTER
Daptomycin is active against many Gram-positive pathogens, including multidrug-resistant organisms (3). Elevated daptomycin MICs have been associated with clinical and microbiologic failures in Staphylococcus aureus and Enterococcus infections (7, 8). The current Clinical and Laboratory Standards Institute (CLSI) as well as European Committee on Antimicrobial Susceptibility Testing (EUCAST) susceptibility breakpoint for streptococci is ≤1 μg/ml (5, 6). To date, resistance to daptomycin in Streptococcus spp. has not been reported.
We describe a case of Streptococcus anginosus with resistance to daptomycin associated with breakthrough S. anginosus bacteremia in a 47-year-old male receiving 6 mg/kg of body weight/day of daptomycin. The patient had an extensive past medical history that included T8 paraplegia, chronic kidney disease, diabetes mellitus, hypertension, neurogenic bladder status post-ileal conduit surgery, and a history of recurrent methicillin-resistant S. aureus (MRSA) infections documented at another institution. Since 2006, he has had recurrent right and left hip ostemyelitis with MRSA, Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens isolated from wounds and bone. No Streptococcus spp. were recovered in any previous culture. During these repeated episodes of infection, he received multiple courses of vancomycin with piperacillin-tazobactam that ranged from 6 to 8 weeks in duration. There was no history of previous therapy with daptomycin. In April 2010, the patient developed MRSA bacteremia, which was treated with vancomycin for 4 weeks. He was admitted to our facility in June 2010 with a recent diagnosis of MRSA bacteremia and left trochanteric osteomyelitis that was being treated with daptomycin at 6 mg/kg/day, which had been initiated at an outside hospital. Twenty-one days after the initiation of daptomycin therapy, the patient was admitted to our medical intensive care unit (MICU) in septic shock with 2 of 3 blood cultures positive for S. anginosus. Urine cultures were positive for Escherichia coli and Proteus spp. Broth microdilution susceptibility tests (5) were performed on the S. anginosus isolate and revealed susceptibility to penicillin (MIC = 0.06 μg/ml), cefotaxime (0.25 μg/ml), ceftriaxone (≤0.5 μg/ml), meropenem (≤0.06 μg/ml), levofloxacin (1 μg/ml), and vancomycin (1 μg/ml) as well as susceptibility to erythromycin, clindamycin, and tetracycline; however, the isolate was not susceptible to daptomycin (MIC = 4 μg/ml; see below). No definitive source of the S anginosus bacteremia was identified. However, we hypothesize that the organism most likely originated from a multiloculated abscess observed on a computed tomography (CT) scan of the pelvis and surrounded the right hip and right greater trochanter that was not cultured at the time of the bacteremia. At the time of S. anginosus bacteremia, his serum creatinine was 1.8 mg/dl (baseline, 0.7 to 1.3 mg/dl), resulting in a creatinine clearance, as determined using the Cockroft-Gault equation, of 72 ml/min. Based upon the susceptibility data above, the patient received therapy with ceftriaxone at 2 g once daily and vancomycin at 1 g every 12 h. He received ceftriaxone for 19 days and vancomycin for 46 days. The right hip abscess was drained after 17 days of ceftriaxone and vancomycin, with recovery of only vancomycin-resistant Enterococcus spp. Repeat blood cultures during therapy remained negative; the patient improved clinically and was discharged from the hospital to complete therapy.
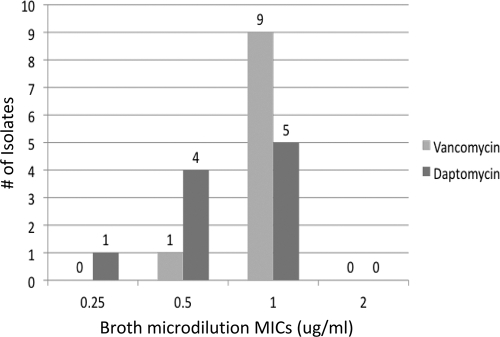
The identification of the S. anginosus isolate was based upon alpha hemolysis, hydrolysis of arginine and esculin, a positive Voges-Proskauer (VP) test result, and lack of fermentation of mannitol and sorbitol (13, 14). The Vitek 2 (bioMérieux, Durham, NC) instrument classified it as Streptococcus intermedius, a member of the S. anginosus group. Susceptibility testing was performed twice by daptomycin Etest; both tests resulted in a daptomycin MIC of 4 μg/ml. Testing by broth microdilution (BMD) also revealed a daptomycin MIC of 4 μg/ml. Further testing performed by a reference laboratory chosen by the manufacturer of daptomycin resulted in a daptomycin MIC, as determined by Etest, of 3 μg/ml and a BMD MIC of 2 μg/ml. Sequence-based identification by an outside reference laboratory (Molecular Epidemiology, Inc., Lake Forest Park, WA) confirmed the isolate as S. anginosus. The isolate retained susceptibility to all other antibiotics tested as described above. Ten previous S. anginosus clinical isolates from our institution were tested for comparison, and all had daptomycin MICs of 0.25 to 1 μg/ml by BMD (Fig. 1).
Fig. 1.
Vancomycin and daptomycint BMD MICs for 10 previous S. anginosus group isolates.
The S. anginosus group (also known as Streptococcus milleri) consists of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (14). S. anginosus is found in the oral cavity and the gastrointestinal (GI) tract as part of the normal flora. Infections from S. anginosus range from minor dental infections to life-threatening invasive infections (13). Bacteremia is usually associated with an identifiable focus of infection, usually associated with the oral cavity or GI tract (9). Complications from S. anginosus bacteremia include endocarditis, myocardial abscess, epidural abscess, meningitis, intra-abdominal abscesses, osteomyelitis, septic arthritis, and, in some patients, aspiration pneumonia, empyema, and mediastinitis, although these complications are more commonly observed with neck and odontogenic infections (2, 4, 9, 10, 11, 12). Our isolate was tested by multiple methods in different laboratories, with all results yielding a nonsusceptible daptomycin MIC of >1 μg/ml (Table 1).
Table 1.
Summary of susceptibility results
| Laboratorya | Method | Result (mg/liter) |
|---|---|---|
| VA | Etest | 4 |
| VA | Etest | 4 |
| UH | Etest | 4 |
| UH | Broth microdilution | 2 |
| UTHSCSA | Broth microdilution | 4 |
| UTHSCSA | Broth microdilution | 4 |
| UTHSCSA | Etest | 4 |
| Outside reference laboratory | Etest | 3 |
| Outside reference laboratory | Broth microdilution | 2 |
VA, South Texas Veterans Affairs Hospital; UH, University Hospital (San Antonio); UTHSCSA, University of Texas Health Science Center at San Antonio.
We invoked the term “breakthrough” bacteremia to describe a bloodstream infection that occurred during treatment with presumably adequate doses of appropriate antibiotics (1). In our patient, the emergence of an S. anginosus isolate with an elevated daptomycin MIC of 4 μg/ml on day 21 of treatment with daptomycin at 6 mg/kg/day for presumed MRSA infection was associated with both clinical and microbiologic failure. Susceptibility testing should be encouraged for isolates that emerge during prolonged daptomycin therapy and are associated with clinical failure.
(These data were presented in part at the 48th Annual Meeting of the Infectious Diseases Society of America, 21 to 24 October 2010 [abstract LB-5].)
Acknowledgments
J.S.L. has served as a consultant for Astellas, Pfizer, and Cubist. J.H.J. has served on advisory boards for BD Microbiology Systems and Rib-X Pharmaceuticals. He has received research support from BD Microbiology Systems, bioMérieux, and Pfizer. All other authors have no conflicts of interest to declare.
Footnotes
Published ahead of print on 18 April 2011.
Contributor Information
Federico Palacio, University of Texas Health Science Center, Department of Medicine, Division of Infectious Diseases, San Antonio, Texas.
James S. Lewis, II, University Health System, Department of Pharmacy, San Antonio, Texas.
Lee Sadkowski, South Texas Veterans Health Care System, San Antonio, Texas.
Kelly Echevarria, South Texas Veterans Health Care System, San Antonio, Texas.
James H. Jorgensen, University of Texas Health Science Center, Departments of Pathology and Medicine, 7703 Floyd Curl Drive, San Antonio, Texas 78229-3900.
REFERENCES
- 1. Anderson E. T., Young L. S., Hewitt W. L. 1976. Simultaneous antibiotic levels in “breakthrough” gram-negative rod bacteremia. Am. J. Med. 61:493–497 [DOI] [PubMed] [Google Scholar]
- 2. Calza L., Manfredi R., Briganti E., Attard L., Chiodo F. 2001. Iliac osteomyelitis and gluteal muscle abscess caused by Streptococcus intermedius. J. Med. Microbiol. 50:480–482 [DOI] [PubMed] [Google Scholar]
- 3. Carpenter C. F., Henry F. C. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis. 38:994–1000 [DOI] [PubMed] [Google Scholar]
- 4. Casariego E., et al. 1996. Prospective study of Streptococcus milleri bacteremia. Eur. J. Clin. Microbiol. 15:194–200 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement M100–S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. EUCAST 2011. Breakpoint tables for interpretation of MICs and zone diameters. Version 1.3. European Committee on Antimicrobial Susceptibility Testing, Vaxjo, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v1.3_pdf.pdf [Google Scholar]
- 7. Lewis J. S., et al. 2005. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob. Agents Chemother. 49:1664–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mangili A., Bica I., Snydman D. R., Hamer D. H. 2005. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 40:1058–1060 [DOI] [PubMed] [Google Scholar]
- 9. Molina J. M., et al. 1991. Clinical and bacterial features of infections caused by Streptococcus milleri. Scand. J. Infect. Dis. 23:659–666 [DOI] [PubMed] [Google Scholar]
- 10. Ortel T. L., Kallianos J., Gallis H. A. 1990. Group C streptococcal arthritis: case report and review. Rev. Infect. Dis. 12:829–837 [DOI] [PubMed] [Google Scholar]
- 11. Ripley R. T., et al. 2006. Streptococcus milleri infections of the pleural space: operative management predominates. Am. J. Surg. 192:817–821 [DOI] [PubMed] [Google Scholar]
- 12. Shishido H., Watanabe K., Matsumoto K., Murakami K., Sato K. 1997. Primary purulent mediastinitis due to Streptococcus milleri. Respiration 64:313–315 [DOI] [PubMed] [Google Scholar]
- 13. Spellerberg B., Brandt C. 2007. Streptococcus, p. 412–429 In Murray P. R., Baron E. J., Jorgensen J. H., Landry M., Pfaller M., (ed.), Manual of clinical microbiology, 9th ed. American Society for Microbiology, Washington, DC [Google Scholar]
- 14. Whiley R. A., Beighton D. 1991. Emended descriptions and recognition of Streptococcus constellatus, Streptococcus intermedius, and Streptococcus anginosus as distinct species. Int. J. Syst. Bacteriol. 41:1–5 [DOI] [PubMed] [Google Scholar]