Abstract
The physiochemical properties of levofloxacin suggest that it is an agent which may exhibit altered pharmacokinetics in obese individuals. The purpose of this study was to describe the pharmacokinetics of a single 750-mg intravenous dose of levofloxacin in both hospitalized and ambulatory obese individuals. The hypothesis was that a standard dose of levofloxacin in obese individuals would achieve serum concentrations likely to be therapeutic. A single levofloxacin dose of 750 mg was infused over 90 min, and seven serial serum samples were subsequently obtained to evaluate the pharmacokinetics after the first dose. The peak concentrations of levofloxacin were comparable to those seen with normal-weight individuals. However, the area under the concentration-time curve and clearance were quite variable. Accelerated clearance was evident in the ambulatory obese individuals. Further investigation of the effects of obesity on the pharmacokinetics of levofloxacin is necessary to ensure optimal dosing.
INTRODUCTION
Medication dosing in obese individuals presents numerous pharmacokinetic challenges, including alterations in the volume of distribution and enhanced renal elimination (3). In general, dosing body weight and volume of distribution are dependent on the lipophilicity of the compound. Obese individuals have higher percentages of adipose tissue per body weight and higher overall amounts of adipose tissue. Thus, lipophilic agents are typically more widely distributed in obese persons and tend to require higher doses to achieve a therapeutic serum concentration. Obesity also accelerates drug elimination due to increased renal blood flow (17). Therefore, estimation of creatinine clearance based on commonly used equations, such as Cockcroft-Gault, may lack accuracy as a means of predicting renal function in the obese population (21). Unfortunately, drug dosing in obese individuals is often difficult to predict despite the knowledge of the physiochemical properties of a given medication, often making educated guesses as to the optimal dose incorrect.
Acute or critical illness may also impact the pharmacokinetics of medications. Specifically, volume of distribution, hepatic biotransformation, and elimination may be altered (8, 14, 23). Patients with active inflammatory processes such as burn, trauma, or severe infection have reductions in plasma proteins such as albumin which allow for a higher fraction of unbound drug. In addition, so-called “third-spacing” of fluids also results in an elevation in drug volume of distribution, as does fluid resuscitation. Hepatic biotransformation and renal elimination may be elevated or decreased depending on the phase of critical illness and the initiating factor (4).
Levofloxacin is a fluoroquinolone antimicrobial agent used for the treatment of community- and hospital-acquired infections. Currently, the daily 750-mg levofloxacin dose is approved by the U.S. Food and Drug Administration for the treatment of community-acquired and ventilator-associated pneumonia, urinary tract infection, and complicated skin and skin structure infections (11). Levofloxacin pharmacokinetics are characterized by consistent absorption, distribution, and elimination in a wide range of populations, including healthy volunteers, elderly subjects and human immunodeficiency virus (HIV)-infected individuals (5, 6, 13, 15). Like many other fluoroquinolones, levofloxacin is primarily renally eliminated and has an extensive volume of distribution (11, 22). Levofloxacin has exhibited altered pharmacokinetics in critical illness, specifically a higher peak concentration (after a 500-mg dose, 7.5 mg/liter versus 6.4 mg/liter [normal]), longer elimination half-life, and increased overall drug exposure (area under the concentration-time curve [AUC], 62.4 mg·h/liter versus 47.5 mg·h/liter [normal]) (6, 16). Based on the physiochemical and pharmacokinetic characteristics of levofloxacin, disposition may also be altered in obese patients due to changes in adipose tissue distribution and increased renal drug elimination (11). However, the effects of obesity on levofloxacin pharmacokinetics have not been described. The present study proposes to address the question of what are the effects of severe obesity and of severe obesity and acute illness on levofloxacin pharmacokinetics.
MATERIALS AND METHODS
This study was a prospective pharmacokinetic analysis of two separate cohorts of subjects receiving levofloxacin at 750 mg intravenously (Table 1). Cohort 1 consisted of hospitalized patients prescribed 750 mg of levofloxacin as part of their medical care. Cohort 2 was composed of ambulatory volunteers. These volunteers were not actively ill during the pharmacokinetic study and had no obvious, significant concomitant disease states that would be likely to affect levofloxacin pharmacokinetics. The hospitalized patients were prescribed levofloxacin for empirical or documented infection. All participants were eligible for inclusion if they were ages 18 to 55, had normal renal function, and had a body-mass index (BMI) > 35 kg/m2. Subjects were excluded if they: were <18 or >55 years of age, had an estimated creatinine clearance of <50 ml/min (according to the Salazar-Corcoran equation), were administered levofloxacin at any dose in the 7 days prior to study, or were pregnant or lactating (19). The study was approved by our institutional review board via full review with informed consent. Informed consent was obtained from each participant (or their designated medical decision maker) prior to any study procedures in all patients. In the case of surrogate consent, informed consent was obtained from the participant once they were suitable for the consent process.
Table 1.
Demographics and pharmacokinetic parameters
| Demographic or pharmacokinetic parametera | Mean ± SDb |
|||
|---|---|---|---|---|
| Ambulatory (n = 3) | Hospitalized (n = 12) | Total (n = 15) | Normal wt in 2001 (n = 4)e | |
| Demographics | ||||
| Age (yr) | 35.3 ± 12.5 | 41.5 ± 11.8 | 38.4 ± 12.8 | NR |
| % Male | 67 | 50 | 53 | NR |
| Wt (kg) | 113.3 ± 12.3 | 161.9 ± 67.1 | 145.5 ± 59.4 | NR |
| BMI (kg/m2) | 37.4 ± 5.9 | 54.8 ± 23.5 | 49.3 ± 20.7 | NR |
| Obese classc | ||||
| Obese class I (n) | 1 | 1 | 2 | NR |
| Obese class II (n) | 1 | 3 | 4 | NR |
| Obese class III (n) | 1 | 8 | 9 | NR |
| SCR (mg/dl) | 0.8 ± 0.1 | 1.0 ± 0.3 | 0.98 ± 0.2 | NR |
| CLCR (ml/min) | ||||
| Estimated (Salazar-Corcoran) | 80.1 ± 13 | 99.2 ± 57.8 | 86.1 ± 41.8 | NR |
| Estimated (Cockroft-Gault/BSA) | 87.2 ± 11.7 | 82.2 ± 41.1 | 83.1 ± 36.9 | NR |
| Measured | 184.3 ± 20.8d | 135.6 ± 71 | 140.7 ± 64.4 | NR |
| Pharmacokinetics | ||||
| Cmax (mg/liter) | 7.84 ± 0.99 | 8.4 ± 1.8 | 8.2 ± 1.8 | 8.12 ± 0.99 |
| Ke (h−1) | 0.264 ± 0.069 | 0.100 ± 0.062 | 0.117 ± 0.0697 | NR |
| Half-life (h) | 2.63 ± 0.687 | 6.93 ± 4.27 | 5.92 ± 3.53 | 6.91 ± 0.83 |
| Vol of distribution (liters) | 79.4 ± 12.8 | 82.7 ± 20.5 | 83.8 ± 21.6 | 106 ± 12 |
| Vol of distribution (liters/kg [ABW]) | 0.7 ± 0.11 | 0.51 ± 0.13 | 0.58 ± 0.15 | NR |
| Vol of distribution (liters/kg [IBW]) | 1.2 ± 0.36 | 1.4 ± 0.76 | 1.3 ± 0.69 | NR |
| Systemic clearance (ml/min) | 348.8 ± 3.5 | 139.7 ± 70.4 | 163.3 ± 70.45 | 186 ± 5 |
| AUC0–24 (mg/liter·h) | 36.8 ± 6.4 | 90.12 ± 40.8 | 76.55 ± 32.96 | 61.1 ± 1.3 |
BMI, body mass index; CLCR, creatinine clearance; SCR, serum creatinine; ABW, actual body weight; IBW, ideal body weight (9).
All values are reported as means ± the standard deviation except as noted in column 1. NR, not reported.
Obese class I, BMI 30 to 35; class II, BMI 35 to 40; class III, BMI >40 (24).
P < 0.05 measured versus the estimated CLCR determined by the Salazar-Corcoran method and by the Cockcroft-Gault method standardized using the body surface area (BSA).
According to Chow et al. (7).
Each study participant received the first dose of levofloxacin at 750 mg intravenously over 90 min. Serum concentrations were sequentially obtained 1. 5 (Cmax), 3, 4, 5, 8, 12, and 24 (trough) hours after the beginning of the intravenous infusion. Serum samples were collected after the first dose only. Urine collection was also performed during this 24-h study period to calculate the creatinine clearance. All patients had more than one site for intravenous access. Hospitalized patients often had central venous catheters as deemed necessary for their medical care. The Cmax was obtained through a different catheter than where the infusion was given in all instances.
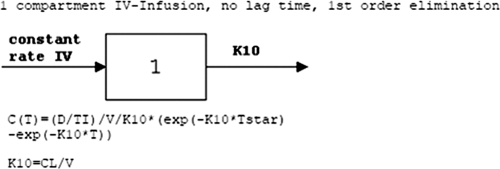
The blood samples were centrifuged for 10 to 15 min at 5,000 rpm, and the serum removed and placed in a plastic vial suitable for freezing. The samples were placed in a −80°C freezer until assay. Levofloxacin concentrations were determined by utilizing high-pressure liquid chromatography (10). The assay was linear from 313 ng/ml to 10 μg/ml. The intraday coefficients of variation at 10 and 5 μg/ml were 4.91 and 4.37%, respectively. The interday coefficients of variation at 5 and 625 μg/ml were 1.49 and 6.78%, respectively. The limit of detection was 0.156 μg/ml, and the limit of quantification was 0.313 μg/ml. All samples analyzed were above the lower limit of detection. Three 24-h samples fell slightly below the lower limit of quantification. The serum pharmacokinetics of levofloxacin were analyzed by standard noncompartmental pharmacokinetic methods, assuming first-order elimination (Fig. 1). All calculations were performed by inputting the data and desired pharmacokinetic model into WinNonlin, version 5.2.1 (Pharsight Corp., Mountain View, CA).
Fig. 1.
Pharmacokinetic model.
RESULTS
A total of 15 participants were included in this pharmacokinetic study. Twelve subjects were hospitalized for medical reasons and received intravenous levofloxacin at 750 mg as part of their medical care. Eight of these patients received levofloxacin for pneumonia, three for a wound infection, and one for a complicated urinary tract infection. Of the 12 hospitalized subjects, three were initially admitted for trauma and three were admitted for acute cardiac events (one each of acute coronary syndrome, acute decompensated heart failure, and elective coronary artery bypass grafting). All of the other subjects were hospitalized for other medical reasons. Only one patient had a history of diabetes. Three ambulatory participants (one of whom had a history of diabetes) were recruited and comprised cohort 2. All of the participants tolerated the infusion and exhibited no adverse effects after the first dose (the study dose). The total study population was relatively young and balanced with regard to gender (Table 1). All participants were obese, with the mean BMI of the study population approaching 50 kg/m2. The ambulatory population was slightly smaller than the hospitalized population (mean BMIs of 37.4 versus 54.8, respectively).
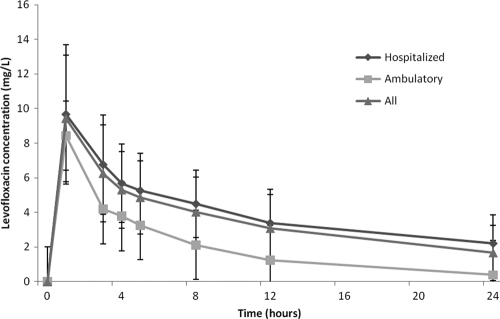
The peak levofloxacin concentrations were similar between the obese subjects (mean of 8.2 mg/liter, Fig. 2) and what has been reported in normal-weight volunteers with normal renal function (8.12 mg/liter) (7). No appreciable difference was seen between ambulatory volunteers and the acutely ill population. Likewise, volume of distribution was similar across both groups and was similar to what has been reported in normal-weight volunteers. The elimination half-life in the overall study population was similar to that for the normal-weight volunteers; however, the three ambulatory obese patients appeared to have much greater levofloxacin elimination compared to the acutely ill participants, as evidenced by the short half-life. This resulted in more than double the levofloxacin clearance in the ambulatory individuals compared to the acutely ill participants. As a consequence, the mean AUC in the ambulatory volunteers was significantly lower (36.8 ± 6.4) than in those that were acutely ill (90.12 ± 40.8). Overall, the mean study population AUC was 76.55 ± 32.96, which is slightly more than has been reported in normal-weight volunteers for this dose.
Fig. 2.
Pharmacokinetic profile of intravenous levofloxacin administered at 750 mg in obese adults.
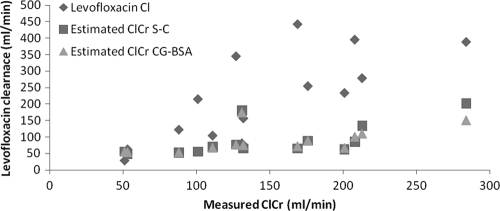
A previously developed model of levofloxacin pharmacokinetics predicted levofloxacin clearance reasonably well (15). The measured levofloxacin clearance was not significantly different than the predicted levofloxacin clearance when using measured creatinine clearance to estimate pharmacokinetic parameters in this model and exhibited a good correlation (r2 = 0.40). The use of calculated creatinine clearance estimates to predict levofloxacin clearance poorly correlated to actual levofloxacin clearance (r2 = 0.04 [Cockcroft-Gault/BSA], where BSA represents the body surface area; r2 = 0.05 [Salazar-Corcoran]) (Fig. 3). The predicted volume of distribution was not statistically significant (predicted 69.5 liters versus actual 93.7 liters, P = 0. 1) and did not exhibit a close correlation with the actual volume of distribution (r2 = 0.009).
Fig. 3.
Comparison of estimated and measured creatinine clearance versus levofloxacin clearance.
Although several participants had comorbid conditions such as diabetes, acute cardiac events, and acute infections which may affect the pharmacokinetic profile of levofloxacin (such as diminished renal clearance as might be seen in diabetes), none of the participants, hospitalized or ambulatory, had a history of renal dysfunction, nor did any participant have renal dysfunction during the study period (as confirmed by the measured creatinine clearance values). The Salazar-Corcoran equation, developed and validated using obese individuals, routinely underestimated the actual creatinine clearance measurements for both ambulatory and hospitalized patients in our study (Table 1). Overall, the mean estimated creatinine clearance ranged from 83.1 ml/min/172 m2 (Cockcroft-Gault standardized to BSA) to 86.1 ml/min (Salazar-Corcoran), whereas the measured 24-h creatinine clearance averaged 140.7 ml/min (P < 0.05).
DISCUSSION
This is the first study to describe the pharmacokinetics of levofloxacin in obese individuals. Overall, the results suggest that levofloxacin pharmacokinetics in obese individuals appear to be similar to normal weight individuals. This is not entirely unexpected, due to the consistency and predictability of levofloxacin pharmacokinetic parameters over a wide range of patient populations such as normal volunteers, elderly, HIV patients, and the critically ill (5, 6, 13). In addition, levofloxacin clearance predicted by the previously developed pharmacokinetic model seemed to have a good correlation with clearance measured in the present study (15). Although the peak concentrations and volume of distribution were similar between the acutely ill and ambulatory cohorts (and comparable to what has been reported in normal-weight volunteers), other pharmacokinetic parameters may have been altered due to obesity. Marked variability in levofloxacin clearance was evident in the obese population. Ambulatory volunteers exhibited exceptionally elevated levofloxacin clearance, likely due to their significantly higher creatinine clearance (as measured by the 24-h urine creatinine). This resulted in a much lower AUC than would be expected in normal-weight individuals (12). Conversely, the acutely ill patients receiving levofloxacin had an AUC similar to those for normal-weight individuals (although there was also significant variability within this acutely ill population).
Another fluoroquinolone agent, ciprofloxacin, has exhibited altered pharmacokinetics in obese subjects (1). Seventeen obese men (mean BMI = 36.4 kg/m2) were compared to 11 normal-weight volunteers (mean BMI = 23.3 kg/m2). Renal and systemic clearance and volume of distribution were significantly greater in the obese group than the normal-weight group. However, when normalized for total body weight, the volume of distribution was greater in the normal-weight individuals, indicating that although ciprofloxacin was distributed to adipose tissue, the distribution was not complete. An alternative dosing strategy using an adjusted dosing body weight (adding 45% of excess body weight) was proposed. The results of this pharmacokinetic evaluation of ciprofloxacin in obese individuals suggest that higher doses of fluoroquinolones may be needed to achieve targeted concentrations.
Variability in fluoroquinolone pharmacokinetics across patient populations has the potential to significantly impact outcomes. The most accurate predictor of fluoroquinolone success appears to be AUC/MIC (although the target AUC/MIC ratio likely depends on the causative pathogen). For infections caused by Streptococcus pneumoniae, an AUC/MIC ratio > 30 has been associated with clinical and microbiologic response (2). The ratio needed to prevent first-step mutations in these bacteria may be higher (20). In addition, the ratio needed to achieve bacterial eradication and clinical success in the treatment of Gram-negative infections is likely >125 (18). Although the hospitalized patients in our study had a mean AUC of ∼90 mg/liters·h, the ambulatory volunteers were significantly lower. In the treatment of infections caused by susceptible pathogens at or near the levofloxacin susceptibility breakpoint of 2 mg/liter, target attainment would be unlikely.
This study has some limitations that may impact the global applicability of the data. First, the number of ambulatory volunteers was low (3). Any degree of variation with one or two patients would greatly impact the results in such a small population. Based on our data, the variation in this cohort seems minimal. Second, the acutely ill cohort was a heterogeneous population. The vast majority of the patients required intensive care services during the 24-h study period due to a variety of medical and surgical problems. All subjects in this cohort had active, acute disease processes, although the severity and impact of these processes are difficult to define. It is likely that a patient in the acute phases of sepsis will demonstrate different pharmacokinetics than a patient recovering from cardiopulmonary bypass or a patient with a history of remote severe trauma with potential hardware and bone infection. We remain unable to predict the precise effect of all of these different physiologic processes on drug pharmacokinetics, although we have some general guides. An important lesson from our data is that this variety of patient illness and phase of disease probably represents a major reason for the wide variability in levofloxacin AUC in the acutely ill cohort.
Conclusion.
Levofloxacin pharmacokinetics in obese individuals may vary from that seen in normal-weight individuals. Although the peak concentrations are similar after a 750-mg intravenous dose, the AUC and levofloxacin clearance appear to be variable. Obese individuals with normal renal function may clear levofloxacin more efficiently than normal-weight individuals, particularly in the absence of acute illness. Practitioners should be mindful of the potential variability in drug exposure in obese individuals and consider the potential impact of underdosing when assessing the response to infection. Further research is necessary to better identify obese patients who may have exceptional renal drug clearance or who have particularly worrisome pathogens that may require higher levofloxacin exposure to maximize the likelihood of treatment success.
ACKNOWLEDGMENTS
We thank Tom Hardin for his guidance in project development and Jared Freml, David Deremer, and Cyndi Mattingly for laboratory assistance.
This investigation was supported by a research grant award by Ortho McNeil Pharmaceuticals, Inc., and by U.S. Public Health Service grant M01RR02.
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Allard S., Kinzig M., Boivin G., Sorgel F., LeBel M. 1993. Intravenous ciprofloxacin disposition in obesity. Clin. Pharmacol. Ther. 54:368–373 [DOI] [PubMed] [Google Scholar]
- 2. Ambrose P. G., et al. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blouin R. A., Warren G. W. 1999. Pharmacokinetic considerations in obesity. J. Pharm. Sci. 88:1–7 [DOI] [PubMed] [Google Scholar]
- 4. Boucher B. A., Hanes S. D. 1998. Pharmacokinetic alterations after severe head injury. Clin. Pharmacokinet. 35:209–221 [DOI] [PubMed] [Google Scholar]
- 5. Chien S.-C., et al. 1997. Absence of age and gender effects on the pharmacokinetics of a single 500-milligram oral dose of levofloxacin in healthy subjects. Antimicrob. Agents Chemother. 41:1562–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chien S.-C., et al. 1997. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob. Agents Chemother. 41:2256–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chow A. T., et al. 2001. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob. Agents Chemother. 45:2122–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook A. M. 2005. Pharmacokinetic alterations of antimicrobials in the critically ill. J. Pharmacy Pract. 18:75–83 [Google Scholar]
- 9. Devine B. J. 1974. Gentamicin therapy. Drug Intell. Clin. Pharmacy 8:650–655 [Google Scholar]
- 10. Djabarouti S., et al. 2004. Determination of levofloxacin in plasma, bronchoalveolar lavage and bone tissues by high-performance liquid chromatography with ultraviolet detection using a fully automated extraction method. J. Chromatogr. B 799:165–172 [DOI] [PubMed] [Google Scholar]
- 11. Ortho-McNeill, Llc 2002. Levofloxacin (Levaquin) package insert. Ortho-McNeill, Titusville, NJ.
- 12. Pea F., et al. 2003. Pharmacokinetics and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia. Clin. Pharmacokinet. 42:589–598 [DOI] [PubMed] [Google Scholar]
- 13. Piscitelli S. C., et al. 1999. Pharmacokinetics and safety of high-dose and extended-interval regimens of levofloxacin in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:2323–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Power B. M., Forbes A. M., van Heerden P. V., Ilett K. F. 1998. Pharmacokinetics of drugs used in critically ill adults. Clin. Pharmacokinet. 34:25–56 [DOI] [PubMed] [Google Scholar]
- 15. Preston S. L., et al. 1998. Levofloxacin population pharmacookinetics and creation of a demographic model for prediction of individual drug clearance in patients with severe community-acquired infections. Antimicrob. Agents Chemother. 42:1098–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rebuck J. A., Fish D. N., Abraham E. 2002. Pharmacokinetics of intravenous and oral levofloxacin in critically ill adults in a medical intensive care unit. Pharmacotherapy 22:1216–1225 [DOI] [PubMed] [Google Scholar]
- 17. Reisin E., Messerli F. G., Ventura H. O., Frohlich E. D. 1987. Renal haemodynamic studies in obesity hypertension. J. Hypertension 5:397–400 [PubMed] [Google Scholar]
- 18. Rodvold K., Neuhauser M. 2001. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Pharmacotherapy 21:233S–252S [DOI] [PubMed] [Google Scholar]
- 19. Salazar D. E., Corcoran G. B. 1988. Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am. J. Med. 84:1053–1060 [DOI] [PubMed] [Google Scholar]
- 20. Schentag J. J., Meagher A. K., Forrest A. 2003. Fluoroquinolone AUIC break points and the link to bacterial killing rates. 1. In vitro and animal models. Ann. Pharmacother. 37:1287–1298 [DOI] [PubMed] [Google Scholar]
- 21. Spinler S. A., et al. 1998. Predictive performance of ten equations for estimating creatinine clearance in cardiac patients. Ann. Pharmacother. 32:1275–1283 [DOI] [PubMed] [Google Scholar]
- 22. Takacs-Novak K., Jozan M., Hermecz I., Szasz G. 1992. Lipophilicity of antibacterial fluoroquinolones. Int. J. Pharmaceut. 79:89–96 [Google Scholar]
- 23. Weinbren M. J. 1999. Pharmacokinetics of antibiotics in burn patients. J. Antimicrob. Chemother. 44:319–327 [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization 31 March 2011, posting date. BMI classification. World Health Organization, Geneva, Switzerland. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html