Abstract
Systemic candidiasis causes significant mortality in patients despite amphotericin B (AMB) therapy. Mycograb C28Y variant, a human recombinant antibody fragment to heat shock protein 90, is closely related to Mycograb, which showed a survival advantage in combination with AMB in a phase III human trial. The Mycograb C28Y variant could potentially increase the antifungal effect of AMB. In our study, the interaction between AMB-desoxycholate (DAMB) and the Mycograb C28Y variant was characterized in vitro by using a checkerboard method. Quantitative cultures of kidneys, livers, and spleens of neutropenic mice with systemic Candida albicans infections were used to assess the in vivo interaction between 1.4 mg/kg of body weight/day of DAMB and 0.15, 1.5, and 15 mg/kg/day of the Mycograb C28Y variant after 1, 3, and 5 days of therapy. DAMB and Mycograb C28Y variant monotherapies, vehicle, and a no-treatment arm served as controls. Also, single- and multidose pharmacokinetics for the Mycograb C28Y variant were determined. Indifference or synergy between DAMB and the Mycograb C28Y variant was seen in two trials by the checkerboard method. The pharmacokinetics of the Mycograb C28Y variant was best described by a 2-compartment model with a median serum t1/2α of ∼0.198 h and a t1/2β of ∼1.77 h. In mice, DAMB together with the Mycograb C28Y variant was no more effective than AMB alone (P > 0.05 by analysis of variance). The Mycograb C28Y variant alone had no antifungal activity. We therefore conclude that the Mycograb C28Y variant in combination with DAMB offered no benefit over DAMB monotherapy in a neutropenic murine model of systemic candidiasis.
INTRODUCTION
Despite antifungal mono- and combination therapies with azoles, echinocandins, and polyene formulations, the mortality associated with systemic candida infections in humans remains above 29% (3, 8, 9, 11–13). Studies have shown that the attributable mortality associated with humans with systemic candidal infections in intensive care units of hospitals can exceed 49% (2, 15). Thus, there is a need to identify adjunct compounds that will improve the efficacy of current antifungal drug therapies.
Heat shock protein 90 (HSP90) is found in the cell wall of Candida species and other fungal pathogens (5). In fungi, HSP90 functions as a molecular chaperone that guides the normal folding, intracellular disposition, and proteolytic turnover of many of the key regulators of cell growth (4). HSP90 is critical to the survival of fungi (14). Further, HSP90 is believed to be involved in the development of resistance to antifungal drugs (5). Observational studies found that patients with high titers of serum antibodies against HSP90 of Candida species had a higher probability for survival. Falling or nondetectable serum antibody levels were associated with a higher probability of death in subjects with candidemia (6, 7).
Mycograb is a 28-kDa human recombinant antibody fragment that binds to the HSP90 of fungi (5). Mycograb consists of the antigen-binding domains of antibody heavy and light chains that are linked together by 2 cross-chain cysteine bonds with a synthetic linker (Fig. 1) to create a recombinant protein that is expressed in Escherichia coli. In vitro, Mycograb is synergistic with amphotericin B (AMB) as shown in experiments using the checkerboard method. A single dose of Mycograb in combination with AMB improved the killing of Candida species in a normal immune mouse model of systemic candidiasis compared with AMB monotherapy (5). In a multinational phase III clinical trial, Mycograb in combination with lipid-associated formulations of AMB decreased the Candida-attributable mortality in patients with invasive candidiasis by >4-fold, substantially improved the overall clinical response rate from 48% with AMB monotherapy to 84% with combination therapy, and increased the rate of culture-confirmed clearance of Candida species compared with the administration of AMB alone (10).
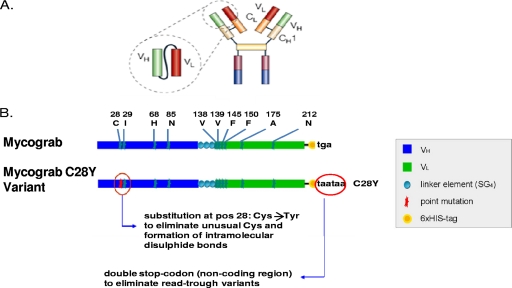
Fig. 1.
(A) Relationship of Mycograb C28Y variant antibody fragment to a full IgG antibody structure. The Mycograb C28Y variant is composed of the variable ends of the heavy (VH) and light (VL) chain from one arm of an antibody that binds to HSP90. (B) Structures of the Mycograb original sequence and the Mycograb C28Y variant. The schematic of the Mycograb and the Mycograb C28Y variant shows that the cysteine (C) at position 28 of the heavy chain fragment that is not paired in the molecule was changed to a tyrosine (Y) to decrease cross-chain binding, which could lead to aggregation. The linker protein sequence connects the two antibody variable region fragments together. The histidine tag at the end of the molecule (six-His tag) was used for protein purification. The end of the molecule stop sequence in the gene used to produce the Mycograb C28Y variant was included to prevent potential readthrough protein synthesis.
A submission of data sets for Mycograb to the European Medicines Agency for licensure for clinical use was rejected on quality aspects and safety concerns (http://www.ema.europa.eu/ema/index.jsp?curl = pages/medicines/human/medicines/000658/human_med_000912.jsp&murl = menus/medicines/medicines.jsp&mid = WC0b01ac058001d124). The quality concerns included an inconsistency in the structure of the compound between manufactured batches, due to heterogeneity, including autoaggregation of the compound. The heterogeneities in molecular weight and conformational structure were believed to be due to the presence of a fifth cysteine at position 28 which was unpaired, was not in the binding site of the protein fragment for HSP90, and did not contribute to the two disulfide bridges normally present in the molecule. Consequently, a modified form of Mycograb (thereafter referred to as the Mycograb C28Y variant) was developed in which the cysteine at position 28 was changed to a tyrosine (Y) (Fig. 1B). It was theorized that this change, along with other process improvements, would markedly reduce heterogeneity and therefore improve interbatch consistency. Manufacturing quality testing has indicated that these goals were largely achieved, since >80% of the Mycograb C28Y variant samples were monomers (Novartis data on file). The purposes of this investigation were to characterize the in vitro interaction of the Mycograb C28Y variant (previously named Myc123 C28Y) with AMB by using a checkerboard assay and to assess the efficacy of this antibody fragment alone and in combination with AMB in a 5-day neutropenic murine model of systemic candidiasis.
(This work was presented, in part, at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, 12 to 15 September 2010, Boston, MA.)
MATERIALS AND METHODS
Materials.
The antibody fragment Mycograb C28Y variant and vehicle were provided by Novartis Pharmaceuticals (East Hanover, NJ). The Mycograb C28Y variant (lot 11894.01SR) was supplied as a lyophilisate at 7.2 mg per vial. The vials of the lyophilisate were stored at 4°C, protected from light. A solution of the Mycograb C28Y variant was freshly prepared on each day of treatment by reconstituting a vial of the lyophilisate with sterile water for injection (Hospira, Inc., Lake Forest, IL). The solution was then passed through a 0.22-μm-pore syringe-driven filter device (Millex-GV Durapore polyvinylidene difluoride [PVDF] membrane; Millipore Corp., Carrigtwohill, County Cork, Ireland). The stock solution of the antibody fragment product consisted of 1.5 mg/ml of the Mycograb C28Y variant in 20 mM Tris, pH 9.2, 250 mM Trehalose, 0.02% polysorbate 80, and 0.00035% sodium laurylsarcosine.
When needed, the stock solution of the Mycograb C28Y variant was serially diluted 10-fold in Falcon polypropylene centrifuge tubes with filter-sterilized vehicle. The vehicle (lot 11549.01SR) used to dilute the antibody fragment solutions also consisted of 20 mM Tris, pH 9.2, 250 mM Trehalose, 0.02% polysorbate 80, and 0.00035% sodium laurylsarcosine. Vials of the vehicle were stored at −80°C. A vial of the vehicle was thawed at room temperature each day, 2 h before use, and then filter sterilized using a 0.22-μm-pore syringe-driven filter device (Millex-GV Durapore PVDF membrane; Millipore Corp.) according to the instructions provided by Novartis. Studies conducted by Novartis (data not shown) demonstrated that the Mycograb C28Y variant did not bind to the materials used in this study for preparing the solutions of this antibody fragment.
The Mycograb C28Y variant solutions and the vehicle were used immediately. Since the rapidity and degree of binding of the Mycograb C28Y variant to the 1-ml tuberculin syringes that were used to administer this compound to mice were unknown, each syringe was filled with the Mycograb C28Y variant and vehicle immediately before the compound was administered to the mice. The contact time of antibody fragment and vehicle with the syringe was no more than 3 min. Any unused solutions of the Mycograb C28Y variant and vehicle were discarded the same day the solutions were prepared.
Pharmaceutical-grade AMB-desoxycholate (DAMB; X-Gen Pharmaceuticals, Inc., Big Flats, NY) was purchased from CuraScriptSD (Lake Mary, FL). A stock solution of 5 mg/liter of DAMB was made at the start of the experiment with water for injection and was stored at 4°C, protected from light. An aliquot of the DAMB was diluted to the desired concentration on the day of use.
RPMI 1640 with glutamine, buffered to pH 7.0 with 0.165 mmol/liter of morpholinepropanesulfuric acid (RPMI 1640 plus MOPS), which was used in the in vitro studies, was purchased from Lonza (Walkersville, MD).
Fungal isolates.
Five C. albicans strains were examined in the in vitro susceptibility and drug interaction studies. The strains were C. albicans ATCC 36082, ATCC 90028, ATCC 24433, ATCC 64548, and ATCC 90029. The isolates were stored at −80°C in skim milk. For each experiment, a sample of a stock culture was passed twice on Saboraud-dextrose agar plates that were incubated overnight at 35°C. For the in vitro experiments, fungal suspensions were made by directly inoculating a few colonies into prewarmed RPMI 1640 plus MOPS. One fungal strain (Candida albicans ATCC 90028) was examined in the in vivo experiments. For the in vivo experiments, the fungal suspensions were made by directly suspending colonies of the fungus that were grown overnight at 35°C on Saboraud-dextrose agar in sterile normal saline. The concentration of fungi in the suspension was determined with a spectrophotometer. Then, the suspension was diluted to the desired concentration with medium for the in vitro studies and in sterile saline for the in vivo experiments. The concentrations of fungi in the final suspensions were confirmed with quantitative cultures.
Susceptibility testing.
The MICs for DAMB alone, DAMB dissolved in vehicle, and the Mycograb C28Y variant for the five C. albicans strains were determined in RPMI 1640 plus MOPS using a broth microdilution method described by the Clinical and Laboratory Standards Institute (1). For the DAMB plus vehicle arm, the stock solution of 5 mg/liter of DAMB (in sterile water for injection) was diluted to a concentration of 1 mg/liter with vehicle. The resultant solution was then serially diluted 2-fold with RPMI 1640 plus MOPS. The final fungal concentration was 103 CFU/well. The MICs were read after 48 h of incubation at 35°C. The MIC was defined as the lowest concentration of drug in which no visible growth was observed. The MICs were determined in triplicate on three different days.
In vitro drug interaction assessment.
The in vitro interactions between DAMB and DAMB plus vehicle with the Mycograb C28Y variant were assessed using the checkerboard method in 96-well microdilution plates. After 48 h of incubation at 35°C, the fractional inhibitory concentrations (FICs) were calculated for each drug by dividing the MIC of the drug in combination with the MIC of the drug as monotherapy. The FIC index (FICI) was derived by adding the FIC values together. Separate FICI values were calculated using the MICs for DAMB monotherapy for drug prepared with and without vehicle. An FICI value of ≤0.5 was defined as synergistic, a value of >0.5 and <4.0 was defined as indifferent, and a value of ≥4 was considered antagonistic, as defined in the Instructions to Authors section of Antimicrobial Agents and Chemotherapy.
Mice.
Female, 5- to 6-week-old, outbred Swiss Webster mice (Taconic Farms, Taconic, NY) weighing 22 to 24 g were used in these studies. The mice were rendered neutropenic with cyclophosphamide at 150 mg/kg of body weight given by the intraperitoneal (i.p.) route 4 days prior to fungal challenge and with 100 mg/kg given 1 day prior to and then on days 1 and 3 after fungal challenge. The neutropenia lasted for the duration of each experiment (data not shown).
All experiments with animals were approved by Ordway Research Institute's Institutional Animal Care and Use Committee.
Assessment of the interaction between the Mycograb C28Y variant and DAMB in the neutropenic mouse model of systemic candidiasis.
Neutropenic mice were infected intravenously with 5 × 104 CFU of C. albicans strain ATCC 90028 via the lateral tail vein. The fungal inoculum was confirmed by quantitative culture. Two hours later, 3 mice from the no-treatment control arm were culled and the kidneys, livers, and spleens of these mice were collected for baseline quantitative culture values. Then treatment was initiated.
The experimental groups and treatment regimens that were used in the in vivo DAMB plus Mycograb C28Y variant interaction study were as follows: (i) no-treatment control; (ii) vehicle control, given once daily; (iii) Mycograb C28Y variant monotherapy control at 15 mg/kg/day; (iv) DAMB at 1.4 mg/kg/day alone; (v) DAMB at 1.4 mg/kg/day and vehicle; (vi) DAMB at 1.4 mg/kg/day plus Mycograb C28Y variant at 0.15 mg/kg/day; (vii) DAMB at 1.4 mg/kg/day plus Mycograb C28Y variant at 1.5 mg/kg/day; (viii) DAMB at 1.4 mg/kg/day plus Mycograb C28Y variant at 15 mg/kg/day.
The DAMB dose of 1.4 mg/kg/day was used in this study because a pilot dose-range trial demonstrated that this regimen resulted in a persistent fungal burden in the kidneys (producing a stasis effect of 3.8 to 4.3 log CFU/g of tissue) and allowed the infected, neutropenic mice to survive the 5-day experiment.
The study design called for five mice per group to be culled after 1, 3, and 5 days of therapy for quantitative cultures of the kidneys, livers, and spleens. Three additional mice were included in groups that received intravenous Mycograb C28Y variant or vehicle to ensure that tissues from at least five fully treated mice would be available for quantitative cultures at the last time point. The DAMB and the Mycograb C28Y variant were administered once daily for 5 days. The DAMB was administered i.p., while the Mycograb C28Y variant and vehicle were administered via the intravenous (i.v.) route. The DAMB and the Mycograb C28Y variant or vehicle injections were given 1 h apart. The same volume of vehicle was administered daily to groups ii, iii, and v through viii.
One mouse in the group that was given DAMB in combination with 0.15 mg/kg/day of the Mycograb C28Y variant did not receive a full dose of antibody fragment on day 4 of treatment. That animal was removed from the study. The remaining animals received all of their antibody fragment or vehicle dosings without difficulty.
The kidneys, livers, and spleens were aseptically collected from euthanized mice after 24, 72, and 120 h of therapy. Tissue homogenates were washed twice with normal saline to prevent drug carryover before they were quantitatively cultured. Quantitative culture results from animals that were euthanized for signs of severe, progressive disease were included in the next time point (i.e., animals euthanized at 48 h were included in the 72-h time point group). The lower limit of reproducible quantification of fungi in tissues was 20 CFU/g (data not shown).
Differences in quantitative culture results among the experimental groups were evaluated by analysis of variance (ANOVA). If significant differences were found between these groups, the Tukey-Kramer test for multiple comparisons was applied to the data. A P value of <0.05 was considered significant. If a portion of the tissues was culture negative, Fisher's exact test was used to determine if combination therapy resulted in a greater proportion of negative cultures than no treatment or DAMB monotherapy. This comparative treatment experiment was conducted once.
Single-dose and 5-day multidose pharmacokinetic studies for the Mycograb C28Y variant.
Because the Mycograb C28Y variant had no intrinsic antifungal activity and C. albicans was virulent in the immunocompromised mice, the pharmacokinetic studies were conducted in noninfected neutropenic mice.
Cyclophosphamide-induced neutropenic mice were separated into three groups. Each group was treated with one of the three dosages of the Mycograb C28Y variant that was examined in the dose-range study described above.
For the single-dose study, sera were collected from 3 mice at 15 and 45 min after the first dose of the Mycograb C28Y variant was administered intravenously to mice. For the multidose study, the Mycograb C28Y variant was administered once daily to mice for 5 doses via the lateral tail vein. Serum was collected from 3 mice per time point at 1 h prior to the administration of the fifth dose of the Mycograb C28Y variant and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, and 8 h after that dose was delivered.
The serum samples were frozen at −80°C until assayed. Samples were analyzed by Novartis with a sandwich immunoassay using a mouse monoclonal anti-Mycograb C28Y variant antibody as a capture antibody, a rabbit polyclonal anti-Mycograb C28Y variant antibody as a second antibody, and a goat anti-rabbit IgG–horseradish peroxidase conjugate as detection antibody (lower limit of quantitation, 0.8 ng/ml of serum; upper limit of quantitation, 30 ng/ml of serum; intraday accuracy and precision, −15.5 to 3.7% and 1.1 to 10.6%, respectively; interday accuracy and precision, −6.4 to 0% and 3.6 to 8.4%, respectively). Data were fit to candidate models (WinNonlin 5.2). Data were weighted by the estimated inverse measure variance, and model discrimination was performed according to Akaike's information criterion.
RESULTS
MICs of the Mycograb C28Y variant and DAMB.
The MICs for DAMB with and without the vehicle were determined simultaneously in three trials. DAMB tested in medium without vehicle produced MICs of 0.5 μg/ml for the 5 C. albicans isolates. The MICs of DAMB with vehicle (which contains 0.02% polysorbate 80, an agent with potential antifungal activity) were the same or one dilution lower than the MICs that were determined simultaneously for DAMB without vehicle.
The MIC for the Mycograb C28Y variant, as a single agent, was >256 μg/ml. Higher concentrations of this anti-HSP90 antibody fragment could not be tested because concentrations of ≥512 μg/ml of the Mycograb C28Y variant diluted in fungal medium were turbid. The Mycograb C28Y variant solutions made at concentrations of ≤256 μg/ml in fungal medium were clear. Also, the stock solution of 1.5 mg/ml of the Mycograb C28Y variant in 100% vehicle was clear.
In vitro interaction between Mycograb C28Y variant and DAMB.
The interaction between the Mycograb C28Y variant and DAMB was assessed by the checkerboard method in two trials. Monotherapies included serial 2-fold dilutions of the Mycograb C28Y variant (in vehicle) alone, DAMB without vehicle, and DAMB with vehicle.
In the checkerboard studies, the MICs of DAMB (without vehicle) were 0.5 and 1 μg/ml and for the Mycograb C28Y variant alone the MICs were >128 μg/ml in two trials for the C. albicans isolates tested (Table 1). In combination, the MICs for DAMB decreased 4-fold to 0.125 μg/ml and the MIC for the Mycograb C28Y variant was 4 or 8 μg/ml in the two trials. The interaction between DAMB without vehicle and the Mycograb C28Y variant was shown to be synergistic in two trials, with FICIs of 0.252 and 0.258 for four of the five strains. The fifth strain, C. albicans ATCC 24433, demonstrated synergy but with greater variability. It had an FICI value of 0.141 in one trial and 0.258 in the second trial.
Table 1.
In vitro interactions of DAMB and Mycograb C28Y variant for 5 C. albicans isolates in two trialsa
| Trial no. | C. albicans strain | Agent | MIC (μg/ml) |
FIC | FICI | Outcome | |
|---|---|---|---|---|---|---|---|
| Agent alone | Two agents in combination | ||||||
| 1 | ATCC 24433 | DAMB | 0.5 | 0.125 | 0.25 | 0.258 | Synergy |
| Myc-C28Y | >128 | 4 | 0.008 | ||||
| ATCC 36082 | DAMB | 0.5 | 0.125 | 0.25 | 0.252 | Synergy | |
| Myc-C28Y | >128 | 1 | 0.002 | ||||
| ATCC 90028 | DAMB | 0.5 | 0.125 | 0.25 | 0.258 | Synergy | |
| Myc-C28Y | >128 | 4 | 0.008 | ||||
| ATCC 90029 | DAMB | 0.5 | 0.125 | 0.25 | 0.258 | Synergy | |
| Myc-C28Y | >128 | 4 | 0.008 | ||||
| ATCC 64548 | DAMB | 0.5 | 0.125 | 0.25 | 0.258 | Synergy | |
| Myc-C28Y | >128 | 4 | 0.008 | ||||
| 2 | ATCC 24433 | DAMB | 1 | 0.125 | 0.125 | 0.141 | Synergy |
| Myc-C28Y | >32 | 8 | 0.016 | ||||
| ATCC 36082 | DAMB | 0.5 | 0.125 | 0.25 | 0.266 | Synergy | |
| Myc-C28Y | >32 | 8 | 0.016 | ||||
| ATCC 90028 | DAMB | 0.5 | 0.125 | 0.25 | 0.258 | Synergy | |
| Myc-C28Y | >32 | 4 | 0.008 | ||||
| ATCC 90029 | DAMB | 0.5 | 0.125 | 0.25 | 0.258 | Synergy | |
| Myc-C28Y | >32 | 4 | 0.008 | ||||
| ATCC 64548 | DAMB | 0.5 | 0.125 | 0.25 | 0.258 | Synergy | |
| Myc-C28Y | >32 | 4 | 0.008 | ||||
DAMB monotherapy was without vehicle. The FICs for the Myc-C28Y variant were calculated using an MIC of 512 μg/ml for the antibody fragment when tested alone.
The MICs for DAMB (with vehicle) alone for the five C. albicans isolates were 0.25 μg/ml in one trial and 0.5 μg/ml in the second trial. In both trials the MIC for DAMB decreased to 0.125 μg/ml when the drug was tested in combination with the Mycograb C28Y variant. The MIC of the Mycograb C28Y variant was 1 to 8 μg/ml when tested in combination in DAMB. The interaction between DAMB (with vehicle) and the Mycograb C28Y variant was synergistic when the FICI was calculated using the DAMB (with vehicle) MIC of 0.5 μg/ml. Indifference was observed for the trial in which the MIC for DAMB of 0.25 μg/ml was used to calculate the FICI values (Table 2).
Table 2.
In vitro interactions of DAMB plus vehicle and the Mycograb C28Y variant for 5 C. albicans isolates in two trialsa
| Trial no. | C. albicans strain | Agent | MIC (μg/ml) |
FIC | FICI | Outcome | |
|---|---|---|---|---|---|---|---|
| Agent alone | Two agents in combination | ||||||
| 1 | ATCC 24433 | DAMB + V | 0.25 | 0.125 | 0.5 | 0.508 | Indifference |
| Myc-C28Y | >128 | 4 | 0.008 | ||||
| ATCC 36082 | DAMB + V | 0.25 | 0.125 | 0.5 | 0.508 | Indifference | |
| Myc-C28Y | >128 | 1 | 0.002 | ||||
| ATCC 90028 | DAMB + V | 0.25 | 0.125 | 0.5 | 0.508 | Indifference | |
| Myc-C28Y | >128 | 4 | 0.008 | ||||
| ATCC 90029 | DAMB + V | 0.25 | 0.125 | 0.5 | 0.508 | Indifference | |
| Myc-C28Y | >128 | 4 | 0.008 | ||||
| ATCC 64548 | DAMB + V | 0.25 | 0.125 | 0.5 | 0.508 | Indifference | |
| Myc-C28Y | >128 | 4 | 0.008 | ||||
| 2 | ATCC 24433 | DAMB + V | 0.5 | 0.125 | 0.25 | 0.266 | Synergy |
| Myc-C28Y | >32 | 8 | 0.016 | ||||
| ATCC 36082 | DAMB + V | 0.5 | 0.125 | 0.25 | 0.266 | Synergy | |
| Myc-C28Y | >32 | 8 | 0.016 | ||||
| ATCC 90028 | DAMB + V | 0.5 | 0.125 | 0.25 | 0.258 | Synergy | |
| Myc-C28Y | >32 | 4 | 0.008 | ||||
| ATCC 90029 | DAMB + V | 0.5 | 0.125 | 0.25 | 0.258 | Synergy | |
| Myc-C28Y | >32 | 4 | 0.008 | ||||
| ATCC 64548 | DAMB + V | 0.5 | 0.125 | 0.25 | 0.258 | Synergy | |
| Myc-C28Y | >32 | 4 | 0.008 | ||||
DAMB + V, DAMB plus vehicle. The FICs for Myc-C28Y groups were calculated using an MIC of 512 μg/ml for the antibody fragment when tested alone.
Interaction between Mycograb C28Y variant and DAMB in the 5-day neutropenic murine systemic candidiasis model.
In the no-treatment control, vehicle control, and the Mycograb C28Y variant monotherapy control arm, the fungal densities in the kidneys and spleens progressively increased while the fungal population remained stable in the liver (Table 3). Animals that survived to the 48-h time point were euthanized for severe, progressive disease. The quantitative culture results for these animals were recorded at the 72-h time point. As shown in Fig. 2, the quantitative culture values for these three experimental arms overlapped one another, suggesting that neither the vehicle nor the Mycograb C28Y variant had intrinsic antifungal activity.
Table 3.
Effects of 1.4 mg/kg/day of DAMB alone or in combination with three dosages of the Mycograb C28Y variant on the fungal densities in the kidneys, livers, and spleens of neutropenic mice with systemic candidiasis
| Organ | Exptl group | Fungal density (log CFU/g ± SD) after indicated duration (h) of treatmenta |
|||
|---|---|---|---|---|---|
| 0 | 24 | 72 | 120 | ||
| Kidney | No-treatment control | 3.52 ± 0.15 (3) | 5.34 ± 0.22 (5) | 5.64 ± 0.18b (10) | |
| Vehicle control | 5.23 ± 0.08 (5) | 5.71 ± 0.27b (13) | |||
| Myc-C28Y, 15 mg/kg/day control | 5.31 ± 0.09 (5) | 5.83 ± 0.28b (13) | |||
| DAMB alone | 4.18 ± 0.12c (5) | 4.29 ± 0.16d (5) | 4.15 ± 0.46d (5) | ||
| DAMB + vehicle | 4.24 ± 0.05c (5) | 4.33 ± 0.31d (5) | 4.19 ± 0.48d (8) | ||
| DAMB + Myc-C28Y at 0.15 mg/kg/day | 4.29 ± 0.16c (5) | 4.19 ± 0.48d (5) | 3.94 ± 0.43d (7)e | ||
| DAMB + Myc-C28Y at 1.5 mg/kg/day | 3.96 ± 0.20c (5) | 4.26 ± 0.37d (5) | 4.03 ± 0.74d (8) | ||
| DAMB + Myc-C28Y at 15 mg/kg/day | 3.74 ± 0.64c (5) | 4.41 ± 0.14d (5) | 3.95 ± 0.64d (8) | ||
| Liver | No-treatment control | 3.81 ± 0.05 (3) | 3.83 ± 0.10 (5) | 3.75 ± 0.21b (10) | |
| Vehicle control | 3.73 ± 0.11 (5) | 3.80 ± 0.23b (13) | |||
| Myc-C28Y, 15 mg/kg/day control | 3.63 ± 0.16 (5) | 3.98 ± 0.36b (13) | |||
| DAMB alone | 2.91 ± 0.42c (5) | 1.18 ± 0.67d (5) | 0.28 ± 0.63d (5) | ||
| DAMB + vehicle | 2.69 ± 0.46c (5) | 1.55 ± 0.90d (5) | 0.81 ± 0.92d (8) | ||
| DAMB + Myc-C28Y at 0.15 mg/kg/day | 2.80 ± 0.44c (5) | 1.06 ± 1.00d (5) | 0.44 ± 0.76d (7)e | ||
| DAMB + Myc-C28Y at 1.5 mg/kg/day | 2.78 ± 0.44c (5) | 1.36 ± 1.35d (5) | 0.83 ± 1.20d (8) | ||
| DAMB + Myc-C28Y at 15 mg/kg/day | 2.53 ± 0.66c (5) | 1.02 ± 0.95d (5) | 0.45 ± 0.83d (8) | ||
| Spleen | No-treatment control | 3.05 ± 0.17 (3) | 3.89 ± 0.28 (5) | 4.05 ± 0.34b (10) | |
| Vehicle control | 3.65 ± 0.34 (5) | 4.12 ± 0.60b (13) | |||
| Myc-C28Y, 15 mg/kg/day control | 3.60 ± 0.15 (5) | 4.04 ± 0.27b (13) | |||
| DAMB alone | 3.21 ± 0.27 (5) | 2.36 ± 0.19d (5) | 1.52 ± 0.87d (5) | ||
| DAMB + vehicle | 3.32 ± 0.24 (5) | 2.67 ± 0.42d (5) | 2.01 ± 0.90d (7)g | ||
| DAMB + Myc-C28Y at 0.15 mg/kg/day | 3.15 ± 0.52 (5) | 2.66 ± 0.16d (5) | 1.32 ± 0.92d (7)e | ||
| DAMB + Myc-C28Y at 1.5 mg/kg/day | 2.93 ± 0.25f (5) | 3.01 ± 0.74d (5) | 2.15 ± 0.55d (8) | ||
| DAMB + Myc-C28Y at 15 mg/kg/day | 2.77 ± 0.82f (5) | 2.65 ± 0.29d (5) | 2.23 ± 0.23d (8) | ||
Numbers in parentheses are the numbers of animals evaluated at each time point.
The 72- and 120-h controls were sacrificed at 48 h because of progressive disease. They were recorded with the 72-h data groups. There were three additional animals in all groups that received vehicle or the antibody fragment by the intravenous route, to increase the probability that at least five mice could be evaluated at the last time point in which the mice in that group survived. This accounts for the higher number of animals at the last evaluable time point in these groups.
The DAMB, DAMB plus vehicle, and the three DAMB plus Myc-C28Y treatment groups were statistically significantly different from the three control groups at 24 h (P < 0.05) but were different from each other (Tukey-Kramer test for multiple comparisons).
The DAMB, DAMB plus vehicle, and the three DAMB plus Myc-C28Y treatment groups were statistically significantly different from the three 72-h control groups (based on the Tukey-Kramer test for multiple comparisons).
One mouse in the DAMB plus Myc-C28Y at 0.15 mg/kg/day group did not receive a full dose of the antibody fragment on day 4 of treatment and was therefore removed from the study.
The DAMB plus Myc-C28Y at 1.5 or 15 mg/kg/day groups were statistically significantly different from the no-treatment controls at 24 h based on the Tukey-Kramer test for multiple comparisons. The DAMB plus Myc-C28Y at 15 mg/kg/day regimen was also statistically different from the vehicle control group at this time point based on the Tukey-Kramer test.
One mouse in this group did not have a spleen. Thus, only 7 of 8 mice in this group were assessed for fungal densities in spleens.
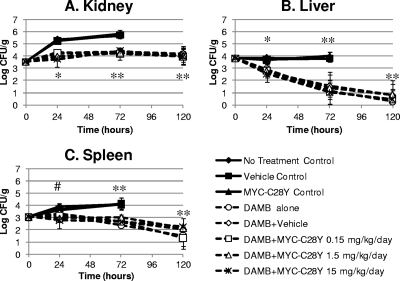
Fig. 2.
Quantitative cultures (log CFU/g ± 1 standard deviation) of kidneys, livers, and spleens of neutropenic mice with systemic candidiasis that were treated for 24, 72, or 120 h with 1.4 mg/kg/day of DAMB alone or in combination with 0.15, 1.5, or 15 mg/kg/day of the Mycograb C28Y variant (Myc-C28Y). Animals in the no-treatment control, vehicle control, and the Myc-C28Y monotherapy control arms that survived beyond the 24-h time point were euthanized after 48 h of infection for progressive disease and were included in the 72-h time point group. The quantitative culture results in the kidneys, livers, and spleens at the 72-h time point of the three control arms were used as comparators for the data derived after 72 and 120 h of therapy for DAMB alone or in combination with Myc-C28Y. Doses of the antibody fragment are in mg/kg/day. In each graph, the no-treatment control, vehicle control, and Myc-C28Y monotherapy control results formed the upper cluster of solid curves, which overlap one another. The DAMB-alone arm, the DAMB plus vehicle arm, and the three DAMB plus Myc-C28Y combination arms are shown as the broken curves and formed the second (lower) cluster of curves. The Tukey-Kramer test for multiple comparisons showed that the DAMB, DAMB plus vehicle, and the three DAMB plus Myc-C28Y groups were statistically significantly different than the three control groups at 24 h (*, P < 0.05), while the DAMB-containing arms were similar to each other (P > 0.05). The Tukey-Kramer test for multiple comparisons showed that the DAMB, DAMB plus vehicle, and the three DAMB plus Myc-C28Y groups were statistically significantly different (P < 0.05) than the three 72-h control groups, while the DAMB-containing arms were similar to each other (**, P > 0.05). The Tukey-Kramer test for multiple comparisons showed that the DAMB plus 1.5 or 15 mg/kg/day of Myc-C28Y groups were significantly different than the three control groups at 24 h (#, P < 0.05), but not from the other experimental groups.
Animals that were treated with DAMB alone or DAMB in combination with 0.15 to 15 mg/kg/day of the antibody fragment survived to their designated time points throughout the 5-day experiment. Thus, at the 24- and 72-h time points, quantitative cultures were conducted for five mice in these experimental groups. At the 120-h time point, the organs of five mice in the DAMB-only group were evaluated. However, since the 3 extra mice that received DAMB in combination with vehicle or the Mycograb C28Y variant survived for the duration of the study, 8 mice were examined in these groups at the last (120-h) time point. Seven animals were examined at the 120-h time point for the group that received DAMB in combination with antibody fragment at 0.15 mg/kg/day, because one animal did not receive a full dose of the antibody fragment on day 4 of therapy and was excluded from analysis.
Treatment of neutropenic mice with 1.4 mg/kg/day of DAMB as monotherapy resulted in approximately a 1.1-log CFU/g reduction in the density of C. albicans in the kidneys compared with the no-treatment controls after 24 h of treatment. The concentrations of fungi in the kidneys of animals treated with DAMB as monotherapy remained between 4.15 and 4.29 log CFU/g for the duration of the 5-day treatment period (Fig. 2A and Table 3). As shown in Fig. 2A, the fungal densities in the kidneys of mice treated with DAMB, DAMB with vehicle, or DAMB in combination with 0.15 to 15 mg/kg/day of the Mycograb C28Y variant overlapped each other after 1, 3, and 5 days of therapy. Statistical analysis (ANOVA and post hoc Tukey-Kramer test for multiple comparisons) confirmed that the quantitative cultures associated with DAMB (with and without vehicle) and DAMB in combination with 0.15 to 15 mg/kg/day of the Mycograb C28Y variant were similar. However, treatment effects of the DAMB-containing regimens were greater than those associated with no therapy, the vehicle alone, or the Mycograb C28Y variant as monotherapy (P < 0.05) (Fig. 2A and Table 3).
The fungal densities in the livers and spleens of mice in the no-treatment control, vehicle control, and Mycograb C28Y variant monotherapy arms formed one cluster of curves that overlapped each other over the course of the experiment (Fig. 2B and C, respectively). The fungal densities in animals treated with DAMB, DAMB with vehicle, or DAMB in combination with 0.15, 1.5, and 15 mg/kg/day of the Mycograb C28Y variant formed a second (lower) cluster of curves (Fig. 2B and C). Statistical analysis (ANOVA followed by the Tukey-Kramer test for multiple comparisons) demonstrated a significant difference between all of the DAMB-containing regimens and the no-treatment control, vehicle control, and Mycograb monotherapy arms (P < 0.05). However, there was no difference in antifungal effect of DAMB when it was given alone, with the vehicle, or in combination with 0.15 to 15 mg/kg/day of the Mycograb C28Y variant (P > 0.05). Further, inspection of the quantitative results in the livers and spleens of mice after 120 h of therapy showed no evidence of a trend toward better outcome when DAMB was administered with higher doses of the Mycograb C28Y variant compared to the antifungal effects of DAMB alone or DAMB plus vehicle (Table 3). Thus, the quantitative culture results showed that DAMB in combination with 0.15 to 15 mg/kg/day of the Mycograb C28Y variant were no better than those associated with DAMB monotherapy.
Application of Fisher's exact test revealed a statistically significant difference in the proportion of negative cultures in the livers of mice treated with DAMB alone, the DAMB with vehicle, and the three DAMB with antibody fragment groups at the 120-h time point compared to the 72-hour no-treatment control, the vehicle control, and antibody fragment monotherapy control groups (P ≤ 0.023) (Table 4). However, the proportion of negative cultures in the livers of the animals that were treated with DAMB alone or in combination with the antibody fragment was similar at this time point. There was no difference in the proportion of negative cultures in the livers between any groups at the 24- and 72-h time points.
Table 4.
Proportion of livers, kidneys, and spleens of infected mice that were culture negative after 24, 72, and 120 h of therapy with DAMB alone or in combination with the Mycograb C28Y variant
| Treatment arm | Proportion of organ samples culture negative at the indicated time (h) posttreatment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Liver |
Kidney |
Spleen |
|||||||
| 24 | 72a | 120 | 24 | 72a | 120 | 24 | 72a | 120 | |
| No-treatment control | 0/5 | 0/10b | 0/5 | 0/10 | 0/5 | 0/10 | |||
| Vehicle control | 0/5 | 0/13 | 0/5 | 0/13 | 0/5 | 0/13 | |||
| Myc-C28Y control | 0/5 | 0/13 | 0/5 | 0/13 | 0/5 | 0/13 | |||
| DAMB alone | 0/5 | 1/5 | 4/5c | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 |
| DAMB + vehicle | 0/5 | 1/5 | 4/8c | 0/5 | 0/5 | 0/8 | 0/5 | 0/5 | 1/7d |
| DAMB + Myc-C28Y, 0.15 mg/kg/day | 0/5 | 2/5 | 5/7c,e | 0/5 | 0/5 | 0/7e | 0/5 | 0/5 | 2/7e |
| DAMB + Myc-C28Y, 1.5 mg/kg/day | 0/5 | 2/5 | 5/8c | 0/5 | 0/5 | 0/8 | 0/5 | 0/5 | 0/8 |
| DAMB + Myc-C28Y, 15 mg/kg/day | 0/5 | 2/5 | 6/8c | 0/5 | 0/5 | 0/8 | 0/5 | 0/5 | 0/8 |
In the animals in the no-treatment control, vehicle control, and Myc-C28Y control groups that were scheduled for sacrifice at the 72- and 120-h time points, animals were culled 48 h after fungal inoculation because of severe, progressive infection. They were included in the 72-h time point in this table.
In groups that were treated with i.v. vehicle or Myc-C28Y fragment, three additional mice were included to increase the probability that at least five mice were evaluable at the last time point, if they survived their infections. This accounts for the higher number of animals in these groups being evaluated at the last time point.
The portions of sterile cultures in these groups were significantly different from the no-treatment control, the vehicle control, and the Myc-C28Y control arms.
The livers and kidneys of 8 animals were quantitatively cultured in the DAMB plus vehicle group. However, only 7 spleens were evaluated, because 1 of animals in this group did not have a spleen.
One animal in the AMB plus Myc-C28Y at 0.15 mg/kg/day group did not receive a full dose of antibody fragment on day 4 of treatment and was excluded from evaluation.
Further, there was no statistical difference in the proportion of negative cultures from the kidneys and spleens of the control groups and the groups that were treated with DAMB alone or in combination with the antibody fragment at any time point (P ≥ 0.05) (Table 4).
Pharmacokinetics of single- and multidose Mycograb C28Y variant adminitration.
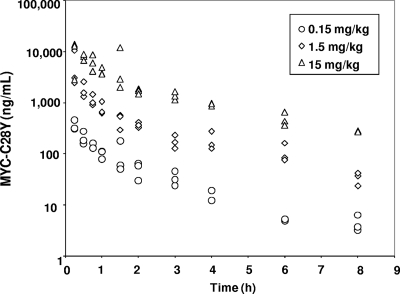
The multidose pharmacokinetic (PK) concentration-time profiles and parameters for intravenously administered Mycograb C28Y variant are shown in Fig. 3 and Table 5, respectively. The pharmacokinetics of Mycograb C28Y variant was best described by a linear 2-compartment model, with i.v. bolus input and no lag time. The median serum t1/2α was ∼0.198 h, and the t1/2β was ∼1.77 h. Model-derived and observed mean PK parameters in the 15-mg/kg group appeared to be lower than dose proportional. Further investigation revealed one animal with a 4-fold-higher maximum concentration of drug in the serum (Cmax) than the other animals in the 1.5-mg/kg dose group, making the apparent group exposure higher than expected, and at the 15-mg/kg dose one animal had an exposure that was approximately half that of the other animals at that time point, decreasing the overall exposure. As biologicals are typically pharmacokinetically “well behaved,” the apparent less-than-dose-proportional behavior was probably secondary to the innate variability due to working with different mice for each time point.
Fig. 3.
Scatter plot of individual time-concentration data for 3 daily doses (in mg/kg/day) of the Mycograb C28Y variant (Myc-C28Y) after intravenous administration.
Table 5.
Serum pharmacokinetic parameters for 5-day, multidose administration of the Mycograb C28Y variant in noninfected neutropenic mice (n = 3 in each cohort)a
| Dose (mg/kg/day) | Type of summary value | Cmax (ng/ml) | AUC (ng·h/ml) | t1/2α (min) | t1/2β (min) |
|---|---|---|---|---|---|
| 0.15 | Mean | 374.2 | 388.0 | 33.0 | 305.4 |
| Median | 330.7 | 360.9 | 28.8 | 119.1 | |
| CV%b | 24.1 | 20.3 | 49.4 | 107.6 | |
| 1.5 | Mean | 14,575.9 | 4,654.7 | 13.5 | 125.1 |
| Median | 5,520.7 | 3,036.2 | 13.1 | 135.9 | |
| CV% | 120.0 | 60.6 | 47.3 | 23.5 | |
| 15 | Mean | 16,969.4 | 18,961.4 | 41.7 | 1,468.9 |
| Median | 22,667.7 | 17,645.3 | 17.7 | 147.0 | |
| CV% | 64.0 | 13.9 | 111.7 | 156.7 |
The Mycograb C28Y variant was administered i.v. once daily for 5 days before the serum samples were collected.
CV%, coefficient of variation.
The concentrations of the Mycograb C28Y variant were similar at the corresponding time points for the single- and multidose pharmacokinetic studies (data not shown).
DISCUSSION
Because of the high morbidity and mortality associated with invasive Candida infections in humans, there is a need to develop novel compounds that can enhance the efficacy of current antifungal agents. In previous studies Mycograb, a human recombinant anti-HSP90 antibody fragment, was shown be synergistic with AMB against several Candida species in in vitro checkerboard studies and in a murine model of systemic candidiasis (5). Also, in a phase III clinical trial, Mycograb in combination with lipid-associated formulations of AMB markedly improved outcomes in patients with invasive candidiasis compared with AMB monotherapy (10). However, Mycograb did not receive licensure from the European Medicines Agency for clinical use, in part because of quality concerns, which included an inconsistency in the structure of the compound between manufactured batches due to heterogeneities, including autoaggregation of the compound.
The Mycograb C28Y variant was produced by replacing the cysteine at position 28 of the heavy chain of Mycograb with a tyrosine, resulting in a balanced number of cysteines in the light and heavy chains. Manufacturing verified the hypothesis that elimination of this unpaired cysteine resulted in a product that had a markedly improved batch-to-batch consistency. However, in contrast to the synergistic in vitro and in vivo interactions that have been demonstrated between Mycograb and AMB (5, 10), our multidose treatment studies in a neutropenic murine model of systemic candidiasis showed indifference between the Mycograb C28Y variant and DAMB: the efficacy of the combination of the Mycograb C28Y variant and DAMB was no better than DAMB monotherapy in clearing C. albicans from the kidneys, livers, and spleens of infected mice.
It is clear from the current investigation that the synergistic interaction between the Mycograb C28Y variant and DAMB observed in the checkerboard assay did not correlate with the outcomes observed in neutropenic mice. Since the FICI values describing the synergistic in vitro interaction between Mycograb and AMB-DMSO and for the Mycograb C28Y variant and DAMB were similar and consistent with prior data for these combinations (Novartis data on file), the discordance between the results of the in vitro checkerboard assay and the murine infection model does not appear to be due to differences in the in vitro testing for this study. Our findings demonstrate that the in vitro checkerboard method cannot be used to predict the in vivo interactions of the Mycograb C28Y variant and AMB.
The disparity in the in vivo outcomes associated with Mycograb and the Mycograb C28Y variant may be due to differences in the compounds themselves and/or the differences in the animal models that were used to evaluate the activities of each antibody fragment, alone and in combination with AMB.
With regard to the compounds themselves, there may be a difference in potency of the Mycograb C28Y variant compared to Mycograb. In vitro, Matthews and colleagues (5) reported that Mycograb had an MIC of 128 to 256 μg/ml for the six Candida isolates that they evaluated. In contrast, we could not identify an MIC value for the Mycograb C28Y variant, because the five C. albicans isolates used in the current project had MICs to this antibody fragment that were >256 μg/ml. Higher concentrations could not be examined because the solutions of the Mycograb C28Y variant were turbid at concentrations of ≥512 μg/ml when the stock preparation (1.5 mg/ml of antibody fragment dissolved in vehicle) was diluted in medium.
Furthermore, in vivo, the original Mycograb formulation as monotherapy demonstrated intrinsic antifungal activity, while the Mycograb C28Y variant did not. In an immunocompetent murine model of systemic candidiasis, single doses of 0.2 and 2 mg/kg of Mycograb, given alone, reduced the densities of C. albicans in the kidneys of infected mice by 0.4 and 0.92 log CFU/g, respectively, 48 h after therapy was administered. Mycograb monotherapy, administered as a single 0.2-mg/kg dose, resulted in negative cultures (defined as <1 CFU/mg [5]) in the kidneys of 60% of the mice that were infected with Candida krusei. Mycograb given alone at 2 mg/kg resulted in negative cultures of the kidneys in 80% of mice that were systemically infected with Candida glabrata. Notably, single doses of 0.2 and 2 mg/kg of Mycograb given in combination with AMB-DMSO resulted in negative cultures of the kidneys in 100% and 90% of mice that were infected with C. albicans, respectively, suggesting that a single dose of Mycograb at 0.2 mg/kg in combination with AMB achieved maximum antifungal effect. Thus, in vivo, Mycograb as monotherapy had intrinsic activity against the Candida strains, and the combination of Mycograb and AMB was synergistic in mice. In contrast, in neutropenic mice, the Mycograb C28Y variant alone had no intrinsic activity against C. albicans and was no better than placebo when it was used in combination with AMB.
In general, the modification of the structure of a compound may change the pharmacokinetics of the molecule and, therefore, alter the length of time the compound may interact with a second drug or a pathogen. The absence of effect of the Mycograb C28Y variant administered alone or in combination with AMB could not be attributed to a difference in the pharmacokinetics of the two antibody fragments. Mycograb at a single dose of 2 mg/kg generated in mice a serum Cmax of 4.7 μg/ml and a 48-h area under the time-concentration curve (AUC) of 155 μg·min/ml (5). The Mycograb C28Y variant at 1.5 mg/kg given once daily generated a serum Cmax of 5.5 μg/ml and 48-h AUC of 364 μg·min/ml, while the dose of 15 mg/kg/day was associated with a Cmax of 22.7 μg/ml and a 48-h AUC of 2,117.4 μg·min/ml. Thus, the Cmax and AUC exposures for these doses of the Mycograb C28Y variant were higher than the values achieved with the doses of Mycograb that were shown to be effective as mono- and combination therapies in the murine model of system candidiasis.
Could differences in the animal models be responsible for the discrepancy in the in vivo efficacies of Mycograb and the Mycograb C28Y variant? The preclinical in vivo benefit of using the original Mycograb formulation together with AMB was demonstrated in a murine model of systemic candidiasis in which single doses of AMB and Mycograb were administered alone or in combination to immunocompetent mice, with efficacy assessed 48 h later (5). In contrast, a multidose treatment design study in mice was chosen for evaluating the potential therapeutic benefit of the Mycograb C28Y variant in order to build upon a phase III human trial which used a multidose treatment regimen to demonstrate the efficacy of the combination of the original Mycograb formulation with AMB in people with invasive candidiasis. Since the original Mycograb formulation was evaluated in normal immune mice and in predominately nonneutropenic human patients, the first multidose study evaluating the interaction of the Mycograb C28Y variant and AMB was conducted in immunocompetent mice by others (Novartis data on file). In that study, normal immune BALB/c mice with systemic candidiasis were treated once daily for 7 days with 0.1 mg/kg of AMB, alone or in combination with 0.16 to 16 mg/kg/day of the Mycograb C28Y variant, and quantitative cultures were conducted on day 8. AMB given as monotherapy lowered the mean concentrations of fungi in the kidneys, spleens, and brains to ≤1.64 log CFU/g. The administration of 0.16 to 16 mg/kg/day of the Mycograb C28Y variant in combination with AMB provided no benefit over AMB monotherapy.
One unlikely hypothesis for why the Mycograb C28Y variant did not produce the same results as the original Mycograb formulation in mice with normal immune systems was that these mice developed anti-human antibody to the Mycograb C28Y variant in response to receiving multiple injections of this human recombination antibody fragment. If mouse antibodies did develop to the Mycograb C28Y variant, the serum concentrations of this human recombinant compound may decrease to extremely low levels. Unfortunately, the concentration of the Mycograb C28Y variant in serum was not measured at the time the multidose study was conducted in immunocompetent mice, because a validated assay for measuring the concentration of the Mycograb C28Y variant in serum did not exist. Although unproven, it is also possible that the very low (and highly efficacious) dose of 0.1 mg/kg/day of AMB employed in that study was associated with a concentration of AMB in the membranes of the fungi that was too low to generate a synergistic (or antagonistic) interaction with the Mycograb C28Y variant.
The neutropenic murine model of systemic candidiasis used in the current project resolved these potential problems. Similar to the infection model used by Matthews et al. (5), a dose of AMB was selected for examination in the neutropenic murine model that resulted in quantitative culture values in the kidneys of infected mice that were stable over the course of 5 days of therapy. As expected, a higher dose of AMB was required to achieve this goal in neutropenic mice than in immune-competent animals. Nevertheless, fungal densities in the kidneys of 4.15 to 4.29 log CFU/g in the neutropenic mice approximated the 3.66 ± 0.82 log CFU/g concentration that was found in the immunocompetent murine infection model described by Matthews et al. (5). Having a fungal population that remained stable with AMB therapy produced the most sensitive and unbiased mouse model that would be able to identify whether the interaction between AMB and the Mycograb C28Y variant was synergistic, indifferent, or antagonistic. Also, the stable fungal densities observed in kidneys of mice treated with AMB monotherapy were well above the lower limit of sensitivity of our quantitative culture assay of 1.3 log CFU/g and, therefore, would enable the investigators to quantify the amount of synergy or antagonism that the Mycograb C28Y variant would have with AMB if one of these interactions occurred. Finally, although we did not specifically measure the production of anti-human antibody in the sera of these neutropenic mice, the serum concentrations of the Mycograb C28Y variant at the 15- and 45-min time points in the single-dose pharmacokinetic study and after the fifth dose of this compound in the multidose pharmacokinetic study were similar. Since the concentration of the Mycograb C28Y variant would be expected to be lower or undetectable if the mice developed antibody against this human recombinant compound, the pharmacokinetic data suggest that the mice did not develop antibodies against the Mycograb C28Y variant in the multidose study. Importantly, since both the original Mycograb formulation and the Mycograb C28Y variant antibody fragments lack an Fc component, their activities are not believed to be due to recruitment of neutrophils to the sites of infection (5). Thus, the neutropenic model of systemic candidiasis overcame the potential problems of the multidose model in immune normal mice and was optimized to characterize whether the interaction between AMB and the Mycograb C28Y variant was synergistic, indifferent, or antagonistic.
Other differences in the mouse infection models employed by Matthews et al. versus those used by our group include the duration of therapy, the use of single versus multidose regimens, and the fungal and the mouse strains that were employed. We believe that none of these differences accounts for the disparities in outcomes for Mycograb and the Mycograb C28Y variant. As discussed earlier, the duration of therapy and the use of single versus multidose regimens did not explain the difference in outcomes for the original Mycograb formulation and the Mycograb C28Y variant, since the single-dose and multidose pharmacokinetics of the Mycograb C28Y variant were similar, suggesting that the neutropenic mice did not produce anti-human antibody against the Mycograb C28Y variant in response to multiple challenges of this compound. Also, since HSP90 is an essential component of the Candida cell wall and is required for fungal survival (4, 14), we do not believe that the different C. albicans strains examined by Matthews et al. and by our group could explain the different in vivo interactions that were observed for Mycograb and the Mycograb C28Y variant when they were administered alone or in combination with AMB. If the interaction between AMB and the Mycograb C28Y variant were dependent on the C. albicans strain examined, the clinical utility of the Mycograb C28Y variant would be substantially diminished.
Finally, differences in the mouse strains used to evaluate the activities of the original Mycograb formulation and the Mycograb C28Y variant could not explain the difference in activity of the two compounds. While Matthews et al. used 5- to 7-week-old 25-g female outbred CD-1 mice in their studies, we used 5- to 6-week-old 22- to 24-g female outbred Swiss Webster mice in our experiments, because we already had preliminary fungal inoculum-range and DAMB dose-range data for the C. albicans isolate in this mouse strain. Thus, consistent with the U.S. Public Health Service guidance for animal experimentation of reducing, refining, and not replicating available in vivo data, we chose to employ the outbred Swiss Webster mouse strain in the studies detailed in this report. CD-1 and outbred Swiss Webster mice are both general, multipurpose, immune-competent strains derived from inbred Swiss mice (based on information from the Charles River and Taconic Farms websites). Hence, the differences in the interaction of AMB with the original Mycograb formulation and the Mycograb C28Y variant were not dependent on the mouse strain that was evaluated. Further, if the efficacy of this investigational antibody fragment differed in closely related mouse strains, this too would markedly diminish the clinical value of this anti-HSP90 compound.
In summary, our study demonstrated that the combination of AMB and up to 15 mg/kg per day of the Mycograb C28Y variant offered no benefit over AMB monotherapy in a multidose neutropenic mouse model of systemic candidiasis. These results contrast with investigations for the original Mycograb formulation, which showed synergy with AMB in vitro, in a model of systemic candidiasis in mice with normal immune systems, and in a phase III clinical trial. Our study suggests that the modification of Mycograb at position 28 of the heavy chain that produced the Mycograb C28Y variant generated a structurally consistent compound that was less active than one or more components of the aggregated mixture of anti-HSP90 antibody structures that can be found in Mycograb solutions. The data also suggest that the checkerboard assay does not predict the in vivo interaction between the Mycograb C28Y variant (and perhaps other anti-HSP90 antibody fragments) and AMB. Therefore, the in vitro checkerboard assay cannot replace in vivo studies in assessing the interaction of anti-HSP90 antibody formulations with AMB for the treatment of invasive Candida infections.
ACKNOWLEDGMENT
This project was supported by Novartis Pharmaceuticals Corp., East Hanover, NJ.
Footnotes
Published ahead of print on 18 April 2011.
REFERENCES
- 1. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeast; approved standard, 3rd ed. M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 2. Gudlaugsson O., et al. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172–1177 [DOI] [PubMed] [Google Scholar]
- 3. Kullberg B. J., et al. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidemia in non-neutropenic patients: a randomized non-inferiority trial. Lancet 366:1435–1442 [DOI] [PubMed] [Google Scholar]
- 4. Matthews R. C., et al. 1998. Stress proteins in fungal diseases. Med. Mycol. 36(Suppl.):45–51 [PubMed] [Google Scholar]
- 5. Matthews R. C., et al. 2003. Preclinical assessment of the efficacy of mycograb, a human recombinant antibody against fungal HSP90. Antimicrob. Agents Chemother. 47:2208–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matthews R. C., Burnie J. P., Tabaqchali S. 1984. Immmunoblot analysis of the serological response in systemic candidosis. Lancet ii:1415–1418 [DOI] [PubMed] [Google Scholar]
- 7. Matthews R. C., Burnie J. P., Tabaqchali S. 1987. Isolation of immunodominant antigens from sera of patients with systemic candidiasis and characterization of serological response to Candida albicans. J. Clin. Microbiol. 25:230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNeil M. M., et al. 2001. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin. Infect. Dis. 33:641–647 [DOI] [PubMed] [Google Scholar]
- 9. Mora-Duarte J., et al. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020–2029 [DOI] [PubMed] [Google Scholar]
- 10. Pachl J., et al. 2006. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 42:1404–1413 [DOI] [PubMed] [Google Scholar]
- 11. Pappas P. G., et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883–893 [DOI] [PubMed] [Google Scholar]
- 12. Rex J. H., et al. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221–1228 [DOI] [PubMed] [Google Scholar]
- 13. Rex J. H., et al. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325–1330 [DOI] [PubMed] [Google Scholar]
- 14. Swoboda R. K., et al. 1995. Structure and regulation of the HSP90 gene from the pathogenic fungus Candida albicans. Infect. Immun. 63:4506–4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wey S. B., Mori M., Pfaller M. A., Woolson R. F., Wenzel R. P. 1988. Hospital-acquired candidemia: the attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642–2645 [DOI] [PubMed] [Google Scholar]