Abstract
Caspofungin (CFG) was tested in neutropenic and corticosteroid-immunosuppressed mice challenged with a lethal sinopulmonary inoculum of Rhizopus oryzae. Compared to untreated controls, CFG administered at 1 mg/kg of body weight/day significantly improved survival (54% versus 19%; P = 0.003) and reduced median R. oryzae fungal burden by 1.5 log10 for conidial equivalent DNA in neutropenic but not corticosteroid-immunosuppressed animals. CFG administered at 16 mg/kg/day was not significantly better than a saline control for treatment of invasive pulmonary mucormycosis (IPM) in either neutropenic or corticosteroid-immunosuppressed animals.
TEXT
Invasive pulmonary mucormycosis (IPM) is a life-threatening infection in immunocompromised patients, with few treatment options. Although preclinical and limited clinical data suggest that echinocandins have modest efficacy administered alone or in combination with other antifungals for treatment of rhinocerebral mucormycosis (2, 7, 8), less is known about the in vivo efficacy of echinocandins for treatment of pulmonary mucormycosis.
We examined the activity of caspofungin (CFG) in neutropenic or corticosteroid-immunosuppressed mice infected with a clinical isolate of Rhizopus oryzae 557969 (caspofungin MIC > 32 μg/ml by Etest; AB bioMérieux, l'Etoile, France). The isolate was previously used in our laboratories to establish IPM in neutropenic (4) and corticosteroid-immunosuppressed (3) animals that could be effectively treated with liposomal amphotericin B.
Eight-week-old female BALB/c mice (Harlan Laboratories, Houston, TX) (weight, 18 to 22 g) were used to establish the IPM model and were cared for in accordance with the highest ethical and humane standards as approved by The University of Texas M.D. Anderson Cancer Center and the University of Houston Institutional Animal Care and Use Committees. Cyclophosphamide and cortisone were prepared and administered to mice as previously reported for the neutropenic model (5), and the corticosteroid-only immunosuppression regimen described by Balloy et al. for invasive pulmonary aspergillosis was used (1). Animals also received doxycycline prophylaxis in drinking water (200 μg/ml) during periods of immunosuppression (4).
Just prior to inoculation, animals were rendered unconscious with 6% isoflurane; then, an inoculum of 3.5 ×104 R. oryzae 557969 conidia prepared the day of the experiment as previously described (4) was slowly pipetted in a volume of 35 μl into the nares of the mouse held in an upright position (4). The animal was then allowed to inhale the inoculum and observed until normal breathing resumed and recovery from the anesthesia was observed. This protocol was found to produce 80% mortality in untreated neutropenic animals at 7 days after inoculation.
Due to the rapidly progressive nature of R. oryzae infection in this model, we administered CFG (1 or 16 mg/kg of body weight) as daily intraperitoneal (i.p.) doses (200 μl) to groups of ≥15 immunosuppressed animals (total, 215 mice) starting 3 days prior to infection to ensure adequate lung tissue concentrations at the time of infection. CFG was continued until day +5 (with a total of 8 daily doses). Control animals received daily 200-μl i.p. saline injections. Animals were euthanized by CO2 narcosis at day +7 or when they appeared moribund according to defined criteria (4). R. oryzae conidial equivalent fungal DNA concentrations in lungs excised from euthanized animals were analyzed using a quantitative real-time PCR assay as previously described by Ibrahim and colleagues (2, 4).
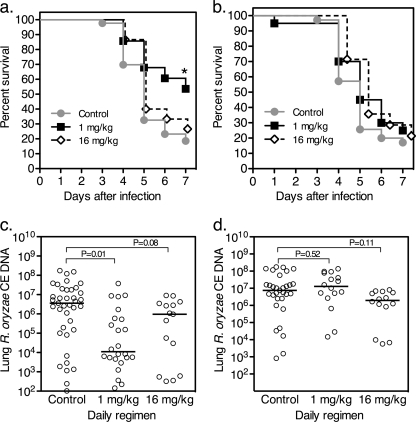
In previous work published by Ibrahim and colleagues, CFG was shown to improve the survival of diabetic ketoacidotic mice challenged with a low intravenous (i.v.) inoculum of R. oryzae when administered at 1 mg/kg of body weight/day divided as two doses but not at 5 mg/kg administered twice daily (2). Similarly, we found that a 1 mg/kg daily regimen of CFG starting 3 days prior to sinopulmonary challenge with R. orzyae significantly improved day +7 survival of neutropenic mice (53% versus 19%, respectively; P = 0.03) (Fig. 1a) but not corticosteroid-immunosuppressed mice (Fig. 1b). Interestingly, the higher-dose CFG 16 mg/kg/day regimen was less effective at prolonging animal survival (Fig. 1a) and reducing median fungal burden (Fig. 1c) in neutropenic animals and was similarly ineffective for corticosteroid-immunosuppressed animals (Fig. 1b and d).
Fig. 1.
Caspofungin improves survival and reduces median lung fungal burden and in neutropenic but not corticosteroid-immunosuppressed mice with invasive pulmonary mucormycosis. (a and b) Survival curves are presented for neutropenic (a) and corticosteroid-immunosuppressed (b) animals. Each group contained ≥15 mice. *, P < 0.05 by Mantel-Cox log-rank test. (c and d) Lung fungal burden was assessed at day +7 after infection or at the time of euthanization of experimentally infected neutropenic (c) or corticosteroid-immunosuppressed (d) animals. Each datum point represents the mean R. oryzae conidial equivalent (CE) concentration of DNA per lung from a single mouse measured in duplicate using a quantitative real-time PCR assay in relation to a 6-point standard curve. Solid bars represent the median fungal burden values. P values were determined by analysis of variance (ANOVA) with Dunn's correction for multiple comparisons.
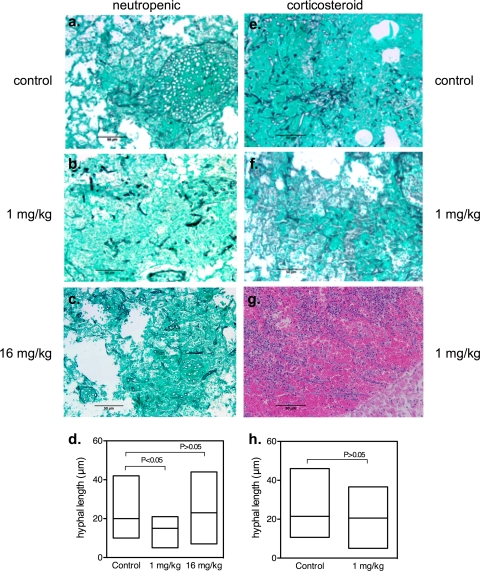
To further assess antifungal effects in vivo, we also compared patterns of hyphal invasion and disease severity at day +3 in groups of 5 mice who were infected and treated as described above. Neutropenic control animals had evidence of extensive angioinvasive hyphae with hemorrhage and alveolar consolidation (Fig. 2a) and median hyphal lengths of 20 μm (range, 9 to 42 μm) per infiltrate field (Fig. 2d). Corticosteroid-immunosuppressed control animals displayed similar patterns of extensive angioinvasive hyphae with extensive consolidation (Fig. 2e) and median hyphal lengths of 23 μm (range, 11 to 46 μm) (Fig. 2h). The similarity of neutropenic and corticosteroid-immunosuppressed animals with respect to patterns of angioinvasion differed from the results of studies of experimental pulmonary aspergillosis in which angioinvasive hyphae were less common in the lungs of corticosteroid-immunosuppressed mice than in those of neutropenic animals (1).
Fig. 2.
Caspofungin impairs early hyphal proliferation in neutropenic but not corticosteroid-immunosuppressed mice with invasive pulmonary mucormycosis. Select experiments were repeated with groups of 5 animals to compare lung histology results at 72 h by comparing the lungs of neutropenic (a to c) and corticosteroid-immunosuppressed (e to g) mice. Lungs were excised and fixed in 10% (vol/vol) formaldehyde and embedded in paraffin wax before being subjected to staining with Grocott's methenamine silver nitrate (a to c, e, and f) or hematoxylin and eosin (g). Photomicrographs were acquired at a magnification of ×200 (scale bar, 25 μm) with 3 separate areas of infiltration per mouse, and hyphal length was calculated for all discernible hyphal elements in the lung field (average, 35 hyphae per field) by the use of calibrated computer image analysis software (Macnification version 1.5; Orbicule BVBA, Heverless, Belgium). Mean hyphal lengths for mice in the neutropenic (d) and corticosteroid-immunosuppressed (h) treatment groups at 72 h were compared using the Kruskal-Wallis test.
CFG treatment at 1 mg/kg/day was associated with disease of lesser severity and decreased angioinvasion in neutropenic (Fig. 2b) but not corticosteroid-immunosuppressed mice (2f). Median hyphal lengths per infiltrate field at 72 h for animals treated with CFG at 1 mg/kg/day were significantly shorter than 72 h controls (20 versus 14 μm; P < 0.05) (Fig. 2d); however, the differences were not statistically significant when animals were compared at later time points (days 5 to 7). As with the fungal burden and survival studies, CFG treatment at 16 mg/kg/day was not associated with appreciable reductions in infection severity or hyphal angioinvasion at 72 h (Fig. 2c and d).
All corticosteroid-immunosuppressed mice had extensive angioinvasive hyphae and infiltrates in the lungs by 72 h for all CFG treatments and doses (Fig. 2e, f, and h). In contrast to the results seen with neutropenic animals, however, we observed extensive infiltration of polymorphonuclear leukocytes (PMN) surrounding hyphal elements that was associated with widespread hemorrhage and coagulative necrosis in surrounding lung fields as early as 72 h (Fig. 2g). These histological findings suggest that extensive PMN pulmonary infiltration resulting in diffuse hemorrhage and coagulative necrosis may have been a factor contributing to the lack of observable CFG activity in the corticosteroid-immunosuppressed animals.
Collectively, our data are consistent with previous work by Ibrahim et al. (2) and demonstrate that lower doses (i.e., 1 mg/kg/day) but not higher doses (16 mg/kg/day) of CFG are modestly effective in vivo at impairing the progression of R. orzyae pulmonary mucormycosis. The poor activity of the echinocandin in the setting of corticosteroid-induced immunosuppression, as well as the profound paradoxical response of the fungus to higher CFG doses, may be a consequence of the tremendous genetic capacity of R. oryzae to adapt to immune cell and drug-associated damage (6). Future studies focusing on these adaptive responses of the fungus could provide insights into mechanisms for enhancing antifungal activity against this notoriously aggressive and multidrug-resistant fungus.
Acknowledgments
Merck & Co. Inc. funded D.P.K. and this study through an investigator-initiated proposal. The funding source was not involved in the design, completion, analysis of data, or writing of the manuscript. R.E.L. has received research funding and served as a consultant to Merck & Co. Inc. and Astellas Inc. D.P.K. has received research funding from Merck & Co. Inc., Astellas Inc., and Pfizer Inc. and serves on consultancy boards or speaker boards for Gilead Inc., Merck & Co. Inc., and Pfizer Inc. K.L. and G.L. have no disclosures to make.
Footnotes
Published ahead of print on 2 May 2011.
REFERENCES
- 1. Balloy V., Huerre M., Latge J. P., Chignard M. 2005. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect. Immun. 73:494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ibrahim A. S., et al. 2005. Caspofungin inhibits Rhizopus oryzae 1,3-β-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 49:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamaris G. A., et al. 2009. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J. Infect. Dis. 199:1399–1406 [DOI] [PubMed] [Google Scholar]
- 4. Lewis R. E., et al. 2010. Comparative pharmacodynamics of amphotericin B lipid complex and liposomal amphotericin B in a murine model of pulmonary mucormycosis. Antimicrob. Agents Chemother. 54:1298–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewis R. E., Wiederhold N. P. 2005. Murine model of invasive aspergillosis. Methods Mol. Med. 118:129–142 [DOI] [PubMed] [Google Scholar]
- 6. Ma L. J., et al. 2009. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 5:e1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reed C., et al. 2008. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect. Dis. 47:364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spellberg B., Fu Y., Edwards J. E., Jr., Ibrahim A. S. 2005. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob. Agents Chemother. 49:830–832 [DOI] [PMC free article] [PubMed] [Google Scholar]