Abstract
The ongoing spread of methicillin-resistant Staphylococcus aureus (MRSA) strains in hospital and community settings presents a great challenge to public health and illustrates the urgency of discovering new antibiotics. Marinopyrrole A is a member of a structurally novel class of compounds identified from a species of marine-derived streptomycetes with evidence of antistaphylococcal activity. We show that marinopyrrole A has potent concentration-dependent bactericidal activity against clinically relevant hospital- and community-acquired MRSA strains, a prolonged postantibiotic effect superior to that of the current first-line agents vancomycin and linezolid, and a favorable resistance profile. Marinopyrrole A showed limited toxicity to mammalian cell lines (at >20× MIC). However, its antibiotic activity against MRSA was effectively neutralized by 20% human serum. A variety of marinopyrrole analogs were isolated from culture or synthetically produced to try to overcome the inhibitory effect of serum. While many of these compounds retained potent bactericidal effect against MRSA, their activities were also inhibited by serum. Marinopyrrole A has significant affinity for plastic and may therefore have potential as a potent anti-MRSA agent in cutaneous, intracatheter, or antibiotic-lock applications.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) infections have reached epidemic proportions in many countries (10) and now represent the most common cause of skin and soft tissue infections in the United States (5). Both hospital-associated (HA) and community-associated (CA) MRSA can exhibit broad resistance to multiple classes of antibiotics (2, 10). Notwithstanding the discovery of the oxazolidinone linezolid in 2000 and the lipopeptide daptomycin in 2003, the small number of new antibiotics discovered over the last half century has defined an urgent need for novel agents to treat MRSA infections (1).
Beginning with the introduction of penicillin, natural products have provided diverse chemical scaffolds leading to 9 of the 12 classes of antibiotics currently used in the clinic (1, 8, 12). The majority of these bioactive secondary metabolites are derived from terrestrial actinomycete bacteria. Although much less explored than terrestrial environments, the ocean has been shown to be a bountiful source of biological and genetic diversity (9) that leads to chemically novel natural products (8, 12).
We recently isolated a novel chemical scaffold from a previously uncharacterized marine actinomycete, designated CNQ-418 (13, 14). This natural product, marinopyrrole A, was found to contain an uncommon 1,3′-bipyrrole pharmacophore, and initial screening revealed potent antistaphylococcal activity (13, 14).
In the current study, we characterize the in vitro activities of marinopyrrole A against a variety of clinically important MRSA strains. Key pharmacological properties of marinopyrrole A that are relevant to anti-MRSA activity, including time-kill kinetics, postantibiotic effect, a tendency to develop resistance in serial passage, cytotoxicity, serum inactivation, and plastic binding, were assessed. Additionally, several derivatives of the novel marinopyrrole A scaffold were evaluated for assessment of structure-activity relationships targeting contemporary strains of MRSA. Our data show that marinopyrrole A exhibits a number of favorable anti-MRSA activities, including rapid killing kinetics, a prolonged postantibiotic effect, and limited eukaryotic cell cytotoxicity.
(A portion of this work was presented previously at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], 2009 [abstract F1-1501] [11].)
MATERIALS AND METHODS
Bacterial strains and media.
A panel of multiply resistant Gram-positive and Gram-negative pathogens was used to probe the antimicrobial activity of marinopyrrole A. We tested the methicillin-sensitive S. aureus (MSSA) reference strain ATCC 29213, the MSSA USA200 strain UAMS-1, the MRSA USA300 strains UAMS-1182 and TCH1516 (ATCC BAA-1717), the CA/HA-MRSA USA700 isolate NRS386, and HA-MRSA strains Sanger 252 and ATCC 33591. To expand the evaluation of marinopyrrole A against multiresistant S. aureus strains, we tested a panel of glycopeptide-intermediate S. aureus (GISA) and two vancomycin-resistant S. aureus (VRSA) strains, including HIP5836 (GISA; New Jersey), A5940 (hetero-GISA), PC-3 (GISA; New York), and VRSA (Michigan) and VRSA (Pennsylvania). Further, our analyses included a wider panel of Gram-positive organisms, including Streptococcus pyogenes (group A streptococcus [GAS] strains M1-5548 and M49-NZ131, Streptococcus agalactiae (group B streptococcus [GBS]) strain COHI, Staphylococcus epidermidis ATCC 12228, Bacillus anthracis (Sterne strain), Bacillus subtilis (strain 3610), and a vancomycin-resistant Enterococcus faecalis (VRE) isolate (ATCC 51299). In addition, we tested marinopyrrole A against four Gram-negative strains, a Pseudomonas aeruginosa blood isolate (ATCC 27853), Haemophilus influenzae (type b; ATCC 1021), Klebsiella pneumoniae (ATCC 700603), and Escherichia coli (ATCC 25922). NRS386 was obtained through the Network of Antimicrobial Resistance in S. aureus (NARSA) program (Chantilly, VA) supported under NIAID/NIH contract HHSN272200700055C. UAMS-1 and UAMS-1182 isolates were provided by G. Somerville at the University of Nebraska, Lincoln, NE, and were originally obtained from Mark Smeltzer at the University of Arkansas Medical Center. Strains designated ATCC were obtained from the American Type Culture Collection (Manassas, VA). B. subtilis (strain 3610) was provided by Pieter Dorrestein, University of California—San Diego (UCSD). Glycopeptide-intermediate and -resistant strains were obtained from George Sakoulas, UCSD. For all strains except H. influenzae, Todd-Hewitt broth (THB) (Sigma, St. Louis, MO) was used for susceptibility, time-kill kinetics, and postantibiotic effect studies, with overnight plating on Todd-Hewitt agar (THA) performed for enumeration of CFU. For H. influenzae, brain heart infusion (BHI) medium was supplemented with hemin (10 μg/ml) and NAD (10 μg/ml; Sigma, St. Louis, MO).
Antimicrobial agents.
The marine natural product marinopyrrole A (see Fig. 4, MPA), was produced by cultivation of the actinomycete strain CNQ-418; methods for its extraction, purification, and structure elucidation were published by Hughes et al. (14). The purified yellow metabolite was solubilized in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO), stored at −20°C, and thawed immediately prior to each use. Derivatives of marinopyrrole A were prepared similarly or were synthesized for susceptibility studies (14). Vancomycin (Novaplus Hospira, Inc., Lake Forest, IL) and linezolid (Zyvox Pfizer, Kalamazoo, MI) (2 mg/ml) were obtained from the pharmacy service at UCSD Medical Center.
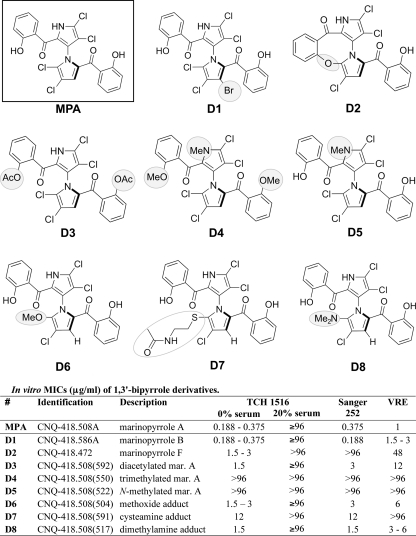
Fig. 4.
1,3′-Bipyrrole derivatives of marinopyrrole.
Susceptibility testing.
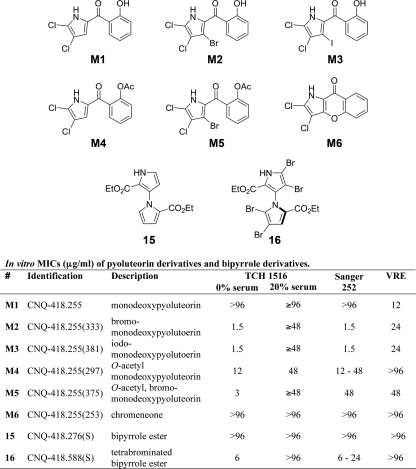
The susceptibility of a bacterial strain to marinopyrrole A was tested by broth macrodilution in duplicate 5-ml polystyrene round-bottom tubes (Falcon, Bedford, MA). Each tube contained a final volume of 4 ml that was first inoculated with 5 × 105 CFU/ml and then incubated with shaking at 37°C for 22 to 24 h. After incubation, 0.2 ml of medium from each of the duplicate tubes, including the growth and blank-medium control tubes, was transferred into a sterile 96-well plate for turbidimetric measurement (optical density at 600 nm [OD600]). Susceptibility testing for the marinopyrrole derivatives (see Fig. 4, D1 to D8, and Fig. 5, M1 to M6, 15, and 16) and for marinopyrrole A against some bacterial strains (Table 1 ) was accomplished by broth microdilution in tissue culture-treated 96-well flat-bottom polystyrene plates (Falcon; Becton Dickinson, Franklin Lakes, NJ). The cultures were inoculated with 5 × 105 CFU/ml and incubated with shaking at 37°C for 22 to 24 h. The MIC was determined to be the lowest concentration of antibiotic that inhibited visible bacterial growth, as measured turbidimetrically (OD600).
Fig. 5.
Pyoluteorin derivatives and synthetic 1,3′-bipyrrole derivatives of marinopyrrole.
Table 1.
In vitro activities (MICs) of marinopyrrole A compared to those of commonly used antibiotics against selected clinical drug-resistant strains
| Strain | Classification | MIC (μg/ml) |
||
|---|---|---|---|---|
| Marinopyrrole A | Vancomycin | Linezolid | ||
| Staphylococcus aureus | ||||
| ATCC 29213 | MSSA | 0.5–1 | 1–2 | NAb |
| UAMS1 (USA200) | MSSA | 0.75 | 1.5 | NA |
| TCH1516 (USA300) | CA-MRSA | 0.188–0.375 | 2 | 3 |
| UAMS1182 (USA300) | CA-MRSA | 0.188–0.375 | 1.56 | 3 |
| NRS386 (USA700) | CA/HA-MRSA | 0.375 | 2–4 | 3 |
| Sanger 252 | HA-MRSA | 0.375 | 1–2 | 3 |
| MRSA ATCC 33591 | HA-MRSA | 0.188–0.375 | 1.56 | NA |
| VRSA (Michigan) | VRSA | 0.375–1.5 | >256 | NA |
| VRSA (Pennsylvania) | VRSA | 0.375–1 | >256 | NA |
| A5940a | Hetero-GISA | 0.25–0.5 | 4 | NA |
| PC-3 (New York)a | VISA | 0.25–0.5 | 8 | NA |
| HIP5836 (New Jersey)a | VISA | 0.25–0.5 | 4-8 | NA |
| Other | ||||
| E. faecalis | vanB | 1 | ||
| S. pyogenes | GAS (MI-5548) | 1 | ||
| S. pyogenes | GAS (M49-NZ131) | 1 | ||
| S. agalactiae | GBS (COHI) | 2 | ||
| S. epidermidis | ATCC 12228 | 0.25–1 | ||
| B. anthracis | Sterne strain | 1–2 | ||
| B. subtilis | Strain 3610 | 0.94–1.87 | ||
| H. influenzae | ATCC 1021 | 2 | ||
| K. pneumoniae | ATCC 700603 | >16 | ||
| P. aeruginosa | ATCC 27853 | >96 | ||
| E. coli | ATCC 25922 | >120 | ||
Reference 17.
NA, not applicable.
Cytotoxicity.
Marinopyrrole A cytotoxicity was assessed by seeding 2 × 104 HeLa or L929 cells per well in sterile 96-well tissue culture-treated plates (Falcon; Becton Dickinson, Franklin Lakes, NJ). After 24 h, the medium was replaced with fresh medium containing increasing concentrations of marinopyrrole A, and the plates were incubated at 37°C in 5% CO2 for 24 h. Cytotoxicity was assayed at 24 h by measuring the reduction of MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] using the CellTiter 96 Aqueous nonradioactive cell proliferation assay according to the manufacturer's instructions (Promega, Madison, WI).
Time-kill analysis.
The bactericidal activities of marinopyrrole A, vancomycin, and linezolid against the CA-MRSA isolate TCH1516 were assessed by time-kill analysis essentially as described previously (3, 7, 20). MRSA was grown overnight in THB at 37°C with shaking, and then fresh THB was inoculated for growth to mid-logarithmic phase. Bacteria were added at a starting inoculum of ∼5 × 105 CFU/ml to duplicate 5 ml polystyrene round-bottom tubes (Falcon, Bedford, MA). Marinopyrrole A, vancomycin, and linezolid were added to final concentrations equal to 0.25, 1.0, 10, or 20 times their experimentally determined MICs of 0.375 μg/ml, 2 μg/ml, and 3 μg/ml, respectively. Bacteria grown in the presence of an equivalent percentage of antibiotic diluent (DMSO) served as the growth control. The cultures were incubated in a 37°C shaking incubator, and aliquots were removed from each tube at 0, 3, 6, 9, and 24 h and serially diluted for CFU enumeration on THA plates. To compare rates of antibiotic killing, the decline of viable bacteria (log10 CFU/ml) was evaluated at the measured time points. The limit of detection for this assay was 1.6 log10 CFU/ml.
Postantibiotic effect.
The postantibiotic effect (PAE) was determined by the viable plate count method (6) using THB. A mid-logarithmic-phase culture of the CA-MRSA strain TCH1516 was prepared as described for the time-kill studies. A starting inoculum of ∼5 × 105 CFU/ml logarithmic-phase bacteria in a final volume of 4 ml was exposed to concentrations of either marinopyrrole A, vancomycin, or linezolid at 1, 10, or 20 times the respective MICs for 1 h at 37°C with shaking. Each solution was prepared in duplicate in 5-ml polystyrene tubes (Falcon, Bedford, MA) with a growth control included as described above. After 1 h, each culture was centrifuged at 4,000 rpm for 10 min. The supernatant was removed, and the bacterial pellet was washed with 4 ml of antibiotic-free THB. The bacterial pellets were then resuspended in fresh THB to a total volume of 4 ml and allowed to recover in a 37°C shaking incubator. Aliquots were removed at −1, 0, 2, 4, 6, 8, and 10 h for CFU enumeration on THA as detailed in the time-kill procedure. The PAE was calculated according to the Craig and Gudmundsson formula: PAE = T − C (6), where T refers to the time required for the treated culture to recover by 1 log10 CFU greater than that observed immediately after drug removal (time zero) and C refers to the corresponding recovery time observed for the untreated control (6).
Resistance studies by serial-passage mutagenesis.
Serial-passage mutagenesis studies were done using the assay described by Silverman et al. (19). Briefly, on day 1, duplicate sets of polystyrene tubes containing THB and marinopyrrole A at either 4× (1.5 μg/ml), 2× (0.75 μg/ml), 1× (0.375 μg/ml), 0.5× (0.189 μg/ml), or 0.25× MIC (0.094 μg/ml) or 0 μg/ml were inoculated with a single colony of S. aureus strains TCH1516 (USA300 CA-MRSA) or Sanger 252 (HA-MRSA). MRSA cultures were incubated at 37°C overnight with shaking. At 16 to 24 h of incubation, the culture at the highest concentration of marinopyrrole A that supported bacterial growth was diluted 1:10,000 into sets of fresh medium and marinopyrrole A at the same concentrations listed above. This serial-passage experiment was continued for 10 days, ending when cultures did not show a change in susceptibility for three successive days (19).
Effect of human serum on antibiotic activity.
The MIC of marinopyrrole A compared to vancomycin against CA-MRSA strain TCH1516 was determined in the presence of 20% pooled human serum in THB. Using 96-well tissue culture-treated plates (Microtest 96; Becton Dickinson, Franklin Lakes, NJ), MICs in the presence and absence of serum were determined using an adaptation of the standard broth microdilution method (21). Due to the opacity produced by addition of serum to the assay, the color change indicator resazurin (Sigma-Adrich, St. Louis, MO) was used as a surrogate indicator of bacterial growth (18). Oxidoreductases of viable bacteria reduce blue resazurin to pink resorufin (18, 21). For this assay, resazurin sodium salt powder was solubilized in sterile water to a final concentration of 6.75 mg/ml (18) and filtered by centrifugation at 10,000 rpm for 10 min using a Costar 8160 Spin-X Centrifuge Tube Filter, and the filtrate was added to each well of the test plate to a final concentration of 10% (21). Mid-logarithmic-phase MRSA was added at a starting inoculum of 5 × 105 CFU/ml to the test plates containing the serially diluted antibiotics plus 10% resazurin solution. The plates were covered with foil and incubated for 24 h with shaking at 37°C. After overnight incubation, the plates were evaluated visually for blue-to-pink color change, indicative of bacterial growth. The MIC was determined to be the lowest antibiotic concentration that did not induce the blue-to-pink color change.
Antibiotic adsorption to plastic.
The degrees of marinopyrrole A adsorption to plastic and glass were compared in similarly sized disposable borosilicate 12- by 75-mm glass test tubes (VWR Culture Tubes) and 5-ml polystyrene round-bottom tubes (Falcon, St. Louis, MO). Marinopyrrole A was added to a final volume of 4 ml THB in each tube at 0, 1, 2, 5, 10, or 20 times the MIC for the MRSA strain TCH1516, 0.375 μg/ml. Each tube was incubated in the absence of bacteria for 22 to 24 h in a 37°C shaking incubator to mimic the experimental conditions of the time-kill kinetics studies. Following incubation, medium containing antibiotic was removed from each tube, and the inside walls of the plastic and glass tubes were washed repeatedly five times with 5 ml water. Then, the adsorbed antibiotic was extracted with 4.0 ml methanol. The resulting sample was analyzed in triplicate via UV spectroscopy at 324 nm. The UV data were averaged to quantify the drug adsorbed to the glass and plastic tubes at the tested concentrations.
RESULTS
In vitro antimicrobial activity.
Marinopyrrole A showed activity against all tested S. aureus strains, including glycopeptide-intermediate and vancomycin-resistant MRSA, and had potent activities against other Gram-positive organisms. In addition, marinopyrrole A was active against H. influenzae but was inactive against other tested Gram-negative strains (Table 1). The MICs of marinopyrrole A against the panel of S. aureus strains (0.188 to 1.5 μg/ml) were generally lower than those measured for vancomycin or linezolid, and the measured MICs against VRE (1 μg/ml) and H. influenzae (2 μg/ml) were also favorably potent.
Concentration-dependent bactericidal killing.
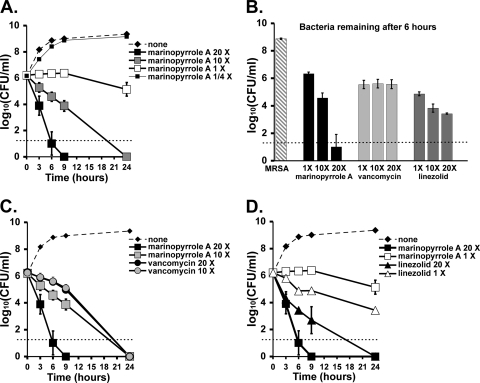
In vitro time-kill studies were used to further characterize marinopyrrole A activity against MRSA. Marinopyrrole A displayed substantial concentration-dependent killing against MRSA strain TCH1516 (Fig. 1 A) and was far more rapid in its antibiotic action than either vancomycin (Fig. 1B and C) or linezolid (Fig. 1B and D). For example, marinopyrrole A at 10× MIC (3.75 μg/ml) showed a 2-log-unit kill of MRSA TCH1516 within 9 h, while the activity of vancomycin at 10× MIC (20 μg/ml) was much slower, reducing the initial inoculum by only about 10-fold. Treatment with marinopyrrole A at 20× MIC (7.5 μg/ml) reduced the initial inoculum by nearly 6-log-fold within 9 h.
Fig. 1.
Time-kill kinetics of marinopyrrole A, vancomycin, and linezolid against MRSA (USA300 strain TCH1516) at multiples of their MICs (0.375 μg/ml, 2 μg/ml, and 3 μg/ml, respectively). (A) Concentration-dependent effects of marinopyrrole A at multiples above and below the MIC. (B) Quantitative cultures of surviving MRSA after 6 h of incubation with 20×, 10×, or 1× MIC of each compound, highlighting the concentration-dependent killing of marinopyrrole A in comparison to equal ratios of linezolid and vancomycin. (C) Marinopyrrole A and vancomycin kinetics at 20× and 10× MIC. (D) Marinopyrrole A and linezolid kinetics at 20× and 1× MIC. Data indicate means ± standard deviations (SD).
Prolonged postantibiotic effect.
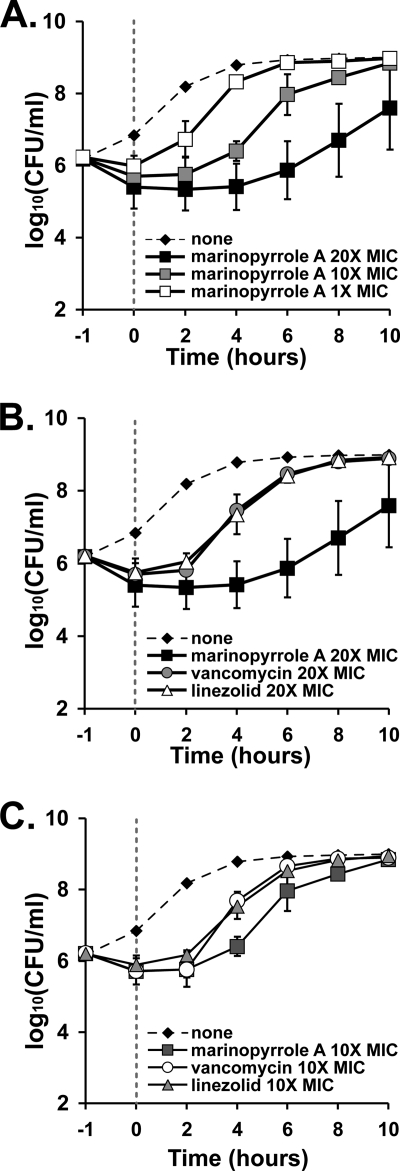
Given its rapid and concentration-dependent activity, we investigated the potential PAE of marinopyrrole A against MRSA. As shown in Fig. 2 A, marinopyrrole at 1×, 10×, or 20× MIC exhibited a concentration-dependent PAE against MRSA strain TCH1516. When vancomycin, linezolid, and marinopyrrole A, each at 20× MIC (Fig. 2B), were compared, a significantly longer recovery of the culture treated with marinopyrrole A was evident, with a calculated PAE (T − C) of between 4 and 6 h. Evidence of this prolonged recovery was also seen at 10× MIC of marinopyrrole A in comparison to the other tested antibiotics (Fig. 2C). Rapid concentration-dependent killing and a prolonged PAE against MRSA are highly favorable and distinguish the pharmacological activity of marinopyrrole A from those of vancomycin and linezolid.
Fig. 2.
PAEs of marinopyrrole A and reference compounds. TCH1516 was incubated for 1 h with the tested compounds at multiples of 20×, 10×, and 1× their respective MICs of 0.375 μg/ml (marinopyrrole A), 2 μg/ml (vancomycin), and 3 μg/ml (linezolid). The antibiotics were subsequently removed, and the bacteria were washed (time zero) and allowed to recover in antibiotic-free medium. The curves show the rates of growth recovery for the bacteria against the tested compounds. (A) Concentration-dependent PAEs for marinopyrrole A at 20×, 10×, and 1× MIC. (B and C) Comparisons of marinopyrrole A, vancomycin, and linezolid at 20× MIC (B) and 10× MIC (C). Data indicate means ± SD.
Resistance studies by serial-passage mutagenesis.
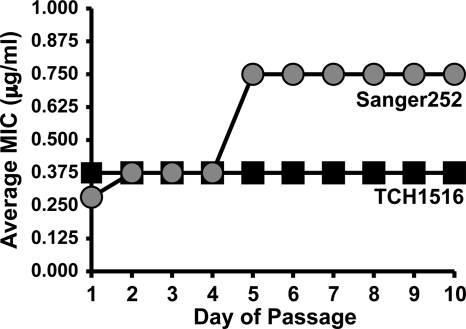
To assess the propensity of MRSA to develop resistance to marinopyrrole A, we tested two MRSA strains, CA-MRSA USA300 TCH1516 and HA-MRSA Sanger 252, in a serial-passage experiment (19). We found that the MIC remained unchanged (MIC = 0.375 μg/ml) during 10 passages (Fig. 3). The MIC of the multidrug-resistant hospital-associated strain Sanger 252 increased by 2-fold to 0.75 μg/ml during 10 passages. Thus, marinopyrrole A exhibited sustained potent inhibitory activities despite repeated bacterial passage in sub-MIC doses of the compound.
Fig. 3.
Serial passage of MRSA strains in sub-MIC concentrations of marinopyrrole A. The tendency for two strains, HA-MRSA Sanger 252 and CA-MRSA TCH1516, to develop resistance to marinopyrrole A was evaluated over the course of 10 days. The average MIC of Sanger 252 increased 2-fold, while that of TCH1516 did not change during serial passage.
Cytotoxicity profile.
In order to estimate a therapeutic index for marinopyrrole A, we tested the compound for cytotoxicity against two mammalian cell lines. Marinopyrrole A exhibited a favorable therapeutic index, with 50% inhibitory concentrations (IC50) in excess of 20× above the MIC in each case: 32 to 64 μg/ml against HeLa cells and 8 to 32 μg/ml against L929 cells.
Activity in the presence of human serum.
In order to determine the potential for marinopyrrole A as a systemic therapeutic, we investigated the MIC in the presence of normal human serum. The MIC of marinopyrrole A in the presence of 20% serum was ∼96 μg/ml, approximately 256-fold higher than the MIC in the absence of serum. This property, presumably reflecting a high degree of protein binding, greatly reduces the activity of marinopyrrole and compromises its prospects as a therapeutic to treat systemic bacterial infections.
Activities of marinopyrrole derivatives.
Following the discovery that the potent anti-MRSA activity of marinopyrrole A was effectively abolished in serum, derivatives of the marinopyrrole A scaffold were tested for the ability to bypass this inherent pharmacologic limitation. A set of 16 derivatives of marinopyrrole A, whose preparation is described in detail elsewhere (13), were analyzed. These derivatives include semisynthetic and synthetic analogs, in addition to a range of minor metabolites produced by the streptomycete strain CNQ-418. Two distinct chemical scaffolds were explored here. The first group consisted of derivatives that contain the 2,2′-salicyloyl-1,3′-bipyrrole structure of the parent compound (Fig. 4, MPA), produced semisynthetically or isolated from culture (Fig. 4, D1 to D8). The second group of derivatives were completely synthetic and include pyoluteorin-like compounds (Fig. 5, M1 to M6), as well as two 1,3′-bipyrroles lacking salicyloyl substituents (Fig. 5, 15 and 16).
The 16 marinopyrrole A derivatives (Fig. 4, D1 to D8, and 5, M1 to M6, 15, and 16) were tested for activity against CA-MRSA TCH1516, HA-MRSA Sanger 252, and vancomycin-resistant E. faecalis. Many of these structural analogs retained potent activities against the three bacterial strains tested in the low μg/ml range. However, the marked inhibitory effect of 20% human serum on the activity of marinopyrrole A could not be overcome with any of the structural modifications (Fig. 4 and 5).
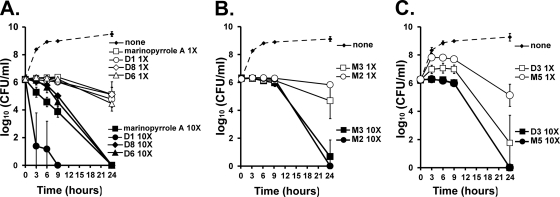
Similar to marinopyrrole A, the majority of the semisynthetic bipyrrole-containing analogs in Fig. 4 (MPA, D1, D6, and D8) showed concentration-dependent killing (Fig. 6 A). For two pyrrole derivatives, bromomonodeoxypyoluteorin (Fig. 5, M2) and iodomonodeoxypyoluteorin (Fig. 5, M3), a noticeable phenomenon of time-dependent killing was observed. For these compounds, treatment of MRSA at 10× and 1× MIC initially inhibited its growth without significant bactericidal activity (Fig. 6B). Within 24 h, however, these compounds displayed significant bactericidal activity at 10× MIC. An interesting time-kill kinetic was shared by O,O′-diacetyl marinopyrrole A (Fig. 4, D3) and O-acetyl bromomonodeoxypyoluteorin (Fig. 5, M5). Both O-acetylated derivatives allowed an initial increase in bacterial growth in the in vitro assay but ultimately inhibited further bacterial growth (Fig. 6C). This effect can likely be attributed to the gradual hydrolysis of the phenolic esters to their free phenolic counterparts, which are bactericidal.
Fig. 6.
In vitro kinetics of the marinopyrrole analogs against MRSA (TCH1516). (A) Concentration-dependent kinetics of marinopyrrole analogs (Fig. 4). (B) Kinetics of pyoluteorin analogs (Fig. 5). (C) Kinetics of two acetylated marinopyrrole A derivatives (the compounds are shown in Fig. 4 and 5). Data indicate means ± SD.
Marinopyrrole A adsorption to plastic.
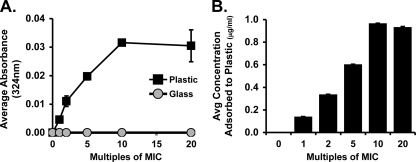
Our initial studies of marinopyrrole A in the macrodilution assay (described above) showed the MIC was 2- to 4-fold lower in glass test tubes than in polystyrene tubes (e.g., 0.094 μg/ml for TCH1516 with glass tubes). We hypothesized that marinopyrrole A in solution was partially binding to plastic, a phenomenon described for other antibiotics, including doxycycline and minocycline (4). This event, called sorption, can occur when a drug is initially adsorbed and subsequently absorbed by its plastic container (4, 15), which may be ascribed to the very hydrophobic and slightly acidic nature of marinopyrrole A. To examine this possibility, the loss of marinopyrrole A to the walls of polystyrene versus glass tubes was measured by methanol extraction of adsorbed drug and UV spectrometric quantification at A324. Our results (Fig. 7 A) showed that the amount of marinopyrrole A that adsorbed to plastic increased in a linear fashion as the tested concentration increased from 1 to 2, 5, and 10 times the MIC, from 0.0046 ± 0.0010 (1× MIC) to 0.0316 ± 0.0007 (10× MIC). At the tested concentrations greater than 10× MIC, the absorbances seem to plateau, possibly indicating saturation of binding (Fig. 7A). Conversely, the absorbance results show that the glass tubes did not retain marinopyrrole A at any of the tested concentrations (Fig. 7A). The resulting amounts (μg) of marinopyrrole A recovered by methanol extraction from plastic tubes were calculated from the measured absorbances (Fig. 7B) and similarly showed a linear increase in adsorbed compound with saturation after 10× MIC.
Fig. 7.
The tendency for marinopyrrole A to adsorb to plastic was tested by incubating plastic and glass test tubes for 22 h with increasing concentrations of marinopyrrole A. After incubation, methanol extraction was used to remove any adsorbed marinopyrrole A. (A) UV spectrometric quantification. Shown are the average absorbances (324 nm) by the plastic and glass tubes at increasing multiples of the MIC. Marinopyrrole A adsorbs to plastic tubes, but not glass. (B) The average concentration of marinopyrrole A removed from plastic tubes via methanol extraction increases as the compound concentration is increased. Data indicate means ± SD.
DISCUSSION
We have shown that the recently discovered marine natural product marinopyrrole A (1) possesses potent in vitro antimicrobial activities against both HA and CA strains of MRSA and VRE at submicromolar levels. Furthermore, marinopyrrole A maintained similar submicromolar MICs against glycopeptide-intermediate and vancomycin-resistant S. aureus strains, such as VRSA from Pennsylvania and Michigan, VISA strains HIP5836 and PC-3, and hetero-GISA A5940. Marinopyrrole A is also active against H. influenzae, but its Gram-negative spectrum does not extend to P. aeruginosa or E. coli. Compared to vancomycin and linezolid, marinopyrrole A demonstrated potent concentration-dependent bactericidal kinetics against MRSA, with a 4 log10 increase in bacterial killing at 20× versus 1× MIC. In contrast, vancomycin showed time-dependent kinetics in which all three concentrations tested (1×, 10×, and 20× MIC) yielded equal quantities of surviving MRSA at 6 h. Such delayed killing kinetics of vancomycin against MRSA have been previously described (16).
Marinopyrrole A also displayed a concentration-dependent PAE profile (Fig. 2), giving a significantly more favorable PAE (4 to 6 h) than either vancomycin or linezolid at concentrations 10× to 20× their respective MICs. At 10× MIC, the concentration of marinopyrrole A, 3.75 μg/ml, is still ∼2× less than the IC50 against L929 cells (8 to 32 μg/ml) and ∼8× less than the IC50 against HeLa cells (32 to 64 μg/ml). From these results, we can conclude that high doses of marinopyrrole A that may cause minimal mammalian in vitro toxicity can retard the regrowth of MRSA for a prolonged period. Furthermore, marinopyrrole A showed sustained and potent MIC activities in serial-passage experiments (Fig. 3). Despite repeated serial passage in sub-MIC concentrations of marinopyrrole A, TCH1516 failed to develop resistance over a 10-day period, and the MIC of Sanger 252 increased by only 1-fold (Fig. 3). Beyond these favorable pharmacokinetic parameters, marinopyrrole A displayed the potential for a reasonable therapeutic index, since mammalian-cell cytotoxicity was observed only at concentrations in excess of 20× the MIC. Further, marinopyrrole A showed sustained and potent MIC activities in serial passage.
Although the mechanism of action of marinopyrrole A remains undefined, the potent and rapid killing kinetics, as well as a low but detectable cytotoxicity profile against mammalian cells, suggests that the compound may nonspecifically kill target bacteria. Although not detailed in this paper, the results of preliminary metabolic-labeling studies indicate that marinopyrrole A acts in a general manner without specifically targeting DNA, RNA, protein, or cell wall synthesis (data not shown). However, it is also possible that marinopyrrole A may specifically target a bacterial process not studied in the metabolic-labeling assay (e.g., fatty acid biosynthesis), and this possibility is currently under investigation.
However, marinopyrrole A was found to have a critical deficiency in its pharmacological profile that will likely prevent its use as a systemic antibacterial therapy, namely, marked inhibition of antibiotic activity by serum. Therefore, we analyzed a series of 16 structural analogs (Fig. 4 and 5) of marinopyrrole A, some retaining the 1,3-bipyrrole scaffold and others possessing a single pyrrole resembling the pyoluteorin structure. While many of these derivatives retained potent anti-MRSA and anti-VRE activity, none was able to overcome the inhibitory effect of serum.
It is conceivable that marinopyrrole A could be used as a topical agent or in local therapy of device-related Gram-positive bacterial infections, which are increasingly common in inpatient settings. In the latter case, e.g., as an antibiotic-lock agent, the affinity of marinopyrrole A for binding to plastic surfaces could prove advantageous, and serum inactivation would limit the potential for systemic toxicity. Further medicinal chemistry analysis of the structure-activity relationships of this potent and rapidly acting bactericidal marine natural-product antibiotic will be required to illuminate its potential therapeutic utility.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant GM084350 (W.F. and V.N.) and National Institutes of Health International Cooperative Biodiversity Groups program TW007401 (W.F. and P.R.J.). N.M.H. was supported by the National Institutes of Health Training Program in Marine Biotechnology (T32 GM067550) and a Ruth L. Kirschstein National Research Service Award (NRSA) from National Institutes of Health grants (5 F31 GM090658-02).
The authors have no conflicts of interest concerning the antimicrobial agents studied.
Footnotes
Published ahead of print on 18 April 2011.
REFERENCES
- 1. Butler M. S., Buss A. D. 2006. Natural products—the future scaffolds for novel antibiotics? Biochem. Pharmacol. 71:919–929 [DOI] [PubMed] [Google Scholar]
- 2. Chambers H. F., Deleo F. R. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chin J. N., Rybak M. J., Cheung C. M., Savage P. B. 2007. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 51:1268–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ciarlone A. E., Fry B. W., Ziemer D. M. 1990. Some observations on the adsorption of tetracyclines to glass and plastic labware. Microchem. J. 42:250–255 [Google Scholar]
- 5. Como-Sabetti K., et al. 2009. Community-associated methicillin-resistant Staphylococcus aureus: trends in case and isolate characteristics from six years of prospective surveillance. Public Health Rep. 124:427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Craig W. A., Gudmundsson S. 1996. Postantibiotic effect, p. 296–329, In Lorian V. (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, MD [Google Scholar]
- 7. Credito K., Lin G., Appelbaum P. C. 2007. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob. Agents Chemother. 51:1504–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fenical W., Jensen P. R. 2006. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat. Chem. Biol. 2:666–673 [DOI] [PubMed] [Google Scholar]
- 9. Gontang E., Fenical W., Jensen P. R. 2007. Phylogenetic diversity of gram-positive bacteria cultured from marine sediments. Appl. Environ. Microbiol. 73:3272–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grundmann H., Aires-de-Sousa M., Boyce J., Tiemersma E. 2006. Emergence and resurgence of methicillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368:874–885 [DOI] [PubMed] [Google Scholar]
- 11. Haste N., et al. 2009. In vitro activity of the novel marine natural product marinopyrrole A against MRSA, abstr F1-1501. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 12. Hughes C. C., Fenical W. 2010. Antibacterials from the sea. Chem. Eur. J. 16:12512–12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hughes C. C., Fenical W. 2010. Structures, reactivities, and antibiotic properties of the marinopyrroles A-F. J. Org. Chem. 75:3240–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes C. C., Prieto-Davo A., Jensen P. R., Fenical W. 2008. The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp. Org. Lett. 10:629–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Illum L., Bundgaard H. B. 1982. Sorption of drugs by plastic infusion bags. Int. J. Pharmaceutics 10:339–351 [Google Scholar]
- 16. Löwdin E., Odenholt I., Cars O. 1998. In vitro studies of pharmacodynamic properties of vancomycin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 42:2739–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakoulas G., et al. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 46:1492–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarker S. D., Nahar L., Kumarasamy Y. 2007. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silverman J. A., Oliver N., Andrew T., Li T. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ueda Y., et al. 2005. In vitro and in vivo antibacterial activities of SM-216601, a new broad-spectrum parenteral carbapenem. Antimicrob. Agents Chemother. 49:4185–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zurenko G. E., et al. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839–845 [DOI] [PMC free article] [PubMed] [Google Scholar]